Occurrence and Determination of Carotenoids and Polyphenols in Different Paprika Powders from Organic and Conventional Production
Abstract
1. Introduction
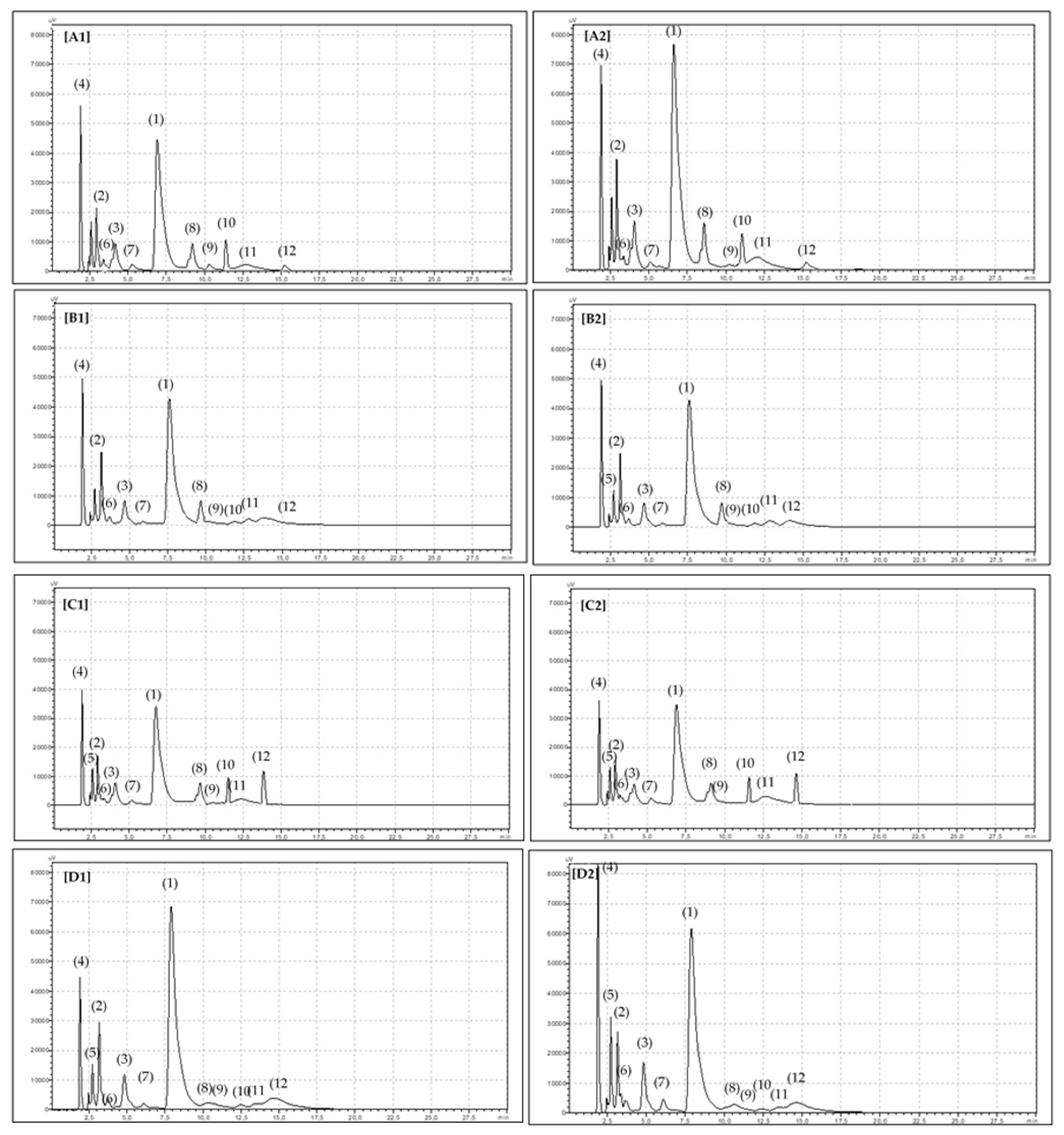
2. Results
3. Discussion
4. Materials and Methods
4.1. Analytical Material Preparation
4.2. Chemicals
4.3. Carotenoid Analysis
4.4. Polyphenols Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campmajó, G.; Rodríguez-Javier, L.R.; Saurina, J.; Núñez, O. Assessment of paprika geographical origin fraud by high-performance liquid chromatography with fluorescence detection (HPLC-FLD) fingerprinting. Food Chem. 2021, 352, 129397. [Google Scholar] [CrossRef] [PubMed]
- Guzman, I.; Hamby, S.; Romero, J.; Bosland, P.W.; O’Connell, M.A. Variability of carotenoid biosynthesis in orange colored Capsicum spp. Plant Sci. 2010, 179, 49–59. [Google Scholar] [CrossRef]
- Cervantes-Paz, B.; Yahia, E.M.; de Jesús Ornelas-Paz, J.; Victoria-Campos, C.I.; Ibarra-Junquera, V.; Pérez-Martínez, J.D.; Escalante-Minakata, P. Antioxidant activity and content of chlorophylls and carotenoids in raw and heat-processed Jalapeño peppers at intermediate stages of ripening. Food Chem. 2014, 146, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira Agostini-Costa, T.; da Silva Gomes, I.; de Melo, L.A.M.P.; Reifschneider, F.J.B.; da Costa Ribeiro, C.S. Carotenoid and total vitamin C content of peppers from selected Brazilian cultivars. J. Food Comp. Anal. 2017, 57, 73–79. [Google Scholar] [CrossRef]
- Rodríguez-Burruezo, A.; González-Mas, M.D.C.; Nuez, F. Carotenoid Composition and Vitamin A Value in Ají (Capsicum baccatum L.) and Rocoto (C. pubescens R. & P.), Pepper Species from the Andean Region. J. Food Sci. 2010, 75, S446–S453. [Google Scholar] [PubMed]
- Daood, H.G.; Palotás, G.; Palotás, G.; Somogyi, G.; Pék, Z.; Helyes, L. Carotenoid and antioxidant content of ground paprika from indoor-cultivated traditional varieties and new hybrids of spice red peppers. Food Res. Internat. 2014, 65, 231–237. [Google Scholar] [CrossRef]
- Rodriguez-Uribe, L.; Guzman, I.; Rajapakse, W.; Richins, R.D.; O’Connell, M.A. Carotenoid accumulation in orange-pigmented Capsicum annuum fruit, regulated at multiple levels. J. Exp. Bot. 2011, 63, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Anjum, F.M.; Khan, M.R.; Saeed, M.; Riaz, A. Antioxidant potential of bell pepper (Capsicum annum L.)—A Review. Pak. J. Food Sci. 2011, 21, 45–51. [Google Scholar]
- Rhim, J.-W.; Hong, S.-I. Effect of water activity and temperature on the color change of red pepper (Capsicum annuum L.) powder. Food Sci. Biotechnol. 2011, 20, 215–222. [Google Scholar] [CrossRef]
- Škrovánková, S.; Mlček, J.; Orsavová, J.; Juríková, J.; Dřímalová, P. Polyphenols content and antioxidant activity of paprika and pepper spices. Potravinarstvo 2017, 11, 52–57. [Google Scholar] [CrossRef]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants—A mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S.; Daglia, M.; Rengasamy, K.R. Dietary carotenoids in cancer chemoprevention and chemotherapy: A review of emerging evidence. Pharmacol. Res. 2020, 157, 104830. [Google Scholar] [CrossRef]
- Toh, D.W.K.; Koh, E.S.; Kim, J.E. Incorporating healthy dietary changes in addition to an increase in fruit and vegetable intake further improves the status of cardiovascular disease risk factors: A systematic review, meta-regression, and meta-analysis of randomized controlled trials. Nutr. Rev. 2020, 78, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, G.; McCartney, D.; Butler, J.S.; Loskutova, E.; Loughman, J. Identification of surrogate biomarkers for the prediction of patients at risk of low macular pigment in Type 2 diabetes. Cur. Eye Res. 2019, 44, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Coppens, J.; Grunert, O.; Van Den Hende, S.; Vanhoutte, I.; Boon, N.; Haesaert, G.; De Gelder, L. The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. J. Appl. Phycol. 2015, 28, 2367–2377. [Google Scholar] [CrossRef]
- Jiwan, M.A.; Duane, P.; O’Sullivan, L.; O’Brien, N.M.; Aherne, S.A. Content and bioaccessibility of carotenoids from organic and non-organic baby foods. J. Food Comp. Anal. 2010, 23, 346–352. [Google Scholar] [CrossRef]
- Ilahy, R.; Tlili, I.; Helyes, L.; Siddiqui, M.W.; Lenucci, M.S.; Pék, Z.; Hdider, C. Organically grown high-lycopene tomatoes: A novel adventure within functional quality. Acta Hort. 2019, 1233, 67–72. [Google Scholar] [CrossRef]
- Ribes-Moya, A.M.; Raigón, M.D.; Moreno-Peris, E.; Fita, A.; Rodríguez-Burruezo, A. Response to organic cultivation of heirloom Capsicum peppers: Variation in the level of bioactive compounds and effect of ripening. PLoS ONE 2018, 13, e0207888. [Google Scholar] [CrossRef]
- Cetó, X.; Serrano, N.; Aragó, M.; Gámez, A.; Esteban, M.; Díaz-Cruz, J.M.; Núñez, O. Determination of HPLC-UV fingerprints of spanish paprika (Capsicum annuum L.) for its classification by linear discriminant analysis. Sensors 2018, 18, 4479. [Google Scholar] [CrossRef]
- Choi, D.Y.; Lee, Y.J.; Hong, J.T.; Lee, H.J. Antioxidant properties of natural polyphenols and their therapeuticpotentials for Alzheimer’s disease. Brain Res. Bull. 2012, 87, 144–153. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Visioli, F. Polyphenol-based nutraceuticals for the prevention and treatment ofcardiovascular disease: Review of human evidence. Phytomedicine 2016, 23, 1145–1174. [Google Scholar] [CrossRef]
- Hallmann, E.; Rembiałkowska, E. Characterization of antioxidant compounds in sweet bell pepper (Capsicum annuum L.) under organic and conventional growing systems. J. Sci. Food Agric. 2012, 92, 2409–2415. [Google Scholar] [CrossRef]
- Hallmann, E.; Marszałek, K.; Lipowski, J.; Jasińska, U.; Kazimierczak, R.; Średnicka-Tober, D.; Rembiałkowska, E. Polyphenols and carotenoids in pickled bell pepper from organic and conventional production. Food Chem. 2019, 278, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Arimboor, R.; Natarajan, R.B.; Menon, K.R.; Chandrasekhar, L.P.; Moorkoth, V. Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: Analysis and stability—A review. J. Food Sci. Tech. 2014, 52, 1258–1271. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Escamilla, J.O.; de la Rosa, L.A.; López-Díaz, J.A.; Rodrigo-García, J.; Núñez-Gastélum, J.A.; Alvarez-Parrilla, E. Effect of the smoking process and firewood type in the phytochemical content and antioxidant capacity of red Jalapeño pepper during its transformation to chipotle pepper. Food Res. Int. 2015, 76, 654–660. [Google Scholar] [CrossRef]
- Sayin, K.; Arslan, D. Antioxidant properties, ascorbic acid and total carotenoid values of sweet and hot red pepper paste: A traditional food in Turkish diet. Int. J. Nutrit. Food Engin. 2015, 9, 834–837. [Google Scholar]
- Topuz, A.; Dincer, C.; Özdemir, K.S.; Feng, H.; Kushad, M. Influence of different drying methods on carotenoids and capsaicinoids of paprika (Cv., Jalapeno). Food Chem. 2011, 129, 860–865. [Google Scholar] [CrossRef]
- Hwang, J.R.; Hwang, I.K.; Kim, S. Quantitative analysis of various carotenoids from different colored paprika using UPLC. Kor. J. Food Sci. Technol. 2015, 47, 1–5. [Google Scholar] [CrossRef][Green Version]
- Velázquez, R.; Hernández, A.; Martín, A.; Aranda, E.; Gallardo, G.; Bartolomé, T.; Córdoba, M.G. Quality assessment of commercial paprikas. Int. J. Food Sci. Tech. 2013, 49, 830–839. [Google Scholar] [CrossRef]
- Deli, J.; Molnár, P. Paprika carotenoids: Analysis, isolation, structure elucidation. Curr. Org. Chem. 2002, 6, 1197–1219. [Google Scholar] [CrossRef]
- Mohd Hassan, N.; Yusof, N.A.; Yahaya, A.F.; Mohd Rozali, N.N.; Othman, R. Carotenoids of capsicum fruits: Pigment profile and health-promoting functional attributes. Antioxidants 2019, 8, 469. [Google Scholar] [CrossRef]
- Bozalan, N.K.; Karadeniz, F. Carotenoid Profile, Total Phenolic Content, and Antioxidant Activity of Carrots. Int. J. Food Prop. 2011, 14, 1060–1068. [Google Scholar] [CrossRef]
- Elvira-Torales, L.I.; García-Alonso, J.; Periago-Castón, M.J. Nutritional Importance of Carotenoids and Their Effect on Liver Health: A Review. Antioxidants 2019, 8, 229. [Google Scholar] [CrossRef]
- Mendelova, A.; Fikselova, A.; Mendel, L. Carotenoids and lycopene content in fresh and dried tomato fruits and tomato juice. Acta Univ. Agric. Silvic. Mendel. Brun. 2013, 61, 1329–1337. [Google Scholar] [CrossRef]
- Yoon, G.-A.; Yeum, K.-J.; Cho, Y.-S.; Chen, O.; Tang, G.; Blumberg, J.B.; Russell, R.M.; Yoon, S.; Lee-Kim, Y. Carotenoids and total phenolic contents in plant foods commonly consumed in Korea. Nutr. Res. Pract. 2012, 6, 481–490. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.A.-A.; Humayoun, A.; Khalid, Z.; Rashida, A. Dietary Sources of Lutein and Zeaxanthin Carotenoids and Their Role in Eye Health. Nutrients 2013, 5, 1169–1185. [Google Scholar] [CrossRef]
- Wall, M.M.; Waddell, C.A.; Bosland, P.W. Variation in β-Carotene and Total Carotenoid Content in Fruits of Capsicum. HortScience 2001, 36, 746–749. [Google Scholar] [CrossRef]
- Park, S.Y.; Choi, S.R.; Lim, S.H.; Yeo, Y.; Kweon, S.J.; Bae, Y.S.; Kim, K.W.; Im, K.H.; Ahn, S.K.; Ha, S.H.; et al. Identification and Quantification of Carotenoids in Paprika Fruits and Cabbage, Kale, and Lettuce Leaves. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 355–358. [Google Scholar] [CrossRef]
- Niewczas, J.; Szweda, D.; Mitek, M. The content of selected pro-healthful components in winter squash (Cucurbita maxima) fruits. Żywność, Nauka. Technologia. Jakość 2005, 2, 147–155. [Google Scholar]
- Martí, R.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a Source of Carotenoids and Polyphenols Targeted to Cancer Prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Pék, Z.; Szuvandzsiev, P.; Daood, H.; Nemenyi, A.; Helyes, L. Effect of irrigation on yield parameters and antioxidant profiles of processing cherry tomato. Central Eur. J. Biol. 2014, 9, 383. [Google Scholar] [CrossRef]
- Kidmose, U.; Knuthsen, P.; Edelenbos, M.; Justesen, U.; Hegelund, E. Carotenoids and flavonoids in organically grown spinach (Spinacia oleracea L) genotypes after deep frozen storage. J. Sci. Food Agric. 2001, 81, 918–923. [Google Scholar] [CrossRef]
- Zhou, W.; Niu, Y.; Ding, X.; Zhao, S.; Li, Y.; Fan, G.; Zhang, S.; Liao, K. Analysis of carotenoid content and diversity in apricots (Prunus armeniaca L.) grown in China. Food Chem. 2020, 330, 1–12. [Google Scholar] [CrossRef]
- Sahabi, M.; Shehu, R.A.; Saidu, Y.; Abdullahi, A.S. Screening for Total Carotenoids and β-Carotene in Some Widely Consumed Vegetables in Nigeria Nigerian. J. Basic Appl. Sci. 2012, 20, 225–227. [Google Scholar]
- Reis, L.C.R.; Oliveira, V.R.; Hagen, M.E.K.; Jablonski, A.; Flôres, S.H.; Rios, A.O. Carotenoids, flavonoids, chlorophylls, phenolic compounds and antioxidant activity in fresh and cooked broccoli (Brassica oleracea var. Avenger) and cauliflower (Brassica oleracea var. Alphina F1). Food Sci. Technol. 2015, 63, 177–183. [Google Scholar]
- Tilahun, S.; Paramaguru, P.; Rajamani, K. Capsaicin and ascorbic acid variability in chilli and paprika cultivars as revealed by HPLC analysis. J. Plant Breed. Gen. 2013, 1, 85–89. [Google Scholar]
- Musfiroh, I.D.A.; Mutakin, M.U.T.A.K.I.N.; Angelina, T.R.E.E.S.Y.E.; Muchtaridi, M.U.C.H.T.A.R.I.D.I. Capsaicin level of various capsicum fruits. Int. J. Pharm. Pharm. Sci. 2013, 5, 248–251. [Google Scholar]
- Bicikliski, O.; Tasev, K.; Trajkova, F.; Mihajlov, L.; Gudeva, L.K. Comparative analysis of capsaicin content in peppers (Capsicum annuum L.) grown in conventional and organic agricultural systems. J. Agric. Plant Sci. 2017, 15, 27–35. [Google Scholar]
- Ornelas-Paz, J.J.; Martínez-Burrola, J.M.; Ruiz-Cruz, S.; Santana-Rodríguez, V.; Ibarra-Junquera, V.; Olivas, G.I.; Pérez-Martínez, J.D. Effect of cooking on the capsaicinoids and phenolics contents of Mexican peppers. Food Chem. 2010, 119, 1619–1625. [Google Scholar]
- Zaki, N.; Hakmaoui, A.; Ouatmane, A.; Hasib, A.; Fernández-Trujillo, J.P. Bioactive components and antioxidant activity of Moroccan Paprika (Capsicum annuum L.) at different period of harvesting and processing. J. Biol. Agric. Health 2013, 3, 1–8. [Google Scholar]
- Cieślik, E.; Gręda, A.; Adamus, W. Contents of polyphenols in fruit and vegetables. Food Chem. 2006, 94, 135–142. [Google Scholar] [CrossRef]
- Chu, Y.H.; Chang, C.L.; Hsu, H.F. Flavonoid content of several vegetables and their antioxidant activity. J. Sci. Food Agric. 2000, 80, 561–566. [Google Scholar] [CrossRef]
- Collera-Zúñiga, O.; Jiménez, F.G.; Gordillo, R.M. Comparative study of carotenoid composition in three mexican varieties of Capsicum annuum L. Food Chem. 2005, 90, 109–114. [Google Scholar] [CrossRef]
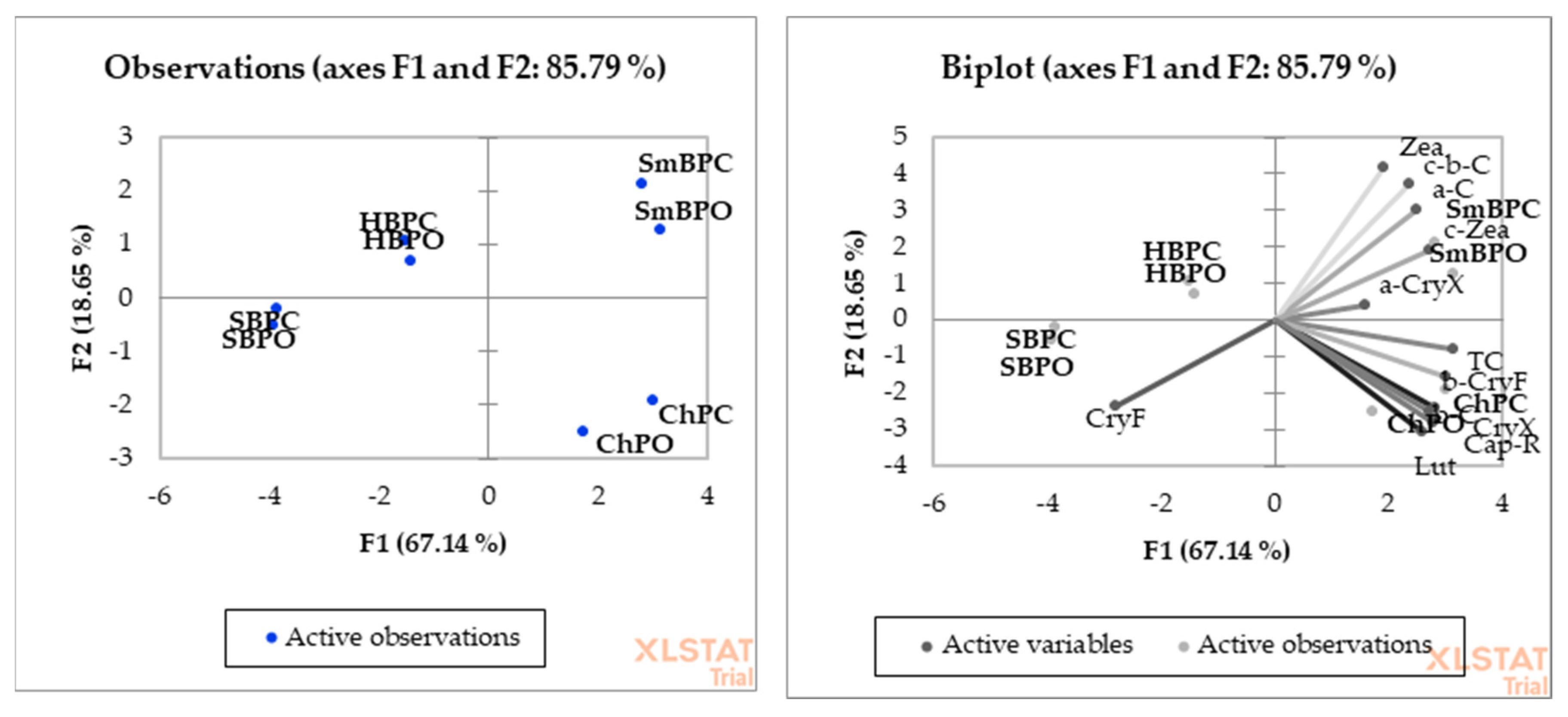
| Production System | Product | Total Carotenoids | beta-Carotene | cis-beta-Carotene | alpha-Carotene | Capsorubin |
|---|---|---|---|---|---|---|
| Organic | Sweet Paprika | 52.4 5± 0.27 c | 4.24 ± 0.09 c | 1.54 ± 0.02 d | 22.34 ± 0.25 c | 2.30 ± 0.02 d |
| Hot Paprika | 66.63 ± 1.11 b | 5.23 ± 0.10 b | 4.15 ± 0.0 c | 29.87 ± 1.01 a | 3.12 ± 0.23 c | |
| Smoked Paprika | 91.25 ± 0.57 a | 6.55 ± 0.15b | 6.45 ± 0.16 a | 35.59 ± 0.40 a | 4.93 ± 0.16 b | |
| Chili Pepper | 87.34 ± 1.29 | 7.35 ± 0.15 a | 3.31 ± 0.08 | 25.30 ± 0.28 b | 5.64 ± 0.13 a | |
| Mean Value | 74.42 ± 3.25 B | 5.85 ± 0.25 A | 3.86 ± 0.36 B | 28.27 ± 1.06 A | 3.99 ± 0.28 A | |
| Conventional | Sweet Paprika | 55.67 ± 0.22 c | 3.43 ± 0.07 c | 2.43 ± 0.01 d | 22.96 ± 0.26 c | 2.54 ± 0.02 d |
| Hot Paprika | 66.18 ± 0.73 b | 4.70 ± 0.11 c | 5.57 ± 0.08 b | 25.85 ± 0.21 b | 2.50 ±0.02 d | |
| Smoked Paprika | 91.20 ± 0.55 a | 5.72 ± 0.10 b | 8.28 ± 0.1 a | 32.08 ± 0.33 a | 3.92 ± 0.14 b | |
| Chili Pepper | 101.67 ± 0.69 a | 7.43 ± 0.06 a | 5.09 ± 0.05 b | 29.15 ± 0.29 a | 5.76 ± 0.18 a | |
| Mean Value | 78.68 ± 3.79 A | 5.32 ± 0.30 B | 5.35 ± 0.43 A | 27.51 ± 0.71 B | 3.68 ± 0.28 B | |
| p-Value | Production System (P) | <0.0001 | <0.0001 | <0.0001 | 0.0363 | 0.0048 |
| Product (Pr) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Interaction (PxPr) | <0.0001 | 0.0013 | <0.0001 | <0.0001 | <0.0001 |
| Production System | Product | Cryptoxanthin | Cryptoflavin | beta-Cryptoflavin | beta-Cryptoxanthin | Lutein | Zeaxanthin | cis-Zeaxanthin |
|---|---|---|---|---|---|---|---|---|
| Organic | Sweet Paprika | 14.88 ± 0.08 c | 1.08 ± 0.03 a | 0.30 ± 0.01 a | 0.23 ± 0.00 a | 3.12 ± 0.11 a | 0.45 ± 0.01 c | 1.98 ± 0.01 |
| Hot Paprika | 17.07 ± 0.23 b | 0.92 ± 0.03 a | 0.44 ± 0.01 a | 0.28 ± 0.00 a | 2.93 ± 0.06 a | 0.54 ±0.00 b | 2.10 ± 0.01 bc | |
| Smoked Paprika | 27.16 ± 0.20 | 0.76 ± 0.01 a | 0.65 ± 0.01 a | 0.53 ± 0.01 a | 5.22 ± 0.10 ba | 0.77 ±0.01 a | 2.63 ± 0.02 a | |
| Chili Pepper | 34.80 ± 1.37 ab | 0.86 ± 0.01 a | 0.71 ± 0.01 a | 0.59 ± 0.02 a | 6.14 ± 0.14 a | 0.46 ±0.01 c | 2.18 ± 0.01 b | |
| Mean Value | 23.48 ± 1.67 B | 0.91 ± 0.03 A | 0.52 ± 0.03 A | 0.41 ± 0.03 B | 4.35 ± 0.28 A | 0.55 ±0.03 A | 2.22 ± 0.05 A | |
| Conventional | Sweet Paprika | 17.95 ± 0.10 c | 0.99 ± 0.02 a | 0.20 ± 0.00 a | 0.31 ± 0.01 a | 2.53 ± 0.02 a | 0.32 ±0.01 d | 2.01 ± 0.00 c |
| Hot Paprika | 20.49 ± 0.15 b | 0.84 ± 0.01 a | 0.34 ± 0.01 a | 1.08 ± 0.13 a | 2.41 ± 0.39 a | 0.49 ±0.01 c | 1.91 ± 0.01 d | |
| Smoked Paprika | 31.73 ± 0.17 b | 0.69 ± 0.02 a | 0.58 ± 0.01 a | 0.71 ± 0.02 a | 4.02 ± 0.16 a | 0.74 ±0.01 a | 2.71 ± 0.02 a | |
| Chili Pepper | 43.57 ± 0.62 a | 0.83 ± 0.01 a | 0.68 ± 0.02 a | 0.86 ± 0.01 a | 5.50 ± 0.16 a | 0.40 ±0.02 cd | 2.40 ± 0.01 b | |
| Mean Value | 28.43 ± 2.08 A | 0.84 ± 0.02 B | 0.45 ± 0.04 B | 0.74 ± 0.14 A | 3.61 ± 0.28 B | 0.49 ± 0.03 B | 2.26 ± 0.07 A | |
| p-Value | Production System (P) | <0.0001 | <0.0001 | <0.0001 | 0.027 | <0.0001 | <0.0001 | N.S. |
| Product (Pr) | <0.0001 | <0.0001 | <0.0001 | N.S. | <0.0001 | <0.0001 | <0.0001 | |
| Interaction (PxPr) | 0.0001 | N.S. | N.S. | N.S. | N.S. | 0.0042 | 0.0412 |
| Production System | Product | Capsaicin | Total polyphenols | Total Phenolic Acids | Gallic | Chlorogenic | Caffeic | p-Coumaric | Ferulic |
|---|---|---|---|---|---|---|---|---|---|
| Organic | Sweet Paprika | 242.67 ± 8.58 c | 226.44 ± 6.51 c | 198.29 ± 6.38 c | 102.58 ± 7.24 b | 66.98 ± 4.45 b | 11.16 ± 1.31 c | 14.59 ± 0.88 c | 2.98 ± 0.23 d |
| Hot Paprika | 731.82 ± 15.96 b | 275.50 ± 5.45 c | 243.49 ± 6.99 b | 85.03 ± 4.59 c | 58.44 ± 1.70 b | 12.09 ± 0.03 c | 73.99 ± 6.26 a | 13.95 ± 0.37 a | |
| Smoked Paprika | 140.50 ± 2.91 c | 323.69 ± 11.62 b | 196.68 ± 9.55 c | 61.37 ± 2.07 d | 66.96 ± 9.70 b | 41.82 ± 4.97 b | 15.32 ± 1.48 c | 11.20 ± 0.19 a | |
| Chili Pepper | 1447.17 ± 79.18 a | 233.65 ± 6.09 c | 190.27 ± 4.80 c | 79.46 ± 4.47 c | 33.23 ± 0.82 c | 65.45 ± 1.82 a | 8.61 ± 0.39 d | 3.52 ± 0.16 c | |
| Mean Value | 640.54 ± 150.49 B | 264.82 ± 11.86 B | 207.18 ± 7.08 B | 82.11 ± 4.91 B | 56.40 ± 4.82 B | 32.63 ± 6.67 A | 28.13 ± 7.85 A | 7.91 ± 1.38 A | |
| Conventional | Sweet Paprika | 852.43 ± 32.15 b | 168.88 ± 2.53 d | 123.77 ± 1.20 d | 62.67 ± 2.75 d | 33.79 ± 1.66 c | 4.70 ± 0.16 d | 13.83 ± 0.79 c | 8.79 ± 0.36 b |
| Hot Paprika | 782.96 ± 58.80 b | 152.64 ± 11.27 d | 122.17 ± 12.27 d | 68.30 ± 10.62 d | 10.55 ± 0.84 d | 5.47 ± 0.14 d | 35.04 ± 1.18 b | 2.82 ± 0.16 d | |
| Smoked Paprika | 119.23 ± 5.52 c | 611.17 ± 35.67 a | 555.67 ± 37.20 a | 107.12 ± 1.96 b | 360.38 ± 37.44 a | 42.42 ± 3.68 b | 41.06 ± 0.38 b | 4.69 ± 0.15 c | |
| Chili Pepper | 1452.87 ± 21.92 a | 798.45 ± 12.57 a | 725.71 ± 10.40 a | 204.21 ± 1.85 a | 474.03 ± 7.15 a | 35.67 ± 2.49 b | 8.16 ± 0.11 d | 3.65 ± 0.26 c | |
| Mean Value | 801.87 ± 137.92 A | 432.78 ± 81.44 A | 381.83 ± 77.38 A | 110.58 ± 16.61 A | 219.69 ± 59.01 A | 22.06 ± 5.07 B | 24.52 ± 4.01 A | 4.99 ± 0.67 B | |
| p-Value | Production System (P) | 0.0002 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0002 | n.s. | <0.0001 |
| Product (Pr) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Interaction (PxPr) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Production System | Product | Total Flavonoids | Quercetin-3-o-Rutinoside | Myricetin | Quercetin | Quercetin-3-o-Glucoside | Kaempferol |
|---|---|---|---|---|---|---|---|
| Organic | Sweet Paprika | 28.14 ± 0.30 c | 5.95 ± 0.63 c | 4.11 ± 0.09 c | 2.73 ± 0.26 b | 13.49 ± 0.60 c | 1.87 ± 0.01 c |
| Hot Paprika | 32.00 ± 1.55 c | 5.08 ± 0.22 c | 3.68 ± 0.12 cd | 2.52 ± 0.12 b | 19.05 ± 1.38 b | 1.67 ± 0.01 c | |
| Smoked Paprika | 127.00 ± 5.97 a | 67.11 ± 5.86 a | 4.15 ± 0.03 c | 2.35 ± 0.03 b | 49.68 ± 0.36 a | 3.70 ± 0.13 a | |
| Chili Pepper | 43.38 ± 3.15 c | 20.16 ± 2.13 bc | 5.02 ± 0.20 b | 2.87 ± 0.10 b | 11.97 ± 0.97 c | 3.37 ± 0.12 a | |
| Mean Value | 57.63 ± 11.80 A | 24.57 ± 7.46 A | 4.24 ± 0.15 B | 2.62 ± 0.09 B | 23.55 ± 4.45 B | 2.65 ± 0.26 B | |
| Conventional | Sweet Paprika | 45.10 ± 1.50 b | 2.16 ± 0.19 d | 7.21 ± 0.08 a | 3.10 ± 0.05 a | 27.74 ± 1.61 b | 4.90 ± 0.17 a |
| Hot Paprika | 30.46 ± 2.85 c | 6.39 ± 0.43 c | 4.40 ± 0.27 c | 3.75 ± 0.07 a | 13.01 ± 2.31 c | 2.90 ± 0.05 b | |
| Smoked Paprika | 55.51 ± 3.04 b | 5.37 ± 0.31 c | 4.01 ± 0.05 c | 2.61 ± 0.13 b | 41.02 ± 2.83 a | 2.49 ± 0.02 b | |
| Chili Pepper | 72.74 ± 3.81 a | 34.54 ± 3.92 b | 4.34 ± 0.09 c | 2.02 ± 0.02 b | 28.94 ± 0.66 b | 2.90 ± 0.06 b | |
| Mean Value | 50.95 ± 4.68 B | 12.11 ± 3.89 B | 4.99 ± 0.38 A | 2.87 ± 0.19 A | 27.68 ± 3.04 A | 3.30 ± 0.27 A | |
| p-Value | Production System (P) | 0.028 | <0.0001 | <0.0001 | 0.027 | 0.0078 | <0.0001 |
| Product (Pr) | <0.0001 | <0.0001 | <0.0001 | 0.0005 | <0.0001 | <0.0001 | |
| Interaction (PxPr) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponder, A.; Kulik, K.; Hallmann, E. Occurrence and Determination of Carotenoids and Polyphenols in Different Paprika Powders from Organic and Conventional Production. Molecules 2021, 26, 2980. https://doi.org/10.3390/molecules26102980
Ponder A, Kulik K, Hallmann E. Occurrence and Determination of Carotenoids and Polyphenols in Different Paprika Powders from Organic and Conventional Production. Molecules. 2021; 26(10):2980. https://doi.org/10.3390/molecules26102980
Chicago/Turabian StylePonder, Alicja, Klaudia Kulik, and Ewelina Hallmann. 2021. "Occurrence and Determination of Carotenoids and Polyphenols in Different Paprika Powders from Organic and Conventional Production" Molecules 26, no. 10: 2980. https://doi.org/10.3390/molecules26102980
APA StylePonder, A., Kulik, K., & Hallmann, E. (2021). Occurrence and Determination of Carotenoids and Polyphenols in Different Paprika Powders from Organic and Conventional Production. Molecules, 26(10), 2980. https://doi.org/10.3390/molecules26102980