Recent Advances on Antimicrobial and Anti-Inflammatory Cotton Fabrics Containing Nanostructures
Abstract
1. Introduction
2. Discussion
2.1. Antibacterial Cotton Fabrics Containing Nanostructures
2.1.1. Metal Nanoparticles (M NPs)
2.1.2. Mixtures of Metal Nanoparticles
2.1.3. Metal Oxide Nanoparticles (MO NPs)
2.1.4. Mixtures of Metal Oxide Nanoparticles
2.1.5. Mixtures of Metal and Metal Oxide Nanoparticles
2.2. Antifungal Cotton Fabrics Containing Nanostructures
2.2.1. Metal Nanoparticles
2.2.2. Metal Oxide Nanoparticles
2.3. Antibacterial and Antifungal Cotton Fabrics Containing Nanostructures
2.3.1. Metal Nanoparticles
2.3.2. Mixtures of Metal Nanoparticles
| Run | Synthesis Method | Source of Silver; Reductant Reagent | Additives | NP Size | Antibacterial Properties against Microorganisms | Other Properties | Ref. Author Year |
| 1 | Pad-dry-cure | AgNO3 Tragacanth gum | - | 17 77 (on cotto) | E. coli; S. aureus | Water absorption | [20] Montazer 2016 |
| 2 | Pad-dry-cure | AgNO3 Dextran | SiO2 VTEOS or APTEOS | 10–25 | E. coli; S. aureus | Thermal stability | [21] Mohamed 2017 |
| 3 | Pad-dry-cure | AgNO3 Carboxymethyl cellulose | GPTMS DMDHEU Fluorochemical (Asahi Guard AG-925) | - | E. coli; S. aureus | Water/oil repellent Thermal stability | [22] Ibrahim 2020 |
| 4 | Immersion (Dip-Dry) | AgNO3 Radiochemical reduction | 2–10 | S. aureus; K. pneumoniae; MRSA; E. coli; P. aeruginosa; S. entérica; V. parahaemolyticus | - | [23] Seino 2016 | |
| 5 | Immersion (Dip-Dry) | AgNO3 Bacillus sp. | N2-plasma treated fabric | 10–17 | E. coli; S. aureus | - | [24] Ibrahim 2017 |
| 6 | Immersion | AgNO3 Aromatic amine (in situ polymerization) | 170 and 3.5 | E. coli; S. aureus | Electrical conductivity Colorimetricsensory effects | [25] Ahmed 2020 | |
| 7 | Immersion | AgNO3 NaBH4 | EugenolSH TAMSH FQPEG | 2–11 | E. coli; S. aureus | - | [26] Vallribera 2019 |
| 8 | Immersion (Dip-Dry) | AgNO3 Black rice | Carboxymethyl chitosan modified cotton | - | E. coli; S. aureus | Hydrophobicity UV protective performance | [27] He 2021 |
| 9 | Ultrasonication | AgNO3 Cellulose mechanoradicals | - | 3–40 | E. coli.; B. subtilis | - | [28] Baytekin 2020 |
| 10 | Aerosol-Based Process | Ag electrode Electrical discharges | - | 10–150 | S. aureus; K. pneumoniae | - | [29] Kruis 2016 |
| 11 | Pad-Dry-Cure | AgNO3 Aspergillus terreus | - | 8–20 | S. aureus; B. subtilis; E. coli; P. aeruginosa; K. pneumoniae; MRSA | Antifungal | [56] Balakumaran 2016 |
| 12 | Pad-Dry-Cure | AgNO3 endophytic actinomycetes strain of Streptomyces laurentii | - | 7–15 | S. aureus; B. subtilis; P. aeruginosa; E. coli | Anticancer Antifungal | [57] Fouda 2020 |
| 13 | Immersion | AgNO3 Ironed at 220 °C | - | E. coli, E. aerogenes; P. mirabilis; K. pneumoniae | Antifungal | [58] Eremenko 2016 | |
| 14 | Impregnation by Pressing at 200 °C | Ag(OAc)2 NaBH4 | Polyvinyl pyrrolidone | 18 | E. coli; S. aureus | Antifungal | [59] Golabiewska 2016 |
2.3.3. Metal Oxide Nanoparticles
2.3.4. Mixtures of Metal Oxide Nanoparticles
2.3.5. Mixtures of Metal and Metal Oxide Nanoparticles
2.3.6. Miscellaneous Nanoparticles
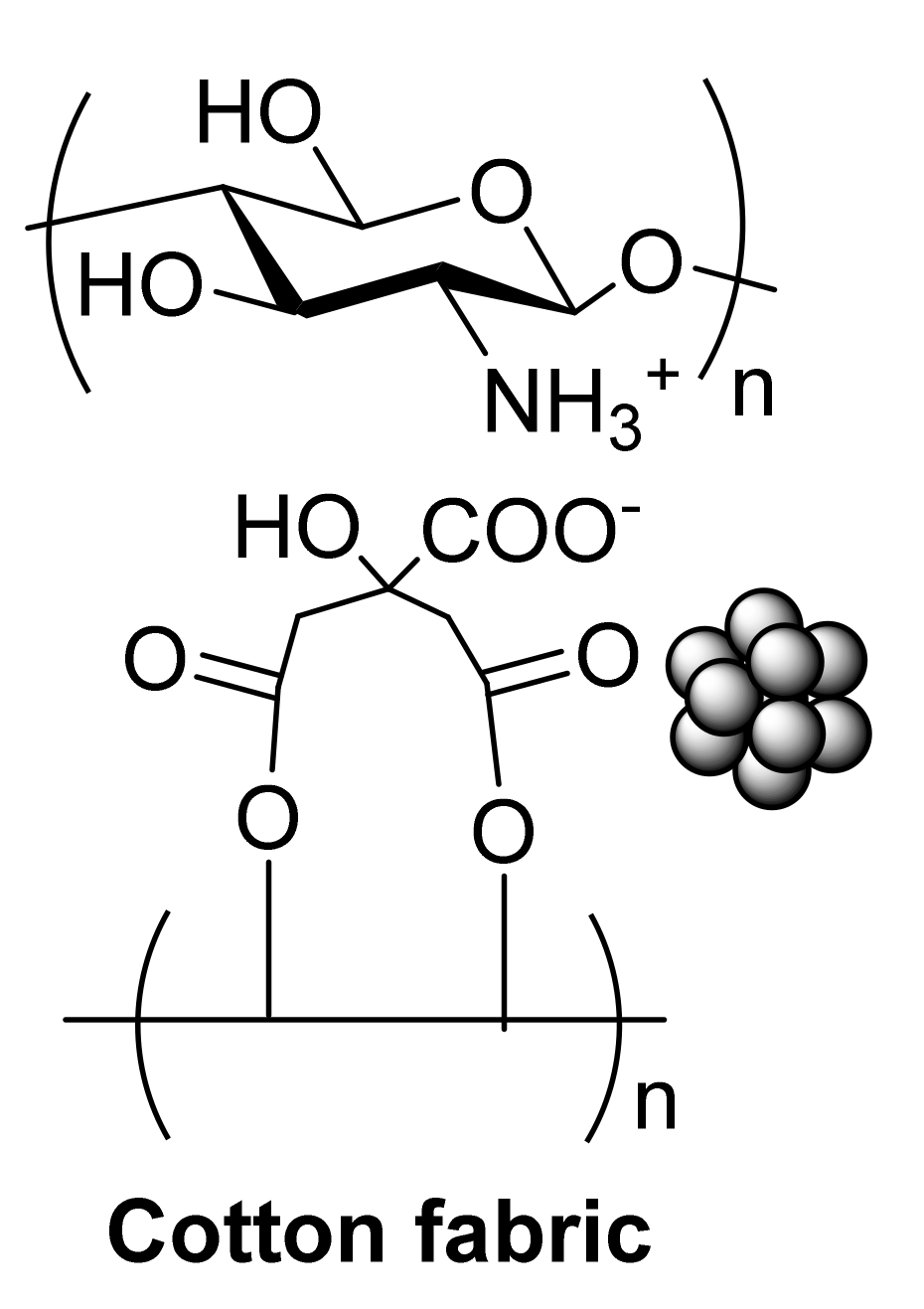
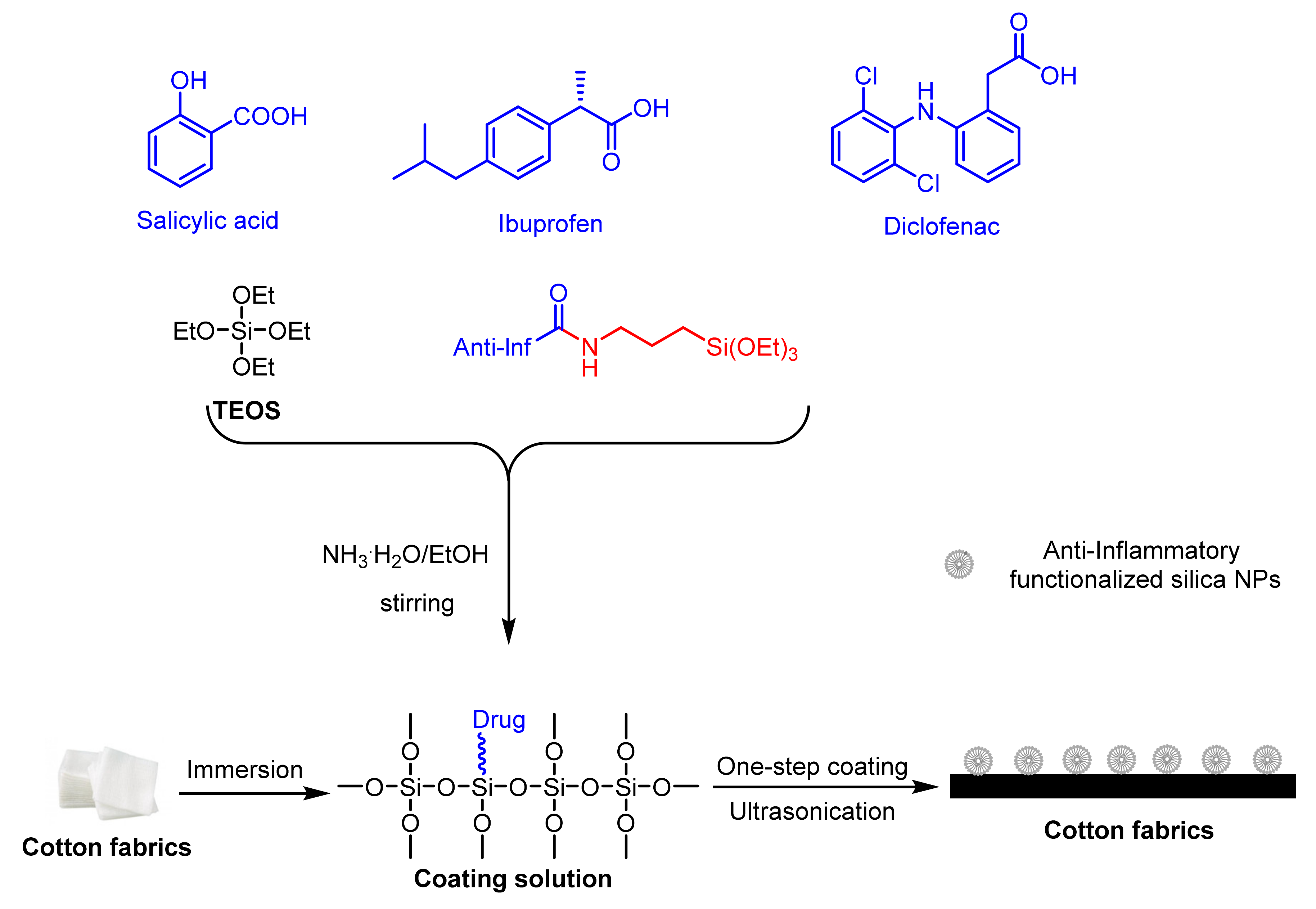
2.4. Anti-Inflammatory Cotton Fabrics Containing Nanostructures
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ibrahim, N.A.; Eid, B.M.; Sharaf, S.M. Functional finishes for cotton-based textiles: Current situation and future trends. In Textiles and Clothing; Shabbir, M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA; Scrivener Publishing LLC.: Beverly, MA, USA, 2019; pp. 131–190. [Google Scholar]
- Mahbubul Bashar, M.; Khan, M.A. An overview on surface modification of cotton fiber for apparel use. J. Polym. Environ. 2013, 21, 181–190. [Google Scholar] [CrossRef]
- Elshafei, A.; El-Zanfaly, H.T. Application of antimicrobials in the development of textiles. Asian J. Appl. Sci. 2011, 4, 585–595. [Google Scholar] [CrossRef]
- Gao, Y.; Cranston, R. Recent advances in antimicrobial treatments of textiles. Text. Res. J. 2008, 78, 60–72. [Google Scholar]
- Simoncic, B.; Tomsic, B. Structures of novel antimicrobial agents for textiles. A review. Text. Res. J. 2010, 80, 1721–1737. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Eid, B.M.; Kafafy, H. Chapter 21-Sustainable colorants for protective textiles. In Advances in Functional and Protective Textiles; Ul-Islam, S., Butola, B.S., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 569–629. [Google Scholar]
- Nada, A.; Al-Moghazy, M.; Soliman, A.A.F.; Rashwan, G.M.T.; Eldawy, T.H.A.; Hassan, A.A.E.; Sayed, G.H. Pyrazole-based compounds in chitosan liposomal emulsion for antimicrobial cotton fabrics. Int. J. Biol. Macromol. 2018, 107, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Gokarneshan, N.; Gopalakrishnan, P.P.; Yeyanthi, B. Influence of nanofinishes on the antimicrobial properties of fabrics. ISRN Nanomater. 2012, 2012, 193836. [Google Scholar] [CrossRef][Green Version]
- Tan, L.-Y.; Sin, L.T.; Bee, S.-T.; Ratnam, C.T.; Woo, K.-K.; Tee, T.-T.; Rahmat, A.R. A review of antimicrobial fabric containing nanostructures metal-based compound. J. Vinyl Add. Technol. 2019, 25, E3–E27. [Google Scholar] [CrossRef]
- Montazer, M.; Maali Amiri, M. ZnO Nano reactor on textiles and polymers: Ex situ and in situ synthesis, application, and characterization. J. Phys. Chem. B 2014, 118, 1453–1470. [Google Scholar] [CrossRef] [PubMed]
- Román, L.E.; Gomez, E.D.; Solís, J.L.; Gómez, M.M. Antibacterial cotton fabric functionalized with copper oxide nanoparticles. Molecules 2020, 25, 5802. [Google Scholar] [CrossRef]
- Gherasim, O.; Puiu, R.A.; Bîrca, A.C.; Burdusel, A.C.; Grumezescu, A.M. An updated review on silver nanoparticles in biomedicine. Nanomaterials 2020, 10, 2318. [Google Scholar] [CrossRef]
- Mahltig, B.; Haufe, H.; Böttcher, H. Functionalisation of textiles by inorganic sol-gel coatings. J. Mater. Chem. 2005, 15, 4385–4398. [Google Scholar] [CrossRef]
- Agafonov, A.V.; Galkina, O.L. Solution process-based technologies: A new way for textile nanofunctionalization. Russ. J. Gen. Chem. 2017, 87, 1412–1417. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Xu, Q.B.; Fu, F.Y.; Liu,, X.D. Durable antimicrobial cotton textiles modified with inorganic nanoparticles. Cellulose 2016, 23, 2791–2808. [Google Scholar] [CrossRef]
- Seth, M.; Jana, S. Nanomaterials based superhydrophobic and antimicrobial coatings. Nano World J. 2020, 6, 26–28. [Google Scholar] [CrossRef]
- Li, H.; Granados, A.; Fernández, E.; Pleixats, R.; Vallribera, A. Anti-inflammatory cotton fabrics and silica nanoparticles with potential topical medical applications. ACS Appl. Mater. Interfaces 2020, 12, 25658–25675. [Google Scholar] [CrossRef]
- Silva, J.; Mesquita, R.; Pinho, E.; Caldas, A.; Real Oliveira, M.E.C.D.; Lopes, C.M.; Lúcio, M.; Soares, G. Incorporation of lipid nanosystems containing omega-3 fatty acids and resveratrol in textile substrates for wound healing and anti-inflammatory applications. SN Appl. Sci. 2019, 1, 1007. [Google Scholar] [CrossRef]
- Syafiuddin, A. Toward a comprehensive understanding of textiles functionalized with silver nanoparticles. J. Chin. Chem. Soc. 2019, 66, 793–814. [Google Scholar] [CrossRef]
- Montazer, M.; Keshvari, A.; Kahali, P. Tragacanth gum/nano silver hydrogel on cotton-fabric: In-situ synthesis and antibacterial properties. Carbohydr. Polym. 2016, 154, 257–266. [Google Scholar] [CrossRef]
- Mohamed, A.L.; El-Naggar, M.E.; Shaheen, T.I.; Hassabo, A.G. Laminating of chemically modified silan based nanosols for advanced functionalization of cotton textile. Int. J. Biol. Macromol. 2017, 95, 429–437. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Amr, A.; Eid, B.M. Multipurpose treatment of cellulose containing fabrics to impart durable antibacterial and repellent properties. Fiber. Polym. 2020, 21, 513–521. [Google Scholar] [CrossRef]
- Seino, S.; Imoto, Y.; Kitigawa, D.; Kubo, Y.; Kosaka, T.; Kojima, T.; Nitani, H.; Nakagawa, T.; Yamamoto, T.A. Radiochemical synthesis of silver nanoparticles onto textile fabrics and the antibacterial activity. J. Nucl. Sci. Technol. 2016, 53, 1021–1027. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Eid, B.M.; Abdel-Aziz, M.S. Effect of plasma superficial treatments on antibacterial functionalization and coloration of cellulosic fabrics. Appl. Surf. Sci. 2017, 392, 1126–1133. [Google Scholar] [CrossRef]
- Ahmed, H.; Khattab, T.A.; Mashaly, H.M.; El-Halwagy, A.A.; Rehan, M. Plasma Activation toward multi-stimuli responsive cotton fabric via in situ development of polyaniline derivatives and silver nanoparticles. Cellulose 2020, 27, 2913–2926. [Google Scholar] [CrossRef]
- Montagut, A.M.; Granados, A.; Ballesteros, A.; Pleixats, R.; Llagostera, M.; Cortes, P.; Sebastian, R.M.; Vallribera, A. Antibiotic protected silver nanoparticles for microbicidal cotton. Tetrahedron 2019, 75, 102–108. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, J.; He, H.; Wang, Y.; Zhao, Y.; Lu, q.; Qin, Y.; Ke, Y.; Peng, Y. Green synthesis of silver nanoparticles with black rice (Oryza sativa L.) extract endowing carboxymethyl chitosan modified cotton with high anti-microbial and durable properties. Cellulose 2021, 28, 1–16. [Google Scholar] [CrossRef]
- Kwiczak-Yiğitbaşı, J.; Demir, M.; Ahan, R.E.; Canlı, S.; Şeker, U.O.Ş.; Baytekin, B. Ultrasonication for environmentally friendly preparation of antimicrobial and catalytically active nanocomposites of cellulosic textiles. ACS Sustain. Chem. Eng. 2020, 8, 18879–18888. [Google Scholar] [CrossRef]
- Hontañon, E.; Meyer, J.; Blanes, M.; Cambra, V.; Guo, X.; Masuhr, M.; Muntean, A.; Santos, L.; Nirschl, H.; Kruis, E. A sustainable route for antibacterial nanofinishing of textile. Int. J. Theor. Appl. Nanotechnol. 2016, 4, 17–27. [Google Scholar] [CrossRef][Green Version]
- Zheng, Y.; Xiao, M.; Jiang, S.; Ding, F.; Wang, J. Coating fabrics with gold nanorods for colouring, UV-protection, and antibacterial function. Nanoscale 2013, 5, 788–795. [Google Scholar] [CrossRef]
- Boomi, P.; Ganesan, R.M.; Pooranic, G.; Prabud, H.G.; Ravikumare, S.; Jeyakanthan, J. Biological synergy of greener gold nanoparticles by using Coleus aromaticus leaf extract. Mater. Sci. Eng. C 2019, 99, 202–210. [Google Scholar] [CrossRef]
- Boomi, P.; Ganesan, R.; Poorani, G.P.; Jegatheeswaran, S.; Balakumar, C.; Prabu, H.G.; Anand, K.; Prabhu, N.M.; Jeyakanthan, J.; Saravanan, M. Phyto-engineered gold nanoparticles (AuNPs) with potential antibacterial, antioxidant, and wound healing activities under in vitro and in vivo conditions. Int. J. Nanomed. 2020, 15, 7553–7568. [Google Scholar] [CrossRef]
- Mamatha, G.; Rajulu, A.V.; Madhukar, K. In situ generation of bimetallic nanoparticles in cotton fabric using aloe vera leaf extract as reducing agent. J. Nat. Fibers 2018, 17, 1121–1129. [Google Scholar] [CrossRef]
- Rao, A.V.; Ashok, B.; Mahesh, M.U.; Subbareddy, V.C.; Sekhar, V.C.; Ramanamurth, G.V.; Rajulu, A.V. Antibacterial cotton fabrics with in situ generated silver copper bimetallic nanoparticles using red sanders power extract as reducing agent. Int. J. Polym. Anal. Charact. 2019, 24, 346–354. [Google Scholar]
- Gao, L.; Feng, J.; Xu, S.; Shi, L.; Wang, L.; Yang, Z. General strategy to prepare single-layered Ag-Au-Pt nanocrystal ternary-coated biomass textiles through polymer-driven self-assembly. Nanomaterials 2020, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Das, M.P.; Rebecca, L.J. Evaluation of antibacterial efficacy of biogenic zinc oxide nanoparticles on cotton fabrics. J. Pharm. Sci. Res. 2017, 9, 2553–2557. [Google Scholar]
- Puvvada, R.U.; Wooding, J.P.; Bellavia, M.C.; Mcguinness, E.K.; Sulchek, T.A.; Losego, M.D. Bacterial growth and death on cotton fabrics conformally coated with ZnO thin films of varying thicknesses via atomic layer deposition (ALD). J. Miner. Met. Mater. Soc. 2019, 71, 178–184. [Google Scholar] [CrossRef]
- Tapas, R.K.; Ashis, K.S.; Mohammed, S.; Runali, K. UV protection and antimicrobial finish on cotton khadi fabric using a mixture of nanoparticles of zinc oxide and poly-hydroxy-amino methyl silicone. Text. Res. J. 2019, 89, 2260–2278. [Google Scholar]
- Belay, A.; Mekuria, M.; Adam, G. Incorporation of zinc oxide nanoparticles in cotton textiles for ultraviolet light protection and antibacterial activities. Nanomater. Nanotechnol. 2020, 10, 1847980420970052. [Google Scholar] [CrossRef]
- Vasantharaja, S.; Sathiyavimal, S.; Saravanana, M.; Senthilkumara, P.; Gnanasekaranc, K.; Shanmugaveld, M.; Manikandane, E.; Pugazhendhi, A. Synthesis of ecofriendly copper oxide nanoparticles for fabrication over textile fabrics: Characterization of antibacterial activity and dye degradation potential. J. Photochem. Photobiol. B 2019, 191, 143–149. [Google Scholar] [CrossRef]
- Perelshtein, I.; Lipovsky, A.; Perkas, N.; Tzanov, T.; Gedanken, A. Sonochemical co-deposition of antibacterial nanoparticles and dyes on textiles. Beilstein J. Nanotechnol. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Mantecca, P.; Kasemets, K.; Deokar, A.; Perelshtein, I.; Gedanken, A.; Bahk, Y.K.; Kianfar, B.; Wang, J. Airborne nanoparticle release and toxicological risk from metal oxide-coated textiles: Toward a multiscale safe-by-design approach. Environ. Sci. Technol. 2017, 51, 9305–9317. [Google Scholar] [CrossRef]
- Nabil, A.; Ibrahim, N.A.; Eid, B.M.; El-Aziz, E.A.; Elmaaty, T.M.A.; Ramadanc, S.M. Multifunctional cellulose-containing fabrics using modified finishing formulations. RSC Adv. 2017, 7, 33219–33230. [Google Scholar]
- Ibrahim, N.A.; Eida, B.M.; El-Aziz, E.A.; Elmaatyc, T.M.A.; Ramadan, S.M. Loading of chitosan-nano metal oxide hybrids onto cotton/polyester fabrics to impart permanent and effective multifunctions. Int. J. Biol. Macromol. 2017, 105, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Eid, B.M.; El-Sayed, G.M.; Ibrahim, H.M.; Habib, N.H. Durable Antibacterial Functionality of Cotton/Polyester Blended Fabrics Using Antibiotic/MONPs Composite. Fiber. Polym. 2019, 20, 2297–2309. [Google Scholar] [CrossRef]
- Attia, N.F.; Moussa, M.; Shetac, A.M.F.; Rehab Taha, R.; Gamal, H. Effect of different nanoparticles based coating on the performance of textile properties. Prog. Org. Coat. 2017, 104, 72–80. [Google Scholar] [CrossRef]
- Hassabo, A.H.; El-Naggar, M.E.; Mohamed, A.L.; Hebeish, A.A. Development of multifunctional modified cotton fabric with tri-component nanoparticle of silver, copper and zinc oxide. Carbohyd. Polym. 2019, 210, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Li, Y.; Lyu, B.; Jim, D.; Ma, J. Silicone quaternary ammonium salt based nanocomposite: A long-acting antibacterial cotton fabric finishing agent with good softness and air permeability. Cellulose 2020, 27, 1055–1069. [Google Scholar] [CrossRef]
- Ansari, M.; Sajjadi, S.A.; Sahebian, S.; Heidari, E.K. Photocatalytic and antibacterial activity of silver/titanium dioxide/zinc oxide nanoparticles coated on cotton fabrics. ChemistrySelect 2020, 5, 8370–8378. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, L.; Dai, Y.; Wu, W.; Sui, X.; Zhong, Y.; Wang, B.; Chen, Z.; Xu, H.; Mao, Z. Construction of a metallic silver nanoparticle-decorated bismuth oxybromide-based composite material as readily recyclable photocatalyst. J. Clean. Prod. 2020, 246, 119007–119020. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Eid, B.M.; Abdel-Aziz, M.S. Green synthesis of AuNPs for eco-friendly functionalization of cellulosic substrates. Appl. Surf. Sci. 2016, 389, 118–125. [Google Scholar] [CrossRef]
- Lim, S.-H.; Hudson, S.M. Application of a fiber-reactive chitosan derivative to cotton fabric as an antimicrobial textile finish. Carbohydr. Polym. 2004, 56, 227. [Google Scholar] [CrossRef]
- Igal, K.; Arreche, R.; Sambeth, J.; Bellotti, N.; Vega-Baudrit, J.; Redondo-Gómez, C.; Vázquez, P. Antifungal activity of cotton fabrics finished modified silica-silver- carbon-based hybrid nanoparticles. Text. Res. J. 2019, 89, 825–833. [Google Scholar]
- Dawoud, T.M.; Yassin, M.A.; El-Samawaty, A.R.M.; Elgorban, A.M. Silver nanoparticles synthesized by Nigrospora oryzae showed antifungal activity. Saudi J. Biol. Sci. 2020, 12, 36–41. [Google Scholar]
- Rilda, Y.; Mahardika, G.; Alif, A.; Agustien, A.; Dachriyanus, D.A. Antifungal property of cotton fabric textile: Modification of cotton fiber functions by coating compounds of TiO2-SiO2 /Chitosan. Der Pharma. Chem. 2016, 8, 124–131. [Google Scholar]
- Balakumaran, M.D.; Ramachandran, R.; Jagadeeswari, S.; Kalaichelvan, P.T. In vitro biological properties and characterization of nanosilver coated fabrics-An application for antimicrobial textile finishing. Int. Biodeterior. Biodegrad. 2016, 107, 48–55. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Niedbala, G.; Hassan, S.E.-D.; Salem, S.S.; Abdo, A.M.; Hetta, H.F.; Shaheen, T.I. Endophytic Streptomyces laurentii mediated green synthesis of Ag-NPs with antibacterial and anticancer properties for developing functional textile fabric properties. Antibiotics 2020, 9, 641. [Google Scholar] [CrossRef] [PubMed]
- Eremenko, A.M.; Petrik, I.S.; Smirnova, N.P.; Rudenko, A.V.; Marikvas, Y.S. Antibacterial and Antimycotic Activity of Cotton Fabrics, Impregnated with Silver and Binary Silver/Copper Nanoparticles. Nanoscale Res. Lett. 2016, 11, 28. [Google Scholar] [CrossRef]
- Paszkiewicz, M.; Golabiewska, A.; Rajski, A.; Kowal, E.; Sajdak, A.; Zaleska-Medynska, A. The antibacterial and antifungal textile properties functionalized by bimetallic nanoparticles of Ag/Cu with different structures. J. Nanomater. 2016, 2016, 6056980. [Google Scholar] [CrossRef]
- Rastgoo, M.; Montazer, M.; Malek, R.M.A.; Harifi, T.; Rad, M.M. Ultrasound mediation for one-pot sonosynthesis and deposition of magnetite nanoparticles on cotton/polyester fabric as a novel magnetic, photocatalytic, sonocatalytic, antibacterial and antifungal textile. Utrason. Sonochem. 2016, 31, 257–266. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Nada, A.A.; Hassabo, A.G.; Eid, B.M.; Noor El-Deen, A.M.; Abou-Zeid, N.Y. Effect of different capping agents on physicochemical and antimicrobial properties of ZnO nanoparticles. Chem. Pap. 2017, 71, 1365–1375. [Google Scholar] [CrossRef]
- El-Nahhal, I.M.; Elmanama, A.A.; El Ashgar, N.M.; Amara, N.; Selmane, M.; Chehimi, M.M. Stabilization of nano-structured ZnO particles onto the surface of cotton fibers using different surfactants and their antimicrobial activity. Ultrason. Sonochem. 2017, 38, 478–487. [Google Scholar] [CrossRef] [PubMed]
- El-Nahhal, I.M.; Elmanama, A.; Amara, N.; Qodih, F.S.; Selmane, M.; Chehimi, M. The efficacy of surfactants in stabilizing coating of nano-structured CuO particles onto the surface of cotton fibers and their antimicrobial activity. Mater. Chem. Phys. 2018, 215, 221–228. [Google Scholar] [CrossRef]
- Roy, T.S.; Shamim, S.U.D.; Rahman, M.K.; Ahmed, F.; Gafur, M.A. The development of ZnO nanoparticle coated cotton fabrics for antifungal and antibacterial applications. Mater. Sci. Appl. 2020, 11, 601–610. [Google Scholar]
- Markovi, D.; Vasiljevi, J.; Ašanin, J.; Ilic-Tomic, T.; Tomšič, B.; Joki, B.; Mitri, M.; Simončič, B.; Miši, D.; Maja Radeti, M. The influence of coating with aminopropyl triethoxysilane and CuO/Cu2O nanoparticles on antimicrobial activity of cotton fabrics under dark conditions. J. Appl. Polym. Sci. 2020, 137, e49194. [Google Scholar] [CrossRef]
- Gowri, S.; Gandhi, R.R.; Senthil, S.; Suresh, J.; Sundrarajan, M. Enhancing antimicrobial activity of biotemplated TiO2 nanoparticles using Aloe Vera plant extract. J. Bionanosci. 2016, 10, 181–190. [Google Scholar] [CrossRef]
- Nazari, A. Superior self-cleaning and antimicrobial properties on cotton fabrics using nano titanium dioxide along with green walnut shell dye. Fiber. Polym. 2019, 20, 2503–2509. [Google Scholar] [CrossRef]
- Montaser, A.S.; Mahmoud, F.A. Preparation of chitosan-graft-poly(vinyl acetate) copolymers and their adsorption of copper ion. Int. J. Biol. Macromol. 2019, 124, 659–666. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Brzezinski, S.; Kaminska, I. Multifunctional nanocoating finishing of polyester/cotton woven fabric by the sol-gel method. Text. Res. J. 2017, 88, 946–956. [Google Scholar] [CrossRef]
- Gao, D.; Liu, J.; Lyu, L.; Li, Y.; Ma, J.; Baig, W. Construct the multifunction of cotton fabric by synergism between nano ZnO.and Ag. Fiber. Polym. 2020, 21, 505–512. [Google Scholar] [CrossRef]
- Tzhayik, O.; Lipovsky, A.; Gedanken, A. Sonochemical fabrication of edible fragrant antimicrobial nano coating on textiles and polypropylene cups. Ultrason. Sonochem. 2017, 38, 614–621. [Google Scholar] [CrossRef]
- Hashemikia, S.; Hemmatinejad, N.; Ahmadi, E.; Montazer, M. Antibacterial and anti-inflammatory drug delivery properties on cotton fabric using betamethasoneloaded mesoporous silica particles stabilized with chitosan and silicone softener. Drug Deliv. 2016, 23, 2946–2955. [Google Scholar] [CrossRef]
- Puoci, F.; Saturnino, C.; Trovato, V.; Iacopetta, D.; Piperopoulos, E.; Triolo, C.; Bonomo, M.G.; Drommi, D.; Parisi, O.I.; Milone, C. Sol-gel treatment of textiles for the entrapping of an antioxidant/anti-inflammatory molecule: Functional coating, morphological characterization and drug release evaluation. Appl. Sci. 2020, 10, 2287. [Google Scholar] [CrossRef]
- Khadeja, L.; Grigoriants, I.; Halperin-Sternfeld, M.; Yona, A.; Adler-Abramovich, L. Sonochemical functionalization of cotton and non-woven fabrics with bio-inspired assembled nanostructures. Isr. J. Chem. 2020, 60, 1190–1196. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granados, A.; Pleixats, R.; Vallribera, A. Recent Advances on Antimicrobial and Anti-Inflammatory Cotton Fabrics Containing Nanostructures. Molecules 2021, 26, 3008. https://doi.org/10.3390/molecules26103008
Granados A, Pleixats R, Vallribera A. Recent Advances on Antimicrobial and Anti-Inflammatory Cotton Fabrics Containing Nanostructures. Molecules. 2021; 26(10):3008. https://doi.org/10.3390/molecules26103008
Chicago/Turabian StyleGranados, Albert, Roser Pleixats, and Adelina Vallribera. 2021. "Recent Advances on Antimicrobial and Anti-Inflammatory Cotton Fabrics Containing Nanostructures" Molecules 26, no. 10: 3008. https://doi.org/10.3390/molecules26103008
APA StyleGranados, A., Pleixats, R., & Vallribera, A. (2021). Recent Advances on Antimicrobial and Anti-Inflammatory Cotton Fabrics Containing Nanostructures. Molecules, 26(10), 3008. https://doi.org/10.3390/molecules26103008







