2D Porphyrinic Metal-Organic Frameworks Featuring Rod-Shaped Secondary Building Units
Abstract
1. Introduction
2. Results and Discussion
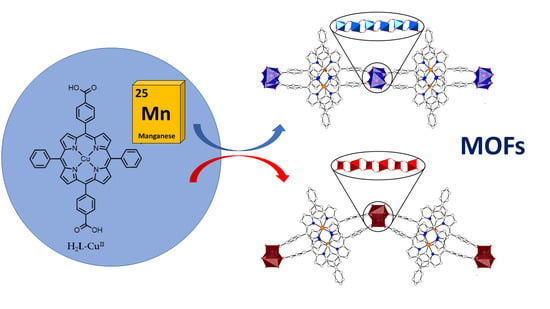
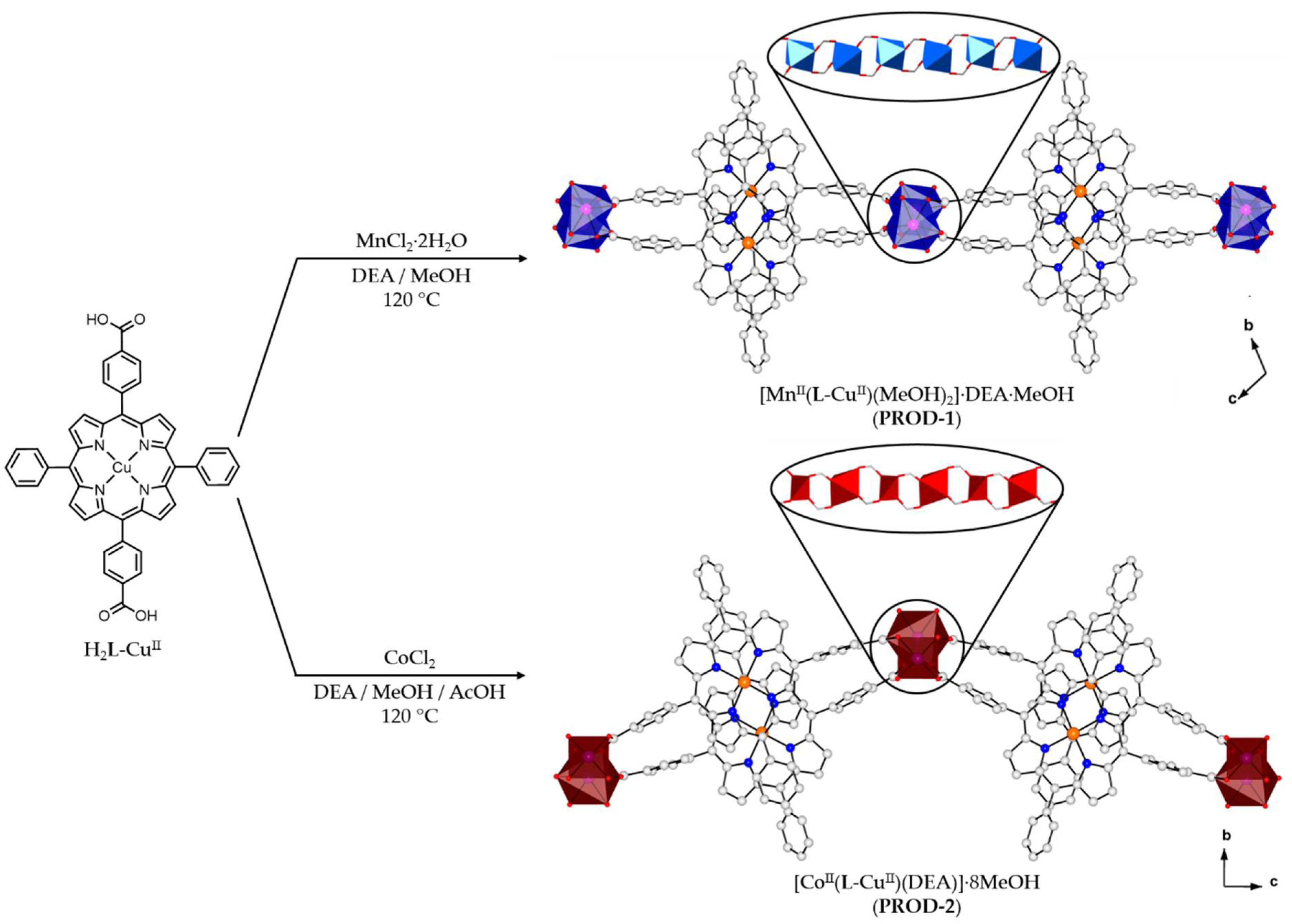
2.1. Synthesis of Metalloporphyrin Rod MOFs
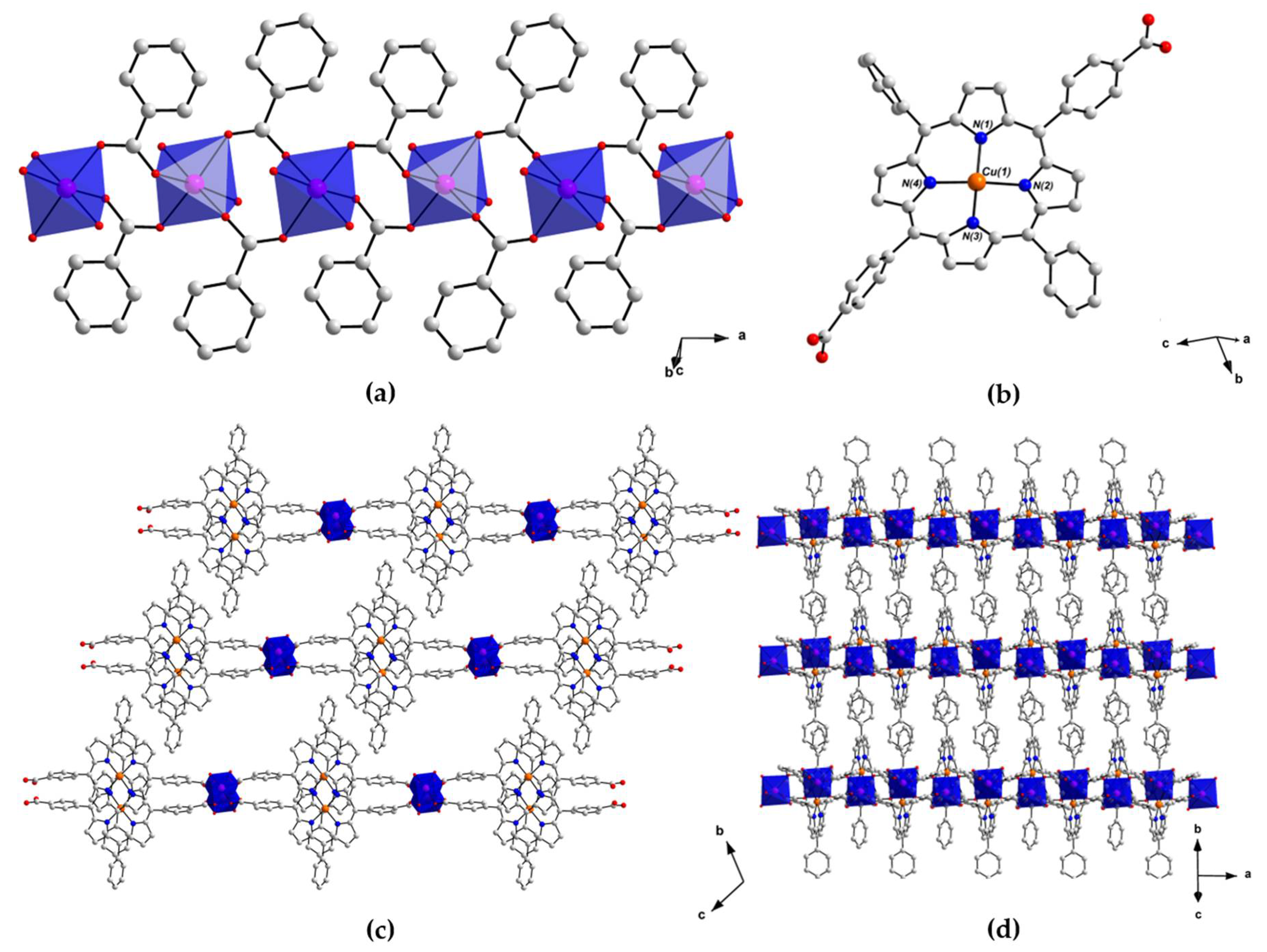
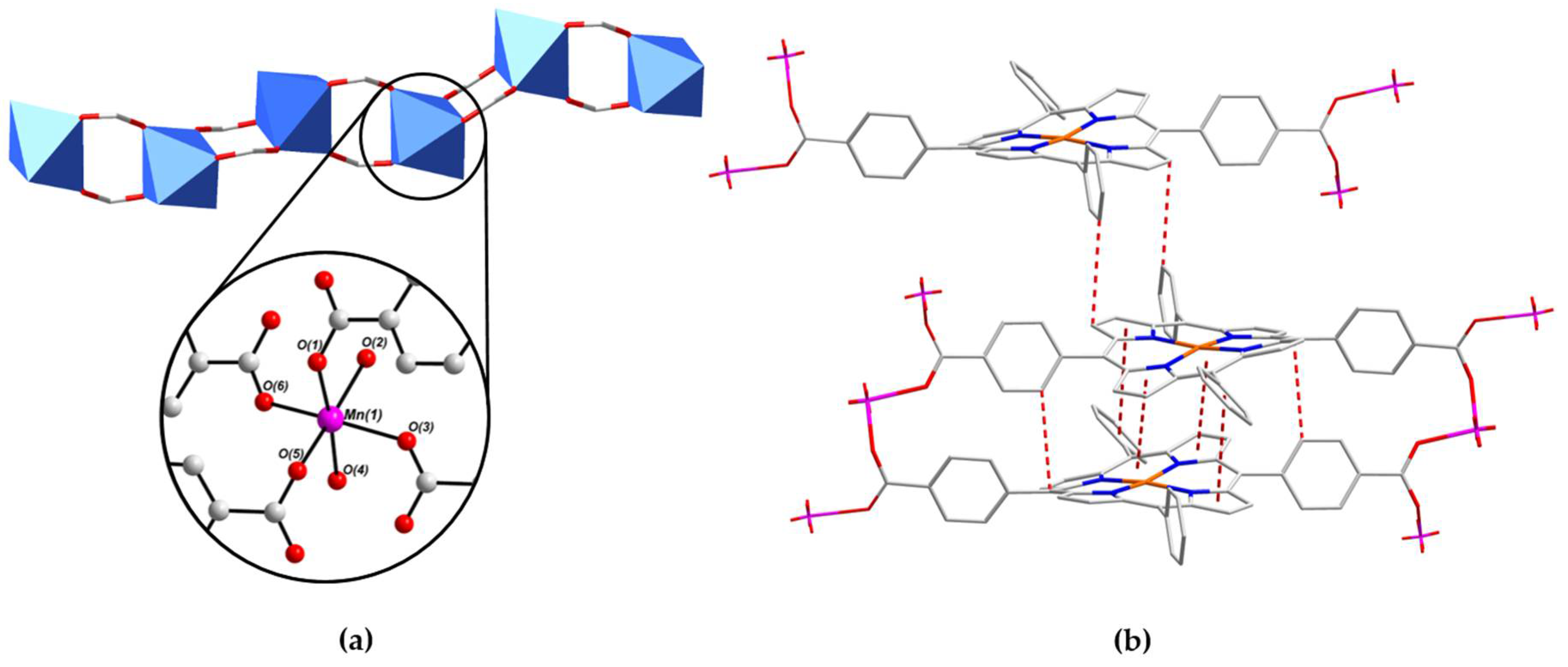
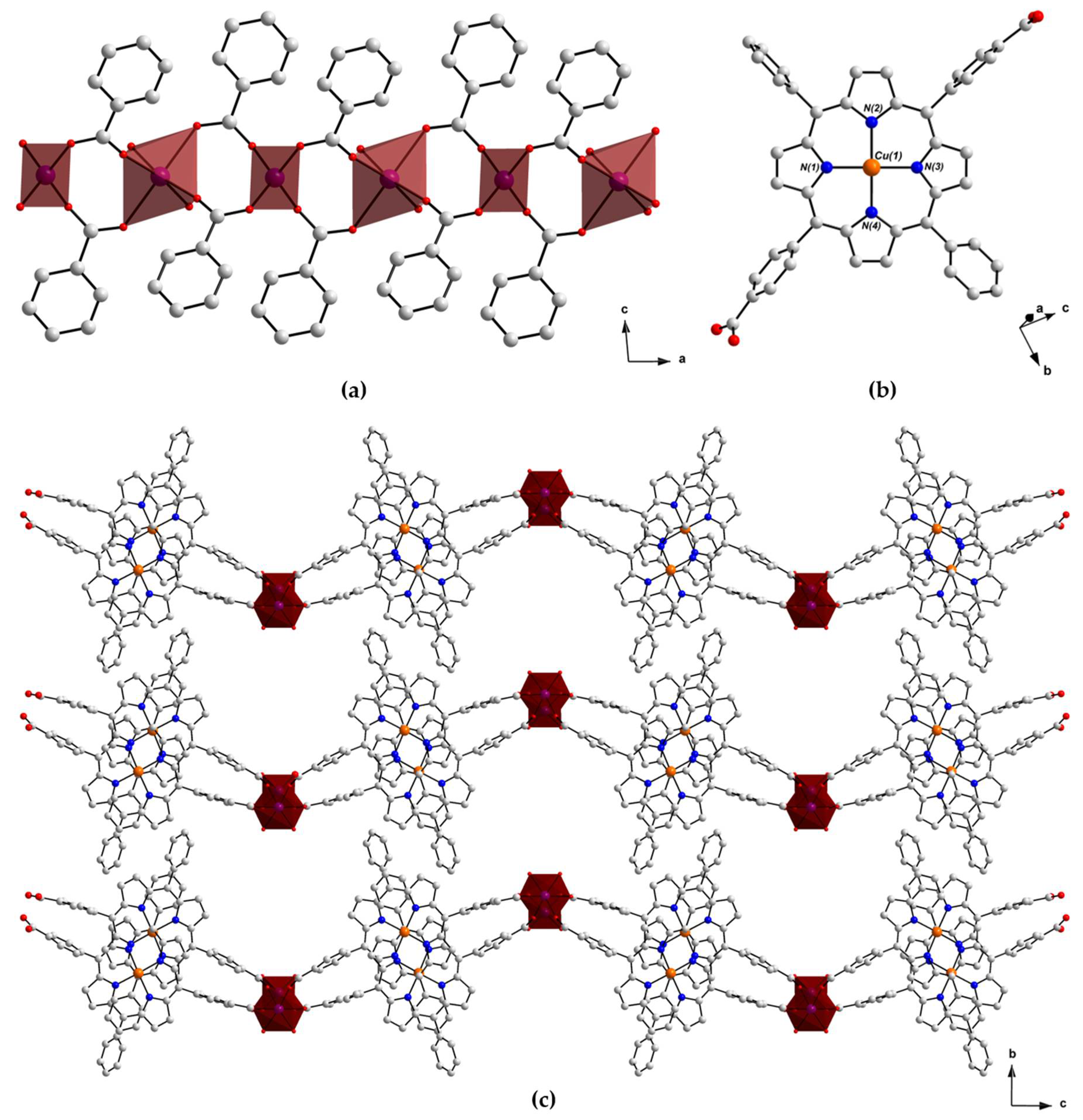
2.2. Crystal Structure of [MnII(L-CuII)(MeOH)2]·DEA·MeOH (PROD-1)
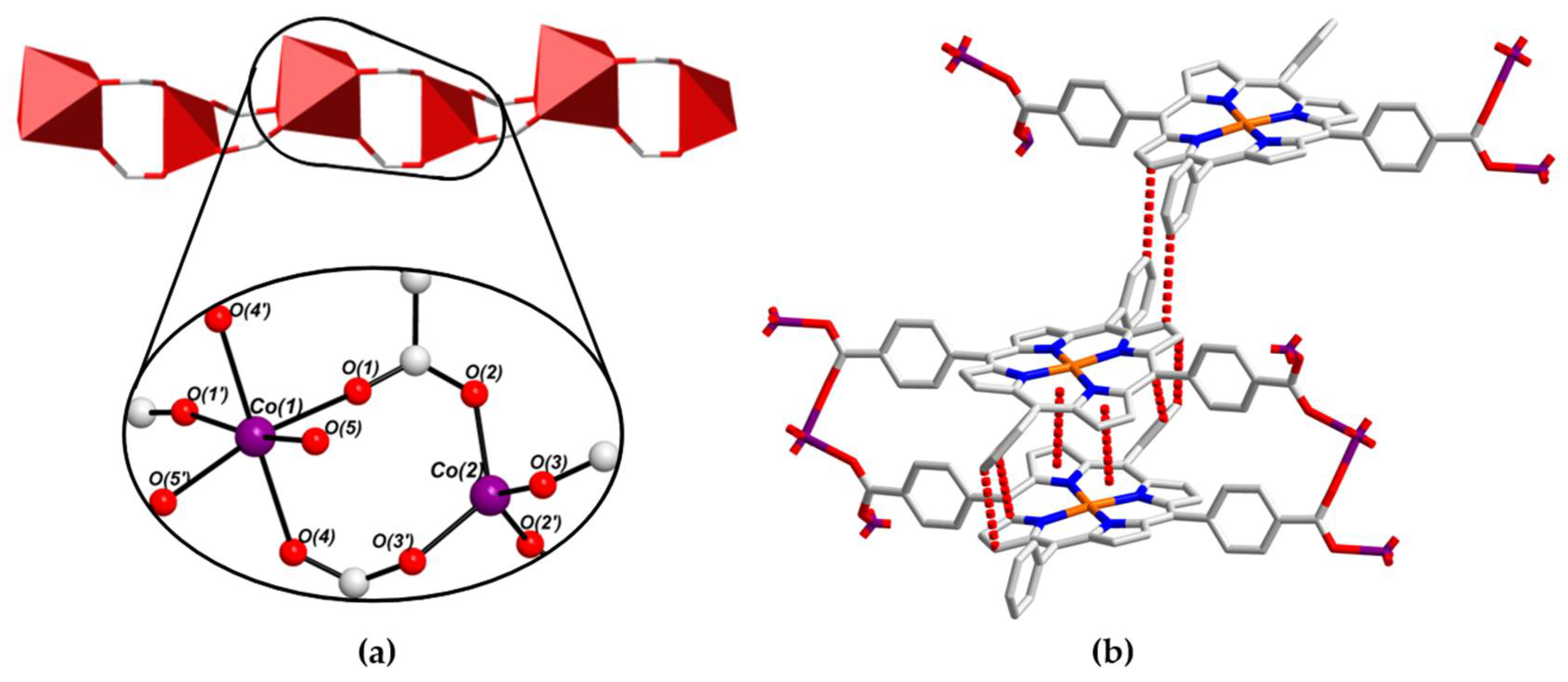
2.3. Crystal Structure of [CoII(L-CuII)(DEA)]·8MeOH (PROD-2)
2.4. Physicochemical Characterisation of PROD-1 & PROD-2
3. Materials and Methods
3.1. Reagents & Analytical Methods
3.2. Synthesis of the Porphyrin Ligand H2L-CuII
3.3. Synthesis of [MnII(L-CuII)(MeOH)2]·DEA·MeOH (PROD-1)
3.4. Synthesis of [CoII(L-CuII)(DEA)]·8MeOH (PROD-2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Cheong, V.F.; Moh, P.Y. Recent Advancement in Metal–Organic Framework: Synthesis, Activation, Functionalisation, and Bulk Production. Mater. Sci. Technol. 2018, 34, 1025–1045. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Jaheon, K.; Nathaniel, R.; David, V.; Joseph, W.; O’Keeffe, M.; Yaghi, O.M. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular Chemistry: Secondary Building Units as a Basis for the Design of Highly Porous and Robust Metal−Organic Carboxylate Frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef]
- Silva, P.; Vilela, S.M.F.; Tomé, J.P.C.; Almeida Paz, F.A. Multifunctional Metal–Organic Frameworks: From Academia to Industrial Applications. Chem. Soc. Rev. 2015, 44, 6774–6803. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Adeel, M.; Rasheed, T.; Iqbal, H.M.N. Multifunctional Metal–Organic Frameworks-Based Biocatalytic Platforms: Recent Developments and Future Prospects. J. Mater. Res. Technol. 2019, 8, 2359–2371. [Google Scholar] [CrossRef]
- Kalmutzki, M.J.; Hanikel, N.; Yaghi, O.M. Secondary Building Units as the Turning Point in the Development of the Reticular Chemistry of MOFs. Sci. Adv. 2018, 4, 9180. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.; Macreadie, L.K.; Sensharma, D.; Vaesen, S.; Zhang, X.; Gough, J.J.; O′Doherty, M.; Zhu, N.-Y.; Rüther, M.; O’Brien, J.E.; et al. Light-Harvesting, 3rd Generation RuII/CoII MOF with a Large, Tubular Channel Aperture. Chem. Commun. 2019, 55, 5013–5016. [Google Scholar] [CrossRef]
- Sensharma, D.; Zhu, N.; Tandon, S.; Vaesen, S.; Watson, G.W.; Schmitt, W. Flexible Metal–Organic Frameworks for Light-Switchable CO2 Sorption Using an Auxiliary Ligand Strategy. Inorg. Chem. 2019, 58, 9766–9772. [Google Scholar] [CrossRef]
- Tobin, G.; Comby, S.; Zhu, N.; Clérac, R.; Gunnlaugsson, T.; Schmitt, W. Towards Multifunctional Lanthanide-Based Metal–Organic Frameworks. Chem. Commun. 2015, 51, 13313–13316. [Google Scholar] [CrossRef]
- Hawes, C.S.; Ó′Máille, G.M.; Byrne, K.; Schmitt, W.; Gunnlaugsson, T. Tetraarylpyrrolo[3,2-b]Pyrroles as Versatile and Responsive Fluorescent Linkers in Metal–Organic Frameworks. Dalton Trans. 2018, 47, 10080–10092. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Eddaoudi, M.; O′Keeffe, M.; Yaghi, O.M. Design and Synthesis of an Exceptionally Stable and Highly Porous Metal-Organic Framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Chui, S.S.-Y.; Lo, S.M.-F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A Chemically Functionalizable Nanoporous Material [Cu3(TMA)2(H2O)3]N. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Kim, D.; Liu, X.; Lah, M.S. Topology Analysis of Metal–Organic Frameworks Based on Metal–Organic Polyhedra as Secondary or Tertiary Building Units. Inorg. Chem. Front. 2015, 2, 336–360. [Google Scholar] [CrossRef]
- Britt, D.; Tranchemontagne, D.; Yaghi, O.M. Metal-Organic Frameworks with High Capacity and Selectivity for Harmful Gases. Proc. Natl. Acad. Sci. USA 2008, 105, 11623–11627. [Google Scholar] [CrossRef]
- Kuppler, R.J.; Timmons, D.J.; Fang, Q.-R.; Li, J.-R.; Makal, T.A.; Young, M.D.; Yuan, D.; Zhao, D.; Zhuang, W.; Zhou, H.-C. Potential Applications of Metal-Organic Frameworks. Coord. Chem. Rev. 2009, 253, 3042–3066. [Google Scholar] [CrossRef]
- Aulakh, D.; Pyser, J.B.; Zhang, X.; Yakovenko, A.A.; Dunbar, K.R.; Wriedt, M. Metal–Organic Frameworks as Platforms for the Controlled Nanostructuring of Single-Molecule Magnets. J. Am. Chem. Soc. 2015, 137, 9254–9257. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Strategies for Hydrogen Storage in Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2005, 44, 4670–4679. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective Gas Adsorption and Separation in Metal-Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Lee, C.Y.; Farha, O.K.; Hong, B.J.; Sarjeant, A.A.; Nguyen, S.T.; Hupp, J.T. Light-Harvesting Metal–Organic Frameworks (MOFs): Efficient Strut-to-Strut Energy Transfer in Bodipy and Porphyrin-Based MOFs. J. Am. Chem. Soc. 2011, 133, 15858–15861. [Google Scholar] [CrossRef] [PubMed]
- Sensharma, D.; Vaesen, S.; Healy, C.; Hartmann, J.; Kathalikkattil, A.C.; Wix, P.; Steuber, F.; Zhu, N.; Schmitt, W. CO2 Adsorption in SIFSIX-14-Cu-i: High Performance, Inflected Isotherms, and Water-Triggered Release via Reversible Structural Transformation. Eur. J. Inorg. Chem. 2018, 1993–1997. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional Porous Coordination Polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Applications of Metal-Organic Frameworks in Heterogeneous Supramolecular Catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; García, H.; Llabrés i Xamena, F.X. Engineering Metal Organic Frameworks for Heterogeneous Catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef] [PubMed]
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal–Organic Frameworks: Opportunities for Catalysis. Angew. Chem. Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef]
- Fujita, M.; Kwon, Y.J.; Washizu, S.; Ogura, K. Preparation, Clathration Ability, and Catalysis of a Two-Dimensional Square Network Material Composed of Cadmium(II) and 4,4’-Bipyridine. J. Am. Chem. Soc. 1994, 116, 1151–1152. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Yang, Y.; Li, L.; Zhang, S.; Ruffley, J.P.; Jarvi, A.G.; Saxena, S.; Veser, G.; Johnson, J.K.; Rosi, N.L. Designing Open Metal Sites in Metal–Organic Frameworks for Paraffin/Olefin Separations. J. Am. Chem. Soc. 2019, 141, 13003–13007. [Google Scholar] [CrossRef]
- Li, B.; Chrzanowski, M.; Zhang, Y.; Ma, S. Applications of Metal-Organic Frameworks Featuring Multi-Functional Sites. Coord. Chem. Rev. 2016, 307, 106–129. [Google Scholar] [CrossRef]
- Rogge, S.M.J.; Bavykina, A.; Hajek, J.; Garcia, H.; Olivos-Suarez, A.I.; Sepúlveda-Escribano, A.; Vimont, A.; Clet, G.; Bazin, P.; Kapteijn, F.; et al. Metal–Organic and Covalent Organic Frameworks as Single-Site Catalysts. Chem. Soc. Rev. 2017, 46, 3134–3184. [Google Scholar] [CrossRef]
- Pereira, C.; Simões, M.; Tomé, J.; Almeida Paz, F. Porphyrin-Based Metal-Organic Frameworks as Heterogeneous Catalysts in Oxidation Reactions. Molecules 2016, 21, 1348. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-Organic Framework Functionalization and Design Strategies for Advanced Electrochemical Energy Storage Devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef]
- Sun, L.; Campbell, M.G.; Dincă, M. Electrically Conductive Porous Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2016, 55, 3566–3579. [Google Scholar] [CrossRef] [PubMed]
- Gordillo, M.A.; Saha, S. Strategies to Improve Electrical and Ionic Conductivities of Metal–Organic Frameworks. Comment. Inorg. Chem. 2020, 40, 86–106. [Google Scholar] [CrossRef]
- Liu, W.; Yin, R.; Xu, X.; Zhang, L.; Shi, W.; Cao, X. Structural Engineering of Low-Dimensional Metal–Organic Frameworks: Synthesis, Properties, and Applications. Adv. Sci. 2019, 6, 1802373. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, D.J.; Foster, J.A. Metal–Organic Framework Nanosheets (MONs): A New Dimension in Materials Chemistry. J. Mater. Chem. A 2018, 6, 16292–16307. [Google Scholar] [CrossRef]
- Nam, K.W.; Park, S.S.; dos Reis, R.; Dravid, V.P.; Kim, H.; Mirkin, C.A.; Stoddart, J.F. Conductive 2D Metal-Organic Framework for High-Performance Cathodes in Aqueous Rechargeable Zinc Batteries. Nat. Commun. 2019, 10, 4948. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, D. The Synthetic Strategies of Metal–Organic Framework Membranes, Films and 2D MOFs and Their Applications in Devices. J. Mater. Chem. A 2019, 7, 21004–21035. [Google Scholar] [CrossRef]
- Majee, A.K.; Kommini, A.; Aksamija, Z. Electronic Transport and Thermopower in 2D and 3D Heterostructures—A Theory Perspective. Ann. Phys. 2019, 531, 1800510. [Google Scholar] [CrossRef]
- Ko, M.; Mendecki, L.; Mirica, K.A. Conductive Two-Dimensional Metal–Organic Frameworks as Multifunctional Materials. Chem. Commun. 2018, 54, 7873–7891. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, P.; Zhang, C.; Wang, Z.; Wang, C. Energy Transfer on a Two-Dimensional Antenna Enhances the Photocatalytic Activity of CO2 Reduction by Metal–Organic Layers. Chem. Commun. 2019, 55, 9657–9660. [Google Scholar] [CrossRef]
- Fujimoto, J.; Hayashi, S.; Kainuma, H.; Manseki, K.; Udagawa, T.; Miyaji, H. Supramolecular Light-Harvesting Antennas of Metal-Coordinated Bis(8-Hydroxyquinoline)-Substituted Porphyrin Networks. Chem. Asian J. 2019, 14, 2567–2572. [Google Scholar] [CrossRef]
- Smykalla, L.; Mende, C.; Fronk, M.; Siles, P.F.; Hietschold, M.; Salvan, G.; Zahn, D.R.T.; Schmidt, O.G.; Rüffer, T.; Lang, H. (Metallo)Porphyrins for Potential Materials Science Applications. Beilstein J. Nanotechnol. 2017, 8, 1786–1800. [Google Scholar] [CrossRef]
- Senge, M.O.; MacGowan, S.A.; O′Brien, J.M. Conformational Control of Cofactors in Nature—The Influence of Protein-Induced Macrocycle Distortion on the Biological Function of Tetrapyrroles. Chem. Commun. 2015, 51, 17031–17063. [Google Scholar] [CrossRef]
- Gao, W.-Y.; Chrzanowski, M.; Ma, S. Metal-Metalloporphyrin Frameworks: A Resurging Class of Functional Materials. Chem. Soc. Rev. 2014, 43, 5841–5866. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, B.F.; Hoskins, B.F.; Robson, R. A New Type of Infinite 3D Polymeric Network Containing 4-Connected, Peripherally-Linked Metalloporphyrin Building Blocks. J. Am. Chem. Soc. 1991, 113, 3606–3607. [Google Scholar] [CrossRef]
- Son, H.-J.; Jin, S.; Patwardhan, S.; Wezenberg, S.J.; Jeong, N.C.; So, M.; Wilmer, C.E.; Sarjeant, A.A.; Schatz, G.C.; Snurr, R.Q.; et al. Light-Harvesting and Ultrafast Energy Migration in Porphyrin-Based Metal–Organic Frameworks. J. Am. Chem. Soc. 2013, 135, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Kosal, M.E.; Chou, J.-H.; Wilson, S.R.; Suslick, K.S. A Functional Zeolite Analogue Assembled from Metalloporphyrins. Nat. Mater. 2002, 1, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, B. Recent Developments in Metal-Metalloporphyrin Frameworks. Dalton Trans. 2015, 44, 14574–14583. [Google Scholar] [CrossRef]
- Xiao, Y.; Guo, W.; Chen, H.; Li, H.; Xu, X.; Wu, P.; Shen, Y.; Zheng, B.; Huo, F.; Wei, W.D. Ultrathin 2D Cu-Porphyrin MOF Nanosheets as a Heterogeneous Catalyst for Styrene Oxidation. Mater. Chem. Front. 2019, 3, 1580–1585. [Google Scholar] [CrossRef]
- Nakagaki, S.; Ferreira, G.; Ucoski, G.; Dias de Freitas Castro, K. Chemical Reactions Catalyzed by Metalloporphyrin-Based Metal-Organic Frameworks. Molecules 2013, 18, 7279. [Google Scholar] [CrossRef] [PubMed]
- Smithenry, D.W.; Wilson, S.R.; Suslick, K.S. A Robust Microporous Zinc Porphyrin Framework Solid. Inorg. Chem. 2003, 42, 7719–7721. [Google Scholar] [CrossRef]
- Castro, K.A.D.; Figueira, F.; Mendes, R.F.; Cavaleiro, J.A.S.; Neves, M.d.G.P.M.; Simões, M.M.Q.; Almeida Paz, F.A.; Tomé, J.P.C.; Nakagaki, S. Copper–Porphyrin–Metal–Organic Frameworks as Oxidative Heterogeneous Catalysts. ChemCatChem 2017, 9, 2939–2945. [Google Scholar] [CrossRef]
- Fateeva, A.; Chater, P.A.; Ireland, C.P.; Tahir, A.A.; Khimyak, Y.Z.; Wiper, P.V.; Darwent, J.R.; Rosseinsky, M.J. A Water-Stable Porphyrin-Based Metal–Organic Framework Active for Visible-Light Photocatalysis. Angew. Chem. Int. Ed. 2012, 51, 7440–7444. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Che, Y.; Zheng, J. Construction of Microporous Metal–Organic Frameworks (MOFs) by Mn–O–C Rod-like Secondary Building Units (SBUs): Solvothermal Synthesis, Structure, Thermostability, and Magnetic Properties. Inorg. Chem. Commun. 2008, 11, 358–362. [Google Scholar] [CrossRef]
- Xiao, Q.; Wu, Y.; Li, M.; O′Keeffe, M.; Li, D. A Metal–Organic Framework with Rod Secondary Building Unit Based on the Boerdijk–Coxeter Helix. Chem. Commun. 2016, 52, 11543–11546. [Google Scholar] [CrossRef]
- Rosi, N.L.; Kim, J.; Eddaoudi, M.; Chen, B.; O′Keeffe, M.; Yaghi, O.M. Rod Packings and Metal−Organic Frameworks Constructed from Rod-Shaped Secondary Building Units. J. Am. Chem. Soc. 2005, 127, 1504–1518. [Google Scholar] [CrossRef]
- Rosi, N.L.; Eddaoudi, M.; Kim, J.; O′Keeffe, M.; Yaghi, O.M. Infinite Secondary Building Units and Forbidden Catenation in Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2002, 41, 284–287. [Google Scholar] [CrossRef]
- Barthelet, K.; Marrot, J.; Riou, D.; Férey, G. A Breathing Hybrid Organic–Inorganic Solid with Very Large Pores and High Magnetic Characteristics. Angew. Chem. Int. Ed. 2002, 41, 281–284. [Google Scholar] [CrossRef]
- Shen, J.-R. The Structure of Photosystem II and the Mechanism of Water Oxidation in Photosynthesis. Annu. Rev. Plant Biol. 2015, 66, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Senge, M.O.; Ryan, A.A.; Letchford, K.A.; MacGowan, S.A.; Mielke, T. Chlorophylls, Symmetry, Chirality, and Photosynthesis. Symmetry 2014, 6, 781–843. [Google Scholar] [CrossRef]
- Chen, D.; Xing, H.; Su, Z.; Wang, C. Electrical Conductivity and Electroluminescence of a New Anthracene-Based Metal–Organic Framework with π-Conjugated Zigzag Chains. Chem. Commun. 2016, 52, 2019–2022. [Google Scholar] [CrossRef] [PubMed]
- Gutzler, R.; Perepichka, D.F. π-Electron Conjugation in Two Dimensions. J. Am. Chem. Soc. 2013, 135, 16585–16594. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.S.; Dincă, M. Novel Topology in Semiconducting Tetrathiafulvalene Lanthanide Metal-Organic Frameworks. Isr. J. Chem. 2018, 58, 1119–1122. [Google Scholar] [CrossRef]
- Xie, L.S.; Alexandrov, E.V.; Skorupskii, G.; Proserpio, D.M.; Dincă, M. Diverse π–π Stacking Motifs Modulate Electrical Conductivity in Tetrathiafulvalene-Based Metal–Organic Frameworks. Chem. Sci. 2019, 10, 8558–8565. [Google Scholar] [CrossRef]
- Narayan, T.C.; Miyakai, T.; Seki, S.; Dincă, M. High Charge Mobility in a Tetrathiafulvalene-Based Microporous Metal–Organic Framework. J. Am. Chem. Soc. 2012, 134, 12932–12935. [Google Scholar] [CrossRef]
- Li, W.-J.; Tu, M.; Cao, R.; Fischer, R.A. Metal–Organic Framework Thin Films: Electrochemical Fabrication Techniques and Corresponding Applications & Perspectives. J. Mater. Chem. A 2016, 4, 12356–12369. [Google Scholar] [CrossRef]
- Jiang, C.; Moniz, S.J.A.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical Devices for Solar Water Splitting—Materials and Challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Zhao, C. Ultrathin Metal-Organic Framework Array for Efficient Electrocatalytic Water Splitting. Nat. Commun. 2017, 8, 15341. [Google Scholar] [CrossRef]
- Haldar, R.; Jakoby, M.; Mazel, A.; Zhang, Q.; Welle, A.; Mohamed, T.; Krolla, P.; Wenzel, W.; Diring, S.; Odobel, F.; et al. Anisotropic Energy Transfer in Crystalline Chromophore Assemblies. Nat. Commun. 2018, 9, 4332. [Google Scholar] [CrossRef]
- Zeng, K.; Tong, Z.; Ma, L.; Zhu, W.-H.; Wu, W.; Xie, Y. Molecular engineering strategies for fabricating efficient porphyrin-based dye-sensitized solar cells. Energy Environ. Sci. 2020, 13, 1617–1657. [Google Scholar] [CrossRef]
- Zeng, K.; Chen, Y.; Zhu, W.-Y.; Tian, H.; Xie, Y. Efficient Solar Cells Based on Concerted Companion Dyes Containing Two Complementary Components: An Alternative Approach for Cosensitization. J. Am. Chem. Soc. 2020, 142, 5154–5161. [Google Scholar] [CrossRef]
- Zou, J.; Yan, Q.; Li, C.; Lu, Y.; Tong, Z.; Xie, Y. Light-Absorbing Pyridine Derivative as a New Electrolyte Additive for Developing Efficient Porphyrin Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 57017–57024. [Google Scholar] [CrossRef]
- Kurumisawa, Y.; Higashino, T.; Nimura, S.; Tsuji, Y.; Iiyama, Y.; Imahori, Y. Renaissance of Fused Porphyrins: Substituted Methylene-Bridged Thiophene-Fused Strategy for High-Performance Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2019, 141, 9910–9919. [Google Scholar] [CrossRef]
- Li, C.; Yin, J.; Chen, R.; Lv, X.; Feng, X.; Wu, Y.; Cao, J. Monoammonium Porphyrin for Blade-Coating Stable Large-Area Perovskite Solar Cells with >18% Efficiency. J. Am. Chem. Soc. 2019, 141, 6345–6351. [Google Scholar] [CrossRef] [PubMed]
- Zha, Q.; Rui, X.; Wei, T.; Xie, Y. Recent advances in the design strategies for porphyrin-based coordination polymers. CrystEngComm. 2014, 16, 7371–7384. [Google Scholar] [CrossRef]
- Lu, W.; Wei, Z.; Gu, Z.-Y.; Liu, T.-F.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Gentle, T., III; et al. Tuning the Structure and Function of Metal–Organic Frameworks via Linker Design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef] [PubMed]
- Schoedel, A.; Li, M.; Li, D.; O′Keeffe, M.; Yaghi, O.M. Structures of Metal–Organic Frameworks with Rod Secondary Building Units. Chem. Rev. 2016, 116, 12466–12535. [Google Scholar] [CrossRef]
- Fang, Q.-R.; Zhu, G.-S.; Jin, Z.; Ji, Y.-Y.; Ye, J.-W.; Xue, M.; Yang, H.; Wang, Y.; Qiu, S.-L. Mesoporous Metal–Organic Framework with Rare Etb Topology for Hydrogen Storage and Dye Assembly. Angew. Chem. Int. Ed. 2007, 46, 6638–6642. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, H.-C. Recent Progress in the Synthesis of Metal–Organic Frameworks. Sci. Technol. Adv. Mater. 2015, 16, 54202. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; He, C.; Lin, W. Nanoscale Metal–Organic Framework for Highly Effective Photodynamic Therapy of Resistant Head and Neck Cancer. J. Am. Chem. Soc. 2014, 136, 16712–16715. [Google Scholar] [CrossRef]
- Verduzco, J.M.; Chung, H.; Hu, C.; Choe, W. Metal−Organic Framework Assembled from T-Shaped and Octahedral Nodes: A Mixed-Linker Strategy to Create a Rare Anatase TiO2 Topology. Inorg. Chem. 2009, 48, 9060–9062. [Google Scholar] [CrossRef]
- Görbitz, C.H. The Development and Use of a Crystallographic Database. Acta Cryst. 2016, B72, 167–168. [Google Scholar] [CrossRef]
- Adler, A.D.; Longo, F.R.; Kampas, F.; Kim, J. On the Preparation of Metalloporphyrins. J. Inorg. Nucl. Chem. 1970, 32, 2443–2445. [Google Scholar] [CrossRef]
- Johnson, J.A.; Luo, J.; Zhang, X.; Chen, Y.-S.; Morton, M.D.; Echeverría, E.; Torres, F.E.; Zhang, J. Porphyrin-Metalation-Mediated Tuning of Photoredox Catalytic Properties in Metal–Organic Frameworks. ACS Catal. 2015, 5, 5283–5291. [Google Scholar] [CrossRef]
- Chen, H.-C.; Reek, J.N.H.; Williams, R.M.; Brouwer, A.M. Halogenated Earth Abundant Metalloporphyrins as Photostable Sensitizers for Visible-Light-Driven Water Oxidation in a Neutral Phosphate Buffer Solution. Phys. Chem. Chem. Phys. 2016, 18, 15191–15198. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, Y.; Zhang, Z.; Zhang, W.; Lai, W.; Wang, Y.; Cao, R. Low Overpotential Water Oxidation at Neutral PH Catalyzed by a Copper(ii) Porphyrin. Chem. Sci. 2019, 10, 2613–2622. [Google Scholar] [CrossRef] [PubMed]
- Uranga, J.; Matxain, J.M.; Lopez, X.; Ugalde, J.M.; Casanova, D. Photosensitization Mechanism of Cu(ii) Porphyrins. Phys. Chem. Chem. Phys. 2017, 19, 20533–20540. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. The Recent Development of Efficient Earth-Abundant Transition-Metal Nanocatalysts. Chem. Soc. Rev. 2017, 46, 816–854. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.-W.; Xie, C.-X.; Yang, H. Poly[[Diaquabis([μ]3-3,5-Dicarboxybenzoato-[κ]3O1:O3:O5)Bis([μ]3-5-Carboxybenzene-1,3-Dicarboxylato-[κ]3O1:O3:O5)Tetrakis(Methylformamide-[κ]O)Trimanganese(II)] Dimethylformamide Tetrasolvate]. Acta Cryst. 2012, E68, 697–698. [Google Scholar] [CrossRef]
- Mondal, K.C.; Sengupta, O.; Nethaji, M.; Mukherjee, P.S. Assembling Metals (CoII and MnII) with Pyridylcarboxylates in the Presence of Azide: Synthesis, Structural Aspects and Magnetic Behavior of Three Coordination Polymers. Dalton Trans. 2008, 767–775. [Google Scholar] [CrossRef]
- Deenadayalan, M.S.; Sharma, N.; Verma, P.K.; Nagaraja, C.M. Visible-Light-Assisted Photocatalytic Reduction of Nitroaromatics by Recyclable Ni(II)-Porphyrin Metal–Organic Framework (MOF) at RT. Inorg. Chem. 2016, 55, 5320–5327. [Google Scholar] [CrossRef]
- Hunter, C.A.; Sanders, J.K.M. The Nature of π-π Interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Kingsbury, C.J.; Senge, M.O. The Shape of Porphyrins. Coord. Chem. Rev. 2021, 431, 213760. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and Analysis of Crystal Structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A Tool for the Calculation of the Disordered Solvent Contribution to the Calculated Structure Factors. Acta Cryst. 2015, C71, 9–18. [Google Scholar] [CrossRef]
- Gutschke, S.O.H.; Price, D.J.; Powell, A.K.; Wood, P.T. Hydrothermal Synthesis, Structure, and Magnetism of [Co2(OH){1,2,3-(O2C)3C6H3}(H2O)]⋅H2O and [Co2(OH){1,2,3-(O2C)3C6H3}]: Magnetic Δ-Chains with Mixed Cobalt Geometries. Angew. Chem. Int. Ed. 2001, 40, 1920–1923. [Google Scholar] [CrossRef]
- Wang, X.-W.; Chen, Y.; Chen, J.-Z.; Liu, J.-H.; Han, L. Structure and Stability of a Linear Trinuclear Cobalt(II) Complex: Co3(PhCH=CHCO2)6(Bpy)2. Z. Naturforschung 2008, 63, 129–133. [Google Scholar] [CrossRef]
- Jiang, W.-H.; Zhang, H.-Z.; Hou, G.-F.; Ma, D.-S.; Liu, B.; Yu, Y.-H. Five Co(ii) Coordination Polymers with Different Counter Anions Based on [3,5-Di(4H-1,2,4-Triazol-4-Yl)Benzoato]—Ligand: Directed Synthesis, Structures and Magnetic Properties. RSC Adv. 2017, 7, 45641–45651. [Google Scholar] [CrossRef]
- Pratik, S.M.; Gagliardi, L.; Cramer, C.J. Boosting Photoelectric Conductivity in Porphyrin-Based MOFs Incorporating C60. J. Phys. Chem. C 2020, 124, 1878–1887. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, W.; Liu, J.; Fujimori, Y.; Higashino, T.; Imahori, H.; Jiang, X.; Zhao, J.; Sakurai, T.; Hattori, Y.; et al. A New Class of Epitaxial Porphyrin Metal–Organic Framework Thin Films with Extremely High Photocarrier Generation Efficiency: Promising Materials for All-Solid-State Solar Cells. J. Mater. Chem. A 2016, 4, 12739–12747. [Google Scholar] [CrossRef]
- Morzyk-Ociepa, B.; Kokot, M.; Różycka-Sokołowska, E.; Giełzak-Koćwin, K.; Filip-Psurska, B.; Wietrzyk, J.; Michalska, D. Crystal Structure, Infrared and EPR Spectra and Anticancer Activity in Vitro of the Novel Manganese(II) Complexes of Indolecarboxylic Acids. Polyhedron 2014, 67, 464–470. [Google Scholar] [CrossRef]
- Boucher, L.J.; Katz, J.J. The Infared Spectra of Metalloporphyrins. J. Am. Chem. Soc. 1967, 89, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Shundalau, M.B.; Komyak, A.I.; Zazhogin, A.P.; Umreiko, D.S. Structure of the Complex UCl4∙2DMF by Vibrational Infrared Spectroscopy and Density Functional Theory. J. Appl. Spectrosc. 2012, 79, 22–30. [Google Scholar] [CrossRef]
- Bruker. Bruker SMART; Version 5.629, 1997-2003; Bruker-Axs Inc: Madison, WI, USA, 2003. [Google Scholar]
- Bruker. Bruker Saint-Plus; Version 6.22, 1997–2003; Bruker-Axs Inc: Madison, WI, USA, 2003. [Google Scholar]
- Sheldrick, G.M. SHELXTL; Version 5.1, 1998; Bruker Axs-Inc: Madison, WI, USA, 1998. [Google Scholar]
- Laha, J.K.; Dhanalekshmi, S.; Taniguchi, M.; Ambroise, A.; Lindsey, J.S. A Scalable Synthesis of Meso-Substituted Dipyrromethanes. Org. Process. Res. Dev. 2003, 7, 799–812. [Google Scholar] [CrossRef]
- Meindl, A.; Ryan, A.; Flanagan, K.; Senge, M. Synthesis of Long-Wavelength Absorbing Porphyrin m-Benzoic Acids as Molecular Tectons for Surface Studies. Heterocycles 2017, 94, 1518–1541. [Google Scholar] [CrossRef]
- Landrou, G.; Panagiotopoulos, A.A.; Ladomenou, K.; Coutsolelos, A.G. Photochemical Hydrogen Evolution Using Sn-Porphyrin as Photosensitizer and a Series of Cobaloximes as Catalysts. J. Porphyr. Phthalocyanines 2016, 20, 534–541. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elliott, R.; Ryan, A.A.; Aggarwal, A.; Zhu, N.; Steuber, F.W.; Senge, M.O.; Schmitt, W. 2D Porphyrinic Metal-Organic Frameworks Featuring Rod-Shaped Secondary Building Units. Molecules 2021, 26, 2955. https://doi.org/10.3390/molecules26102955
Elliott R, Ryan AA, Aggarwal A, Zhu N, Steuber FW, Senge MO, Schmitt W. 2D Porphyrinic Metal-Organic Frameworks Featuring Rod-Shaped Secondary Building Units. Molecules. 2021; 26(10):2955. https://doi.org/10.3390/molecules26102955
Chicago/Turabian StyleElliott, Rory, Aoife A. Ryan, Aviral Aggarwal, Nianyong Zhu, Friedrich W. Steuber, Mathias O. Senge, and Wolfgang Schmitt. 2021. "2D Porphyrinic Metal-Organic Frameworks Featuring Rod-Shaped Secondary Building Units" Molecules 26, no. 10: 2955. https://doi.org/10.3390/molecules26102955
APA StyleElliott, R., Ryan, A. A., Aggarwal, A., Zhu, N., Steuber, F. W., Senge, M. O., & Schmitt, W. (2021). 2D Porphyrinic Metal-Organic Frameworks Featuring Rod-Shaped Secondary Building Units. Molecules, 26(10), 2955. https://doi.org/10.3390/molecules26102955