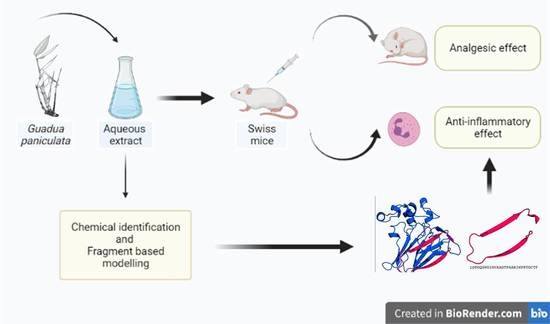
Identification of the Active Principle Conferring Anti-Inflammatory and Antinociceptive Properties in Bamboo Plant
Abstract
:1. Introduction
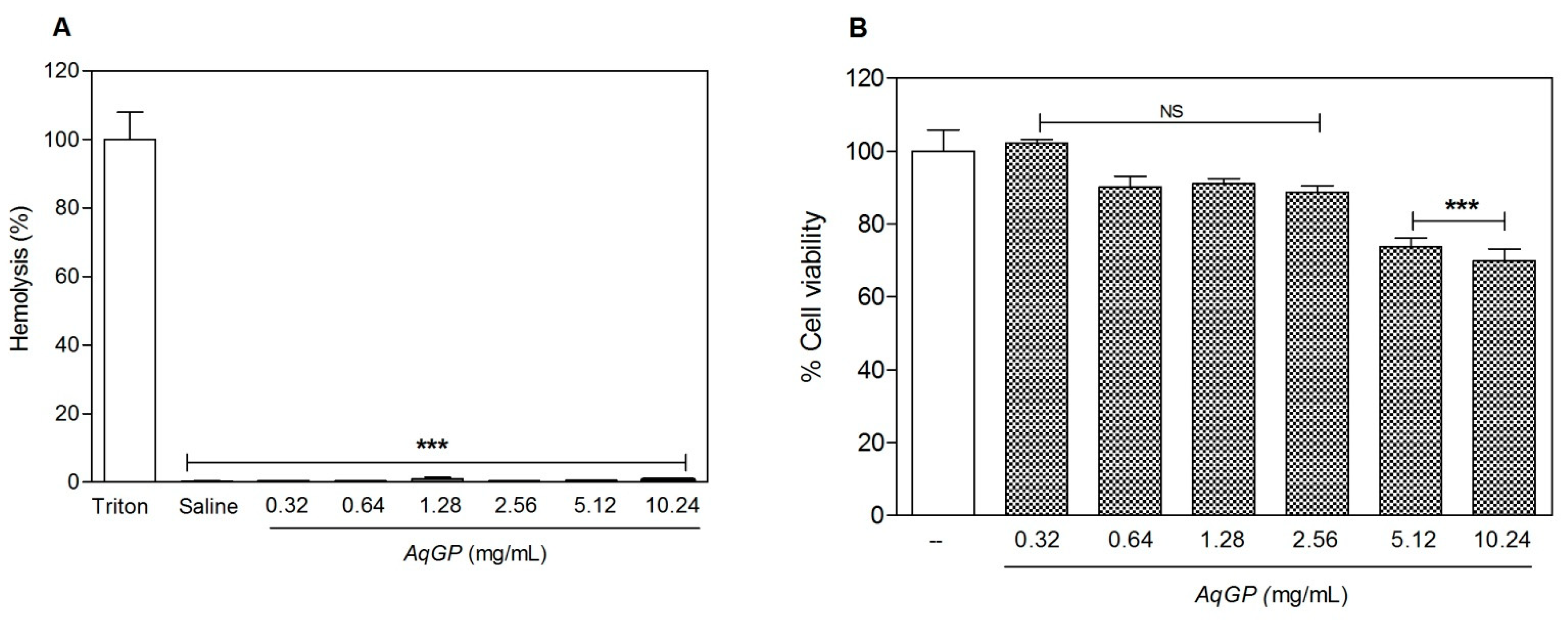
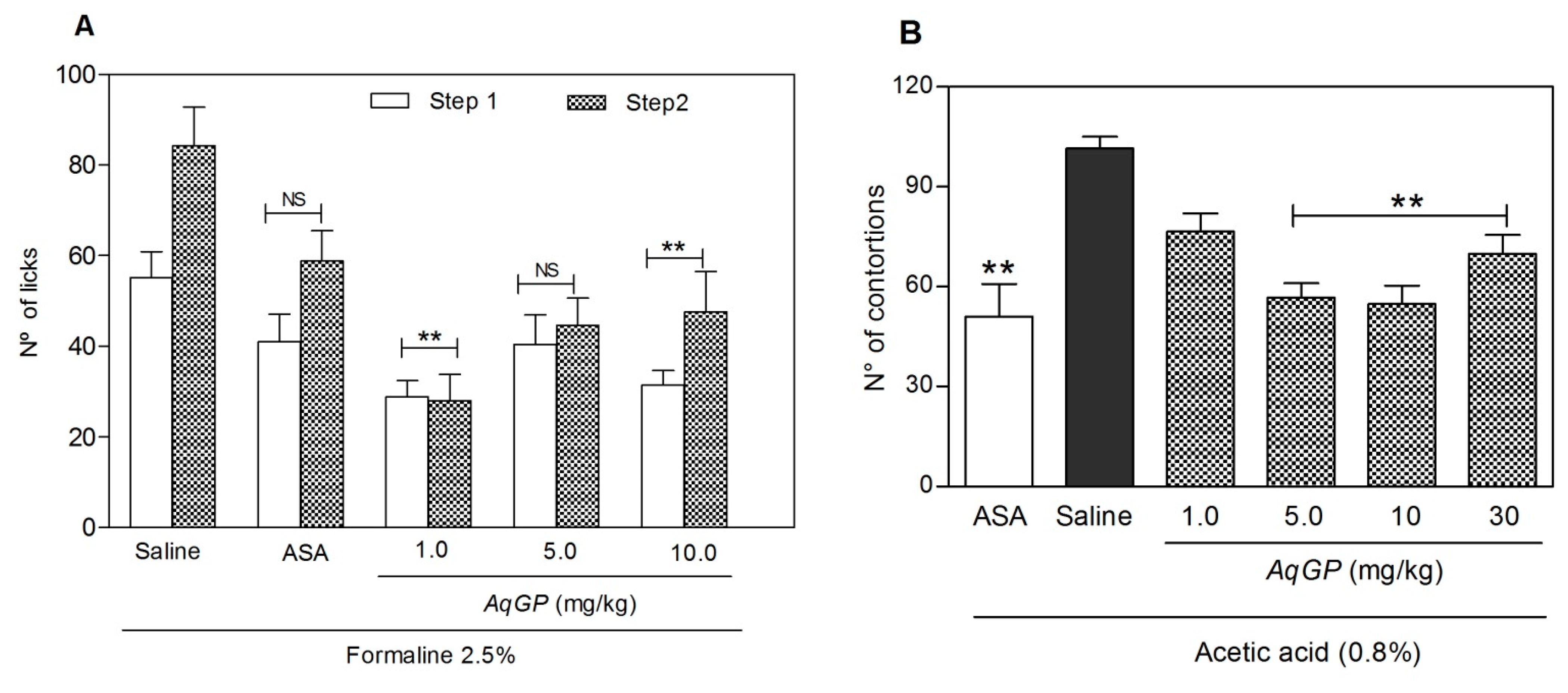
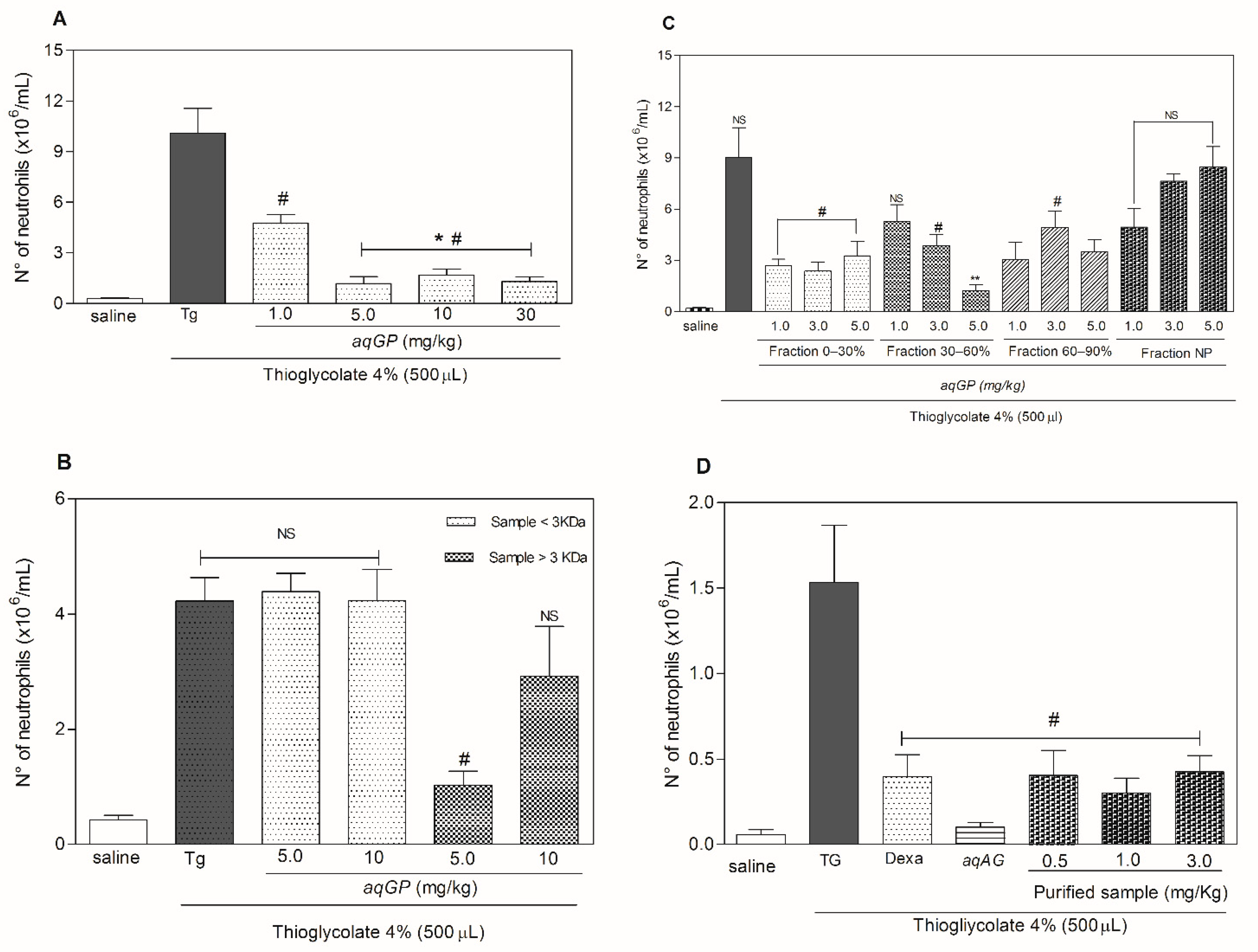
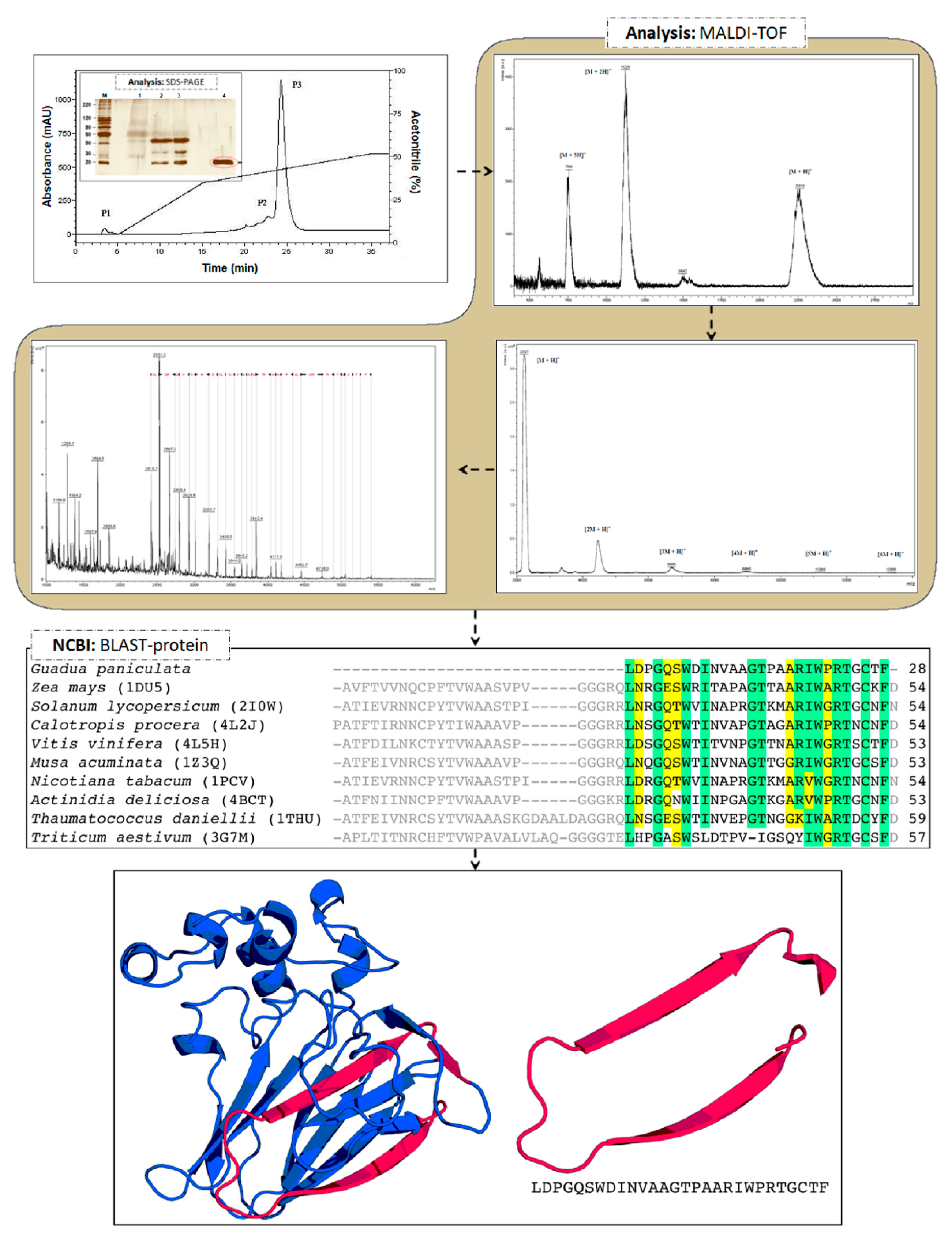
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material
3.2. Plant Extract Preparation
3.3. Evaluation of Cell Viability
3.3.1. Hemolytic Activity Test
3.3.2. MTT Assay
3.4. Animals
3.5. Evaluation of Nociceptive Response
3.5.1. Formalin Testsed
3.5.2. Acetic Acid-Induced Abdominal Contortions
3.6. Anti-Inflammatory Assay: Neutrophil Migration
3.7. Anti-Inflammatory Bioassay Guided Fractionation
3.7.1. Dialysis Fractionation
3.7.2. Fractionation by Ammonium Sulfate Precipitation
3.7.3. Fractionation by High Performance Liquid Chromatography
3.8. Chemical Identification Methods
3.8.1. SDS-PAGE Analysis
3.8.2. Mass Spectrometry Analysis by MALDI-TOF
3.9. Fragment-Based Modeling
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.; Rosenbloom, B.N.; Fashler, S. Chronic Pain, Psychopathology, and DSM-5 Somatic Symptom Disorder. Can. J. Psychiatry. 2015, 60, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Jaillon, S.; Galdiero, M.R.; Del Prete, D.; Cassatella, M.A.; Garlanda, C.; Mantovani, A. Neutrophils in innate and adaptive immunity. Semin. Immunopathol. 2013, 35, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Brown, K.A.; Brain, S.D.; Pearson, J.D.; Edgeworth, J.D.; Lewis, S.M.; Treacher, D.F. Neutrophils in development of multiple organ failure in sepsis. Lancet 2006, 368, 157–169. [Google Scholar] [CrossRef]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Batlouni, M. Nonsteroidal anti-inflammatory drugs: Cardiovascular, cerebrovascular and renal effects. Arq. Bras. Cardiol. 2010, 94, 556–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, G.M.; Frautschy, S.A. Mechanisms of action of non-steroidal anti-inflammatory drugs for the prevention of Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2010, 9, 140–148. [Google Scholar] [CrossRef]
- Odabasoglu, F.; Cakir, A.; Suleyman, H.; Aslan, A.; Bayir, Y.; Halici, M.; Kazaz, C. Gastroprotective and antioxidant effects of usnic acid on indomethacin-induced gastric ulcer in rats. J. Ethnopharmacol. 2006, 103, 59–65. [Google Scholar] [CrossRef]
- Adebayo, S.A.; Dzoyem, J.P.; Shai, L.J.; Eloff, J.N. The anti-inflammatory and antioxidant activity of 25 plant species used traditionally to treat pain in southern African. BMC Complement. Altern. Med. 2015, 15, 159. [Google Scholar] [CrossRef] [Green Version]
- Pinto, M.F.S.; Silva, O.N.; Viana, J.C.; Porto, W.F.; Migliolo, L.; Da Cunha, N.B.; Gomes, N.; Fensterseifer, I.C.M.; Colgrave, M.L.; Craik, D.J.; et al. Characterization of a Bioactive Acyclotide from Palicourea rigida. J. Nat. Prod. 2016. [Google Scholar] [CrossRef] [PubMed]
- Nürnberger, T.; Brunner, F. Innate immunity in plants and animals: Emerging parallels between the recognition of general elicitors and pathogen-associated molecular patterns. Curr. Opin. Plant Biol. 2002, 5, 318–324. [Google Scholar] [CrossRef]
- Katiyar, C.; Gupta, A.; Kanjilal, S.; Katiyar, S. Drug discovery from plant sources: An integrated approach. Ayu 2012, 33, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.F.S.; Almeida, R.G.; Porto, W.F.; Fensterseifer, I.C.M.; Lima, L.A.; Dias, S.C.; Franco, O.L. Cyclotides: From Gene Structure to Promiscuous Multifunctionality. J. Evid. Based. Complement. Altern. Med. 2012, 17, 40–53. [Google Scholar] [CrossRef]
- Silva, O.N.; Porto, W.F.; Migliolo, L.; Mandal, S.M.; Gomes, D.G.; Holanda, H.H.S.; Silva, R.S.P.; Dias, S.S.C.; Costa, M.P.; Costa, C.R.; et al. Cn-AMP1: A new promiscuous peptide with potential for microbial infections treatment. Biopolymers 2012, 98, 322–331. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente-Núez, C.; Mansour, S.; Wang, Z.; Jiang, L.; Breidenstein, E.; Elliott, M.; Reffuveille, F.; Speert, D.; Reckseidler-Zenteno, S.; Shen, Y.; et al. Anti-Biofilm and Immunomodulatory Activities of Peptides That Inhibit Biofilms Formed by Pathogens Isolated from Cystic Fibrosis Patients. Antibiotics 2014, 3, 509–526. [Google Scholar] [CrossRef] [Green Version]
- De la Fuente-Núñez, C.; Reffuveille, F.; Fernández, L.; Hancock, R.E.W. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 2013, 16, 580–589. [Google Scholar] [CrossRef]
- Silva, O.N.; Fensterseifer, I.C.M.; Rodrigues, E.A.; Holanda, H.H.S.; Novaes, N.R.F.; Cunha, J.P.A.; Rezende, T.M.B.; Magalhães, K.G.; Moreno, S.E.; Jerônimo, M.S.; et al. Clavanin A Improves Outcome of Complications from Different Bacterial Infections. Antimicrob. Agents Chemother. 2014. [Google Scholar] [CrossRef] [Green Version]
- Mansour, S.C.; de la Fuente-Núñez, C.; Hancock, R.E.W. Peptide IDR-1018: Modulating the immune system and targeting bacterial biofilms to treat antibiotic-resistant bacterial infections. J. Pept. Sci. 2015, 21, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Miele, M.; Costantini, S.; Colonna, G. Structural and functional similarities between osmotin from Nicotiana tabacum seeds and human adiponectin. PLoS ONE 2011, 6, e16690. [Google Scholar] [CrossRef]
- Arsenescu, V.; Narasimhan, M.L.; Halide, T.; Bressan, R.A.; Barisione, C.; Cohen, D.A.; de Villiers, W.J.S.; Arsenescu, R. Adiponectin and Plant-Derived Mammalian Adiponectin Homolog Exert a Protective Effect in Murine Colitis. Dig. Dis. Sci. 2011, 56, 2818–2832. [Google Scholar] [CrossRef]
- Fangkrathok, N.; Junlatat, J.; Sripanidkulchai, B. In vivo and in vitro anti-inflammatory activity of Lentinus polychrous extract. J. Ethnopharmacol. 2013, 147, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Buapool, D.; Mongkol, N.; Chantimal, J.; Roytrakul, S.; Srisook, E.; Srisook, K. Molecular mechanism of anti-inflammatory activity of Pluchea indica leaves in macrophages RAW 264.7 and its action in animal models ofinflammation. J. Ethnopharmacol. 2013, 146, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Hanáková, Z.; Hošek, J.; Kutil, Z.; Temml, V.; Landa, P.; Vaněk, T.; Schuster, D.; Dall’Acqua, S.; Cvačka, J.; Polanský, O.; et al. Anti-inflammatory Activity of Natural Geranylated Flavonoids: Cyclooxygenase and Lipoxygenase Inhibitory Properties and Proteomic Analysis. J. Nat. Prod. 2017, 80, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.D.L.; Teixeira, S.S.; Monteiro, M.H.D.; De-Oliveira, A.C.A.X.; Paumgartten, F.J.R. Traditional use and safety of herbal medicines. Rev. Bras. Farmacogn. 2014, 24, 248–257. [Google Scholar] [CrossRef]
- Calixto, J.B. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents). Brazilian J. Med. Biol. Res. 2000, 33, 179–189. [Google Scholar] [CrossRef] [PubMed]
- de Abreu Matos, F.J. Farmaácias Vivas: Sistema de Utilizaçaão de Plantas Medicinais Projetado Para Pequenas Comunidades, 4th ed.; UFC: Fortaleza-CE, Brazil, 2002; ISBN 8572820086. [Google Scholar]
- Smith, U. Dietary fibre, diabetes and obesity. Int. J. Obes. 1987, 11 (Suppl. 1), 27–31. [Google Scholar] [CrossRef]
- Chongtham, N.; Bisht, M.S.; Haorongbam, S. Nutritional Properties of Bamboo Shoots: Potential and Prospects for Utilization as a Health Food. Compr. Rev. Food Sci. Food Saf. 2011, 10, 153–168. [Google Scholar] [CrossRef]
- Lu, B.; Wu, X.; Tie, X.; Zhang, Y.; Zhang, Y. Toxicology and safety of anti-oxidant of bamboo leaves. Part 1: Acute and subchronic toxicity studies on anti-oxidant of bamboo leaves. Food Chem. Toxicol. 2005, 43, 783–792. [Google Scholar] [CrossRef]
- Lehto, M.; Airaksinen, L.; Puustinen, A.; Tillander, S.; Hannula, S.; Nyman, T.; Toskala, E.; Alenius, H.; Lauerma, A. Thaumatin-like protein and baker’s respiratory allergy. Ann. Allergy Asthma Immunol. 2010, 104, 139–146. [Google Scholar] [CrossRef]
- Tschannen, M.P.; Glück, U.; Bircher, A.J.; Heijnen, I.; Pletscher, C. Thaumatin and gum arabic allergy in chewing gum factory workers. Am. J. Ind. Med. 2017, 60, 664–669. [Google Scholar] [CrossRef]
- Tjølsen, A.; Berge, O.G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The formalin test: An evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef]
- de Oliveira Júnior, J.O.; Portella Junior, C.S.A.; Cohen, C.P. Inflammatory mediators of neuropathic pain. Rev. Dor 2016, 17, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Serbina, N.V.; Jia, T.; Hohl, T.M.; Pamer, E.G. Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 2008, 26, 421–452. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, R.; Jakschik, B.A. The role of mast cells in thioglycollate-induced inflammation. J. Immunol. 1988, 141, 2090–2096. [Google Scholar]
- Ness, T.J. Models of Visceral Nociception. ILAR J. 1999, 40, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, S. Peripheral analgesia: Mechanism of the analgesic action of aspirin-like drugs and opiate-antagonists. Br. J. Clin. Pharmacol. 1980, 10, 237S–245S. [Google Scholar] [CrossRef] [Green Version]
- Batalia, M.A.; Monzingo, A.F.; Ernst, S.; Roberts, W.; Robertus, J.D. The crystal structure of the antifungal protein zeamatin, a member of the thaumatin-like, PR-5 protein family. Nat. Struct. Biol. 1996, 3, 19–23. [Google Scholar] [CrossRef]
- Ghosh, R.; Chakrabarti, C. Crystal structure analysis of NP24-I: A thaumatin-like protein. Planta 2008, 228, 883–890. [Google Scholar] [CrossRef]
- Ramos, M.V.; de Oliveira, R.S.B.; Pereira, H.M.; Moreno, F.B.M.B.; Lobo, M.D.P.; Rebelo, L.M.; Brandão-Neto, J.; de Sousa, J.S.; Monteiro-Moreira, A.C.O.; Freitas, C.D.T.; et al. Crystal structure of an antifungal osmotin-like protein from Calotropis procera and its effects on Fusarium solani spores, as revealed by atomic force microscopy: Insights into the mechanism of action. Phytochemistry 2015, 119, 5–18. [Google Scholar] [CrossRef]
- Marangon, M.; Van Sluyter, S.C.; Waters, E.J.; Menz, R.I. Structure of Haze Forming Proteins in White Wines: Vitis vinifera Thaumatin-Like Proteins. PLoS ONE 2014, 9, e113757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leone, P.; Menu-Bouaouiche, L.; Peumans, W.J.; Payan, F.; Barre, A.; Roussel, A.; Van Damme, E.J.M.; Rougé, P. Resolution of the structure of the allergenic and antifungal banana fruit thaumatin-like protein at 1.7-Å. Biochimie 2006, 88, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Ha, S.C.; Hasegawa, P.M.; Bressan, R.A.; Yun, D.-J.; Kim, K.K. Crystal structure of osmotin, a plant antifungal protein. Proteins Struct. Funct. Bioinform. 2003, 54, 170–173. [Google Scholar] [CrossRef]
- Ko, T.P.; Day, J.; Greenwood, A.; McPherson, A. Structures of three crystal forms of the sweet protein thaumatin. Acta Crystallogr. Sect. D Biol. Crystallogr. 1994, 50, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Vandermarliere, E.; Lammens, W.; Schoepe, J.; Rombouts, S.; Fierens, E.; Gebruers, K.; Volckaert, G.; Rabijns, A.; Delcour, J.A.; Strelkov, S.V.; et al. Crystal structure of the noncompetitive xylanase inhibitor TLXI, member of the small thaumatin-like protein family. Proteins Struct. Funct. Bioinform. 2010, 78, 2391–2394. [Google Scholar] [CrossRef]
- Shah, S.A.; Lee, H.Y.; Bressan, R.A.; Yun, D.J.; Kim, M.O. Novel osmotin attenuates glutamate-induced synaptic dysfunction and neurodegeneration via the JNK/PI3K/Akt pathway in postnatal rat brain. Cell Death Dis. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Pang, C.; Cao, T.; Li, J.; Jia, M.; Zhang, S.; Ren, S.; An, H.; Zhan, Y. Combining fragment homology modeling with molecular dynamics aims at prediction of Ca2+ binding sites in CaBPs. J. Comput. Aided. Mol. Des. 2013, 27, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Kelm, S.; Vangone, A.; Choi, Y.; Ebejer, J.-P.; Shi, J.; Deane, C.M. Fragment-based modeling of membrane protein loops: Successes, failures, and prospects for the future. Proteins Struct. Funct. Bioinform. 2014, 82, 175–186. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Lee, H.G.; Kim, H.-S.; Vaas, A.P.J.P.; De Silva, H.I.C.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, D.-S.; Lee, J.-S.; et al. Fucoidan Purified from Sargassum polycystum Induces Apoptosis through Mitochondria-Mediated Pathway in HL-60 and MCF-7 Cells. Mar. Drugs 2020, 18, 196. [Google Scholar] [CrossRef] [Green Version]
- Kamada, T.; Kang, M.-C.; Phan, C.-S.; Zanil, I.; Jeon, Y.-J.; Vairappan, C. Bioactive Cembranoids from the Soft Coral Genus Sinularia sp. in Borneo. Mar. Drugs 2018, 16, 99. [Google Scholar] [CrossRef] [Green Version]
- Aboudy, Y.; Mendelson, E.; Shalit, I.; Bessalle, R.; Fridkin, M. Activity of two synthetic amphiphilic peptides and magainin-2 against herpes simplex virus types 1 and 2. Int. J. Pept. Protein Res. 1994, 43, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Koster, R.; Anderson, M.; De-Beer, E. Acetic acid for analgesic screening. Fed. Proc. 1959, 18, 412–418. [Google Scholar]
- Roriz, B.C.; Buccini, D.F.; dos Santos, B.F.; Silva, S.R.D.S.; Domingues, N.L.D.C.; Moreno, S.E. Synthesis and biological activities of a nitro-shiff base compound as a potential anti-inflammatory agent. Eur. J. Pharm. Sci. 2020, 148, 105300. [Google Scholar] [CrossRef]
- Coldebella, P.F.; Gomes, S.D.; Evarini, J.A.; Cereda, M.P.; Coelho, S.R.M.; Coldebella, A. Evaluation of protein extraction methods to obtain protein concentrate from cassava leaf. Eng. Agric. 2013, 33, 1223–1233. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- EnCor Biotechnology Inc. Ammonium Sulfate Calculator. Available online: https://www.encorbio.com/protocols/AM-SO4.htm (accessed on 16 April 2018).
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [Green Version]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. In Current Protocols in Protein Science; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; Volume 86, pp. 2.9.1–2.9.37. [Google Scholar]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef] [Green Version]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Sumathi, K.; Ananthalakshmi, P.; Roshan, M.N.A.M.; Sekar, K. 3dSS: 3D structural superposition. Nucleic Acids Res. 2006, 34, W128–W132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, R.B.; Barton, G.J. Multiple protein sequence alignment from tertiary structure comparison: Assignment of global and residue confidence levels. Proteins Struct. Funct. Genet. 1992, 14, 309–323. [Google Scholar] [CrossRef] [PubMed]
- DeLano, W.L. The PyMOL Molecular Graphics System. Schrödinger LLC wwwpymolorg 2002. Version 1. Available online: https://pymol.org/2/ (accessed on 10 September 2018).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araujo Sousa, B.; Nascimento Silva, O.; Farias Porto, W.; Lima Rocha, T.; Paulino Silva, L.; Ferreira Leal, A.P.; Buccini, D.F.; Oluwagbamigbe Fajemiroye, J.; de Araujo Caldas, R.; Franco, O.L.; et al. Identification of the Active Principle Conferring Anti-Inflammatory and Antinociceptive Properties in Bamboo Plant. Molecules 2021, 26, 3054. https://doi.org/10.3390/molecules26103054
Araujo Sousa B, Nascimento Silva O, Farias Porto W, Lima Rocha T, Paulino Silva L, Ferreira Leal AP, Buccini DF, Oluwagbamigbe Fajemiroye J, de Araujo Caldas R, Franco OL, et al. Identification of the Active Principle Conferring Anti-Inflammatory and Antinociceptive Properties in Bamboo Plant. Molecules. 2021; 26(10):3054. https://doi.org/10.3390/molecules26103054
Chicago/Turabian StyleAraujo Sousa, Bruna, Osmar Nascimento Silva, William Farias Porto, Thales Lima Rocha, Luciano Paulino Silva, Ana Paula Ferreira Leal, Danieli Fernanda Buccini, James Oluwagbamigbe Fajemiroye, Ruy de Araujo Caldas, Octávio Luiz Franco, and et al. 2021. "Identification of the Active Principle Conferring Anti-Inflammatory and Antinociceptive Properties in Bamboo Plant" Molecules 26, no. 10: 3054. https://doi.org/10.3390/molecules26103054
APA StyleAraujo Sousa, B., Nascimento Silva, O., Farias Porto, W., Lima Rocha, T., Paulino Silva, L., Ferreira Leal, A. P., Buccini, D. F., Oluwagbamigbe Fajemiroye, J., de Araujo Caldas, R., Franco, O. L., Grossi-de-Sá, M. F., de la Fuente Nunez, C., & Moreno, S. E. (2021). Identification of the Active Principle Conferring Anti-Inflammatory and Antinociceptive Properties in Bamboo Plant. Molecules, 26(10), 3054. https://doi.org/10.3390/molecules26103054