Effects of Kynurenic Acid on the Rat Aorta Ischemia—Reperfusion Model: Pharmacological Characterization and Proteomic Profiling
Abstract
1. Introduction
2. Results
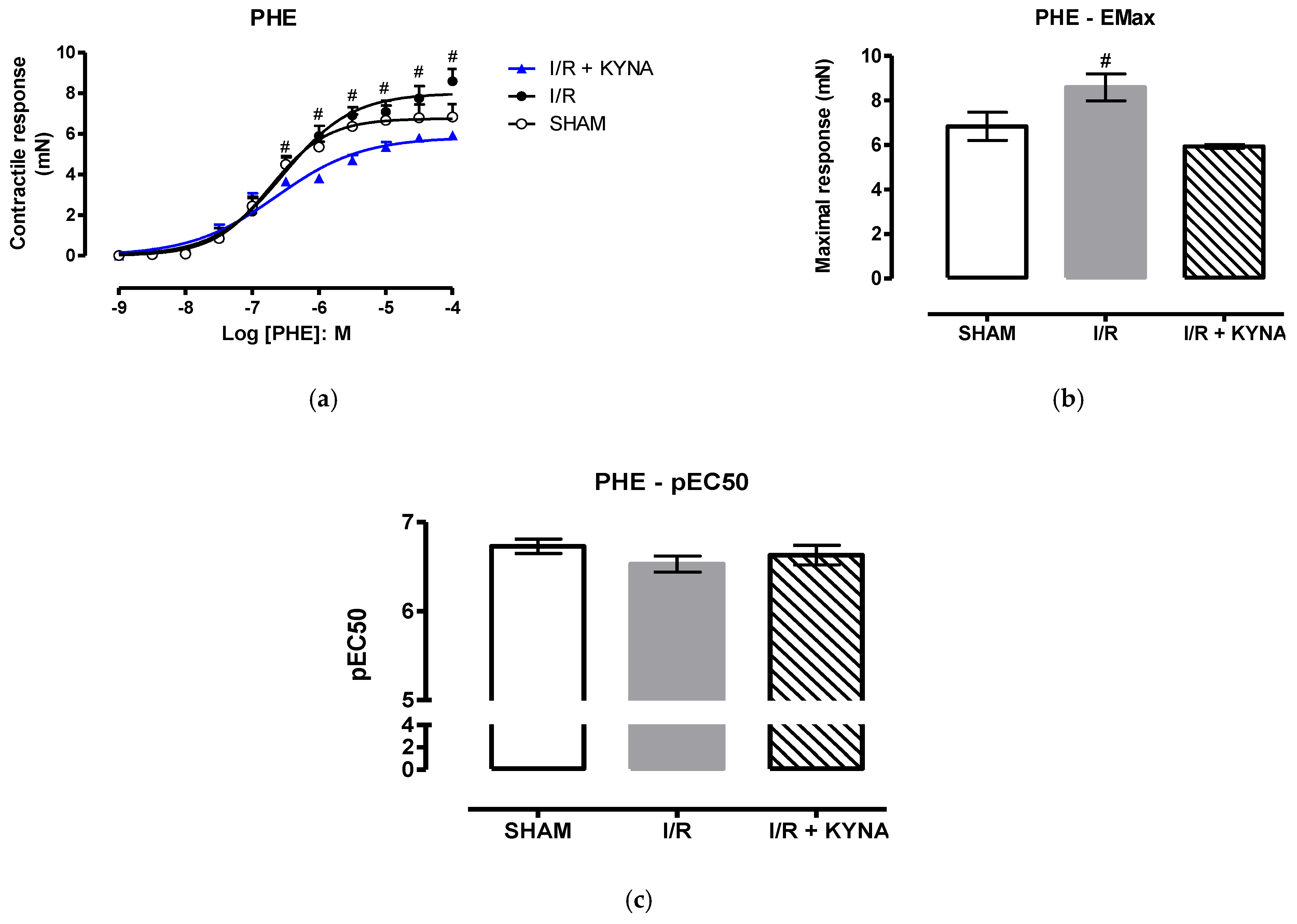
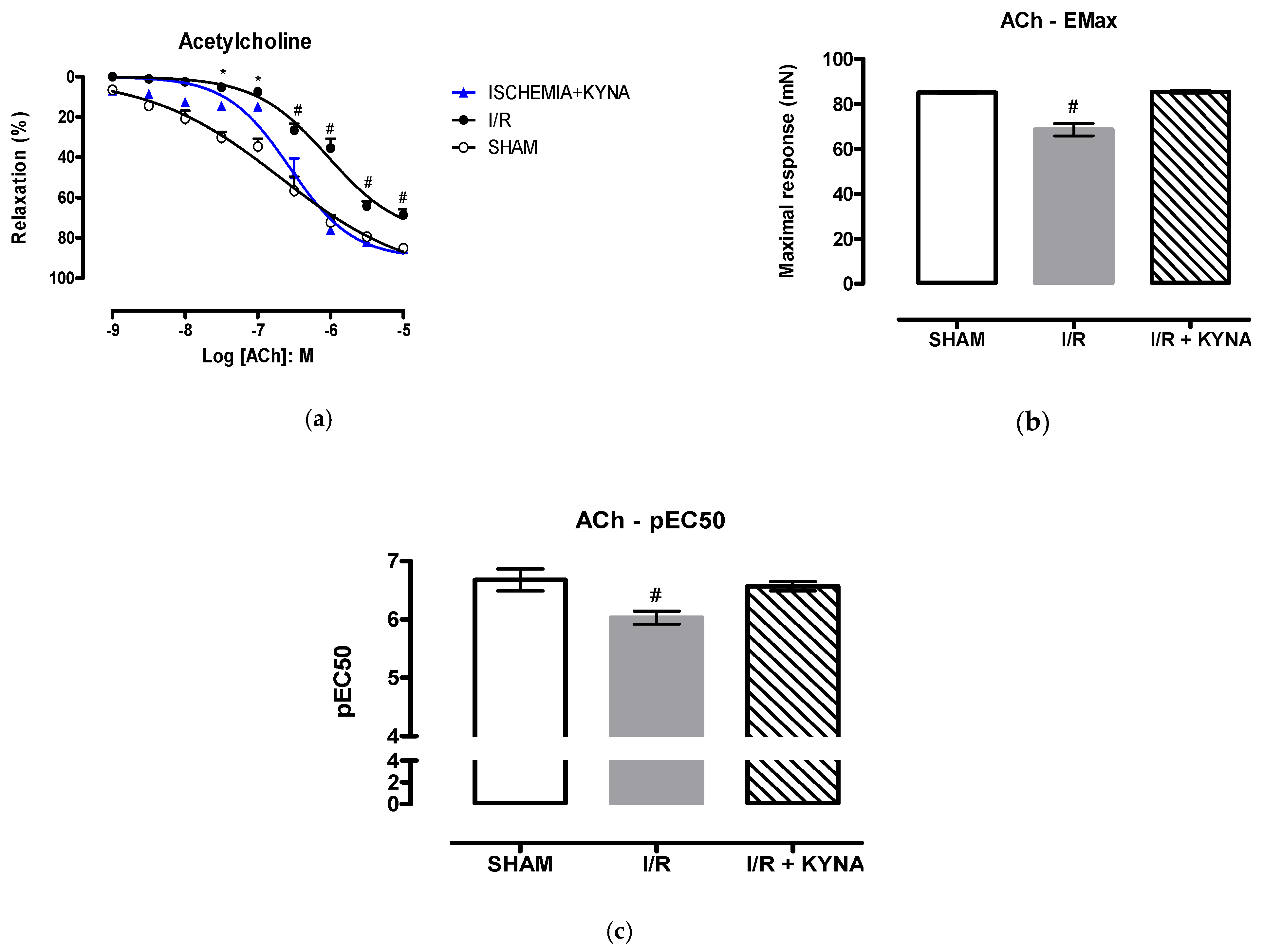
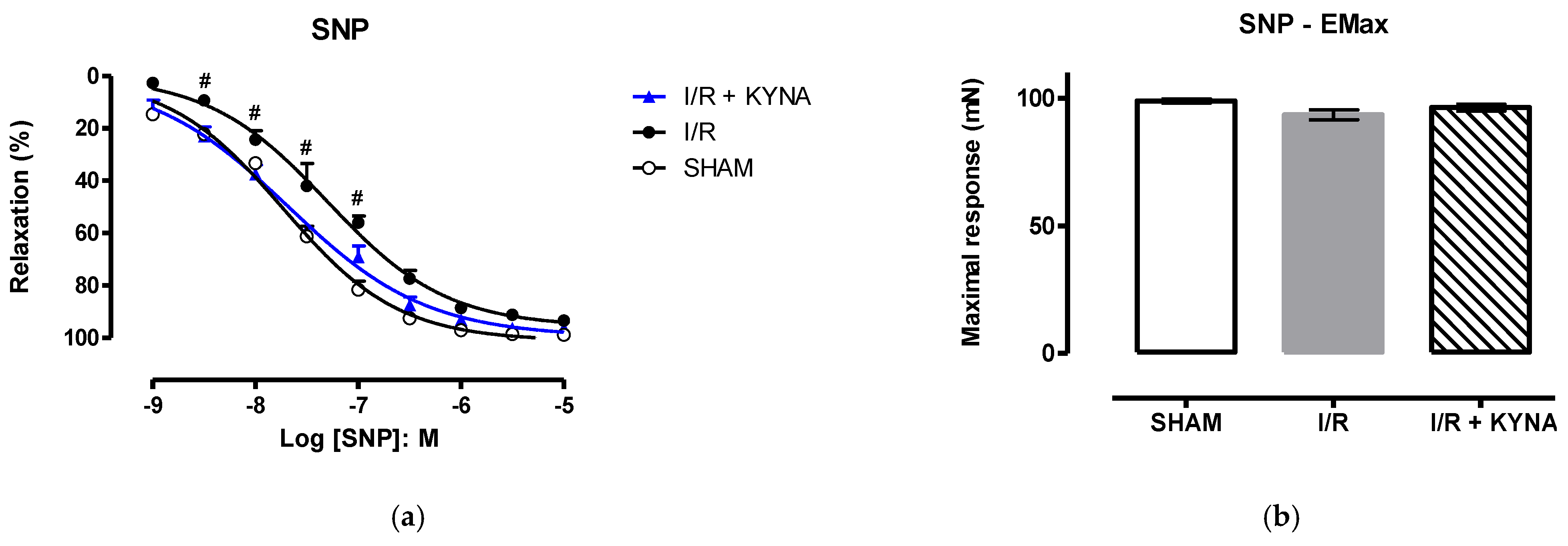
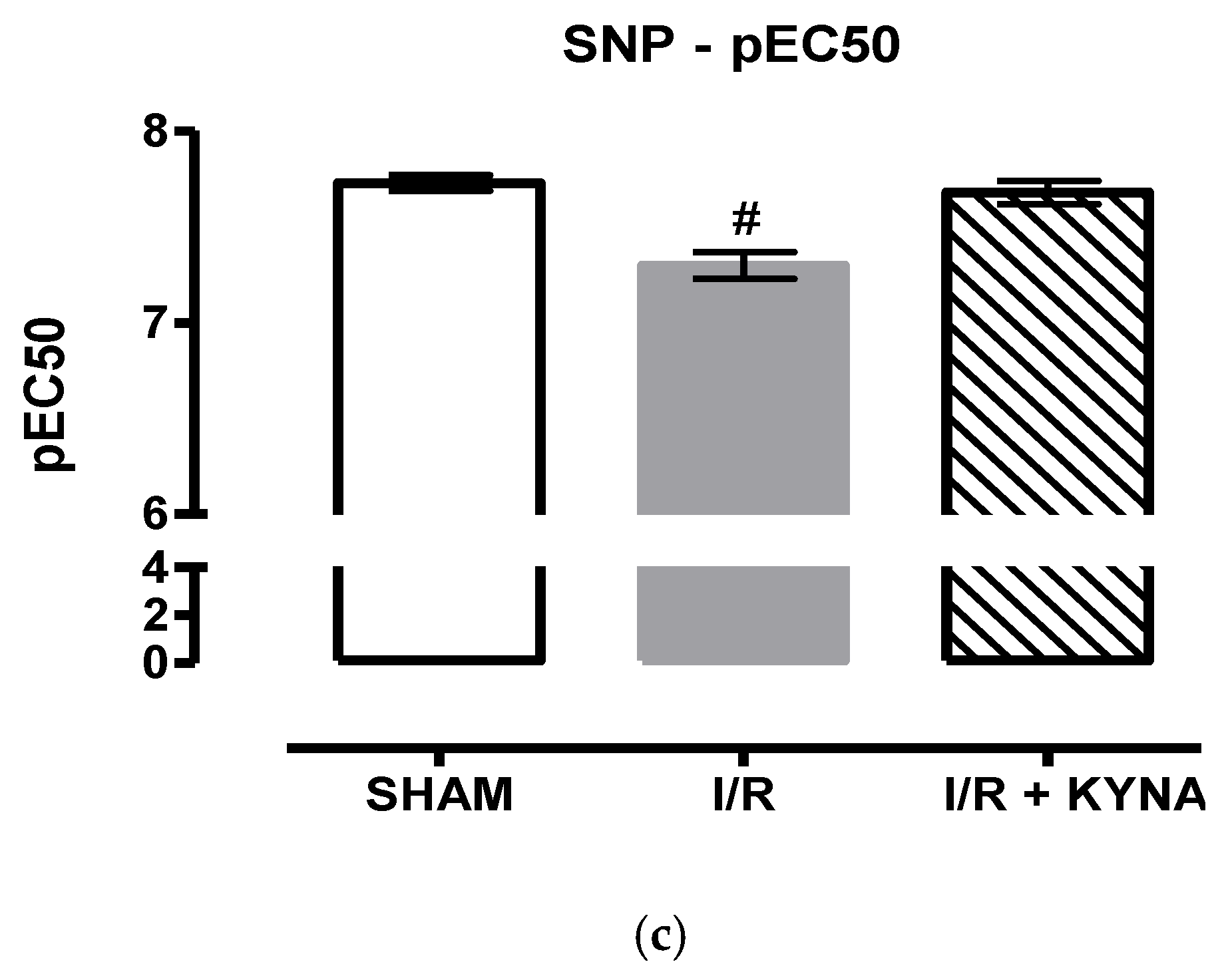
2.1. I/R Model
2.2. Effect of KYNA on Ischemic Aorta Contraction and Relaxation
2.3. Protein Profile f Ischemic Aorta Treated with KYNA
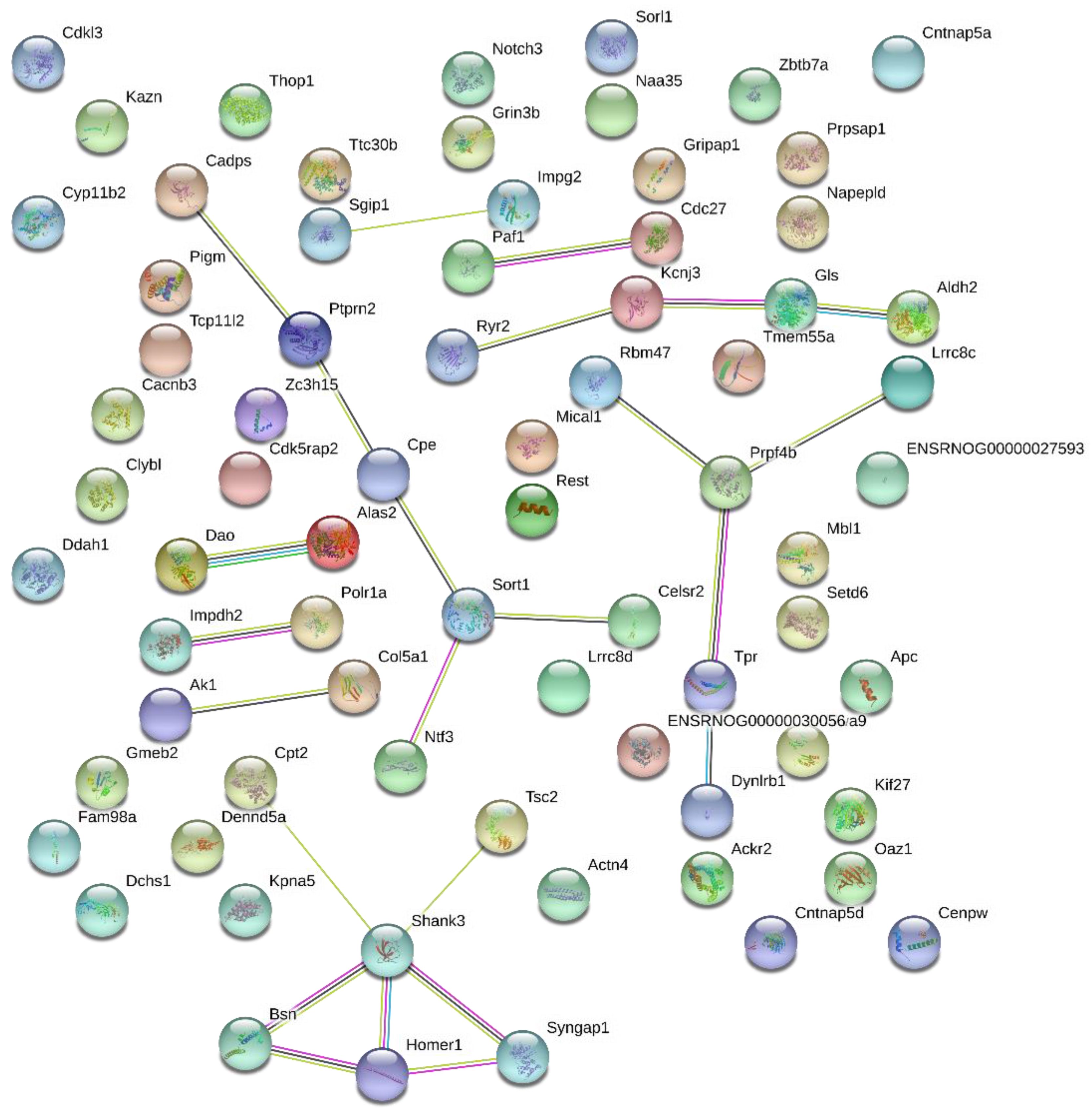
2.4. Protein-Protein Interaction
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. Ischemia/Reperfusion Model
4.4. Functional Studies
4.5. Protein Identification
4.6. Bioinformatic Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Badawy, A.A.-B. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Moroni, F. Tryptophan Metabolism and Brain Function: Focus on Kynurenine and Other Indole Metabolites. Eur. J. Pharmacol. 1999, 375, 87–100. [Google Scholar] [CrossRef]
- Rossi, F.; Schwarcz, R.; Rizzi, M. Curiosity to Kill the KAT (Kynurenine Aminotransferase): Structural Insights into Brain Kynurenic Acid Synthesis. Curr. Opin. Struct. Biol. 2008, 18, 748–755. [Google Scholar] [CrossRef]
- Noguchi, T.; Minatogawa, Y.; Okuno, E.; Nakatani, M.; Morimoto, M.; Kido, R. Purification and Characterization of Kynurenine-2-Oxoglutarate Aminotransferase from the Liver, Brain and Small Intestine of Rats. Biochem. J. 1975, 151, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Stążka, J.; Luchowski, P.; Wielosz, M.; Kleinrok, Z.; Urbańska, E.M. Endothelium-Dependent Production and Liberation of Kynurenic Acid by Rat Aortic Rings Exposed to l-Kynurenine. Eur. J. Pharmacol. 2002, 448, 133–137. [Google Scholar] [CrossRef]
- Ramos-Chávez, L.A.; Lugo Huitrón, R.; González Esquivel, D.; Pineda, B.; Ríos, C.; Silva-Adaya, D.; Sánchez-Chapul, L.; Roldán-Roldán, G.; Pérez de la Cruz, V. Relevance of Alternative Routes of Kynurenic Acid Production in the Brain. Oxidative Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Moroni, F.; Cozzi, A.; Sili, M.; Mannaioni, G. Kynurenic Acid: A Metabolite with Multiple Actions and Multiple Targets in Brain and Periphery. J. Neural Transm. 2012, 119, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Lapin, I.P. Depressor Effect of Kynurenine and Its Metabolites in Rats. Life Sci. 1976, 19, 1479–1483. [Google Scholar] [CrossRef]
- Pocivavsek, A.; Wu, H.-Q.; Potter, M.C.; Elmer, G.I.; Pellicciari, R.; Schwarcz, R. Fluctuations in Endogenous Kynurenic Acid Control Hippocampal Glutamate and Memory. Neuropsychopharmacology 2011, 36, 2357–2367. [Google Scholar] [CrossRef]
- Stone, T.W. Kynurenines in the CNS: From Endogenous Obscurity to Therapeutic Importance. Prog. Neurobiol. 2001, 64, 185–218. [Google Scholar] [CrossRef]
- Resta, F.; Masi, A.; Sili, M.; Laurino, A.; Moroni, F.; Mannaioni, G. Kynurenic Acid and Zaprinast Induce Analgesia by Modulating HCN Channels through GPR35 Activation. Neuropharmacology 2016, 108, 136–143. [Google Scholar] [CrossRef]
- Rothhammer, V.; Quintana, F.J. The Aryl Hydrocarbon Receptor: An Environmental Sensor Integrating Immune Responses in Health and Disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef]
- DiNatale, B.C.; Murray, I.A.; Schroeder, J.C.; Flaveny, C.A.; Lahoti, T.S.; Laurenzana, E.M.; Omiecinski, C.J.; Perdew, G.H. Kynurenic Acid Is a Potent Endogenous Aryl Hydrocarbon Receptor Ligand That Synergistically Induces Interleukin-6 in the Presence of Inflammatory Signaling. Toxicol. Sci. 2010, 115, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Ramprasath, T.; Wang, H.; Zou, M.-H. Abnormal Kynurenine Pathway of Tryptophan Catabolism in Cardiovascular Diseases. Cell. Mol. Life Sci. 2017, 74, 2899–2916. [Google Scholar] [CrossRef]
- Zuo, H.; Ueland, P.M.; Ulvik, A.; Eussen, S.J.P.M.; Vollset, S.E.; Nygård, O.; Midttun, Ø.; Theofylaktopoulou, D.; Meyer, K.; Tell, G.S. Plasma Biomarkers of Inflammation, the Kynurenine Pathway, and Risks of All-Cause, Cancer, and Cardiovascular Disease Mortality. Am. J. Epidemiol. 2016, 183, 249–258. [Google Scholar] [CrossRef]
- Pedersen, E.R.; Tuseth, N.; Eussen, S.J.; Ueland, P.M.; Strand, E.; Svingen, G.F.T.; Midttun, Ø.; Meyer, K.; Mellgren, G.; Ulvik, A.; et al. Associations of Plasma Kynurenines With Risk of Acute Myocardial Infarction in Patients With Stable Angina Pectoris. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Bakhta, O.; Pascaud, A.; Dieu, X.; Beaumont, J.; Kouassi Nzoughet, J.; Kamel, R.; Croyal, M.; Tamareille, S.; Simard, G.; de la Barca, J.M.C.; et al. Tryptophane–Kynurenine Pathway in the Remote Ischemic Conditioning Mechanism. Basic Res. Cardiol. 2020, 115, 13. [Google Scholar] [CrossRef]
- Kato, A.; Suzuki, Y.; Suda, T.; Suzuki, M.; Fujie, M.; Takita, T.; Furuhashi, M.; Maruyama, Y.; Chida, K.; Hishida, A. Relationship between an Increased Serum Kynurenine/Tryptophan Ratio and Atherosclerotic Parameters in Hemodialysis Patients. Hemodial. Int. 2010, 14, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.; Chen, R.; Yeh, Y.; Lin, M.; Hsieh, J.; Chen, S. Kynurenic Acid Attenuates Multiorgan Dysfunction in Rats after Heatstroke. Acta Pharmacol. Sin. 2011, 32, 167–174. [Google Scholar] [CrossRef]
- Nahomi, R.B.; Nam, M.-H.; Rankenberg, J.; Rakete, S.; Houck, J.A.; Johnson, G.C.; Stankowska, D.L.; Pantcheva, M.B.; MacLean, P.S.; Nagaraj, R.H. Kynurenic Acid Protects Against Ischemia/Reperfusion-Induced Retinal Ganglion Cell Death in Mice. Int. J. Mol. Sci. 2020, 21, 1795. [Google Scholar] [CrossRef]
- Colpo, G.D.; Venna, V.R.; McCullough, L.D.; Teixeira, A.L. Systematic Review on the Involvement of the Kynurenine Pathway in Stroke: Pre-Clinical and Clinical Evidence. Front. Neurol. 2019, 10, 778. [Google Scholar] [CrossRef]
- Zaragoza, C.; Gomez-Guerrero, C.; Martin-Ventura, J.L.; Blanco-Colio, L.; Lavin, B.; Mallavia, B.; Tarin, C.; Mas, S.; Ortiz, A.; Egido, J. Animal Models of Cardiovascular Diseases. J. Biomed. Biotechnol. 2011, 2011. [Google Scholar] [CrossRef]
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; al Suwaidi, S.K.B.M.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef] [PubMed]
- Zammert, M.; Gelman, S. The Pathophysiology of Aortic Cross-Clamping. Best Pract. Res. Clin. Anaesthesiol. 2016, 30, 257–269. [Google Scholar] [CrossRef]
- Omer, K.; Nermin, G.; Ali, A.; Mehmet, A.; Unal, D.; Sezen, K.S.; Hakan, K. Lesão de Isquemia-reperfusão Induzida Por Torniquete: Comparação Dos Efeitos Antioxidantes de Propofol e Cetamina Em Doses Baixas. Braz. J. Anesthesiol. 2017, 67, 246–250. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yellon, D.M.; Hausenloy, D.J. Myocardial Reperfusion Injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Cochain, C.; Channon, K.M.; Silvestre, J.-S. Angiogenesis in the Infarcted Myocardium. Antioxid. Redox Signal. 2013, 18, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Gourdin, M.J.; Bree, B.; de Kock, M. The Impact of Ischaemia–Reperfusion on the Blood Vessel. Eur. J. Anaesthesiol. 2009, 26, 537–547. [Google Scholar] [CrossRef]
- Sas, K.; Csete, K.; Vécsei, L.; Papp, J.G. Effect of Systemic Administration of L-Kynurenine on Corticocerebral Blood Flow under Normal and Ischemic Conditions of the Brain in Conscious Rabbits. J. Cardiovasc. Pharmacol. 2003, 42, 403–409. [Google Scholar] [CrossRef]
- Gleadle, J.M.; Mazzone, A. Remote Ischaemic Preconditioning: Closer to the Mechanism? F1000Research 2016, 5, 2846. [Google Scholar] [CrossRef] [PubMed]
- Parada-Turska, J.; Rzeski, W.; Zgrajka, W.; Majdan, M.; Kandefer-Szerszeń, M.; Turski, W. Kynurenic Acid, an Endogenous Constituent of Rheumatoid Arthritis Synovial Fluid, Inhibits Proliferation of Synoviocytes in Vitro. Rheumatol. Int. 2006, 26. [Google Scholar] [CrossRef] [PubMed]
- Zapolski, T.; Kamińska, A.; Kocki, T.; Wysokiński, A.; Urbanska, E.M. Aortic Stiffness—Is Kynurenic Acid a Novel Marker? Cross-Sectional Study in Patients with Persistent Atrial Fibrillation. PLoS ONE 2020, 15, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Bulkley, G.B. Free Radical-Mediated Reperfusion Injury: A Selective Review. Br. J. Cancer Suppl. 1987, 8, 66–73. [Google Scholar] [PubMed]
- Lee, H.J.; Suh, J.K.; Song, H.H.; Jeong, M.A.; Yeom, J.H.; Kim, D.W. Antioxidant Effects of Methylprednisolone and Hydrocortisone on the Impairment of Endothelium Dependent Relaxation Induced by Reactive Oxygen Species in Rabbit Abdominal Aorta. Korean J. Anesthesiol. 2013, 64, 54–60. [Google Scholar] [CrossRef][Green Version]
- Yassin, M.M.I.; Harkin, D.W.; Barros D’Sa, A.A.B.; Halliday, M.I.; Rowlands, B.J. Lower Limb Ischemia-Reperfusion Injury Triggers a Systemic Inflammatory Response and Multiple Organ Dysfunction. World J. Surg. 2002, 26, 115–121. [Google Scholar] [CrossRef]
- Gross, G.J.; O’Rourke, S.T.; Pelc, L.R.; Warltier, D.C. Myocardial and Endothelial Dysfunction after Multiple, Brief Coronary Occlusions: Role of Oxygen Radicals. Am. J. Physiol.-Heart Circ. Physiol. 1992, 263, H1703–H1709. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.S.; Schmitz, F.; Marques, E.P.; Siebert, C.; Wyse, A.T.S. Intrastriatal Quinolinic Acid Administration Impairs Redox Homeostasis and Induces Inflammatory Changes: Prevention by Kynurenic Acid. Neurotox. Res. 2020, 38, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Huitrón, R.; Blanco-Ayala, T.; Ugalde-Muñiz, P.; Carrillo-Mora, P.; Pedraza-Chaverrí, J.; Silva-Adaya, D.; Maldonado, P.D.; Torres, I.; Pinzón, E.; Ortiz-Islas, E.; et al. On the Antioxidant Properties of Kynurenic Acid: Free Radical Scavenging Activity and Inhibition of Oxidative Stress. Neurotoxicol. Teratol. 2011, 33, 538–547. [Google Scholar] [CrossRef]
- Moroni, F.; Fossati, S.; Chiarugi, A.; Cozzi, A. Kynurenic Acid Actions in Brain and Periphery. Int. Congr. Ser. 2007, 1304, 305–313. [Google Scholar] [CrossRef]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; di Paola, R. Focus on the Role of NLRP3 Inflammasome in Diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef]
- Fusco, R.; Scuto, M.; Cordaro, M.; D’Amico, R.; Gugliandolo, E.; Siracusa, R.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. N-Palmitoylethanolamide-Oxazoline Protects against Middle Cerebral Artery Occlusion Injury in Diabetic Rats by Regulating the SIRT1 Pathway. Int. J. Mol. Sci. 2019, 20, 4845. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.-Q. Kynurenines in the Mammalian Brain: When Physiology Meets Pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef]
- Rubanyi, G.M.; Vanhoutte, P.M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am. J. Physiol. Circ. Physiol. 1986, 250, H822–H827. [Google Scholar] [CrossRef] [PubMed]
- Laude, K.; Thuillez, C.; Richard, V. Coronary Endothelial Dysfunction after Ischemia and Reperfusion: A New Therapeutic Target? Braz. J. Med Biol. Res. 2001, 34. [Google Scholar] [CrossRef]
- Tennant, M.; McGeachie, J.K. Blood vessel structure and function: A brief update on recent advances. Anz J. Surg. 1990, 60, 747–753. [Google Scholar] [CrossRef]
- Tandon, A.; Bannykh, S.; Kowalchyk, J.A.; Banerjee, A.; Martin, T.F.J.; Balch, W.E. Differential Regulation of Exocytosis by Calcium and CAPS in Semi-Intact Synaptosomes. Neuron 1998, 21, 147–154. [Google Scholar] [CrossRef]
- Cai, T.; Hirai, H.; Zhang, G.; Zhang, M.; Takahashi, N.; Kasai, H.; Satin, L.S.; Leapman, R.D.; Notkins, A.L. Deletion of Ia-2 and/or Ia-2β in Mice Decreases Insulin Secretion by Reducing the Number of Dense Core Vesicles. Diabetologia 2011, 54, 2347–2357. [Google Scholar] [CrossRef]
- Wang, X.-J.; Zhang, D.-L.; Xu, Z.-G.; Ma, M.-L.; Wang, W.-B.; Li, L.-L.; Han, X.-L.; Huo, Y.; Yu, X.; Sun, J.-P. Understanding Cadherin EGF LAG Seven-Pass G-Type Receptors. J. Neurochem. 2014, 131, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Song, R.; Ao, L.; Cleveland, J.C.; Fullerton, D.A.; Meng, X. Neurotrophin 3 Upregulates Proliferation and Collagen Production in Human Aortic Valve Interstitial Cells: A Potential Role in Aortic Valve Sclerosis. Am. J. Physiol.-Cell Physiol. 2017, 312, C697–C706. [Google Scholar] [CrossRef] [PubMed]
- Dong-Ryul, L.; Kondo, H.; Furukawa, S.; Nakano, K. Stimulation of NGF Production by Tryptophan and Its Metabolites in Cultured Mouse Astroglial Cells. Brain Res. 1997, 777, 228–230. [Google Scholar] [CrossRef] [PubMed]
- di Serio, C.; Cozzi, A.; Angeli, I.; Doria, L.; Micucci, I.; Pellerito, S.; Mirone, P.; Masotti, G.; Moroni, F.; Tarantini, F. Kynurenic Acid Inhibits the Release of the Neurotrophic Fibroblast Growth Factor (FGF)-1 and Enhances Proliferation of Glia Cells, in Vitro. Cell. Mol. Neurobiol. 2005, 25, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Verpelli, C.; Dvoretskova, E.; Vicidomini, C.; Rossi, F.; Chiappalone, M.; Schoen, M.; di Stefano, B.; Mantegazza, R.; Broccoli, V.; Böckers, T.M.; et al. Importance of Shank3 Protein in Regulating Metabotropic Glutamate Receptor 5 (MGluR5) Expression and Signaling at Synapses*. J. Biol. Chem. 2011, 286, 34839–34850. [Google Scholar] [CrossRef]
- Deibert, E.; Crenshaw, M.; Miller, M.S. A Patient with Phelan-McDermid Syndrome and Dilation of the Great Vessels. Clin. Case Rep. 2019, 7, 607–611. [Google Scholar] [CrossRef]
- Shiraishi-Yamaguchi, Y.; Furuichi, T. The Homer Family Proteins. Genome Biol. 2007, 8, 206. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Chen, S.-S.; Jing, W.; Tan, Q.; Yu, M.-X.; Tu, J.-C. Diagnostic Potential of Differentially Expressed Homer1, IL-1β, and TNF-α in Coronary Artery Disease. Int. J. Mol. Sci. 2014, 16, 535–546. [Google Scholar] [CrossRef]
- Dieck, S.; Sanmartí-Vila, L.; Langnaese, K.; Richter, K.; Kindler, S.; Soyke, A.; Wex, H.; Smalla, K.-H.; Kämpf, U.; Fränzer, J.-T.; et al. Bassoon, a Novel Zinc-Finger CAG/Glutamine-Repeat Protein Selectively Localized at the Active Zone of Presynaptic Nerve Terminals. J. Cell Biol. 1998, 142, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.-X.; Andres, D.A.; Cai, W. Ras Family Small GTPase-Mediated Neuroprotective Signaling in Stroke. Cent. Nerv. Syst. Agents Med. Chem. 2011, 11. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, V.S.S.; Mariano, D.O.C.; Vigerelli, H.; Janussi, S.C.; Baptista, T.V.L.; Claudino, M.A.; Pimenta, D.C.; Sciani, J.M. Effects of Kynurenic Acid on the Rat Aorta Ischemia—Reperfusion Model: Pharmacological Characterization and Proteomic Profiling. Molecules 2021, 26, 2845. https://doi.org/10.3390/molecules26102845
Lima VSS, Mariano DOC, Vigerelli H, Janussi SC, Baptista TVL, Claudino MA, Pimenta DC, Sciani JM. Effects of Kynurenic Acid on the Rat Aorta Ischemia—Reperfusion Model: Pharmacological Characterization and Proteomic Profiling. Molecules. 2021; 26(10):2845. https://doi.org/10.3390/molecules26102845
Chicago/Turabian StyleLima, Viviane Soares Souza, Douglas Oscar Ceolin Mariano, Hugo Vigerelli, Sabrina Cardoso Janussi, Thayz Vanalli Lima Baptista, Mário Angelo Claudino, Daniel Carvalho Pimenta, and Juliana Mozer Sciani. 2021. "Effects of Kynurenic Acid on the Rat Aorta Ischemia—Reperfusion Model: Pharmacological Characterization and Proteomic Profiling" Molecules 26, no. 10: 2845. https://doi.org/10.3390/molecules26102845
APA StyleLima, V. S. S., Mariano, D. O. C., Vigerelli, H., Janussi, S. C., Baptista, T. V. L., Claudino, M. A., Pimenta, D. C., & Sciani, J. M. (2021). Effects of Kynurenic Acid on the Rat Aorta Ischemia—Reperfusion Model: Pharmacological Characterization and Proteomic Profiling. Molecules, 26(10), 2845. https://doi.org/10.3390/molecules26102845






