Adsorptive Cathodic Stripping Voltammetry for Quantification of Alprazolam
Abstract
1. Introduction
2. Results and Discussion
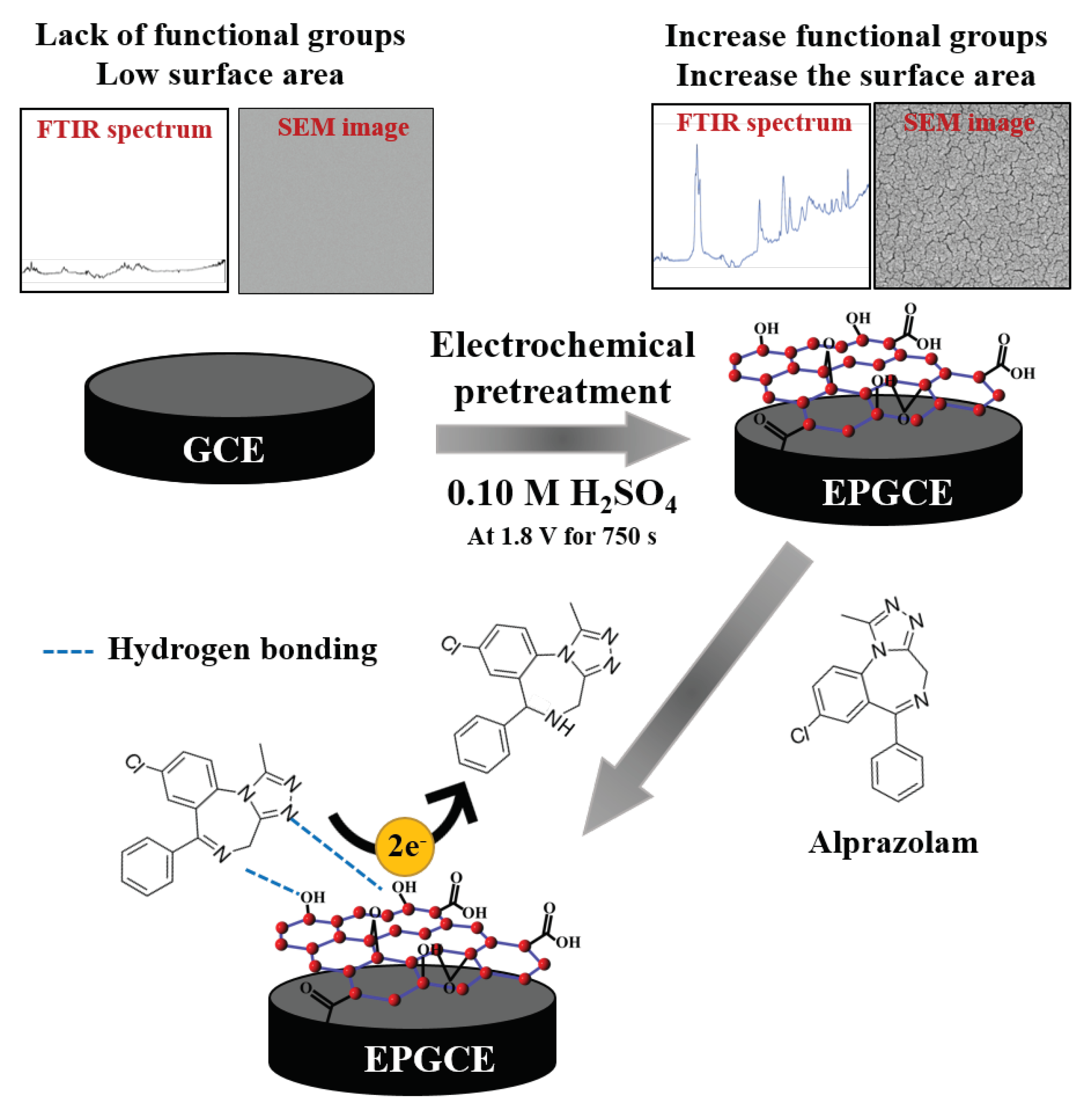
2.1. Chemicals for the Electrochemical Pretreatment
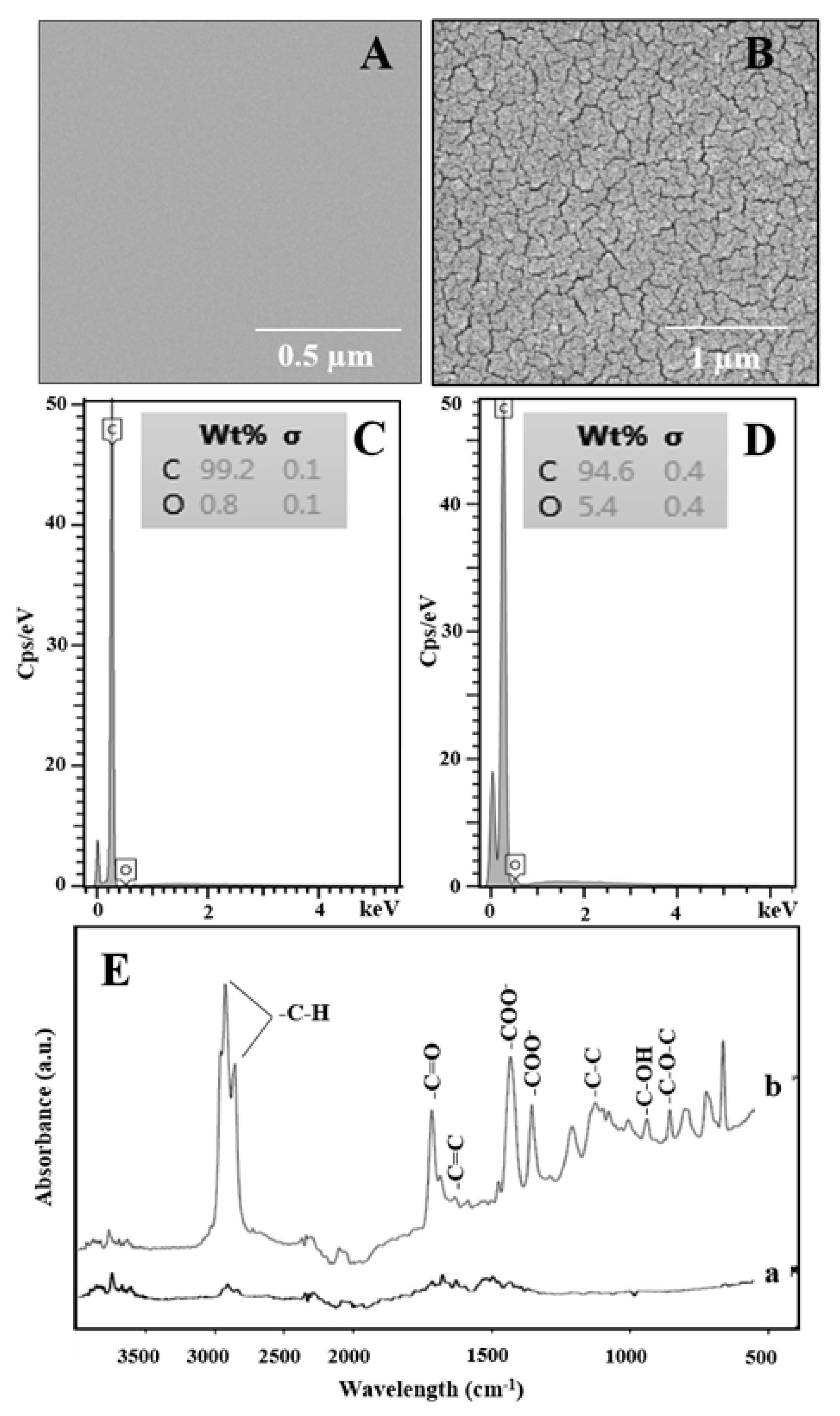
2.2. Characterization
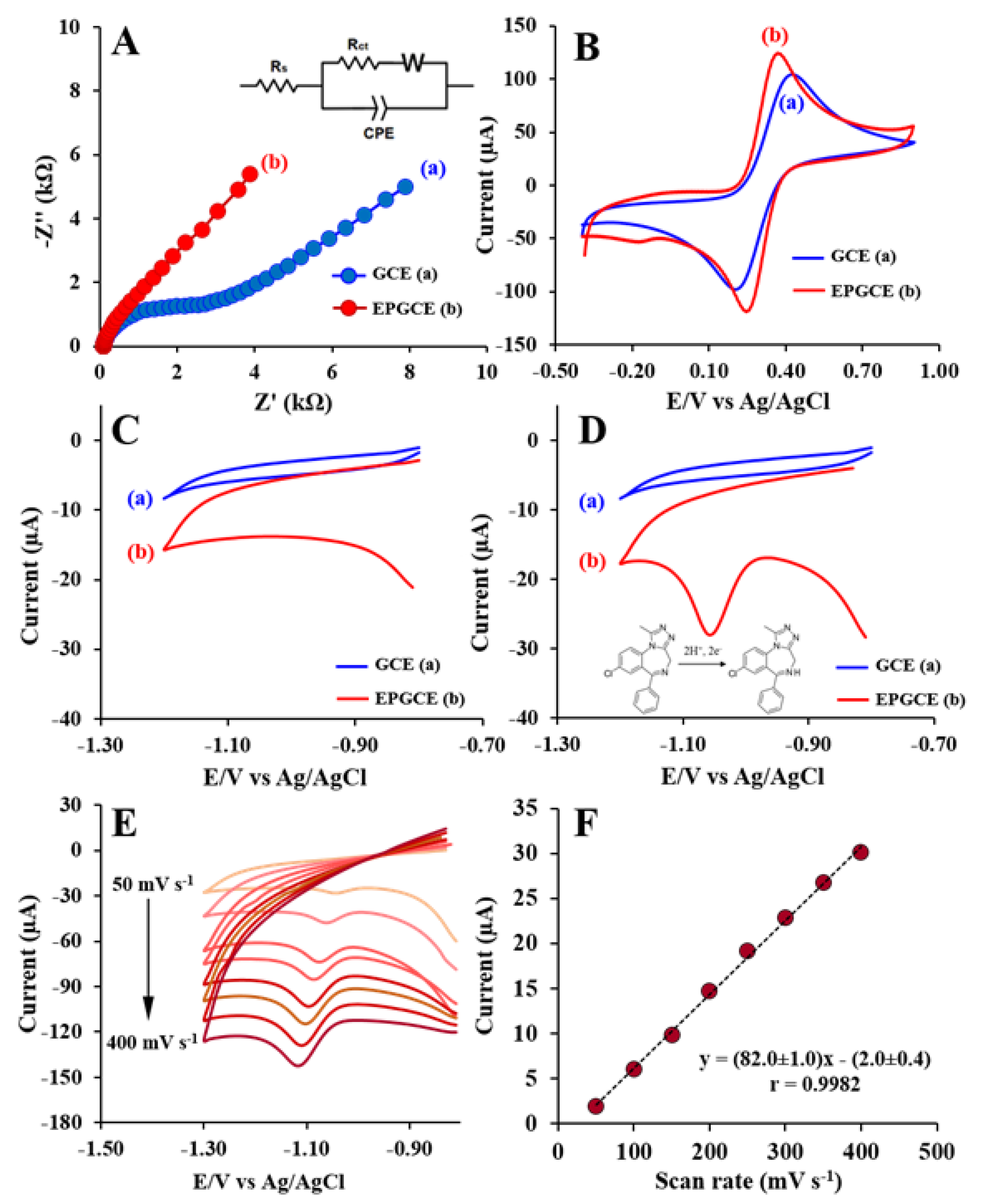
2.3. Electrochemical Behavior
2.4. Effect of Scan Rate
2.5. Optimization
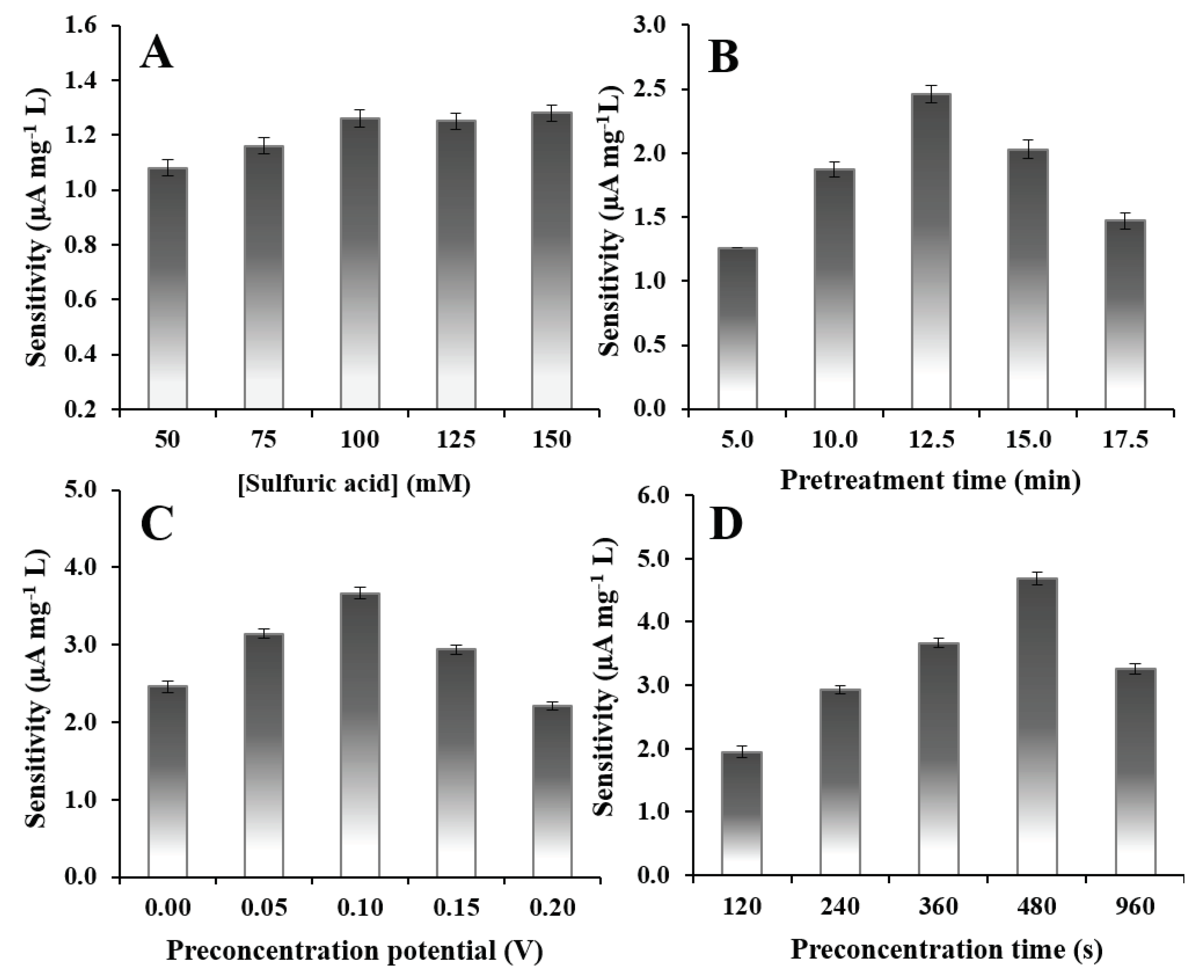
2.5.1. Electrochemical Pretreatment
2.5.2. Preconcentration Potential and Preconcentration Time
2.6. Analytical Performance
2.6.1. Linearity, Limit of Detection, and Limit of Quantification
2.6.2. Repeatability
2.6.3. Selectivity
2.6.4. Real Sample Analysis
3. Materials and Methods
3.1. Materials
3.2. Apparatus
3.3. Electrode Preparation
3.4. Electrochemical Measurements
3.5. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ganjali, M.R.; Haji-Hashemi, H.; Faridbod, F.; Norouzi, P.; Qomi, M. Potentiometric Determination of Alprazolam based on carbon paste and PVC membrane electrodes. Int. J. Electrochem. Sci. 2012, 7, 1470–1481. [Google Scholar]
- Nunes, C.N.; Pauluk, L.E.; dos Anjos, V.E.; Lopes, M.C.; Quináia, S.P. New approach to the determination of contaminants of emerging concern in natural water: Study of alprazolam employing adsorptive cathodic stripping voltammetry. Anal. Bioanal. Chem. 2015, 407, 6171–6179. [Google Scholar] [CrossRef]
- Narang, J.; Malhotra, N.; Singhal, C.; Mathur, A.; Pn, A.K.; Pundir, C.S. Detection of alprazolam with a lab on paper economical device integrated with urchin like Ag@ Pd shell nano-hybrids. Mater. Sci. Eng. C 2017, 80, 728–735. [Google Scholar] [CrossRef]
- Kakkar, A.; Kumar, S. Alprazolam poisoning. J. Indian Acad. Forensic Med. 2014, 36, 432–433. [Google Scholar]
- Chouinard, G. Issues in the clinical use of benzodiazepines: Potency, withdrawal, and rebound. J. Clin. Psychiatry 2004, 65 (Suppl. 5), 7–12. [Google Scholar] [PubMed]
- Donoghue, J.; Lader, M. Usage of benzodiazepines: A review. Int. J. Psychiatry Clin. Pr. 2010, 14, 78–87. [Google Scholar] [CrossRef]
- Westbury, J.; Jackson, S.; Gee, P.; Peterson, G. An effective approach to decrease antipsychotic and benzodiazepine use in nursing homes: The RedUSe project. Int. Psychogeriatr. IPA 2009, 22, 26–36. [Google Scholar] [CrossRef]
- Madhusoodanan, S.; Bogunovic, O.J. Safety of benzodiazepines in the geriatric population. Expert Opin. Drug Saf. 2004, 3, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Madea, B.; Musshoff, F. Knock-out drugs: Their prevalence, modes of action, and means of detection. Dtsch. Arztebl. Int. 2009, 106, 341–347. [Google Scholar] [PubMed]
- Samiec, P.; Navrátilová, Z. Electrochemical behaviour of bromazepam and alprazolam and their determination in the pharmaceutical tablets Lexaurin and Xanax on carbon paste electrode. Mon. Für Chem. Chem. Mon. 2017, 148, 449–455. [Google Scholar] [CrossRef]
- Samiec, P.; Švorc, Ľ.; Stanković, D.M.; Vojs, M.; Marton, M.; Navrátilová, Z. Mercury-free and modification-free electroanalytical approach towards bromazepam and alprazolam sensing: A facile and efficient assay for their quantification in pharmaceuticals using boron-doped diamond electrodes. Sens. Actuators B Chem. 2017, 245, 963–971. [Google Scholar] [CrossRef]
- Honeychurch, K.C. Review of Electroanalytical-Based Approaches for the Determination of Benzodiazepines. Biosensors 2019, 9, 130. [Google Scholar] [CrossRef]
- Samoson, K.; Thavarungkul, P.; Kanatharana, P.; Limbut, W. A Nonenzymatic Glucose Sensor Based on the Excellent Dispersion of a Graphene Oxide-Poly(acrylic acid)-Palladium Nanoparticle-Modified Screen-Printed Carbon Electrode. J. Electrochem. Soc. 2019, 166, B1079–B1087. [Google Scholar] [CrossRef]
- Rodsud, S.; Limbut, W. A Simple Electrochemical Sensor Based on Graphene Nanoplatelets Modified Glassy Carbon Electrode (GrNPs/GCE) for Highly Sensitive Detection of Yohimbine (YOH). J. Electrochem. Soc. 2019, 166, B771–B779. [Google Scholar] [CrossRef]
- Soleh, A.; Saisahas, K.; Promsuwan, K.; Thavarungkul, P.; Kanatharana, P.; Limbut, W. N-Doped Graphene Nanoplatelets for Direct Capsaicin Detection in Chili Pepper Samples. ACS Appl. Nano Mater. 2020, 3, 10094–10104. [Google Scholar] [CrossRef]
- Saisahas, K.; Soleh, A.; Promsuwan, K.; Phonchai, A.; Mohamed Sadiq, N.S.; Teoh, W.K.; Chang, K.H.; Lim Abdullah, A.F.; Limbut, W. A portable electrochemical sensor for detection of the veterinary drug xylazine in beverage samples. J. Pharm. Biomed. Anal. 2021, 198, 113958. [Google Scholar] [CrossRef] [PubMed]
- Cotchim, S.; Promsuwan, K.; Dueramae, M.; Duerama, S.; Dueraning, A.; Thavarungkul, P.; Kanatharana, P.; Limbut, W. Development and Application of an Electrochemical Sensor for Hydroquinone in Pharmaceutical Products. J. Electrochem. Soc. 2020, 167, 155528. [Google Scholar] [CrossRef]
- Promsuwan, K.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. Subnanomolar detection of promethazine abuse using a gold nanoparticle-graphene nanoplatelet-modified electrode. Microchim. Acta 2020, 187, 646. [Google Scholar] [CrossRef]
- Saichanapan, J.; Promsuwan, K.; Limbut, W. Adsorption and determination of sibutramine in illegal slimming product using porous graphene ink-modified electrode. Talanta 2020, 212, 120788. [Google Scholar] [CrossRef]
- Torrarit, K.; Promsuwan, K.; Soleh, A.; Saisahas, K.; Thiagchanya, A.; Phonchai, A.; Limbut, W. Adsorptive Anodic Stripping Voltammetric Determination of Atropine in Urine Sample. J. Electrochem. Soc. 2021, 168, 037512. [Google Scholar] [CrossRef]
- Tyszczuk-Rotko, K.; Pietrzak, K.; Sasal, A. Adsorptive stripping voltammetric method for the determination of caffeine at integrated three-electrode screen-printed sensor with carbon/carbon nanofibers working electrode. Adsorption 2019, 25, 913–921. [Google Scholar] [CrossRef]
- Promsuwan, K.; Kaewjunlakan, C.; Saichanapan, J.; Soleh, A.; Saisahas, K.; Thipwimonmas, Y.; Kongkaew, S.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. Poly(phenol red) hierarchical micro-structure interface enhanced electrode kinetics for adsorption and determination of hydroquinone. Electrochim. Acta 2021, 377, 138072. [Google Scholar] [CrossRef]
- Kaewnu, K.; Promsuwan, K.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. A Simple and Sensitive Electrochemical Sensor for Chloramphenicol Detection in Pharmaceutical Samples. J. Electrochem. Soc. 2020, 167, 087506. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Zhuang, Q. Simple self-referenced ratiometric electrochemical sensor for dopamine detection using electrochemically pretreated glassy carbon electrode modified by acid-treated multiwalled carbon nanotube. J. Electroanal. Chem. 2019, 851, 113446. [Google Scholar] [CrossRef]
- Promsuwan, K.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. Nitrite amperometric sensor for gunshot residue screening. Electrochim. Acta 2020, 331, 135309. [Google Scholar] [CrossRef]
- Promsuwan, K.; Kachatong, N.; Limbut, W. Simple flow injection system for non-enzymatic glucose sensing based on an electrode modified with palladium nanoparticles-graphene nanoplatelets/mullti-walled carbon nanotubes. Electrochim. Acta 2019, 320, 134621. [Google Scholar] [CrossRef]
- Promsuwan, K.; Thongtawat, J.; Limbut, W. Porous palladium-poly(3,4-ethylenedioxythiophene)–coated carbon microspheres/graphene nanoplatelet–modified electrode for flow-based-amperometric hydrazine sensor. Microchim. Acta 2020, 187, 539. [Google Scholar] [CrossRef] [PubMed]
- McCreery, R.L. Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef]
- Poon, M.; McCreery, R.L. In situ laser activation of glassy carbon electrodes. Anal. Chem. 1986, 58, 2745–2750. [Google Scholar] [CrossRef]
- Schreurs, J.; Berg, J.; Wonders, A.; Barendrecht, E. Characterization of a glassy-carbon-electrode surface pretreated with rf-plasma. Recl. Des Trav. Chim. Des Pays-Bas 2010, 103, 251–259. [Google Scholar] [CrossRef]
- Zhai, H.; Wang, S.; Zhou, J.; Pan, J.; Tong, Y.; Mei, Q.; Zhou, Q. A Simple and Sensitive Electrochemical Sensor for 3-Nitrotyrosine Based on Electrochemically Anodic Pretreated Glassy Carbon Electrode in Anionic Surfactant Medium. J. Electrochem. Soc. 2019, 166, B1426–B1433. [Google Scholar] [CrossRef]
- Wang, S.; Zhai, H.; Chen, Z.; Wang, H.; Tan, X.; Sun, G.; Zhou, Q. Constructing a Sensitive Electrochemical Sensor for Tyrosine Based on Graphene Oxide-ε-MnO2 Microspheres/Chitosan Modified Activated Glassy Carbon Electrode. J. Electrochem. Soc. 2017, 164, B758–B766. [Google Scholar] [CrossRef]
- Yi, Y.; Weinberg, G.; Prenzel, M.; Greiner, M.; Heumann, S.; Becker, S.; Schlögl, R. Electrochemical corrosion of a glassy carbon electrode. Catal. Today 2017, 295, 32–40. [Google Scholar] [CrossRef]
- Santhiago, M.; Maroneze, C.M.; Silva, C.C.C.; Camargo, M.N.L.; Kubota, L.T. Electrochemical Oxidation of Glassy Carbon Provides Similar Electrochemical Response as Graphene Oxide Prepared by Tour or Hummers Routes. ChemElectroChem 2015, 2, 761–767. [Google Scholar] [CrossRef]
- Kongkaew, S.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. A preparation of homogeneous distribution of palladium nanoparticle on poly (acrylic acid)-functionalized graphene oxide modified electrode for formalin oxidation. Electrochim. Acta 2017, 247, 229–240. [Google Scholar] [CrossRef]
- Xu, X.; Feng, Y.; Li, J.; Li, F.; Yu, H. A novel protocol for covalent immobilization of thionine on glassy carbon electrode and its application in hydrogen peroxide biosensor. Biosens. Bioelectron. 2010, 25, 2324–2328. [Google Scholar] [CrossRef] [PubMed]
- Samiec, P.; Navrátilová, Z.; Fischer, J. Voltammetry of benzodiazepines on meniscus-modified silver solid amalgam electrode. Mon. Für Chem. Chem. Mon. 2016, 147, 127–134. [Google Scholar] [CrossRef]
- Ma, X.; Chen, M.; Li, X.; Purushothaman, A.; Li, F. Electrochemical Detection of Norepinephrine in the Presence of Epinephrine, Uric Acid and Ascorbic Acid Using a Graphene- modified Electrode. Int. J. Electrochem. Sci. 2012, 7, 991–1000. [Google Scholar]
- Cotchim, S.; Thavarungkul, P.; Kanatharana, P.; Limbut, W. A new strategy for 2,4,6-Trinitrotoluene adsorption and electrochemical reduction on poly(melamine)/graphene oxide modified electrode. Electrochim. Acta 2015, 184, 102–110. [Google Scholar] [CrossRef]
- AOAC. Guidelines for Single-Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals; AOAC: Rockville, MD, USA, 2012; pp. 1–32. [Google Scholar]
- Mynttinen, E.; Wester, N.; Lilius, T.; Kalso, E.; Koskinen, J.; Laurila, T. Simultaneous electrochemical detection of tramadol and O-desmethyltramadol with Nafion-coated tetrahedral amorphous carbon electrode. Electrochim. Acta 2018, 295, 347–353. [Google Scholar] [CrossRef]
- Acikkol, M.; Mercan, S.; Karadayi, Ş. Simultaneous Determination of Benzodiazepines and Ketamine from Alcoholic and Nonalcoholic Beverages by GC-MS in Drug Facilitated Crimes. Chromatographia 2009, 70, 1295–1298. [Google Scholar] [CrossRef]
- Sarfaraz, S.; Readdy, C.V.R.; Shareef, K.M.A. Method development, Validation and determination of Alprazolam in its pharmaceutical dosage by 2,3-dichloro 5,6-dicyano−1,4-benzoquinone. J. Chem. Pharm. Res. 2014, 6, 411–418. [Google Scholar]
- Afkhami, A.; Ghaedi, H.; Madrakian, T.; Ahmadi, M.; Mahmood-Kashani, H. Fabrication of a new electrochemical sensor based on a new nano-molecularly imprinted polymer for highly selective and sensitive determination of tramadol in human urine samples. Biosens. Bioelectron. 2013, 44, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Atta, N.F.; Galal, A.; Hassan, S.H. Ultrasensitive determination of nalbuphine and tramadol narcotic analgesic drugs for postoperative pain relief using nano-cobalt oxide/ionic liquid crystal/carbon nanotubes-based electrochemical sensor. J. Electroanal. Chem. 2019, 839, 48–58. [Google Scholar] [CrossRef]


| Modified Electrode | Technique | Linear Range (mg L−1) | LOD (mg L−1) | Sample | Reference |
|---|---|---|---|---|---|
| a CPE | Potentiometry | 0.310–3087.70 | 0.300 | Pharmaceutical tablets | [1] |
| b BDDE | f DPV | 0.250–30.9 | 0.198 | Pharmaceutical tablets | [11] |
| c m-AgSAE | DPV | 0.185–30.9 | 0.155 | Urine | [37] |
| CPE | DPV | 0.247–30.9 | 0.130 | Pharmaceutical tablets | [10] |
| d EPGCE | g AdCSV | 0.100–20.0 | 0.03 | Pepsi, coke, orange Juice, beer, wine, vodka | This work |
| Other method | |||||
| e GC–MS | - | 50–1000 | 7.00 | Beer and peach juice | [42] |
| UV visible spectrometry | - | 1.00–20.0 | 0.400 | Pharmaceutical tablets | [43] |
| Sample | %Recovery of Proposed Method (n = 3) | ||
|---|---|---|---|
| Concentration of Spiking (mg L−1) | |||
| 4 | 8 | 16 | |
| Pepsi Max Taste | 97.0 ± 0.2 | 99.1 ± 0.3 | 104.3 ± 0.7 |
| Smirnoff Black Ice | 91.8 ± 0.3 | 100.2 ± 0.1 | 106.8 ± 0.4 |
| Eristoff vodka | 82.0 ± 0.2 | 107.5 ± 0.3 | 101.8 ± 0.3 |
| Coke Light | 86.90 ± 0.04 | 95.4 ± 0.4 | 109.0 ± 0.3 |
| Orange Big | 98.1 ± 0.5 | 102.9 ± 0.4 | 98.8 ± 1.2 |
| Full Moon Wine | 87.4 ± 0.2 | 93.5 ± 0.1 | 106.8 ± 0.5 |
| Sample | Alprazolam Spike (mg L−1) | GC–MS Method Found (mg L−1) (n = 3) | Proposed Method Found (mg L−1) (n = 3) |
|---|---|---|---|
| Pepsi Max Taste | 4 | 4.4 ± 0.2 | 3.46 ± 0.15 |
| Eristoff vodka | 16 | 17.3 ± 0.8 | 17.35 ± 0.17 |
| Smirnoff Black Ice | 40 | 36.9 ± 3.5 | 39.84 ± 0.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonmee, W.; Samoson, K.; Yodrak, J.; Thiagchanya, A.; Phonchai, A.; Limbut, W. Adsorptive Cathodic Stripping Voltammetry for Quantification of Alprazolam. Molecules 2021, 26, 2958. https://doi.org/10.3390/molecules26102958
Boonmee W, Samoson K, Yodrak J, Thiagchanya A, Phonchai A, Limbut W. Adsorptive Cathodic Stripping Voltammetry for Quantification of Alprazolam. Molecules. 2021; 26(10):2958. https://doi.org/10.3390/molecules26102958
Chicago/Turabian StyleBoonmee, Waree, Kritsada Samoson, Janjira Yodrak, Adul Thiagchanya, Apichai Phonchai, and Warakorn Limbut. 2021. "Adsorptive Cathodic Stripping Voltammetry for Quantification of Alprazolam" Molecules 26, no. 10: 2958. https://doi.org/10.3390/molecules26102958
APA StyleBoonmee, W., Samoson, K., Yodrak, J., Thiagchanya, A., Phonchai, A., & Limbut, W. (2021). Adsorptive Cathodic Stripping Voltammetry for Quantification of Alprazolam. Molecules, 26(10), 2958. https://doi.org/10.3390/molecules26102958





