Synthesis of Zinc Oxide Nanoparticles Using Rubus fairholmianus Root Extract and Their Activity against Pathogenic Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection, Extraction and Biosynthesis of ZnO NPs
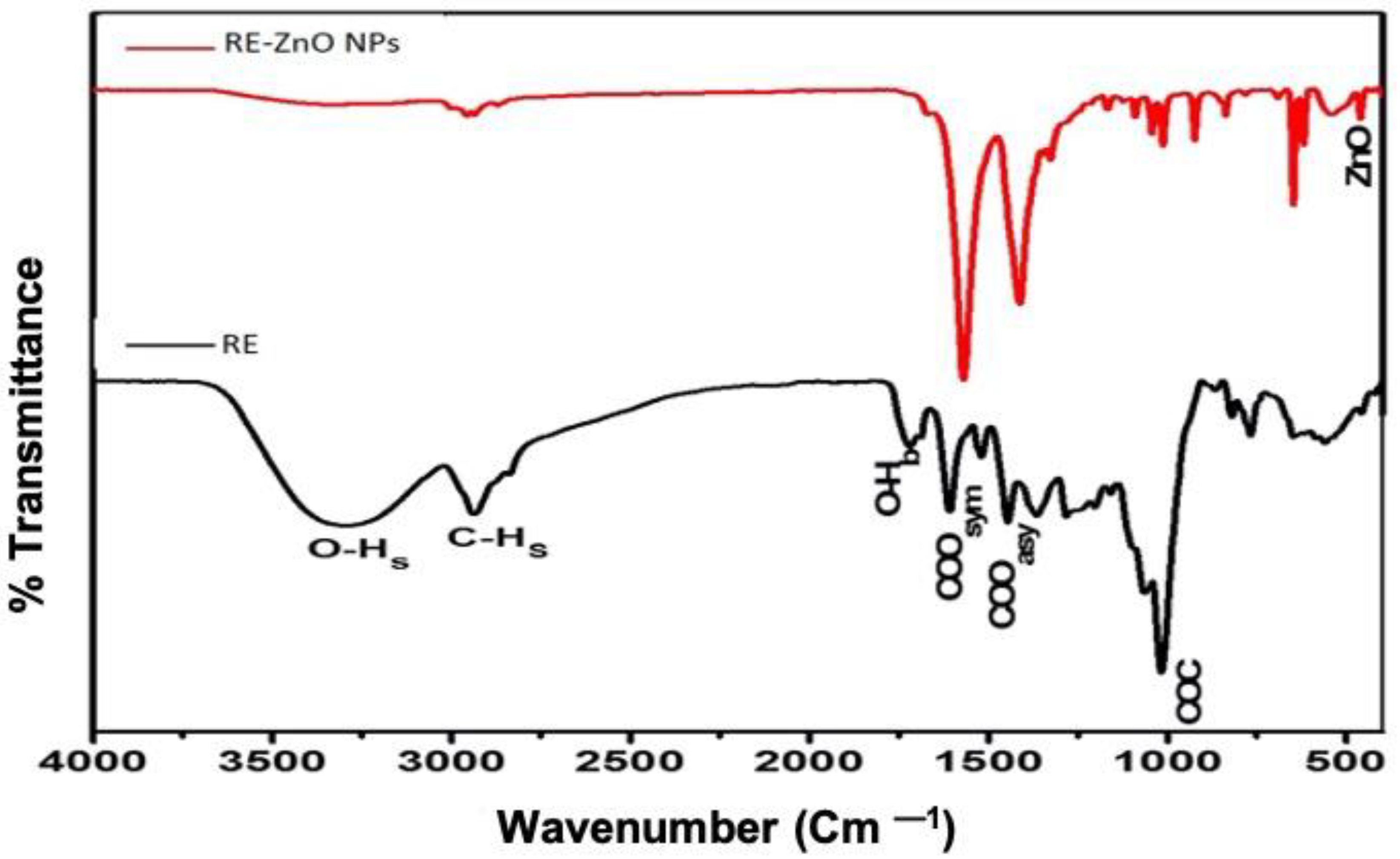
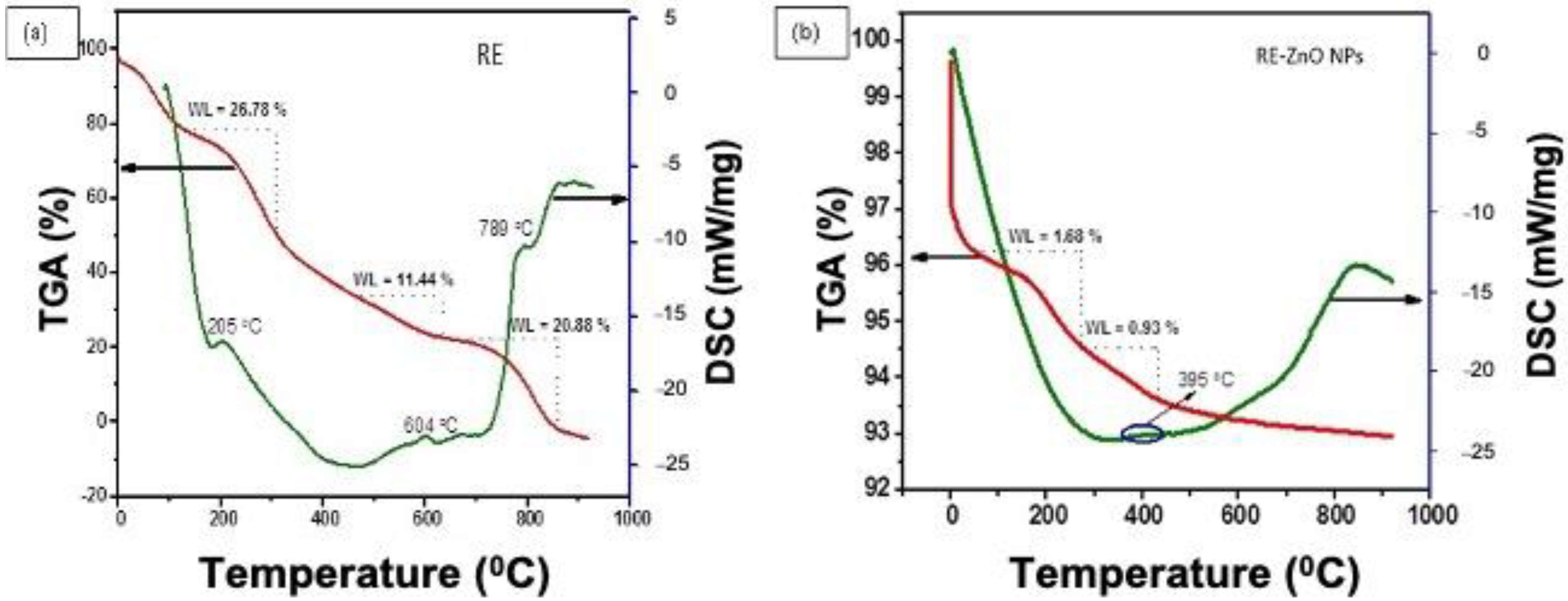
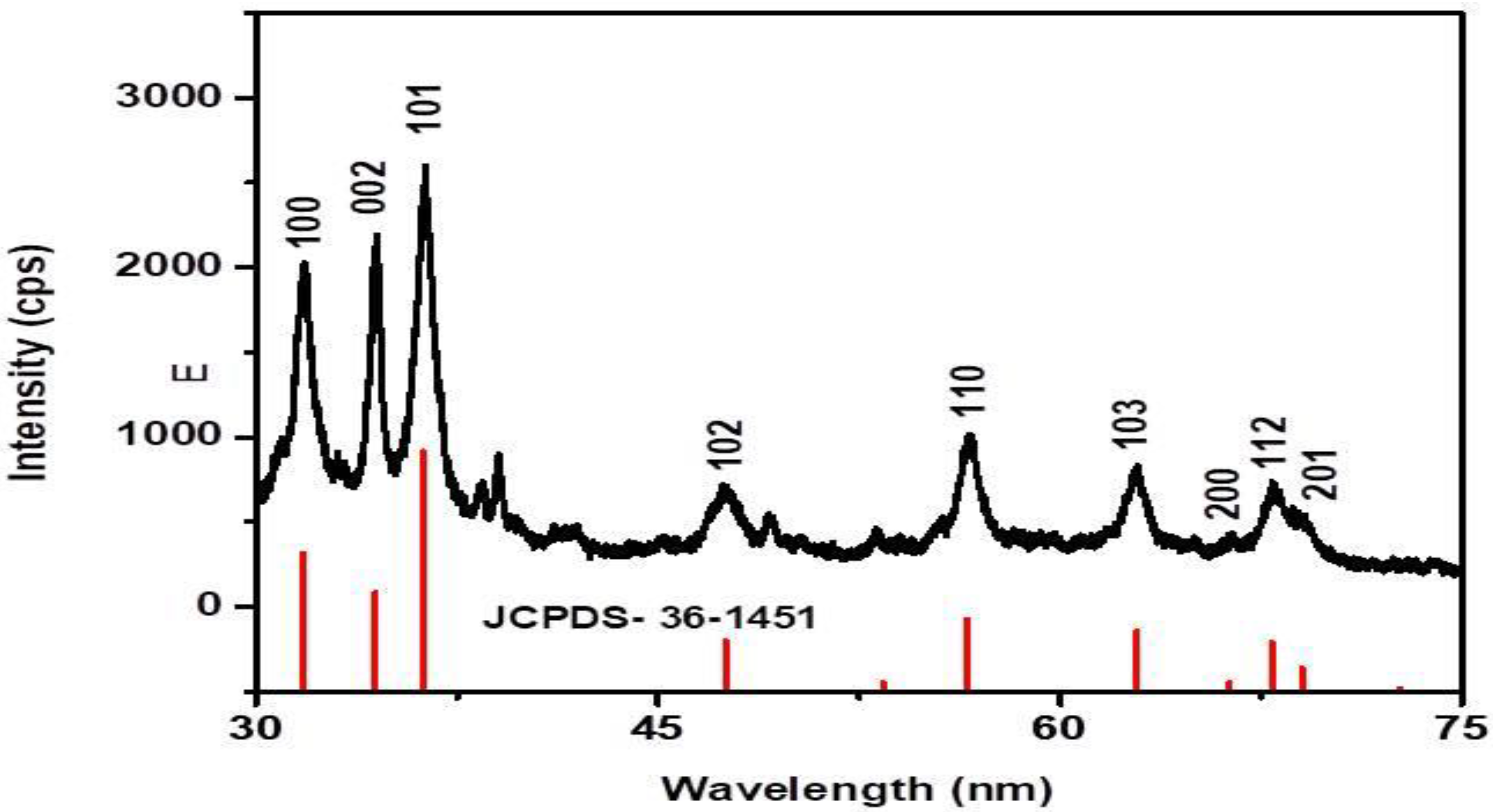
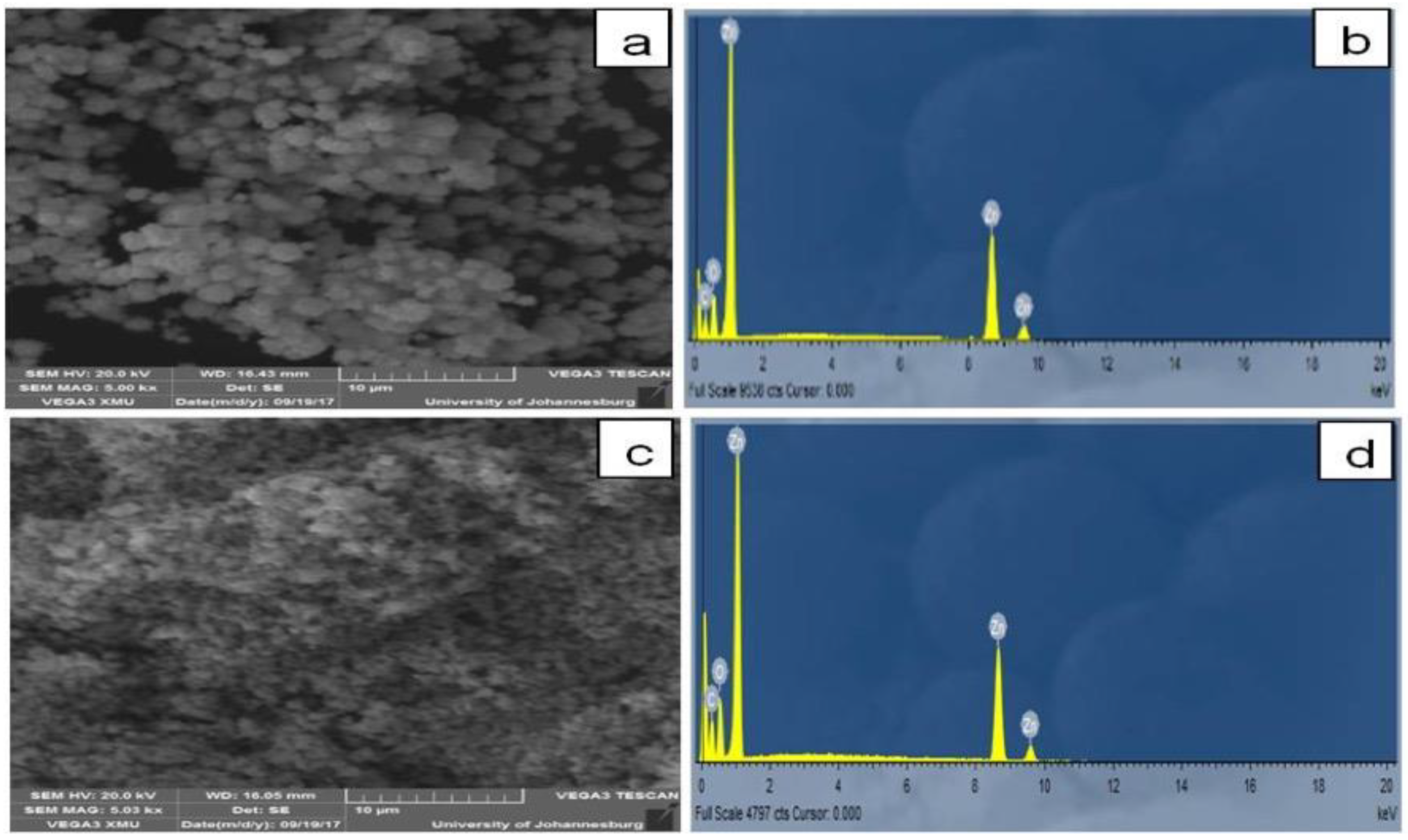
2.2. Characterization of Biosynthesized RE-ZnO NPs
2.3. Agar Well Diffusion Method for Antimicrobial Activity
2.4. Minimum Inhibitory Concentrations
2.5. Bacterial Growth in Different Concentrations of RE-ZnO NPs
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sivaraj, R.; Pattanathu, K.; Rajiv, P.; Hasna, S.; Venckatesh, R. Spectrochim. Biogenic copper oxide nanoparticles synthesis using Tabernaemontana divaricate leaf extract and its antibacterial activity against urinary tract pathogen. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Rudramurthy, G.R.; Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Nanoparticles: Alternatives Against Drug-Resistant Pathogenic Microbes. Molecules 2016, 21, 836–842. [Google Scholar] [CrossRef]
- Hamelian, M.; Zangeneh, M.M.; Amisama, A.; Varmira, K.; Veisi, H. Green synthesis of silver nanoparticles using Thymus kotschyanus extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Appl. Organomet. Chem. 2018, 32, e4458. [Google Scholar] [CrossRef]
- Hemmati, S.; Rashtiani, A.; Zangeneh, M.M.; Mohammadi, P.; Zangeneh, A.; Veisi, H. Green synthesis and characterization of silver nanoparticles using Fritillaria flower extract and their antibacterial activity against some human pathogens. Polyhedron 2019, 158, 8–14. [Google Scholar] [CrossRef]
- Zangeneh, M.M.; Bovandi, S.; Gharehyakheh, S.; Zangeneh, A.; Irani, P. Green synthesis and chemical characterization of silver nanoparticles obtained using Allium saralicum aqueous extract and survey of in vitro antioxidant, cytotoxic, antibacterial and antifungal properties. Appl. Organomet. Chem. 2019. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Seydi, N.; Mahdavi, B.; Paydarfard, S.; Zangeneh, A.; Zangeneh, M.M.; Najafi, F.; Jalalvand, A.R.; Pirabbasi, E. Preparation, characterization, and assessment of cytotoxicity, antioxidant, antibacterial, antifungal, and cutaneous wound healing properties of titanium nanoparticles using aqueous extract of Ziziphora clinopodioides Lam leaves. Appl. Organomet. Chem. 2019, 33. [Google Scholar] [CrossRef]
- Zhaleh, F.; Zangeneh, A.; Goorani, S.; Seydi, N.; Zangeneh, M.M.; Tahvilian, R.; Pirabbasi, E. In vitro and in vivo evaluation of cytotoxicity, antioxidant, antibacterial, antifungal, and cutaneous wound healing properties of gold nanoparticles produced via a green chemistry synthesis using Gundelia tournefortii L. as a capping and reducing agent. Appl. Organomet. Chem. 2019, 33. [Google Scholar] [CrossRef]
- Zangeneh, M.M.; Joshani, Z.; Zangeneh, A.; Miri, E. Green synthesis of silver nanoparticles using aqueous extract of Stachys lavandulifolia flower, and their cytotoxicity, antioxidant, antibacterial and cutaneous wound-healing properties. Appl. Organomet. Chem. 2019, 33. [Google Scholar] [CrossRef]
- Rouhi, J.; Mahmud, S.; Naderi, N.; Ooi, C.R.; Mahmood, M.R. Physical properties of fish gelatin-based bio-nanocomposite films incorporated with ZnO nanorods. Nanoscale Res. Let. 2013, 8, 364–371. [Google Scholar] [CrossRef]
- Kolekar, T.V.; Bandgar, S.; Shirguppikar, S.S.; Ganachari, V.S. Synthesis and characterization of ZnO nanoparticles for efficient gas sensors. Arch. Appl. Sci. Res. 2013, 5, 20–28. [Google Scholar]
- Jayaseelan, C.; Abdul, R.A.; Vishnu, K.A.; Marimuthu, S.; Santhoshkumar, T.; Bagavan, A.; Gaurav, K.; Karthik, L.; Rao, B.K.V. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim. Acta A 2012, 90, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, G.; Rajeshwari, S.; Venckatesh, R. Green Synthesis of Zinc Oxide Nanoparticles by Aloe Barbadensis Miller Leaf Extract: Structure and Optical Properties. Mater. Res. Bull. 2011, 46, 2560–2566. [Google Scholar] [CrossRef]
- Gunalan, S.; Sivaraj, R.; Rajendran, V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci. Mater. Int. 2012, 22, 693–700. [Google Scholar] [CrossRef]
- Ladj, R.; Bita, A.; Eissa, M.; Mugnier, Y.; Le Dantec, R.; Fessi, H.; Elaissari, A. Individual inorganic nanoparticles: Preparation, functionalization and in vitro biomedical diagnostic applications. J. Mater. Chem. B 2013, 1, 1381–1396. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, H.; Fu, L. Preparation gold nanoparticles using herb leaf extract for electro-oxidation determination of ascorbic acid. Inorg. Nano Met. Chem. 2018, 48, 449–453. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Y.; Shi, Y.; Fu, Y. Green biosynthesis of ZnO nanoparticles by plectranthus amboinicus leaf extract and their application for electrochemical determination of norfloxacin. Inorg. Nano Met. Chem. 2019, 49, 277–282. [Google Scholar] [CrossRef]
- Ying, J.; Zheng, Y.; Zhang, H.; Fu, L. Room temperature biosynthesis of gold nanoparticles with Lycoris aurea leaf extract for the electrochemical determination of aspirin. Rev. Mex. Ing. Quim. 2020, 19, 585–592. [Google Scholar] [CrossRef]
- Jalalvand, A.R.; Zhaleh, M.; Goorani, S.; Zangeneh, M.M.; Seydi, N.; Zangeneh, A.; Moradi, R. Chemical characterization and antioxidant, cytotoxic, antibacterial, and antifungal properties of ethanolic extract of Allium Saralicum, R.M. Fritsch leaves rich in linolenic acid, methyl ester. J. Photochem. Photobiol. B 2019, 192, 103–112. [Google Scholar] [CrossRef]
- Sayyedrostami, T.; Pournaghi, P.; Ebrahimi Vosta-Kalaeea, S.; Zangeneh, M.M. Evaluation of the wound healing activity of Chenopodium botrys leaves essential oil in rats (a short-term study). J. Essent. Oil Bear. Plants 2018, 21, 164–174. [Google Scholar] [CrossRef]
- Zhaleh, M.; Sohrabi, N.; Zangeneh, M.M.; Zangeneh, A.; Moradi, R.; Zhaleh, H. Chemical composition and antibacterial effects of essential oil of Rhus coriaria fruits in the west of Iran (Kermanshah). J. Essent. Oil Bear. Plants 2018, 21, 493–501. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911–920. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- George, B.P.; Thangaraj, P.; Sulaiman, C.; Piramanayagam, S.; Ramaswamy, S.K. Bioassay Directed Isolation and Biological Evaluation of Compounds Isolated from Rubus fairholmianus Gard. Biomed. Res. Int. 2014, 204340, 1–15. [Google Scholar] [CrossRef]
- George, B.P.; Abrahamse, H.; Parimelazhagan, T. Caspase dependent apoptotic inhibition of melanoma and lung cancer cells by tropical Rubus extracts. Biomed. Pharmacother. 2016, 80, 193–199. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, F.; Fuigui, H.; Aiwu, W.; Wen, C.; Jinping, Y.; Feng, P. Green biosynthesis and characterization of zinc oxide nanoparticles using Corymbia citriodora leaf extract and their photocatalytic activity. Green. Chem. Lett. Rev. 2015, 8, 59–63. [Google Scholar] [CrossRef]
- Jayappa, M.; Ramaiah, C.; PavanKumar, M.; Suresh, D.; Prabhu, A.; Devasya, R.; Sheikh, S. Green synthesis of zinc oxide nanoparticles from the leaf, stem and in vitro grown callus of Mussaenda frondosa L.: Characterization and their applications. Appl. Nanosci. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, A.; Aswathy, T.R.; Nair, A. Green synthesis and characterization of zinc oxide nanoparticles using Cayratia pedata leaf extract. Biochem. Biophys. Rep. 2021, 26, 100995. [Google Scholar] [CrossRef]
- Barziniy, A.; Azeez, H. Green synthesis and characterization of zinc oxide nanoparticles using Eucalyptus globulus Labill. leaf extract and zinc nitrate hexahydrate salt. SN Appl. Sci. 2020, 2, 991. [Google Scholar] [CrossRef]
- Balogun, S.W.; James, O.; Sanusi, Y.; Olayinka, O. Green synthesis and characterization of zinc oxide nanoparticles using bashful (Mimosa pudica), leaf extract: A precursor for organic electronics applications. SN App. Sci. 2020, 2, 504. [Google Scholar] [CrossRef]
- Pillai, M.; Sivasankarapillai, V.; Rahdar, A.; Joseph, J.; Sadeghfar, F.; Anu, R. Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. J. Mol. Struct. 2020, 1211, 128107. [Google Scholar] [CrossRef]
- Mandell, G.; Douglas, J.; Bennett, R. Principles and Practice of Infectious Diseases, 4th ed.; Churchill Livingstone Ltd.: Edinburgh, UK, 1995. [Google Scholar]
- Oladiran, A.A.; Olabisi, I.A. Synthesis and characterization of ZnO nanoparticles with zinc chloride as zinc source. Asian J. Nat. Appl. Sci. 2013, 2, 41–44. [Google Scholar]
- Senthilkumar, N.; Nandhakumar, E.; Priya, P.; Soni, C.; Vimalan, M.; Vetha, N. Synthesis of ZnO nanoparticles using leaf extract of Tectona grandis (L.) and their anti-bacterial, anti-arthritic, anti-oxidant and in vitro cytotoxicity activities. New J. Chem. 2017, 41, 10347–10356. [Google Scholar] [CrossRef]
- Tamuly, C.; Hazarika, M.; Borah, S.; Das, M.R.; Boruah, M.P. The synthesis of citrate-modified silver nanoparticles in an aqueous suspension of graphene oxide nanosheets and their antibacterial activity. Colloids Surf. B Biointerfaces 2013, 102, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Darezereshki, E.; Alizadeh, M.; Bakhtiari, F.; Schaffie, M.; Ranjbar, M. A novel thermal decomposition method for the synthesis of ZnO nanoparticles from low concentration ZnSO 4 solutions. Appl. Clay Sci. 2011, 54, 107–111. [Google Scholar] [CrossRef]
- Matinise, N.; Fuku, X.G.; Kaviyarasu, K.; Mayedwa, N.; Maaza, M. ZnO nanoparticles via Moringa oleifera green synthesis: Physical properties & mechanism of formation. Appl. Surf. Sci. 2017, 406, 339–347. [Google Scholar] [CrossRef]
- El-Kader, F.H.A.; Hakeem, N.A.; Elashmawi, I.S.; Ismail, A.M. Structural, optical and thermal characterization of ZnO nanoparticles doped in PEO/PVA blend films. Nanosci. Nanotechnol. 2013, 7, 179–188. [Google Scholar]
- Fakhari, S.; Jamzad, M.; Fard, H.K. Green synthesis of zinc oxide nanoparticles: A comparison. Green Chem. Lett. Rev. 2019, 12, 19–24. [Google Scholar] [CrossRef]
- Azizi, S.; Ahmad, M.B.; Namvar, F.; Mohamad, R. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater. Lett. 2013, 116, 275–277. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Dingh, Y.; Daskalakis, K.; Povey, J.L.; O’Neil, A.J. Mechanistic investigation into antibacterial behaviour of suspensions of ZnO nanoparticles against E. coli. J. Nanopart. Res. 2010, 12, 1625–1636. [Google Scholar] [CrossRef]
- Dutta, R.K.; Nenavathu, B.P.; Gangishetty, M.K.; Reddy, A.V.R. Studies on antibacterial activity of ZnO nanoparticles by ROS induced lipid peroxidation. Colloids Surf. B Biointerfaces 2012, 94, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Ahamed, M.; Kumar, S.; Khan, M.M.; Ahmad, J.; Alrokayan, S.A. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int. J. Nanomed. 2012, 7, 845–857. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Nel, A.E. Impairment of mitochondrial function by particulate matter (PM) and their toxic components: Implications for PM-induced cardiovascular and lung disease. Front. Biosci. 2007, 12, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.B.V.; Saila, E.S.; Narang, P.; Aishwarya, M.; Raina, R.; Gautam, M.; Shankar, E.G. Biofunctionalization and biological synthesis of the ZnO nanoparticles: The effect of Raphanus sativus (white radish) root extract on antimicrobial activity against MDR strain for wound healing applications. Inorg. Chem. Comm. 2019, 100, 101–106. [Google Scholar] [CrossRef]


| Plant Name | Biosynthesis Conditions | Nature | References |
|---|---|---|---|
| Mussaenda frondosa L. | Continuous stirring at 70 °C for 15 min + drying at 400 °C 30 min + calcination at 400 °C | Crystal | [27] |
| Cayratia pedata | Continuous stirring at 65 °C for 20 min + drying at 65 °C for overnight + calcination at 400 °C for 2 h | Fine powder | [28] |
| Eucalyptus globulus Labill | Continuous stirring at 60 °C for 1 h + drying at 100 °C + calcination 400 °C for 2 h | Fine powder | [29] |
| Mimosa pudica | Continuous stirring at room temperature for 4 h + drying at 300 °C for 45 min + calcination 400 °C | Crystal | [30] |
| Beta vulgaris, Cinnamomum tamala, Cinnamomum verum, Brassica oleracea var. | Continuous stirring at 70 °C until white paste formation + calcination 400 °C for 2 h | Coroase powder | [31] |
| Samples | Susceptibility (μg/mL) |
|---|---|
| S. aureus | |
| MIC | |
| RE | 337.86 |
| RE-ZnO NPs | 157.22 |
| Ampicillin (positive control) | 0.79 |
| Groups | 10 μg/mL | 20 μg/mL | 30 μg/mL | 40 μg/mL | 50 μg/mL |
|---|---|---|---|---|---|
| RE | + | + | + | + | + |
| RE-ZnO NPs | + | + | - | - | - |
| Positive Control | + | + | + | + | + |
| Negative Control | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajendran, N.K.; George, B.P.; Houreld, N.N.; Abrahamse, H. Synthesis of Zinc Oxide Nanoparticles Using Rubus fairholmianus Root Extract and Their Activity against Pathogenic Bacteria. Molecules 2021, 26, 3029. https://doi.org/10.3390/molecules26103029
Rajendran NK, George BP, Houreld NN, Abrahamse H. Synthesis of Zinc Oxide Nanoparticles Using Rubus fairholmianus Root Extract and Their Activity against Pathogenic Bacteria. Molecules. 2021; 26(10):3029. https://doi.org/10.3390/molecules26103029
Chicago/Turabian StyleRajendran, Naresh Kumar, Blassan P. George, Nicolette N. Houreld, and Heidi Abrahamse. 2021. "Synthesis of Zinc Oxide Nanoparticles Using Rubus fairholmianus Root Extract and Their Activity against Pathogenic Bacteria" Molecules 26, no. 10: 3029. https://doi.org/10.3390/molecules26103029
APA StyleRajendran, N. K., George, B. P., Houreld, N. N., & Abrahamse, H. (2021). Synthesis of Zinc Oxide Nanoparticles Using Rubus fairholmianus Root Extract and Their Activity against Pathogenic Bacteria. Molecules, 26(10), 3029. https://doi.org/10.3390/molecules26103029








