Chemical and Genotypic Variations in Aniba rosiodora from the Brazilian Amazon Forest
Abstract
1. Introduction
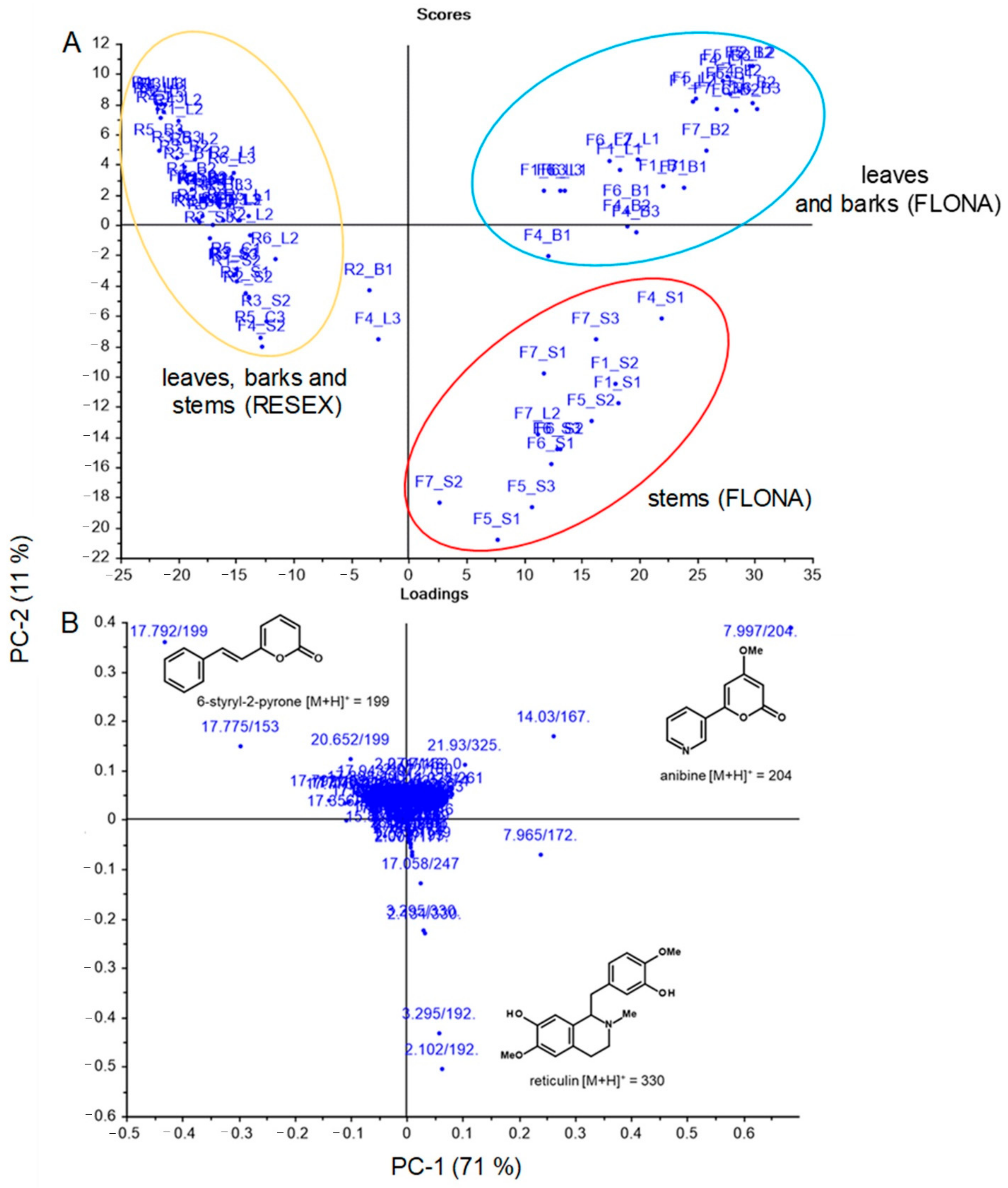
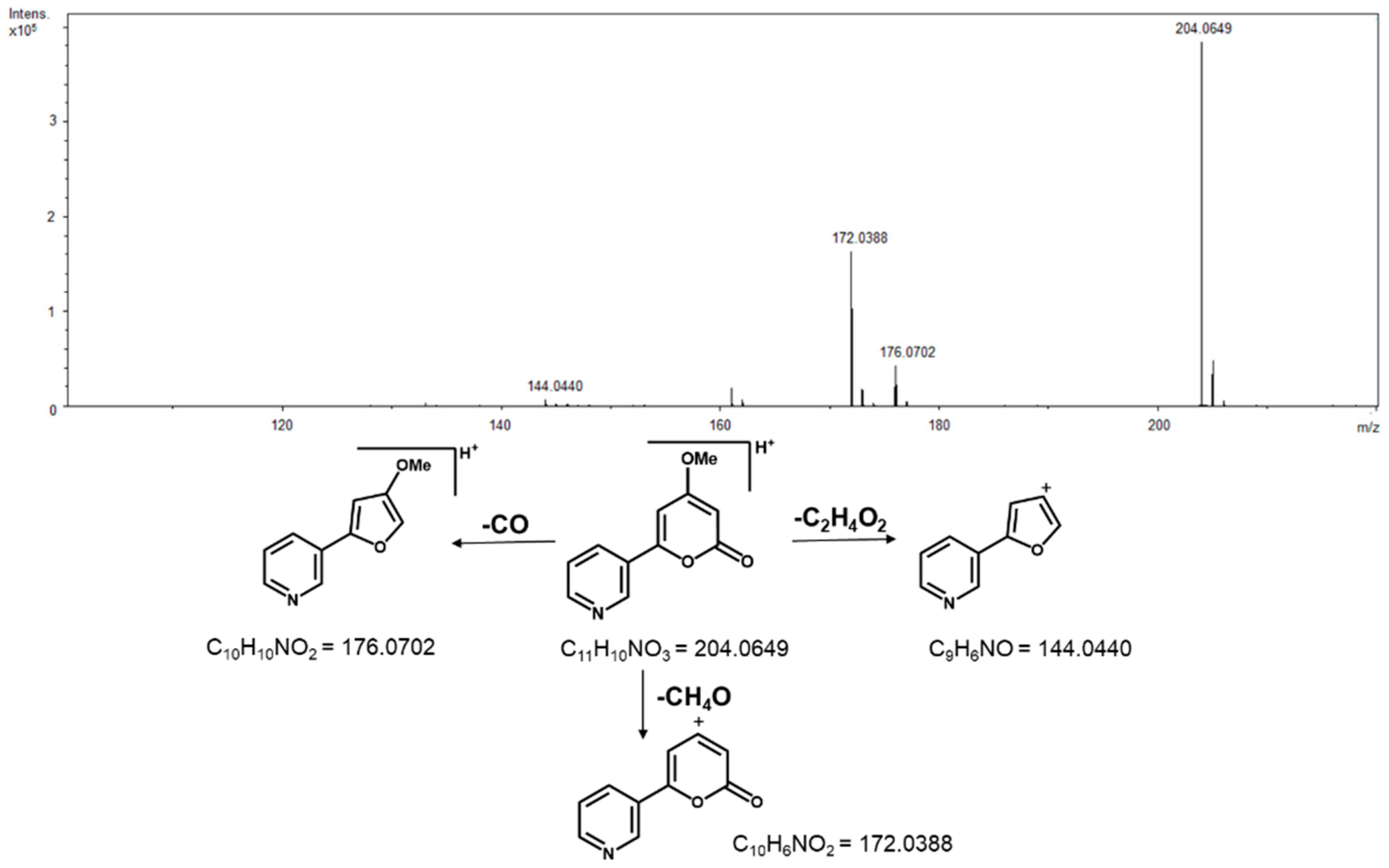
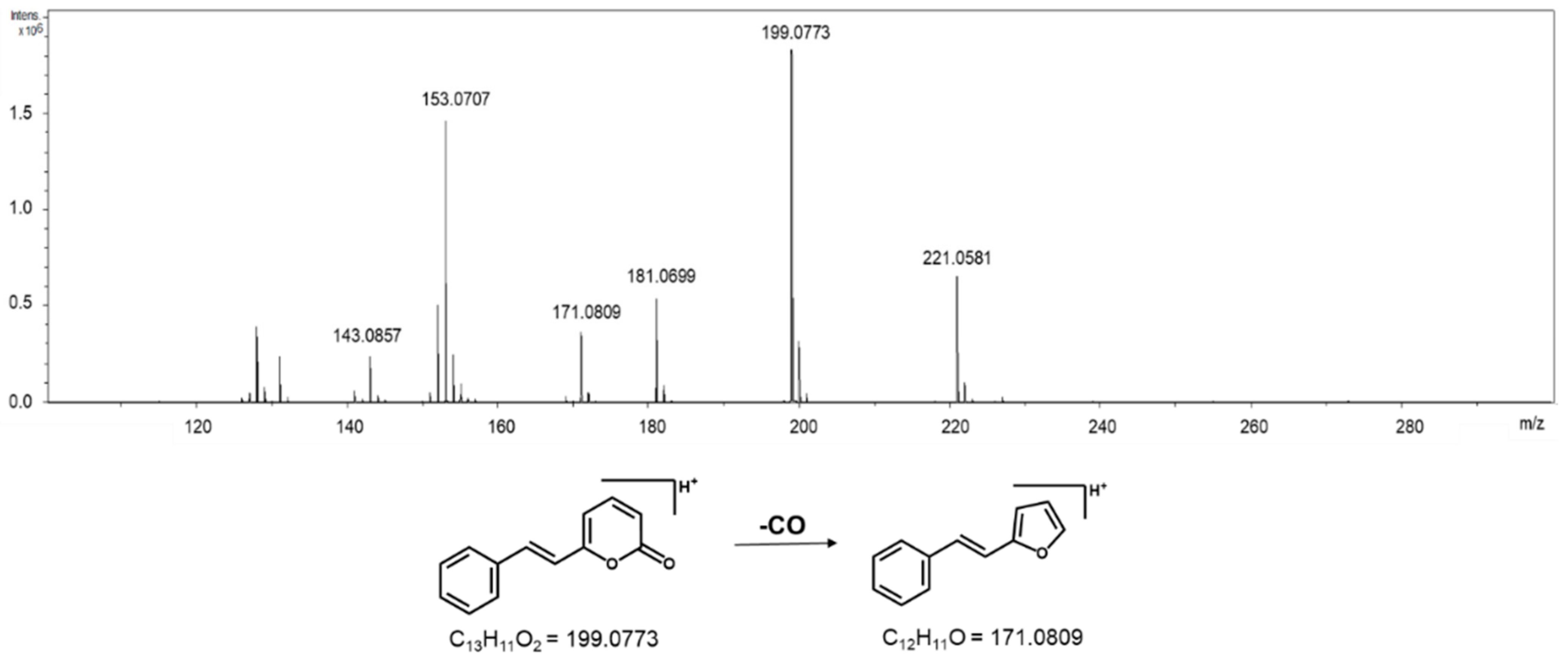
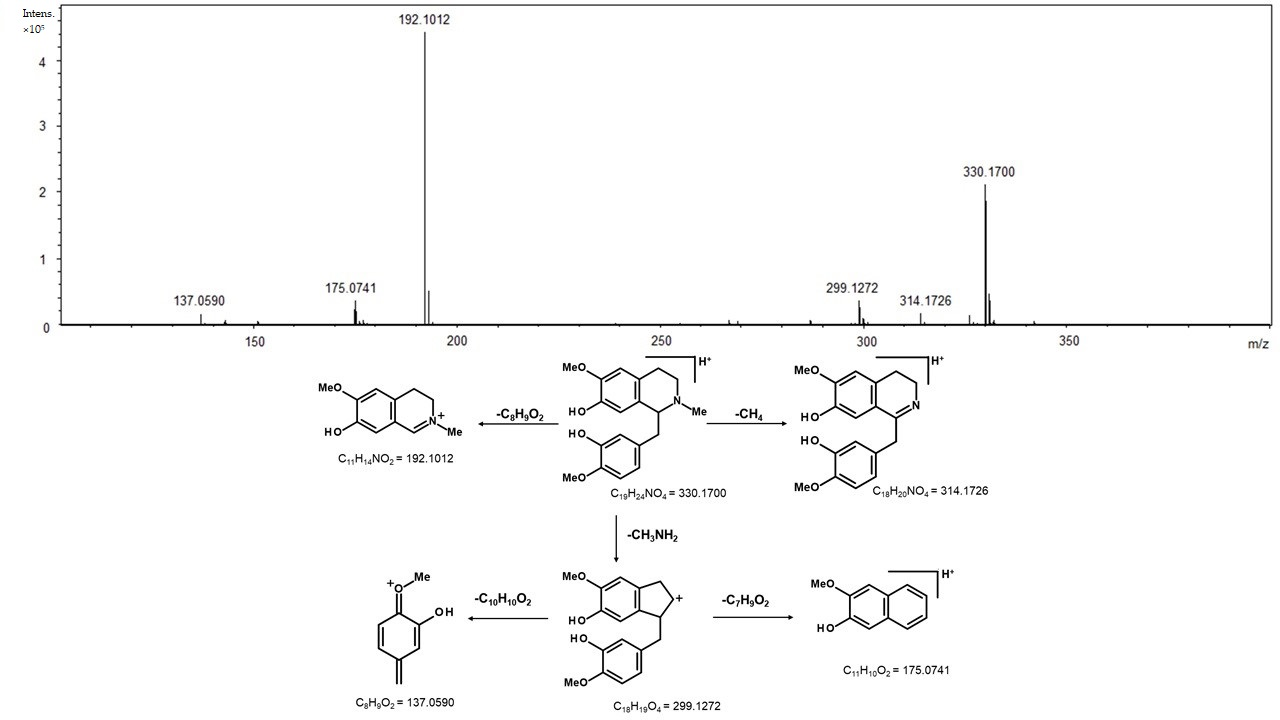
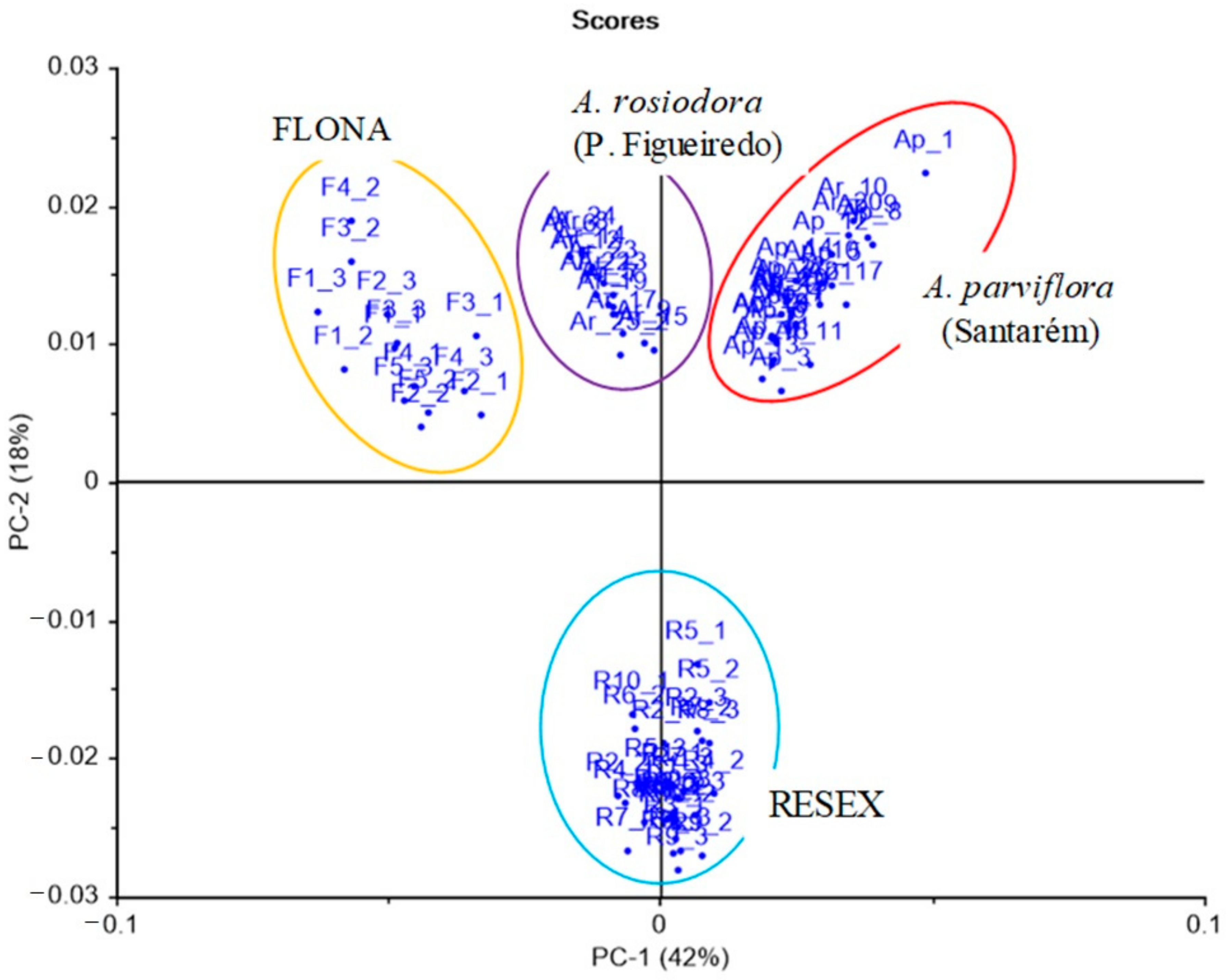
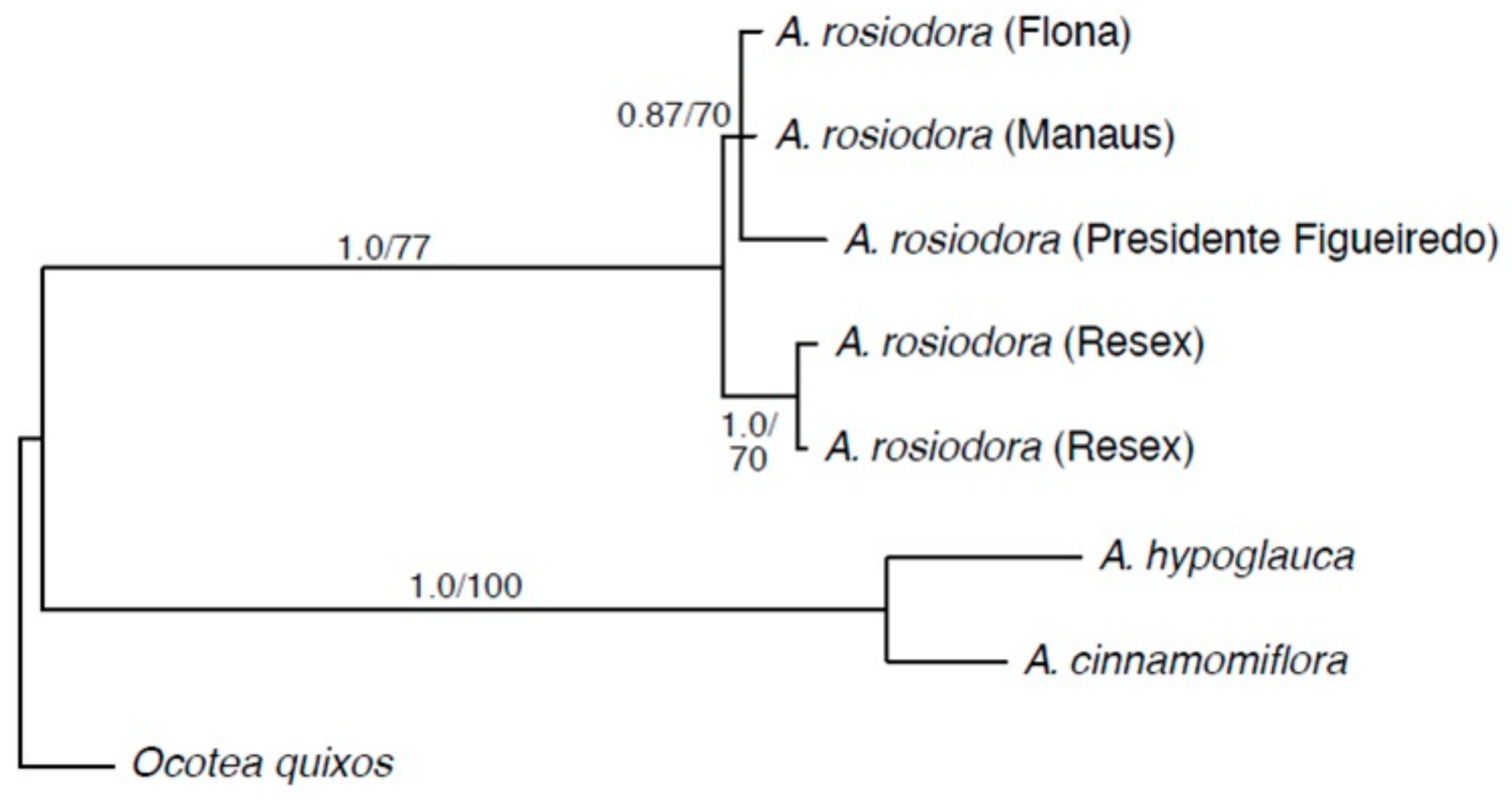
2. Results
3. Discussion
4. Materials and Methods
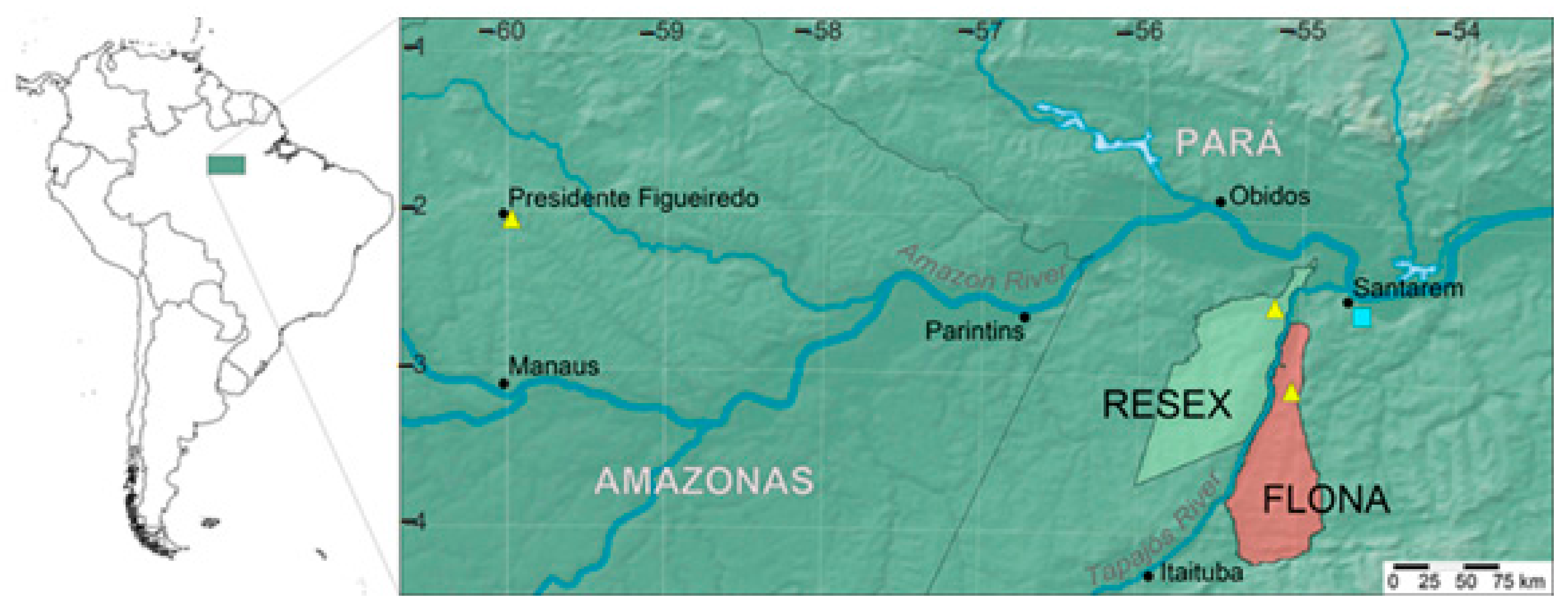
4.1. Plant Material
4.2. Chemical Analysis
4.2.1. Essential Oil Extraction and GC-MS Analysis
4.2.2. Sample Preparation for Non-Volatile Compounds
4.2.3. HPLC-ESI-Q-Tof/MS Analyses
4.2.4. NMR Analysis
4.3. Phylogenetic Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribeiro, J.E.L.S.; Hopkins, M.J.G.; Vicentini, A.; Sothers, C.A.; Costa, M.A.S.; Brito, J.M.; Souza, M.A.D.; Martins, L.H.P.; Lohmann, L.G.; Assunção, P.A.C.L.; et al. Flora da Reserva Ducke: Guia de Identificação das Plantas Vasculares de uma Floresta de Terra-firme na Amazônia Central; INPA-DFID: Manaus, Brazil, 1999; p. 800. [Google Scholar]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.-H.; Li, D.-Z.; Marhold, K.; et al. International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017; Regnum Vegetabile 159; Koeltz Botanical Books: Glashütten, Germany, 2018. [Google Scholar] [CrossRef]
- Mors, W.B.; Gottlieb, O.R.; Djerassi, C. The Chemistry of Rosewood. Isolation and Structure of Anibine and 4-Methoxyparacotoin1. J. Am. Chem. Soc. 2007, 79, 4507–4511. [Google Scholar] [CrossRef]
- Gottlieb, O.R.; Fineberg, M.; Guimarães, M.L.; Magalhães, M.T.; Maravalhas, N. Notes on Brazilian rosewood. Perfum. Essent. Oil Rec. 1964, 55, 253–257. [Google Scholar]
- Kubitzki, K.; Renner, S. Lauraceae I (Aniba and Aiouea). Flora Neotropica. 1982, 31, 1–124. [Google Scholar]
- Mitja, D.; Lescure, J.P. Madeira para Perfume: Qual Será o Destino do Pau-Rosa? In A Floresta em Jogo. O Extrativismo na Amazônia Central; Emperaire, L., Ed.; Editora Unesp: São Paulo, Brazil, 2000; pp. 93–302. [Google Scholar]
- Franciscon, C.H.; Miranda, I.S. Distribution and conservation of Aniba Aubl. (Lauraceae Jussieu) species in Brazil. Biota Neotrop. 2018, 18, e20170362. [Google Scholar]
- Chantraine, J.-M.; Dhenin, J.-M.; Moretti, C. Chemical variability of rosewood (Aniba rosaeodora Ducke) essential oil French Guiana. J. Essent. Oil Res. 2009, 21, 486–495. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Andrade, E.H.A.; Couto, H.A.R.; Silva, A.C.M.; Marx, F.; Henke, C. Plant sources of Amazon rosewood oil. Quim. Nova 2007, 30, 1906–1910. [Google Scholar] [CrossRef]
- Lara, C.S.; Barata, L.E.S.; Sampaio, P.d.T.B.; Eberlin, M.N.; Fidelis, C.H.d.V. Linalool enantiomeric distribution in rosewood-reminiscent populations in Central Amazon. J. Essent. Oil Res. 2018, 30, 464–469. [Google Scholar] [CrossRef]
- Galaverna, R.S.; Sampaio, P.T.B.; Barata, L.E.S.; Eberlin, M.N.; Fidelis, C.H.V. Differentiation of two morphologically similar Amazonian Aniba species by mass spectrometry leaf fingerprinting. Anal. Methods. 2015, 7, 1984–1990. [Google Scholar] [CrossRef]
- Santos, R.P.; Ângelo, P.C.S.; Sampaio, P.T.B.; Quisen, R.C.; Leite, A.M.C.; Oliveira, C.L. Geographic pattern of genetic diversity in natural populations of rosewood (Aniba rosaeodora), in the Central Amazonia. Acta Amaz. 2008, 38, 459–466. [Google Scholar] [CrossRef]
- May, P.H.; Barata, L.E.S. Rosewood exploitation in the Brazilian Amazon: Options for sustainable production. Econ. Bot. 2004, 58, 257–265. [Google Scholar] [CrossRef]
- Sandasi, M.; Kamatou, G.P.P.; Viljoen, A.M. An untargeted metabolomic approach in the chemotaxonomic assessment of two Salvia species as a potential source of α-bisabolol. Phytochemistry 2012, 84, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Gallon, M.E.; Monge, M.; Casoti, R.; Da Costa, F.B.; Semir, J.; Gobbo-Neto, L. Metabolomic analysis applied to chemosystematics and evolution of megadiverse Brazilian Vernonieae (Asteraceae). Phytochemistry 2018, 150, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, A.M.; Gottlieb, O.R.; Mors, W.B.; Magalhães, M.T.; Mageswaran, S.; Ollis, W.D.; Sutherland, I.O. The natural occurrence of 6-styryl-2-pyrones and their synthesis. Tetrahedron 1971, 27, 1043–1048. [Google Scholar] [CrossRef]
- Rossi, M.H.; Yoshida, M.; Maia, J.G.S. Neolignans, styrylpyrones and flavonoids from Aniba species. Phytochemistry 1997, 45, 1263–1269. [Google Scholar] [CrossRef]
- Ferreira, Z.S.; Gottlieb, O.R.; Roque, N.F. Chemosystematic implications of benzyltetrahydroisoquinolines in Aniba. Biochem. System. Ecol. 1980, 8, 51–54. [Google Scholar] [CrossRef]
- Schmidt, J.; Raith, K.; Boettcher, C.; Zenk, M.H. Analysis of benzylisoquinoline-type alkaloids by electrospray tandem mass spectrometry and atmospheric pressure photoionization. Eur. J. Mass Spectrom. 2005, 11, 325–333. [Google Scholar] [CrossRef]
- Gottlieb, O.R.; Kubitzki, K. Plant chemosystematics and phylogeny. Part XII. Chemosystematics of Aniba. Biochem. Syst. Ecol. 1981, 9, 5–12. [Google Scholar] [CrossRef]
- Andrade, E.H.A.; Zoghbi, M.G.B.; Maia, J.G.S. Volatiles from Aniba terminalis Ducke. J. Essent. Oil Res. 2003, 15, 81–82. [Google Scholar] [CrossRef]
- Luz, A.I.R.; Domingos Da Silva, J.; Zoghbi, M.G.B.; Andrade, E.H.A.; Maia, J.G.S. Essential oil from Aniba riparia (Nees) Mez. J. Essent. Oil Res. 2000, 14, 218–219. [Google Scholar] [CrossRef]
- Da Silva, J.K.R.; Maia, J.G.S.; Dosoky, N.S.; Setzer, W.N. Antioxidant, antimicrobial, and cytotoxic properties of Aniba parviflora essential oils from the Amazon. Nat. Prod. Commun. 2016, 11, 1025–1028. [Google Scholar] [CrossRef]
- Gottlieb, O.R.; Mors, W.B. The chemistry of rosewood. II. Isolation and identification of cotoin and pinocembrin. JACS 1958, 80, 2263–2265. [Google Scholar]
- Santos, A.S.; Alves, S.M.; Figueirêdo, F.J.C.; Neto, O.G.R. Descrição de Sistema e de Métodos de Extração de Óleos Essenciais e Determinação de Umidade de Biomassa em Laboratório, 1st ed.; Ministério da Agricultura, Pecuária e Abastecimento: Belém-PA, Brazil, 2004. [Google Scholar]
- Sandra, P.; Bicchi, C. Capillary Gas Chromatography in Essential oil Analysis; Hüthig: Heidelberg, Germany, 1987. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases. J. Chrom. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; p. 804. [Google Scholar]
- Matsuda, F.; Nakabayashi, R.; Sawada, Y.; Suzuki, M.; Hirai, M.Y.; Kanaya, S.; Saito, K. Mass spectra-based framework for automated structural elucidation of metabolome data to explore phytochemical diversity. Front. Plant. Sci. 2011, 2, 40. [Google Scholar] [CrossRef] [PubMed]
- Sang, T.; Crawford, D.J.; Stuessy, T.F. Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae). Am. J. Bot. 1997, 84, 1120–1136. [Google Scholar] [CrossRef]
- Shaw, J.; Lickey, E.B.; Schilling, E.E.; Small, R.L. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: The tortoise and the hare III. Am. J. Bot. 2007, 94, 275–288. [Google Scholar] [CrossRef]
- Shaw, J.; Lickey, E.B.; Beck, J.T.; Farmer, S.B.; Liu, W.; Miller, J.; Siripun, K.C.; Winder, C.T.; Schilling, E.E.; Small, R.L. The tortoise and the hare II: Relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. Am. J. Bot. 2005, 92, 142–166. [Google Scholar] [CrossRef]
- Hamilton, M.B. Four primer pairs for the amplification of chloroplast intergenic regions with intraspecific variation. Mol. Ecol. 1999, 8, 521–523. [Google Scholar]
- Huelsenbeck, J.P.; Ronquist, F. MrBayes: Bayesian inference of phylogeny. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest v2. Program Distributed by the Author. Evolutionary Biology Centre, Uppsala University. 2004. Available online: www.csit.fsu.edu/~nylander/ (accessed on 1 November 2020).
- Swofford, D.L. PAUP*: Phylogenetic Analysis Using Parsimony (* and Other Methods), Version 4.0a147. Program Distributed by the Author. Biology Department, Duke University, 2016. Available online: http://people.sc.fsu.edu/~dswofford/paup_test/ (accessed on 1 November 2020).

| Compounds | RT (min) | FLONA (%) | RESEX (%) | ||||
|---|---|---|---|---|---|---|---|
| (5 Individuals) | SD | R.I. | (10 Individuals) | SD | R.I. | ||
| α-pinene | 2.7 | nd | - | - | 1.7 | 1.00 | 931 |
| α-phellandrene | 3.6 | nd | - | - | 22.8 | 6.76 | 930 |
| p-cymene | 3.9 | nd | - | - | 7.0 | 1.77 | 936 |
| β-thujene | 4.0 | nd | - | - | 6.0 | 3.22 | 951 |
| β-ocimene | 4.4 | nd | - | - | 2.5 | 0.81 | 915 |
| cis-linalool oxide | 4.8 | 1.0 | 0.55 | 878 | nd | - | - |
| trans-linalool oxide | 5.1 | 1.1 | 0.47 | 832 | nd | - | - |
| linalool | 5.4 | 83.7 | 2.63 | 967 | 39.6 | 9.03 | 962 |
| α-terpineol | 7.4 | nd | - | - | 1.7 | 0.60 | 890 |
| γ-elemene | 14.7 | nd | - | - | 3.5 | 2.08 | 897 |
| spathulenol | 16.5 | 1.6 | 0.98 | 834 | 3.5 | 1.07 | 913 |
| guaiol | 17.0 | nd | - | - | 1.8 | 0.51 | 838 |
| cis α-santalol | 19.5 | 1.4 | 0.59 | 717 | nd | - | - |
| aromadendrene oxide | 19.6 | 2.5 | 0.86 | 783 | nd | - | - |
| Species | Voucher | Locality | psbA–trnH | psbD–trnT | trnC–rpoB | trnS–trnG |
|---|---|---|---|---|---|---|
| Aniba rosiodora Ducke | D. Amazonas 22 (SPSF) | BRAZIL. Pará: Floresta Nacional do Tapajós (“FLONA”) | MT679556 | MT679562 | MT679566 | MT679571 |
| Aniba rosiodora Ducke | D. Amazonas 226 (SPSF) | BRAZIL. Pará: Reserva Extrativista Tapajós-Arapiuns (“RESEX”) | MT679557 | MT679563 | MT679567 | MT679572 |
| Aniba rosiodora Ducke | D. Amazonas 278 (SPSF) | BRAZIL. Pará: Reserva Extrativista Tapajós-Arapiuns (“RESEX”) | MT679558 | MT679564 | MT679568 | MT679573 |
| Aniba rosiodora Ducke | Kato 1193 | BRAZIL. Amazonas: Presidente Figueiredo | MT679559 | MT679565 | — | — |
| Aniba rosiodora Ducke | Kato 1446 | BRAZIL. Amazonas: Manaus | MT679560 | — | MT679569 | MT679574 |
| Aniba cinnamomiflora C.K. Allen | N. Cuello 955 (MO) | VENEZUELA. Trujillo: Boconó, Parque Nacional Guaramacal | AF268770 | — | — | — |
| Aniba hypoglauca Sandwith | A. Chanderbali 165 (MO) | GUYANA. Upper Demerara-Berbice: Iwokrama Reserve | AF268771 | — | — | — |
| Ocotea quixos (Lam.) Kosterm. | D. Neill 9487 (MO) | ECUADOR. Napo: Jatun Sacha Biological Reserve | AF261999 | — | — | — |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amazonas, D.R.; Oliveira, C.; Barata, L.E.S.; Tepe, E.J.; Kato, M.J.; Mourão, R.H.V.; Yamaguchi, L.F. Chemical and Genotypic Variations in Aniba rosiodora from the Brazilian Amazon Forest. Molecules 2021, 26, 69. https://doi.org/10.3390/molecules26010069
Amazonas DR, Oliveira C, Barata LES, Tepe EJ, Kato MJ, Mourão RHV, Yamaguchi LF. Chemical and Genotypic Variations in Aniba rosiodora from the Brazilian Amazon Forest. Molecules. 2021; 26(1):69. https://doi.org/10.3390/molecules26010069
Chicago/Turabian StyleAmazonas, Diana R., Celso Oliveira, Lauro E. S. Barata, Eric J. Tepe, Massuo J. Kato, Rosa H. V. Mourão, and Lydia F. Yamaguchi. 2021. "Chemical and Genotypic Variations in Aniba rosiodora from the Brazilian Amazon Forest" Molecules 26, no. 1: 69. https://doi.org/10.3390/molecules26010069
APA StyleAmazonas, D. R., Oliveira, C., Barata, L. E. S., Tepe, E. J., Kato, M. J., Mourão, R. H. V., & Yamaguchi, L. F. (2021). Chemical and Genotypic Variations in Aniba rosiodora from the Brazilian Amazon Forest. Molecules, 26(1), 69. https://doi.org/10.3390/molecules26010069







