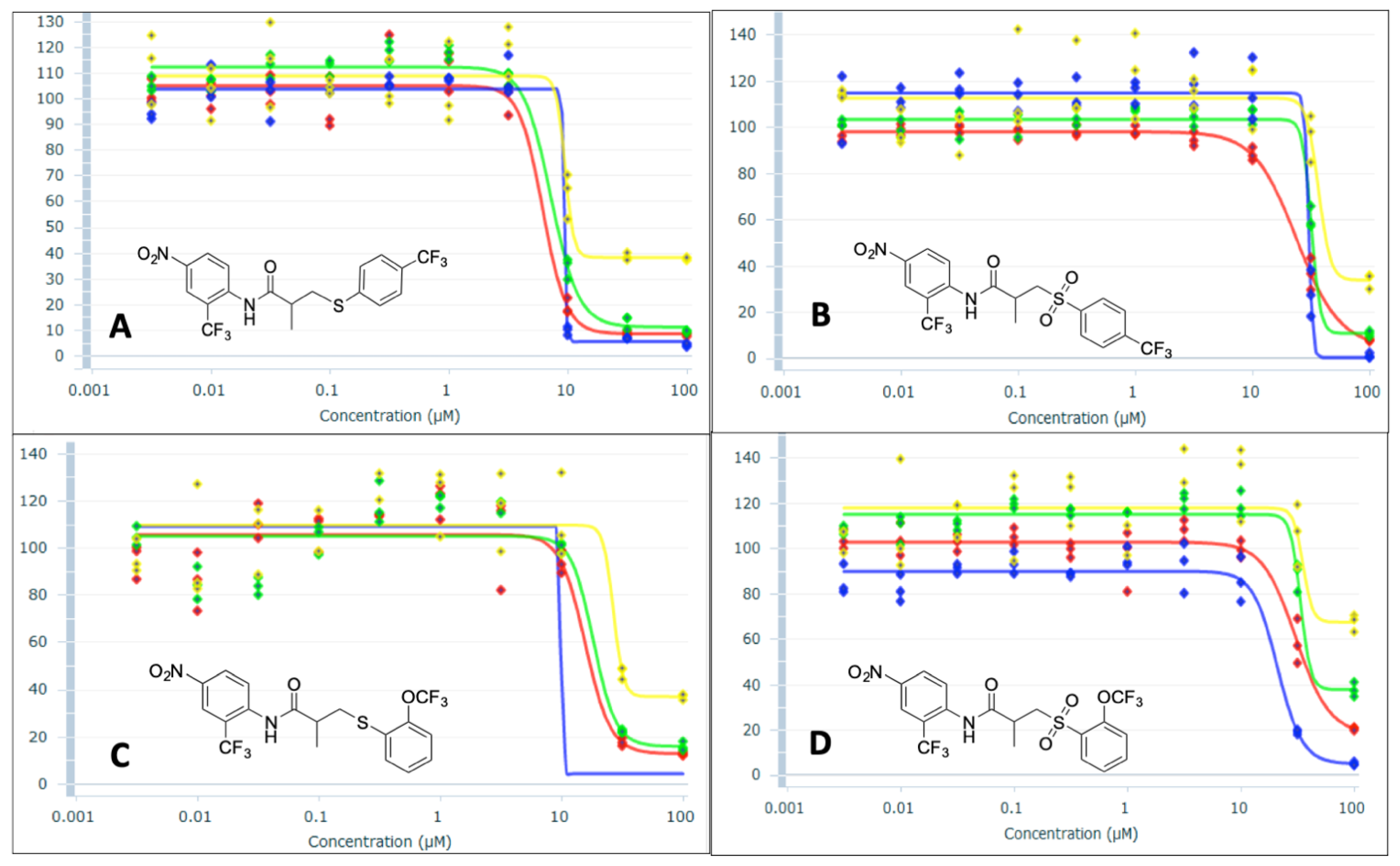
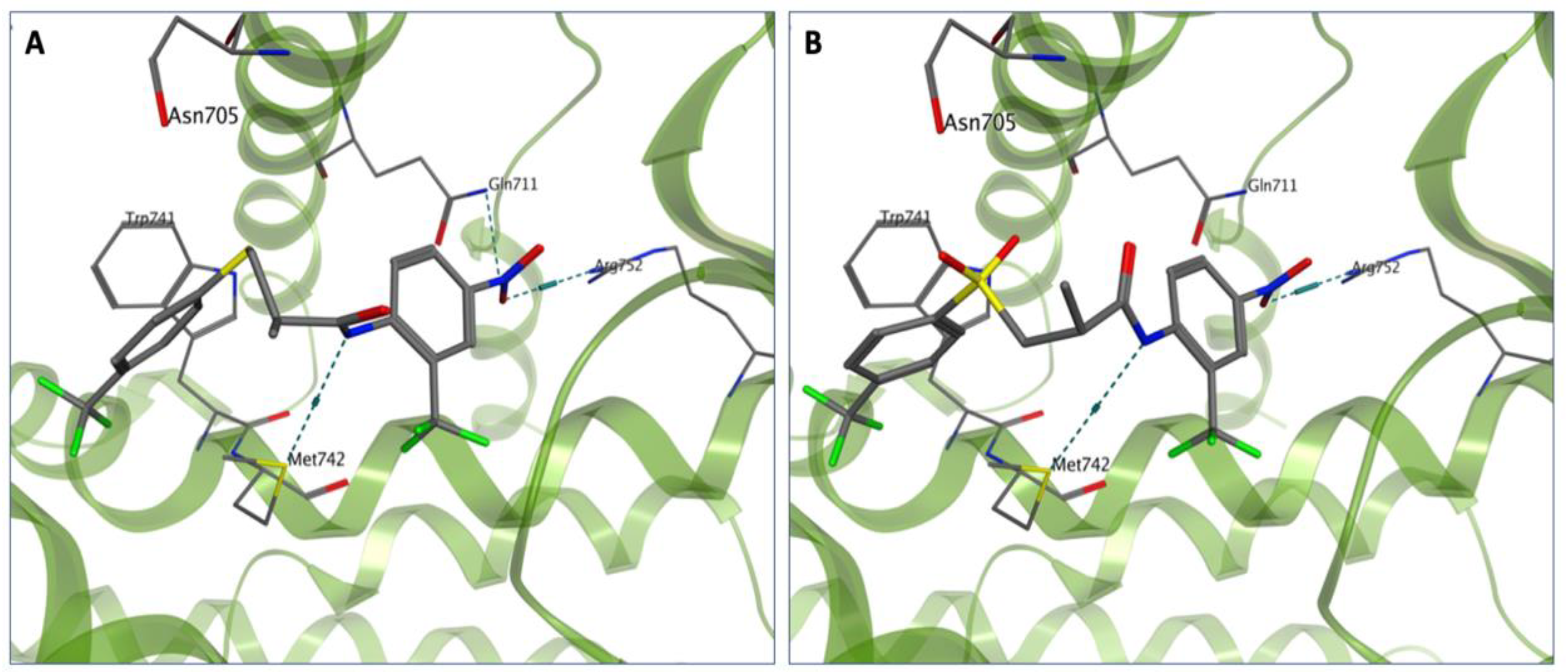
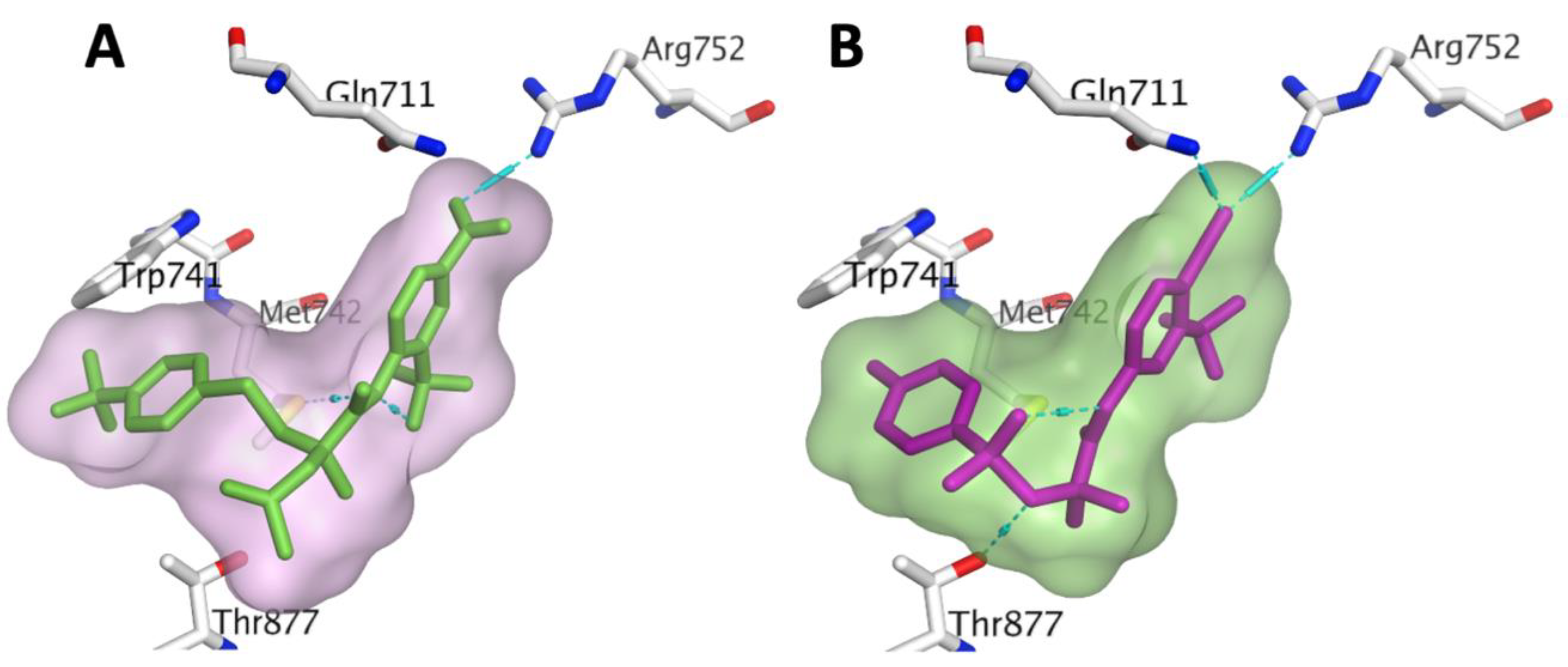
3.1. Chemistry
All chemicals were purchased from Sigma-Aldrich or Alfa Aesar and were used without further purification. Thin-layer chromatography (TLC): precoated aluminum-backed plates (60 F254, 0.2 mm thickness, Merck) were visualized under both short- and long-wave UV light (254 and 366 nm, respectively). Flash column chromatography was carried out using silica gel supplied by Fisher (60A, 35–70 mm); 1H-NMR (500 MHz), 13C-NMR (125 MHz), and 19F-NMR (470 MHz) spectra were recorded on a Bruker Avance 500 MHz spectrometer at 25 °C. Chemical shifts (δ) are expressed in parts per million (ppm) and coupling constants (J) are given in hertz (Hz). The following abbreviations are used in the assignment of NMR signals: s (singlet), bs (broad singlet), d (doublet), t (triplet), q (quartet), qn (quintet), m (multiplet), dd (doublet of doublet), dt (doublet of triplet), td (triple doublet), dq (double quartet), m (multiplet), dm (double multiplet). Reverse-phase HPLC, eluting with H2O/CH3CN from 90/10 to 0/100 in 30 min; flow = 1 mL/min, tR = 20.39 min.
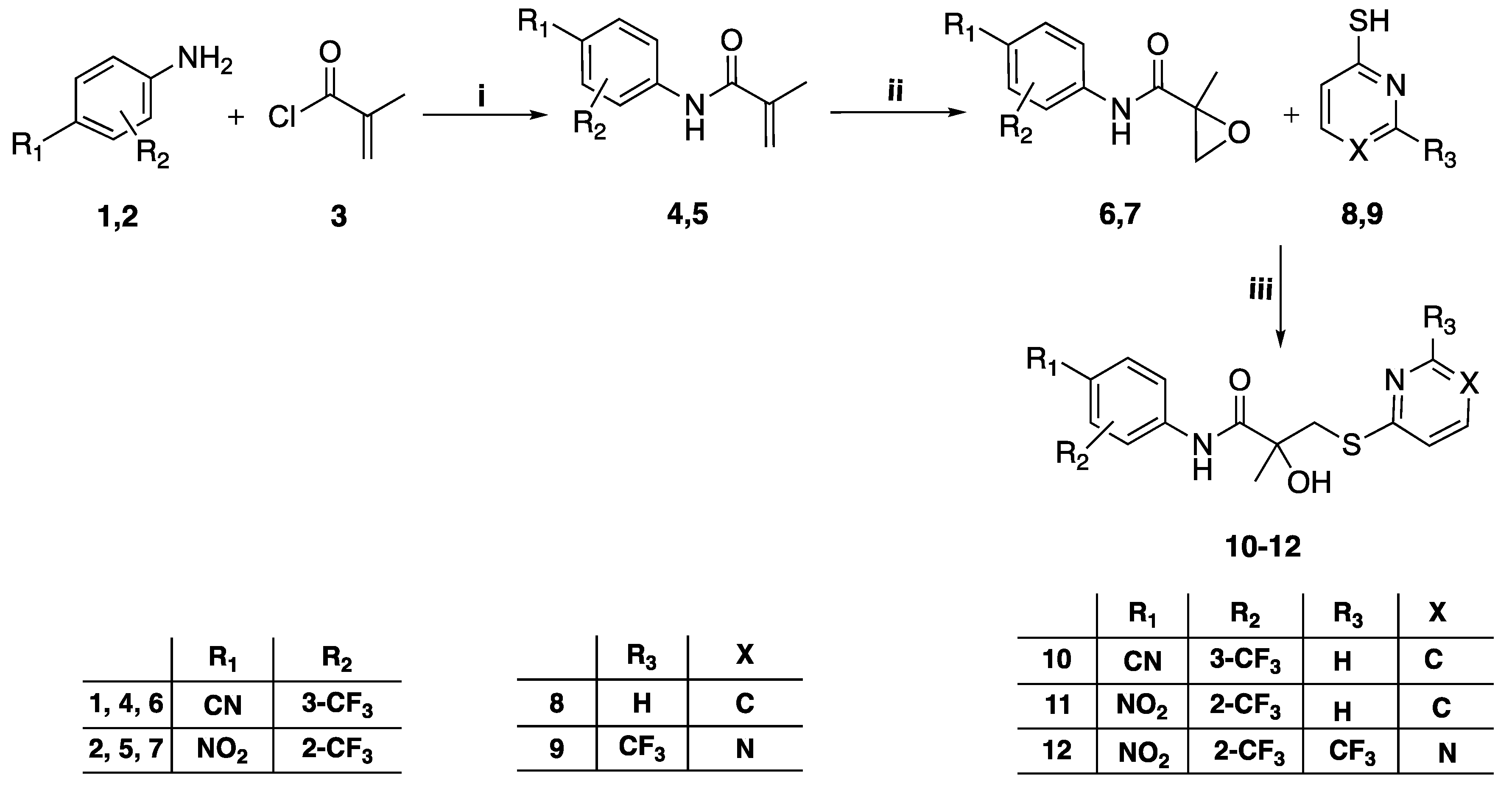
3.1.1. General Method for the Preparation of Intermediates 4–5
Methacryloyl chloride 3 (8.4 mL, 85.96 mmol) was added over the course of 10 min to a stirring solution of the appropriate trifluoromethylaniline 1–2 (10.75 mmol) in N,N-dimethylacetamide (10 mL) at room temperature for 24 h. After the reaction was complete, the mixture was diluted with ethyl acetate (100 mL) and extracted with saturated NaHCO3 solution (2 × 50 mL), then cold brine (2 × 50 mL). The combined organic layer was dried over anhydrous Na2SO4 and the solvent was removed under reduced pressure. The crude oil residue was purified by flash column chromatography eluting with chloroform-ethyl acetate 95:5 v/v to obtain the titled compounds.
N-(4-cyano-3-(trifluoromethyl)phenyl)methacrylamide (4) [
22], Yield 92%,
1H-NMR (CDCl
3) δ 8.10 (d,
J = 2Hz, 1H, Ar
H), 8.06 (bs, 1H, N
H), 8.01 (dd, J = 2, 8.5 Hz, 1H, Ar
H), 7.81 (d,
J = 8.5Hz, 1H, Ar
H), 5.89 (d,
J = 1Hz, 1H, C
H2), 5.62 (q,
J = 1.5Hz, 1H, C
H2), 2.10 (dd,
J = 0.5, 1.5 Hz, 3H, C
H3).
19F-NMR: (CDCl
3) δ −62.23
. N-(4-Nitro-2-(trifluoromethyl)phenyl)methacrylamide (5) [
22], Yield 94%,
1H-NMR (CDCl
3) δ 8.73 (d,
J = 9 Hz, 1H, Ar
H), 8.46 (d,
J = 3 Hz, 1H, Ar
H), 8.37 (dd,
J = 9 Hz, 2.5 Hz, 1H, Ar
H), 8.17 (bs, 1H, N
H), 5.85 (q,
J= 0.5 Hz, 1H, C
H2), 5.58 (q,
J = 1.5 Hz, 1H, C
H2), 2.15-2.13 (dd, J = 1, 1.5 Hz, 1H, C
H3).
19F-NMR: (CDCl
3) d-61.31.
3.1.2. General Method for the Preparation of Intermediates 6–7
To a stirred solution of the intermediate 4–5 (3 mmol) in DCM (7 mL) was added 30% hydrogen peroxide (3.6 mL, 32.03 mmol). The reaction mixture was put in a water bath at rt and trifluoroacetic anhydride (3.7 mL, 26.7 mmol) was added slowly to the mixture, which was then stirred for 24 h. The reaction mixture was transferred to a separating funnel using DCM (30 mL). The organic layer was washed with distilled water (20 mL), sat. aq. Na2S2O3 (4 × 20 mL), sat. aq. NaHCO3 (3 × 20 mL), and brine (20 mL), dried over Na2SO4, and concentrated at reduced pressure.
N-(4-Cyano-3-(trifluoromethyl)phenyl)-2-methyloxirane-2-carboxamide (6) [
22].The data are in accordance with literature data. Obtained in 86% yield as a yellow solid.
1H-NMR (CDCl
3): d 8.38 (bs, 1H), 8.00 (d,
J = 2.1 Hz, 1H), 7.88 (dd,
J = 8.5 Hz, 2.1 Hz, 1H), 7.78 (d,
J = 8.5 Hz, 1H), 3.00 (s, 2H), 1.68 (s, 3H).
2-Methyl-N-(4-nitro-2-(trifluoromethyl)phenyl)oxirane-2-carboxamide (7). Obtained in 71% yield as a yellow wax. 1H-NMR (CDCl3): d 8.92 (bs, 1H), 8.74 (d, J = 9.6 Hz, 1H), 8.53 (d, J = 2.5 Hz, 1H), 8.44 (dd, J = 9.6 Hz, 2.5 Hz, 1H), 3.04 (d, J= 4.6 Hz, 1H), 3.02 (d, J = 4.6 Hz, 1H), 1.72 (s, 3H). 19F-NMR (CDCl3): d −61.69 (s, 3F). 13C-NMR (CDCl3): d 169.2, 142.9, 140.4, 128.35 (m), 123.7, 122.3 (m), 121.6, 119.2 (m), 56.5, 53.9, 16.4.
3.1.3. General Method for the Preparation of Compounds 10–12, 22–29
To a mixture of sodium hydride (NaH) (60% in mineral oil, 0.050 g, 1.23 mmol) in anhydrous THF (2 mL) at 0 °C under Ar atmosphere was added a solution of the differently substituted thiophenol 8, 9, 19–21 (1.11 mmol) in 1 mL of anhydrous THF. This mixture was stirred at rt for 20 min. A solution of the different intermediate 17–21 (0.74 mmol) in anhydrous THF (3 mL) was added slowly. The reaction mixture was stirred at rt for 24h.The mixture was then diluted with ethyl acetate (30 mL), washed with brine (15 mL) and water (30 mL), dried over Na2SO4, and concentrated under vacuum. The crude residue was purified by flash column chromatography.
N-(4-cyano-3-(trifluoromethyl)phenyl)-2-hydroxy-2-methyl-3-(pyridin-2-ylthio) propanamide (10), yield 79%, 1H-NMR (CDCl3) δ 9.64 (s, 1H, NH), 8.89 (s, 1H, ArH), 8.39 (ddd, J = 1, 1.5, 5 Hz, 1H, ArH), 8.13 (d, J = 2 Hz, 1H, ArH), 8.00 (dd, J = 2, 8.5 Hz, 1H, ArH), 7.91 (d, J = 8.5 Hz, 1H, ArH), 7.61 (ddd, J = 2, 8, 8.5 Hz, 1H, ArH), 7.37 (dt, J = 1, 8 Hz, 1H, ArH), 7.17 (ddd, J = 1, 5, 7.5 Hz, 1H, ArH), 3.61 (d, J = 15.5 Hz, 1H, CH2), 3.50 (d, J = 15 Hz, 1H, CH2), 1.63 (s, 3H, CH3); 19F-NMR (CDCl3) δ −62.16 (s, 3F); 13C-NMR (CDCl3) δ 175.18 (C=O), 158.85 (ArC), 148.32 (ArCH), 141.69 (ArC), 137.45 (ArCH), 135.80 (ArCH), 133.92 (q, 2JC-F = 32.5 Hz, ArC), 123.55 (ArCH), 122.19 (q, 1JC-F = 271.3 Hz, CF3), 121.66 (ArCH), 120.92 (ArCH), 117.16 (q, 3JC-F = 5 Hz, ArCH), 115.67 (ArC), 104.15 (CN), 77.01 (COH), 41.48 (CH2), 26.81 (CH3). MS [ESI, m/z]: 382.1 [M+H]+, 404.1 [M+Na]+. Reverse-phase HPLC, eluting with H2O/CH3CN from 90/10 to 0/100 in 30 min; flow = 1 mL/min, tR = 21.17 min 99.5%.
2-Hydroxy-2-methyl-N-(4-nitro-2-(trifluoromethyl)phenyl)-3-(pyridin-2-ylthio) propenamide (11), yield 75%, 1H-NMR (CDCl3) δ 10.26 (s, 1H, NH), 9.08 (s, 1H, ArH), 8.84 (d, J = 9 Hz, 1H, ArH), 8.52 (d, J = 3 Hz, 1H, ArH), 8.42 (m, 2H, ArH), 7.61 (m, 1H, ArH), 7.37 (d, J = 8.5 Hz, 1H, ArH), 7.18 (m, 1H, ArH), 3.60 (d, J = 15 Hz, 1H, CH2), 3.52 (d, J = 15 Hz, 1H, CH2), 1.65 (s, 3H, CH3); 19F-NMR (CDCl3) δ −62.01 (s, 3F); 13C-NMR (CDCl3) δ 175.24 (C=O), 158.68 (ArC), 148.31 (ArCH), 142.62 (ArC), 141.00 (ArC), 137.40 (ArCH), 128.27 (ArCH), 123.36 (ArCH), 122.78 (q, 1JC-F = 272 Hz, CF3), 122.36 (q, 3JC-F = 5.5 Hz, ArCH), 122.04 (ArCH), 120.86 (ArCH), 119.28 (q, 2JC-F = 32.5 Hz, ArC), 77.13 (COH), 41.46 (CH2), 26.54 (CH3). MS (ES+) m/z: 402.1 [M + H]+ 424.1 [M + Na]+. Reverse-phase HPLC, eluting with H2O/CH3CN from 90/10 to 0/100 in 30 min; flow = 1 mL/min, tR = 23.98 min 99.75%.
2-Hydroxy-2-methyl-N-(4-nitro-2-(trifluoromethyl)phenyl)-3-(4-(trifluoromethyl) pyrimidin-2-ylthio)propanamide (12), yield 73%, 1H-NMR (CDCl3) δ 10.02 (s, 1H, NH), 8.83 (d, J = 5 Hz, 1H, ArH), 8.80 (d, J = 9.5 Hz, 1H, ArH), 8.53 (d, J = 2.5 Hz, 1H, ArH), 8.45 (dd, J = 2.5, 9 Hz, 1H, ArH), 7.46 (d, J = 5 Hz, 1H, ArH), 6.43 (s, 1H, OH), 3.69 (s, 2H, CH2), 1.68 (s, 3H, CH3); 19F-NMR (CDCl3) δ −62.00, -70.26; 13C-NMR (CDCl3) δ 174.21 (ArC), 173.91 (C=O), 159.79 (ArCH), 156.02 (q, 2JC-F = 36.3 Hz, ArC), 142.87 (ArC), 140.67 (ArC), 128.31 (ArCH), 122.76 (q, 1JC-F = 271.9 Hz, CF3), 122.39 (q, 3JC-F = 5.5 Hz, ArCH), 122.30 (ArCH), 119.79 (q, 1JC-F = 274 Hz, CF3), 119.49 (q, 2JC-F = 31.6 Hz, ArC), 112.97 (q, 3JC-F = 2.6 Hz, ArCH), 77.82 (COH), 40.67 (CH2), 26.38 (CH3).MS (ES+) m/z: 471.1 [M + H]+, 493.1 [M + Na]+. tR = 23.16 min 95.22%.
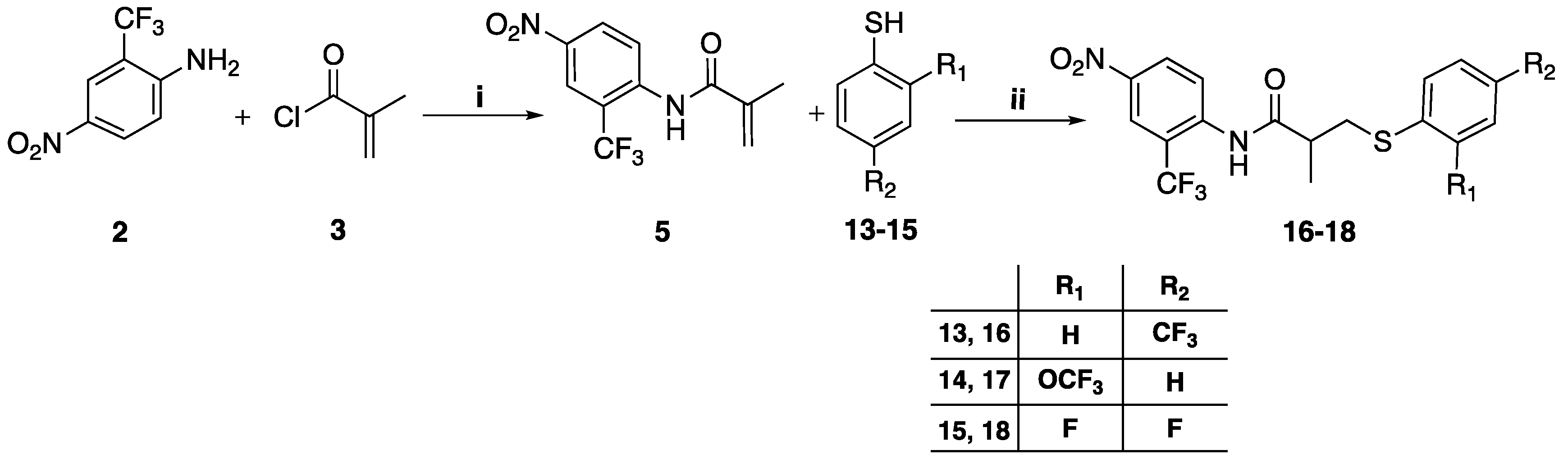
3.1.4. General Method for the Preparation of Compounds 16–18
To a mixture of 60% sodium hydride (NaH) in mineral oil (2.36 mmol) in 5 mL of anhydrous tetrahydrofuran at 0 °C under anhydrous THF under nitrogen atmosphere was added dropwise the corresponding thiophenol
10–
13 (2.05 mmol). This mixture was stirred at room temperature for 20 min. A solution of the intermediate
5 (1.57 mmol in 5 mL anhydrous tetrahydrofuran) was added slowly to the thiophenol mixture and stirred at room temperature for 24h. The mixture was concentrated under vacuum, then diluted with 30 mL ethyl acetate washed with 20 mL brine and 30 mL water, dried over anhydrous sodium sulfate, and concentrated under vacuum. The crude residue was purified by column chromatography eluting with chloroform-ethyl acetate gradually increasing from 95:5 to 90:10
v/v [
12].
2-Methyl-N-(4-nitro-2-(trifluoromethyl) phenyl)-3-(4-(trifluoromethyl)phenylthio) propanamide (16), yield 71%, 1H-NMR (CDCl3) δ 8.64 (d, J = 9 Hz, 1H, ArH), 8.54 (d, J = 3 Hz, 1H, ArH), 8.42 (dd, J = 3, 9.5 Hz, 1H, ArH), 7.80 (s, 1H, OH), 7.55 (d, J = 8 Hz, 2H, ArH), 7.44 (d, J = 8 Hz, 2H, ArH), 3.43 (dd, J = 8.5, 14 Hz, 1H, CH2), 3.16 (dd, J = 5.5, 13.5 Hz, 1H, CH2), 2.73 (m, 1H, CH), 1.44 (d, J = 7 Hz, 3H, CH3); 19F-NMR (CDCl3) δ: −62.63, −61.00; 13C-NMR (CDCl3) δ: 172.73 (C=O), 143.04 (ArC), 141.71 (ArC), 140.56 (ArC), 128.49 (ArCH), 128.26 (ArCH), 128.02 (m, ArC), 125.93 (q, 3JC-F = 3.7 Hz, ArCH), 125.87, 123.30 (ArCH), 123.73 (m, 2CF3), 122.31 (q, 3JC-F = 5 Hz, ArCH), 120.65 (m, ArC), 43.09 (CH), 36.32 (CH2), 17.46 (CH3). MS (ES+) m/z: 453.1 [M + H]+, 475.1 [M + Na]+. Reverse-phase HPLC, eluting with H2O/CH3CN from 90/10 to 0/100 in 30 min; flow = 1 mL/min, tR = 24.45 min 98.0%.
2-Methyl-N-(4-nitro-2-(trifluoromethyl)phenyl)-3-(2-(trifluoromethoxy)phenylthio) propanamide (17), yield 69%, 1H-NMR (CDCl3) δ 8.65 (d, J = 9.5 Hz, 1H, ArH), 8.54 (d, J = 2.5 Hz, 1H, ArH), 8.43 (dd, J = 3, 9.5 Hz, 1H, ArH), 7.80 (s, 1H, NH), 7.48 (m, 1H, ArH), 7.23 (m, 3H, ArH), 3.37 (dd, J = 8, 13.5 Hz, 1H, CH2), 3.08 (dd, J = 5.5, 13.5 Hz, 1H, CH2), 2.68 (m, 1H, CH), 1.42 (d, J = 7 Hz 3H, CH3); 19F-NMR (CDCl3) δ −61.13, −57.29; 13C-NMR (CDCl3) δ 172.90 (C=O), 160.91 (ArC), 143.04 (ArC), 140.30 (ArC), 133.91 (ArCH), 131.48 (ArCH), 129.45 (m, CF3), 128.22 (ArCH), 127.36 (ArCH), 123.96 (m, CF3), 123.48 (ArCH), 122.30 (q, 3JC-F = 5 Hz, ArCH), 121.55 (m, ArC), 121.25 (ArCH), 120.58 (ArC), 43.12 (CH), 36.58 (CH2), 17.37 (CH3).MS (ES+) m/z: 469.1 [M + H]+, 491.1 [M + Na]+. tR = 24.22 min 95.02%.
3-(2,4-Difluorophenylthio)-2-methyl-N-(4-nitro-2-(trifluoromethyl)phenyl) propanamide (18), yield 75%, 1H-NMR (CDCl3) δ 8.68 (d, J = 9.5 Hz, 1H, ArH), 8.55 (d, J = 2.5 Hz, 1H, ArH), 8.44 (dd, J = 2.5, 9 Hz, 1H, ArH), 7.85 (s, 1H, NH), 7.47 (m, 1H, ArH), 6.88 (m, 2H, ArH), 3.27 (dd, J = 8.5, 14 Hz, 1H, CH2), 2.99 (dd, J = 5.5, 14 Hz, 1H, CH2), 2.64 (m, 1H, CH), 1.38 (d, J = 7 Hz, 3H, CH3); 19F-NMR (CDCl3) δ −61.06 (3F), −102.93 (1F), −108.49 (1F); 13C-NMR (CDCl3) δ 172.98 (C=O), 164.15 (ArC), 158.55 (ArC), 142.99 (ArC), 140.77 (ArC), 135.81 (m, ArCH), 135.73, 128.25 (ArCH), 124.89 (q, 1JC-F = 228 Hz, ArC), 123.99 (ArC), 123.29 (ArCH), 122.29 (q, 3JC-F = 6.3 Hz, ArCH), 121.87 (m, ArC), 112.14 (dd, 4JC-F = 3.6 Hz, 2JC-F = 22.5 Hz, ArCH), 104.82 (t, 2JC-F = 25.9 Hz, ArCH), 43.54 (CH), 38.28 (CH2), 17.38 (CH3). MS (ES+) m/z: 421.1 [M + H]+, 443.1 [M + Na]+. Reverse-phase HPLC, eluting with H2O/CH3CN from 90/10 to 0/100 in 30 min; flow = 1 mL/min, tR = 24.92 min 95.10%.
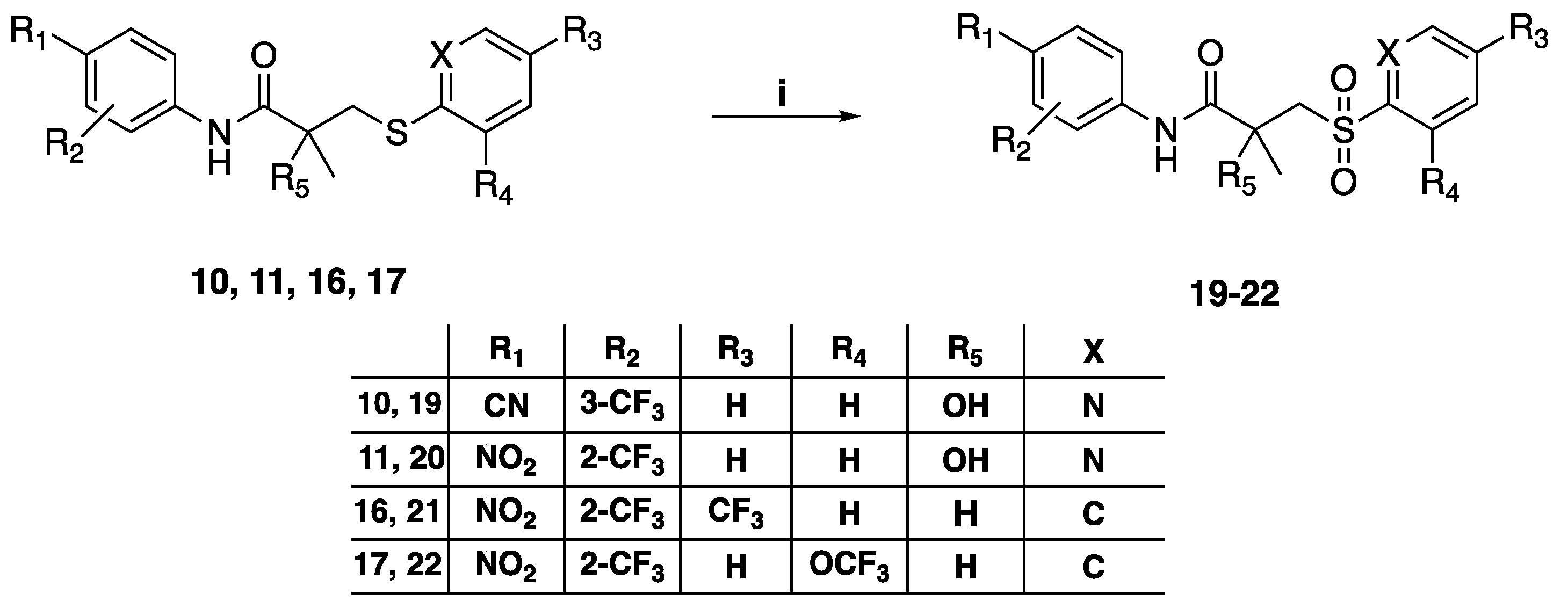
3.1.5. General Method for the Preparation of Sulfone Compounds 19–22, 31
To a stirring solution of the different sulfide 10, 11, 16, 17, 27 (0.7 mmol) in 5 mL anhydrous dichloromethane (DCM) was added m-chloroperbenzoic acid (mCPBA) (1.4 mmol). The solution was stirred at room temperature for 24 h. The reaction mixture was neutralized with 1M sodium hydroxide. Distilled water (50 mL) was added to the reaction mixture and was extracted with 2 × 50 mL of dichloromethane. The combined organic layers were washed, dried over anhydrous sodium sulfate, and concentrated in vacuo. The crude residue was purified by column chromatography, preparative TLC, or crystallization from methanol.
N-(4-cyano-3-(trifluoromethyl)phenyl)-2-hydroxy-2-methyl-3-(pyridin-2-ylsulfonyl)propanamide (19), yield 76%, 1H-NMR (CDCl3) δ 9.32 (s, 1H, NH), 8.74 (d, J = 5 Hz, 1H, ArH), 8.13 (d, J = 8 Hz, 1H, ArH), 8.07 (m, 2H, ArH), 7.96 (d, J = 7.5 Hz, 1H, ArH), 7.81 (d, J = 8 Hz, 1H, ArH), 7.68 (m, 1H, ArH), 6.86 (s, 1H, OH), 4.38 (d, J = 15.5 Hz, 1H, CH2), 3.76 (d, J = 15 Hz, 1H, CH2), 1.64 (s, 3H, CH3); 19F-NMR (CDCl3) δ −62.17; 13C-NMR (CDCl3) δ: 172.72 (C=O), 155.12 (ArC), 149.46 (ArCH), 141.50 (ArC), 139.43 (ArCH), 135.82 (ArCH), 136.55 (m, CF3), 128.21 (ArCH), 121.98 (ArCH), 121.93 (ArCH), 121.42 (ArC), 117.37 (q, 3JC-F = 5 Hz, ArCH), 115.54 (CN), 99.99 (ArC), 73.45 (COH), 60.99 (CH2), 27.77 (CH3). MS (ES+) m/z: 414.1 [M + H]+, 436.1 [M + Na]+. Reverse-phase HPLC, eluting with H2O/CH3CN from 90/10 to 0/100 in 30 min; flow = 1 mL/min, tR = 15.42 min 98.7%.
2-Hydroxy-2-methyl-N-(4-nitro-2-(trifluoromethyl)phenyl)-3-(pyridin-2-yl sulfonyl) propanamide, (20), yield 81%, 1H-NMR (CDCl3) δ 9.88 (s, 1H, NH), 8.77 (d, J = 5 Hz, 1H, ArH), 8.71 (d, J = 9 Hz, 1H, ArH), 8.55 (d, J = 2.5 Hz, 1H, ArH), 8.44 (dd, J = 3, 9.5 Hz, 1H, ArH), 8.14 (d, J = 7.5 Hz, 1H, ArH), 7.70 (m, 1H, ArH), 8.09 (m, 1H, ArH), 7.09 (s, 1H, OH), 4.39 (d, J = 15.5 Hz, 1H, CH2), 3.81 (d, J = 15.5 Hz, 1H, CH2), 1.65 (s, 3H, CH3); 19F-NMR (CDCl3) δ −61.68; 13C-NMR (CDCl3) δ 172.90 (C=O), 157.37 (ArC), 149.35 (ArCH), 142.95 (ArC), 140.82 (ArC), 139.55 (ArCH), 128.25 (ArCH), 123.91 (m, ArC), 123.01 (ArCH), 122.72 (ArCH), 122.38 (m, CF3) 122.34 (q, 3JC-F = 5.6 Hz, ArCH), 121.79 (ArCH), 73.37 (COH), 61.37 (CH2), 27.64 (CH3).MS (ES+) m/z: 434.1 [M + H]+, 456.1 [M + Na]+. tR = 17.44 min 99.47%.
2-Methyl-N-(4-nitro-2-(trifluoromethyl)phenyl)-3-((4-(trifluoromethyl)phenyl) sulfonyl) propenamide (21), yield 74.7%, 1H-NMR (CDCl3) δ 8.57 (d, J = 2 Hz, 1H, ArH), 8.46 (d, J = 9 Hz, 1H, ArH), 8.43 (dd, J = 9.5, 2.5 Hz, 1H, ArH), 8.10 (d, J = 8 Hz, 2H, ArH), 7.86 (m, 3H, ArH, NH), 3.78 (dd, J = 4, 9.5 Hz, 1H, CH2), 3.70 (dd, J = 4.5, 10.5 Hz, 1H, CH2), 3.12 (m, 1H, CH), 1.39 (d, J = 7.5 Hz, 3H, CH3); 19F-NMR (CDCl3) δ −60.92, −63.33; 13C-NMR (CDCl3) δ 171.48 (C=O), 150.72 (ArC), 143.42 (ArC), 140.31 (ArC), 135.77, 128.69 (ArCH), 128.21 (ArCH), 126.64 (q, 3JC-F = 3.6 Hz, ArCH), 124.06 (ArCH), 124.74, 124.70, 124.67, 124.47, 124.42, 124.39, 122.40, 122.36 (q, 3JC-F = 5.4 Hz, ArCH), 120.8 (q, 1JC-F = 232.8 Hz, CF3), 120.32, 58.70, 37.14 (CH2), 18.57 (CH3). MS (ES+) m/z: 485.1 [M + H]+, 507.1 [M + Na]+. Reverse-phase HPLC, eluting with H2O/CH3CN from 90/10 to 0/100 in 30 min; flow = 1 mL/min, tR = 21.79 min 97.30%.
2-Methyl-N-(4-nitro-2-(trifluoromethyl)phenyl)-3-(2-(trifluoromethoxy) phenylsulfonyl) propanamide (22), yield 71.6%, 1H-NMR (CDCl3) δ 8.55 (d, J = 2.5 Hz, 1H, ArH), 8.49 (d, J = 9 Hz, 1H, ArH), 8.42 (dd, J = 2.5, 9 Hz, 1H, ArH), 8.08 (dd, J = 2, 8 Hz, 1H, ArH), 7.88 (s, 1H, NH), 7.73 (ddd, J = 2, 7.5, 8.5 Hz, 1H, ArH), 7.49 (2m, 1H, ArH), 7.45 (td, J = 1, 7.5 Hz, 1H, ArH), 4.01 (dd, J = 8.5, 14 Hz, 1H, CH2), 3.39 (dd, J = 4, 14 Hz, 1H, CH2), 3.12 (m, 1H, CH), 1.48 (d, J = 7.5 Hz, 3H, CH3); 19F-NMR (CDCl3) δ −60.99, −56.24; 13C-NMR (CDCl3) δ 171.28 (C=O), 160.35 (ArC), 143.61 (ArC), 140.61 (ArC), 135.96 (ArCH), 130.98 (ArCH), 129.50 (ArC), 129.16 (ArC), 128.13 (ArCH), 126.79 (ArCH), 123.90 (ArCH), 123.27 (CF3), 121.45 (ArC), 120.26 (ArCH), 120.24 (ArCH), 58.28 (CH2), 37.21 (CH), 18.37 (CH3). MS (ES+) m/z: 501.1 [M + H]+, 523.0 [M + Na]+. Reverse-phase HPLC, eluting with H2O/CH3CN from 90/10 to 0/100 in 30 min; flow = 1 mL/min, tR = 24.22 min 95.00%.
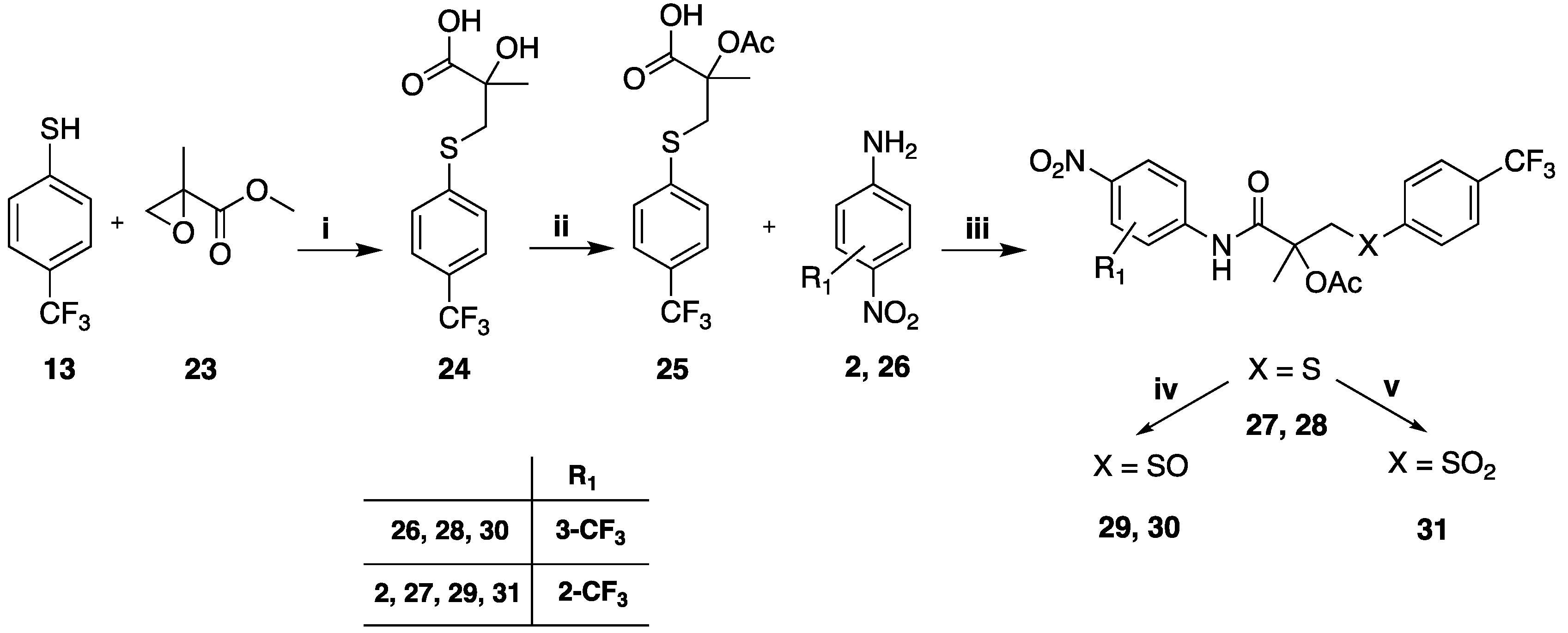
3.1.6. General Method for the Preparation of Acetylated Compounds 27, 28
4-trifluoromethyl thiophenol (
13, 0.283 g, 0.0178 moles) was placed in a round-bottomed flask and heated to 55–60 °C.; methyl 2-methylglycidate (
23, 2.11 g, 0.0181 moles) was then added slowly over an hour at 55–60 °C after a further 60 min at 55–60 °C. TLC showed that the reaction was complete with major presence of the methyl ester intermediate. Water was added, followed by 30% NaOH solution (2.61 g, 0.0195 moles) and the mixture was heated at 60 °C for 2 h. TLC showed only the spot of the desired 2-hydroxy 2-methyl-3-(4-trifluoromethylphenylthio) propionic acid (
24). To a solution of 24 (1.36 g, 0.0593 moles) in 7 mL of anhydrous toluene was added acetic anhydride (0.601 mL, 0.00623 moles) and the resulting solution was heated at 100 °C for 5 h. After cooling to room temperature, the solution was washed twice with 2 mL of water, and the combined organic phases were dried and removed under reduced pressure. A dense oil was obtained, which solidified spontaneously (
25). To a solution of the acetoxy derivative (
25) (0.4 g, 0.00146 moles) in 20 mL of anhydrous toluene at rt was added 0.127 mL (0.00148 moles) of thionyl chloride, and the mixture was heated at 85–90 °C for 3–4 h. The solvent was completely evaporated under reduced pressure. The product was obtained as an oil which was used as is in the successive reaction. To a solution of the acid chloride (0.21g, 0.00072 moles) in 1.2 mL of anhydrous toluene was added 0.09 g (0.00072 moles) of DMAP, maintaining the solution at room temperature; the suspension was allowed to react for 10 min, then 0.115 g (0.00062 moles) of the corresponding 4-amino-benzonitrile derivative (
2,
26) dissolved in toluene was added and the mixture was heated at 75–80 °C for 8–10 h following the disappearance of the amine by TLC (eluent: ethyl acetate/toluene 75/25). After cooling to room temperature, the solution was diluted with toluene and treated with a 5% solution of HCl, separated, and washed with 5% bicarbonate solution. The organic phase was cured and evaporated under reduced pressure. Crude propionamide derivatives (
48 and
49) were obtained in quantitative yield [
21].
2-Methyl-l-(4-nitro-2-(trifluoromethyl) phenylamino)-l-oxo-3-(4-(trifluoromethyl) phenylthio)propan-2-yl acetate (27), yield 30%, 1H-NMR (CDCl3) δ 9.10 (s, 1H, NH), 8.62 (d, J = 9 Hz, 1H, ArH), 8.54 (d, J = 2.5 Hz, 1H, ArH), 8.40 (dd, J = 3, 9 Hz, 1H, ArH), 7.45 (m, 4H, ArH), 4.07 (d, J = 14.5 Hz, 1H, CH2), 3.75 (d, J = 14 Hz, 1H, CH2), 1.99 (s, 3H, CH3), 1.89 (s, 3H, CH3); 19F-NMR (CDCl3) δ −61.29, −62.78; 13 C-NMR (CDCl3) δ 169.90 (C=O), 168.21 (C=O), 143.05 (ArC), 140.03 (ArC), 139.45 (ArC), 130.22 (ArCH), 128.90 (q, 2JC-F = 32.6 Hz, ArC), 128.49 (ArCH), 125.65 (q, 3JC-F = 3.8 Hz, ArCH), 123.79 (q, 1JC-F = 270.6 Hz, CF3), 123.03 (q, 1JC-F = 272.1 Hz, CF3), 122.34 (q, 3JC-F = 6.3 Hz, ArCH) 121.93, 121.89 (ArCH), 118.82 (q, 2JC-F = 31.3 Hz, ArC), 84.34 (COAc), 39.06 (CH2), 22.94 (CH3), 21.14 (CH3). MS (ES+) m/z: 511.1 [M + H]+, 533.1 [M + Na]+. Reverse-phase HPLC, eluting with H2O/CH3CN from 90/10 to 0/100 in 30 min; flow = 1 mL/min, tR = 25.50 min 97.3%.
2-Methyl-l-(4-nitro-3-(trifluoromethyl) phenylamino)-l-oxo-3-(4-(trifluoromethyl) phenylthio) propan-2-yl acetate (28), yield 84%, 1H-NMR (CDCl3) δ 1.90 (s, 3H), 2.10 (s, 3H), 3.77 (d, J = 14 Hz, 1H), 4.04 (d, J = 14 Hz, 1H), 7.44 (d, J = 8.5 Hz, 2H), 7.48 (d, J = 8.5 Hz, 2H), 7.88 (d, J = 2 Hz, 1H), 7.92 (dd, J = 2, 8.5 Hz, 1H), 7.97 (d, J = 9 Hz, 1H), 8.40 (s, 1H); 19F-NMR (CDCl3) δ −62.80, −60.14; 13C-NMR (CDCl3) δ 169.84 (C=O), 168.59 (C=O), 144.75 (ArC), 139.59 (ArC), 129.53 (ArCH), 129.11 (q, 2JC-F = 31.5 Hz, ArC), 126.95 (ArCH), 125.75 (q, 3JC-F = 3.8 Hz, ArCH), 124.84 (q, 1JC-F = 270.6 Hz, CF3), 123.43 (q, 1JC-F = 271.2 Hz, CF3), 122.56 (ArCH) 118.51 (ArCH), 118.50 (q, 2JC-F = 30.4 Hz, ArC), 83.89 (COAc), 60.39 (CH2), 22.95 (CH3), 21.69 (CH3). MS (ES+) m/z: 511.1 [M + H]+, 533.1 [M + Na]+. Reverse-phase HPLC, eluting with H2O/CH3CN from 90/10 to 0/100 in 30 min; flow = 1 mL/min, tR = 24.40 min 96.31%.
2-Methyl-l-(4-nitro-2-(trifluoromethyl) phenylamino)- l-oxo-3-(4-(trifluoromethyl) phenylsulfinyl)propan-2-yl acetate (29), yield 69%, 1H-NMR (CDCl3) δ [9.10 (s), 9.26 (s), 1H, NH], [8.73 (s), 8.75 (s), 1H, ArH], [8.57 (d, J = 2.5 Hz), 8.61 (d, J = 2.5 Hz), 1H, ArH], [8.47 (dd, J = 2.5, 9.5 Hz), 8.51 (dd, J = 2.5, 9.5 Hz), 1H, ArH), 7.81 (m, 4H, ArH), [3.62 (d, J = 14 Hz), 3.82 (s), 3.98 (d, J = 14 Hz), 2H, CH2], [2.25 (s), 2.36 (s), 3H, CH3], [1.93 (s), 1.96 (s), 3H, CH3]; 19F-NMR (CDCl3) δ −61.29, −62.92; 13 C-NMR (CDCl3) δ 168.98 (C=O), 168.88 (C=O), 147.83 (ArC), (143.32, 142.47, ArC), (140.30, 139.97, ArC), 133.44 (m, ArC), 128.52 (ArCH), 126.47 (m, ArCH), 125.84 (q, 1JC-F = 263.4 Hz, CF3), [124.49, 124.43, ArCH], [123.13, 123.00, ArCH], [122.54 (q, 3JC-F = 5 Hz), 122.40 (q, 3JC-F = 6.3 Hz), ArCH], 121.02 (q, 1JC-F = 226.9 Hz, CF3), (82.06, 81.37, COCH3), (62.65, 61.75, CH2), (24.34, 23.98, CH3), (21.64, 21.61, CH3). MS (ES+) m/z: 527.1 [M + H]+, 549.1 [M + Na]+. Reverse-phase HPLC, eluting with H2O/CH3CN from 90/10 to 0/100 in 30 min; flow = 1 mL/min, tR = 22.54 min 98.81%.
2-Hydroxy-2-methyl-N-(4-nitro-3-(trifluoromethyl)phenyl)-3-(4-(trifluoromethyl) phenylsulfinyl) propanamide (30), yield 59%, 1H-NMR (CDCl3) δ 9.47 (s, 1H, NH), 8.26 (d, J = 1.5 Hz, 1H, ArH), 8.06 (m, 2H, ArH), 7.89 (d, J = 8.5 Hz, 2H, ArH), 7.83 (d, J = 8 Hz, 2H, ArH), 5.89 (s, 1H, OH), 3.61 (d, J = 13 Hz, 1H, CH2), 3.08 (d, J = 13 Hz, 1H, CH2), 1.62 (s, 3H, CH3); 19F-NMR (CDCl3) δ −60.10, −63.02; 13C-NMR (CDCl3) δ 173.26 (C=O), 149.51 (ArC), 145.87 (ArC), 141.21 (ArC), 137.77 (m, ArC), 126.84 (q, 3JC-F = 3.4 Hz, ArCH), 124.64 (ArCH), 124.35 (ArC), 124.24 (ArCH), 122.59 (ArC), 122.32 (ArCH), 120.64 (m, ArC), 118.50 (q, 3JC-F = 5 Hz, ArCH), 76.80 (COH), 62.25 (CH2), 28.25 (CH3). MS (ES+) m/z: 485.1 [M + H]+, 507.1 [M + Na]+. Reverse-phase HPLC, eluting with H2O/CH3CN from 90/10 to 0/100 in 30 min; flow = 1 mL/min, tR = 18.95 min 96.76%.
2-Methyl-l-(4-nitro-2-(trifluoromethyl) phenylamino)-l-oxo-3-(4-(trifluoromethyl) phenylsulfonyl) propan-2-yl acetate (31), yield 83%, 1H-NMR (CDCl3) δ 9.22 (s, 1H, NH), 8.71 (d, J = 9.5 Hz, 1H, ArH), 8.60 (d, J = 2.5 Hz, 1H, ArH), 8.49 (dd, J = 2.5, 9 Hz, 1H, ArH), 8.06 (d, J = 8 Hz, 2H, ArH), 7.84 (d, J = 8.5 Hz, 2H, ArH), 4.41 (d, J = 14.5 Hz, 1H, CH2), 4.07 (d, J = 14.5 Hz, 1H, CH2), 2.26 (s, 3H, CH3), 1.90 (s, 3H, CH3); 19F-NMR (CDCl3) δ −63.31, −61.29; 13 C-NMR (CDCl3) δ 169.05 (C=O), 168.72 (C=O), 143.39 (ArC), 143.02 (ArC), 140.14 (ArC), 136.06 (m, ArC), 128.75 (ArCH), 128.54 (ArCH), 126.57 (q, 3JC-F = 3.8 Hz, ArCH), 124.07 (m, 2CF3), 122.82 (ArCH), 122.47 (q, 3JC-F = 5 Hz, ArCH), 121.09 (m, ArC), 80.16 (COAc), 57.74 (CH2), 24.36 (CH3), 21.72 (CH3). MS (ES+) m/z: 543.1 [M + H]+, 565.1 [M + Na]+ Reverse-phase HPLC, eluting with H2O/CH3CN from 90/10 to 0/100 in 30 min; flow = 1 mL/min, tR = 24.08 min 96.52%.