Evaluation of Anthocyanin Profile and Color in Sweet Cherry Wine: Effect of Sinapic Acid and Grape Tannins during Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Cherry Wines
- CK: normal winemaking, no copigment added.
- BFSA: 300 mg/L sinapic acid added before alcohol fermentation.
- AFSA: 300 mg/L sinapic acid added after alcohol fermentation.
- BFGT: 300 mg/L grape tannins added before alcohol fermentation.
- AFGT: 300 mg/L grape tannins added after alcohol fermentation.
2.3. Total Monomeric Anthocyanins and Polymeric Color
2.4. Determination of the Copigmentation Effect
2.5. Color Evaluation
2.6. UPLC-MS Analysis of Cherry Wine Anthocyanins
2.7. Data Analysis
3. Results
3.1. Effects of Copigments on Total Monomeric Anthocyanins in Cherry Wine
3.2. Evaluation of the Copigmentation Effect of Copigments on Anthocyanins in Cherry Wine
3.3. Evaluation of the Color Effect of Copigments on Anthocyanins in Cherry Wine
3.4. Changes in Anthocyanins and Anthocyanin-Derived Compound in the Cherry Wines
3.5. UV–Vis Spectra of Anthocyanins and Anthocyanin-Derived Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Blando, F.; Oomah, B.D. Sweet and Sour Cherries: Origin, Distribution, Nutritional Composition and Health Benefits. Trends Food Sci. Technol. 2019, 86, 517–529. [Google Scholar] [CrossRef]
- Ballistreri, G.; Continella, A.; Gentile, A.; Amenta, M.; Fabroni, S.; Rapisarda, P. Fruit Quality and Bioactive Compounds Relevant to Human Health of Sweet Cherry (Prunus avium L.) Cultivars Grown in Italy. Food Chem. 2013, 140, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic Compounds Profile and Antioxidant Properties of Six Sweet Cherry (Prunus avium) Cultivars. Food Res. Int. 2017, 97, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Zhong, F.; Tian, R.; Zhang, K.; Zhang, X.; Li, T. Comparative Study of Phenolic Compounds and Antioxidant Activity in Different Species of Cherries. J. Food Sci. 2011, 76, C633–C638. [Google Scholar] [CrossRef] [PubMed]
- Čakar, U.; Petrović, A.; Pejin, B.; Čakar, M.; Živković, M.; Vajs, V.; Đorđević, B. Fruit as a Substrate for a Wine: A Case Study of Selected Berry and Drupe Fruit Wines. Sci. Hortic. 2019, 244, 42–49. [Google Scholar] [CrossRef]
- Xiao, Z.; Fang, L.; Niu, Y.; Yu, H. Effect of Cultivar and Variety on Phenolic Compounds and Antioxidant Activity of Cherry Wine. Food Chem. 2015, 186, 69–73. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and Their Variation in Red Wines I. Monomeric Anthocyanins and Their Color Expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Palencia, L.A.; Talcott, S.T. Chemical Stability of Açai Fruit (Euterpe oleracea Mart.) Anthocyanins as Influenced by Naturally Occurring and Externally Added Polyphenolic Cofactors in Model Systems. Food Chem. 2010, 118, 17–25. [Google Scholar] [CrossRef]
- Turturica, M.; Oancea, A.M.; Rapeanu, G.; Bahrim, G. Anthocyanins: Naturally Occuring Fruit Pigments with Functional Properties. Ann. Univ. Dunarea Jos Galati Fascicle VI Food Technol. 2015, 39, 9–24. [Google Scholar]
- Malaj, N.; Simone, B.; Quartarolo, A.; Russo, N. Spectrophotometric Study of the Copigmentation of Malvidin 3-O-glucoside with p-coumaric, Vanillic and Syringic Acids. Food Chem. 2013, 141, 3614–3620. [Google Scholar] [CrossRef] [PubMed]
- Escribano, T.; Santos-Buelga, C. Anthocyanin Copigmentation–Evaluation, Mechanisms and Implications for the Colour of Red Wines. Curr. Org. Chem. 2012, 16, 715–723. [Google Scholar]
- Boulton, R. The Copigmentation of Anthocyanins and Its Role in the Color of Red Wine: A Critical Review. Am. J. Enol. Vitic 2001, 52, 67–87. [Google Scholar]
- Blanco-Vega, D.; López-Bellido, F.; Alía-Robledo, J.; Hermosín-Gutiérrez, I. HPLC-DAD-ESI-MS/MS Characterization of Pyranoanthocyanins Pigments Formed in Model Wine. J. Agric. Food Chem. 2011, 59, 9523–9531. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Vega, D.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Identification, Content and Distribution of Anthocyanins and Low Molecular Weight Anthocyanin-derived Pigments in Spanish Commercial Red Wines. Food Chem. 2014, 158, 449–458. [Google Scholar] [CrossRef]
- Rentzsch, M.; Schwarz, M.; Winterhalter, P. Pyranoanthocyanins–an Overview on Structures, Occurrence, and Pathways of Formation. Trends Food Sci. Technol. 2007, 18, 526–534. [Google Scholar] [CrossRef]
- Oliveira, J.; Fernandes, V.; Miranda, C.; Santos-Buelga, C.; Silva, A.; Freitas, V.; Mateus, N. Color Properties of Four Cyanidin−Pyruvic Acid Adducts. J. Agric. Food Chem. 2006, 54, 6894–6903. [Google Scholar] [CrossRef]
- De Freitas, V.; Mateus, N. Formation of Pyranoanthocyanins in Red Wines: A New and Diverse Class of Anthocyanin Derivatives. Anal. Bioanal. Chem. 2011, 401, 1463–1473. [Google Scholar] [CrossRef]
- Pina, F.; Oliveira, J.; de Freitas, V. Anthocyanins and Derivatives are More Than Flavylium Cations. Tetrahedron 2015, 71, 3107–3114. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Luo, H.; Ding, L.; Jiang, X.; Li, X.; Jiao, R.; Bai, W. Comparative Study on the Stability and Antioxidant Activity of Six Pyranoanthocyanins Based on Malvidin-3-glucoside. J. Agric. Food Chem. 2020, 68, 2783–2794. [Google Scholar] [CrossRef]
- Heras-Roger, J.; Díaz, C.; Darias-Martín, J. What Gives a Wine Its Strong Red Color? Main Correlations Affecting Copigmentation. J. Agric. Food Chem. 2016, 64, 6567–6574. [Google Scholar] [CrossRef]
- Yawadio, R.; Morita, N. Color Enhancing Effect of Carboxylic Acids on Anthocyanins. Food Chem. 2007, 105, 421–427. [Google Scholar] [CrossRef]
- Ko, A.; Lee, J.S.; Nam, H.; Lee, H. Stabilization of Black Soybean Anthocyanin by Chitosan Nanoencapsulation and Copigmentation. J. Food Biochem. 2016, 41. [Google Scholar] [CrossRef]
- Neves, A.C.; Spranger, M.I.; Zhao, Y.; Leandro, M.C.; Sun, B. Effect of Addition of Commercial Grape Seed Tannins on Phenolic Composition, Chromatic Characteristics, and Antioxidant Activity of Red Wine. J. Agric. Food Chem. 2010, 58, 11775–11782. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Liang, N.N.; Wang, J.; Pan, Q.H.; Duan, C.Q. Effect of the Prefermentative Addition of Five Enological Tannins on Anthocyanins and Color in Red Wines. J. Food Sci. 2013, 78, C25–C30. [Google Scholar] [CrossRef] [PubMed]
- Vivas, N.; Nonier, M.F.; Gaulejac, N.; Absalon, C.; Bertrand, A.; Mirabel, M. Differentiation of Proanthocyanidin Tannins from Seeds, Skins and Stems of Grapes (Vitis vinifera) and Heartwood of Quebracho (Schinopsis balansae) by Matrix-assisted Laser Desorption/Ionization Time-of-flight Mass Spectrometry and Thioacidolysis/Liquid Chromatography/Electrospray Ionization Mass Spectrometry. Anal. Chim. Acta 2004, 513, 247–256. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 0, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Boulton, R. A Method for the Assessment of Copigmentation in Red Wines. In Proceedings of the 47th Annual Meeting of the American Society for Enology and Viticulture, Reno, NV, USA, 26–28 June 1996. [Google Scholar]
- Li, X.; Zhang, L.; Peng, Z.; Zhao, Y.; Wu, K.; Zhou, N.; Yan, Y.; Ramaswamy, H.S.; Sun, J.; Bai, W. The Impact of Ultrasonic Treatment on Blueberry Wine Anthocyanin Color and Its in vitro Anti-oxidant Capacity. Food Chem. 2020, 333, 127455. [Google Scholar] [CrossRef] [PubMed]
- Han, F.L.; Zhang, W.N.; Pan, Q.H.; Zheng, C.R.; Chen, H.Y.; Duan, C.Q. Principal Component Regression Analysis of the Relation Between CIELAB Color and Monomeric Anthocyanins in Young Cabernet Sauvignon Wines. Molecules 2008, 13, 2859–2870. [Google Scholar] [CrossRef] [PubMed]
- Kanha, N.; Surawang, S.; Pitchakarn, P.; Regenstein, J.M.; Laokuldilok, T. Copigmentation of cyanidin 3-O-glucoside with Phenolics: Thermodynamic Data and Thermal Stability. Food Biosci. 2019, 30, 100419. [Google Scholar] [CrossRef]
- Kalisz, S.; Oszmiański, J.; Hładyszowski, J.; Mitek, M. Stabilization of Anthocyanin and Skullcap Flavone Complexes–Investigations with Computer Simulation and Experimental Methods. Food Chem. 2013, 138, 491–500. [Google Scholar] [CrossRef]
- Sinela, A.; Rawat, N.; Mertz, C.; Achir, N.; Fulcrand, H.; Dornier, M. Anthocyanins Degradation during Storage of Hibiscus sabdariffa Extract and Evolution of Its Degradation Products. Food Chem. 2017, 214, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Mao, Y.; Sui, L.; Yang, N.; Li, S.; Zhu, Z.; Wang, C.; Yin, S.; He, J.; He, Y. Degradation of Anthocyanins and Polymeric Color Formation during Heat Treatment of Purple Sweet Potato Extract at Different pH. Food Chem. 2019, 274, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Ertan, K.; Türkyılmaz, M.; Özkan, M. Effects of Natural Copigment Sources in Combination with Sweeteners on the Stability of Anthocyanins in Sour Cherry Nectars. Food Chem. 2019, 294, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Ertan, K.; Türkyılmaz, M.; Özkan, M. Effect of Sweeteners on Anthocyanin Stability and Colour Properties of Sour Cherry and Strawberry Nectars during Storage. J. Food Sci. Technol. 2018, 55, 4346–4355. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, H.; Lou, L.; Chen, Y.; Ye, X.; Chen, J. Copigmentation Effect of Three Phenolic Acids on Color and Thermal Stability of Chinese Bayberry Anthocyanins. Food Sci. Nutr. 2020, 8, 3234–3242. [Google Scholar] [CrossRef] [PubMed]
- Chatonnet, P.; Dubourdieu, D.; Boidron, J.; Lavigne, V. Synthesis of Volatile Phenols by Saccharomyces cerivisae in Wines. J. Sci. Food Agric. 1993, 62, 191–202. [Google Scholar] [CrossRef]
- Akdemir, H.; Silva, A.; Zha, J.; Zagorevski, D.V.; Koffas, M.A.G. Production of Pyranoanthocyanins Using Escherichia coli Co-cultures. Metab. Eng. 2019, 55, 290–298. [Google Scholar] [CrossRef]
- Klisurova, D.; Petrova, I.; Ognyanov, M.; Georgiev, Y.; Denev, P. Co-pigmentation of Black Chokeberry (Aronia melanocarpa) Anthocyanins with Phenolic Co-pigments and Herbal Extracts. Food Chem. 2019, 279, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Czyżowska, A.; Pogorzelski, E. Changes to Polyphenols in the Process of Production of Must and Wines from Blackcurrants and Cherries. Part II. Anthocyanins and Flavanols. Eur. Food Res. Technol. 2004, 218, 355–359. [Google Scholar] [CrossRef]
- Liu, S.; Laaksonen, O.; Yang, W.; Zhang, B.; Yang, B. Pyranoanthocyanins in Bilberry (Vaccinium myrtillus L.) Wines Fermented with Schizosaccharomyces Pombe and Their Evolution during Aging. Food Chem. 2020, 305. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Oliveira, J.; Silva, A.M.; Mateus, N.; De Freitas, V. Oxovitisins: A New Class of Neutral Pyranone-anthocyanin Derivatives in Red Wines. J. Agric. Food Chem. 2010, 58, 8814–8819. [Google Scholar] [CrossRef] [PubMed]





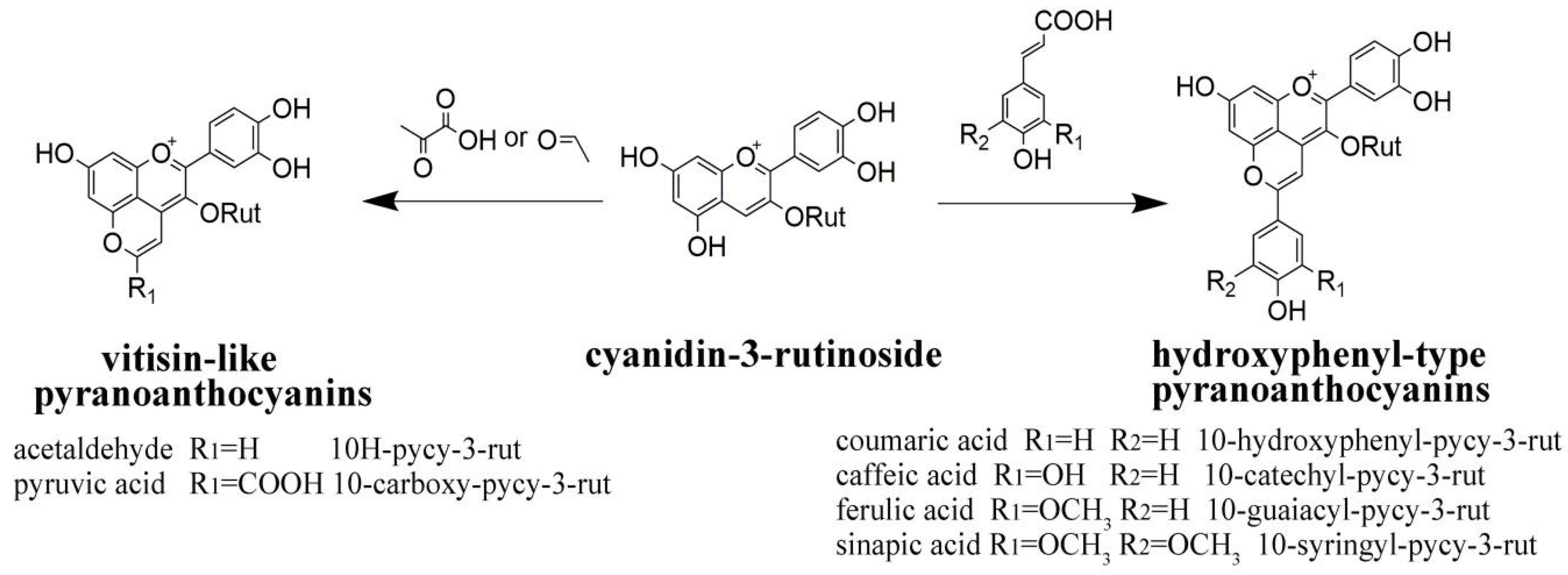


| Time (Month) | Index Tested | CK | BFSA | AFSA | BFGT | AFGT |
|---|---|---|---|---|---|---|
| 0 | λmax | 506.67 ± 1.15 bc | 512.00 ± 0.00 a | 507.33 ± 1.15 b | 504.00 ± 2.00 c | 506.67 ± 2.31 bc |
| Amax | 1.11 ± 0.07 b | 2.18 ± 0.04 a | 1.13 ± 0.06 b | 1.22 ± 0.06 b | 1.12 ± 0.05 b | |
| 3 | λmax | 500.00 ± 3.00 b | 508.00 ± 1.00 a | 503.00 ± 2.65 b | 500.00 ± 0.00 b | 500.33 ± 2.52 b |
| Amax | 0.95 ± 0.03 c | 1.98 ± 0.07 a | 1.07 ± 0.06 b | 1.09 ± 0.03 b | 1.17 ± 0.06 b | |
| 6 | λmax | 501.67 ± 1.53 bc | 506.33 ± 0.58 a | 502.00 ± 0.00 bc | 502.33 ± 2.08 b | 499.33 ± 2.08 c |
| Amax | 0.86 ± 0.03 d | 1.81 ± 0.02 a | 1.02 ± 0.01 bc | 1.00 ± 0.04 c | 1.07 ± 0.04 b | |
| 12 | λmax | 493.00 ± 2 c | 505.67 ± 0.58 a | 500.67 ± 2.89 b | 497.67 ± 1.15 b | 499.67 ± 3.21 b |
| Amax | 0.83 ± 0.02 c | 1.78 ± 0.10 a | 0.94 ± 0.04 b | 0.95 ± 0.02 b | 1.02 ± 0.07 b |
| Peak No. | Compound | RT (min) | [M.H]+ (m/z) | Fragment (m/z) | λvis-max |
|---|---|---|---|---|---|
| 1 | Cyanidin-3-O-rutinoside | 10.7 | 595 | 287 | 519 ± 0 |
| 2 | 10-Carboxy-pyranocyanidin-3-O-rutinoside (vitisin A type) | 11.0 | 663 | 355 | 512 ± 0 |
| 3 | 10H-Pyranocyanidin-3-O-rutinoside (vitisin B type) | 11.8 | 619 | 311 | nd |
| 4 | Peonidin-3-O-rutinoside | 12.2 | 609 | 301 | 519.67 ± 0.58 |
| 5 | 10-Catechyl-pyranocyanidin-3-O-rutinoside | 15.1 | 727 | 419 | 506.67 ± 0.94 |
| 6 | 10-Hydroxyphenyl-pyranocyanidin-3-O-rutinoside | 15.7 | 711 | 403 | 503.33 ± 1.15 |
| 7 | 10-Guaiacyl-pyranocyanidin-3-O-rutinoside | 16.0 | 741 | 433 | 509.67 ± 0.58 |
| 8 | 10-Syringyl-pyranocyanidin-3-rutinoside | 16.2 | 771 | 463 | 514 ± 0 |
| Model | R2 | DW | F | P |
|---|---|---|---|---|
| CI = 1.397 × pe-3-rut | 0.830 | 0.902 | 22.277 | <0.001 |
| H = −0.424 × 10-carboxy-pycy-3-rut + 0.728 × 10-guaiacyl-pycy-3-rut − 0.698 × 10-syringyl-pycy-3-rut | 0.949 | 1.332 | 122.371 | <0.001 |
| Amax = 0.201 × 10-carboxy-pycy + 0.121 × pe-3-rut + 0.180 × 10-syringyl-pycy-3-rut + 0.678 × TA | 0.984 | 1.575 | 404.704 | <0.001 |
| λmax = 0.728 × 10-carboxy-pycy-3-rut − 0.528 × 10H-pycy-3-rut + 0.644 × pe-3-rut | 0.746 | 1.885 | 16.301 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zhao, X.; Sun, Y.; Yang, Z.; Han, G.; Yang, X. Evaluation of Anthocyanin Profile and Color in Sweet Cherry Wine: Effect of Sinapic Acid and Grape Tannins during Aging. Molecules 2021, 26, 2923. https://doi.org/10.3390/molecules26102923
Li M, Zhao X, Sun Y, Yang Z, Han G, Yang X. Evaluation of Anthocyanin Profile and Color in Sweet Cherry Wine: Effect of Sinapic Acid and Grape Tannins during Aging. Molecules. 2021; 26(10):2923. https://doi.org/10.3390/molecules26102923
Chicago/Turabian StyleLi, Mingyue, Xinjie Zhao, Yuxia Sun, Zhen Yang, Guomin Han, and Xue Yang. 2021. "Evaluation of Anthocyanin Profile and Color in Sweet Cherry Wine: Effect of Sinapic Acid and Grape Tannins during Aging" Molecules 26, no. 10: 2923. https://doi.org/10.3390/molecules26102923
APA StyleLi, M., Zhao, X., Sun, Y., Yang, Z., Han, G., & Yang, X. (2021). Evaluation of Anthocyanin Profile and Color in Sweet Cherry Wine: Effect of Sinapic Acid and Grape Tannins during Aging. Molecules, 26(10), 2923. https://doi.org/10.3390/molecules26102923





