Bacterial Nanocellulose in Dentistry: Perspectives and Challenges
Abstract
1. Introduction
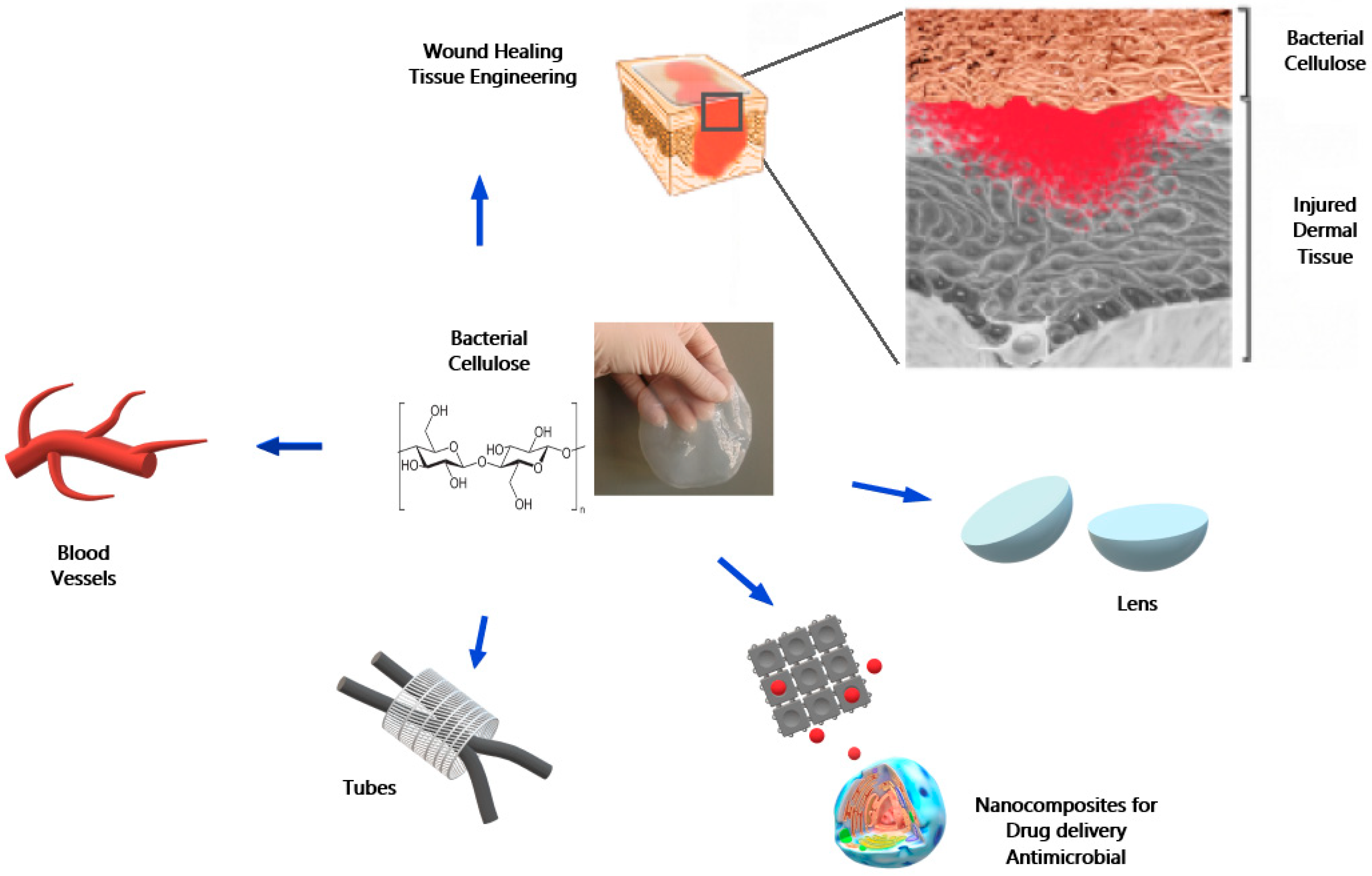
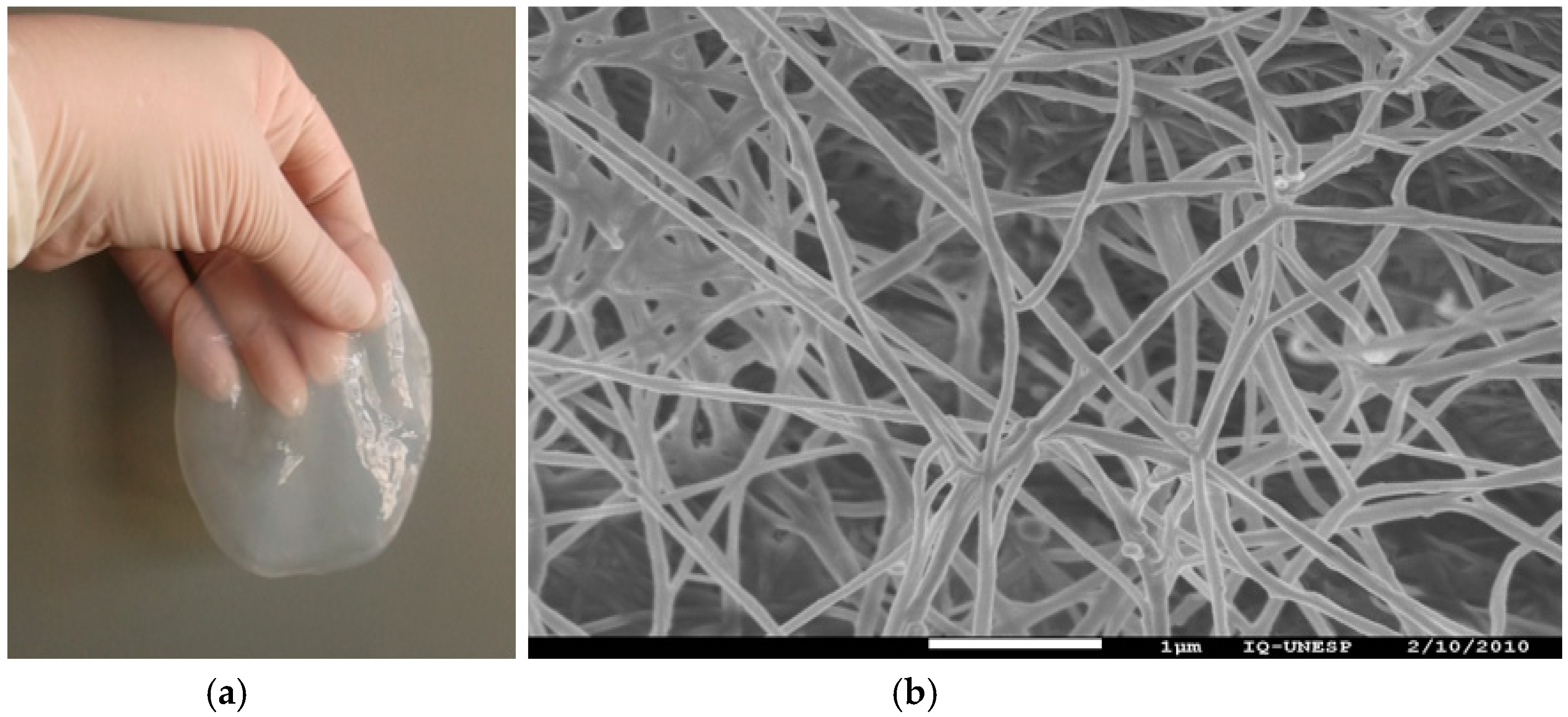
2. Bacterial Cellulose
3. A Brief Overview of BC Uses in Biomedicine Applications
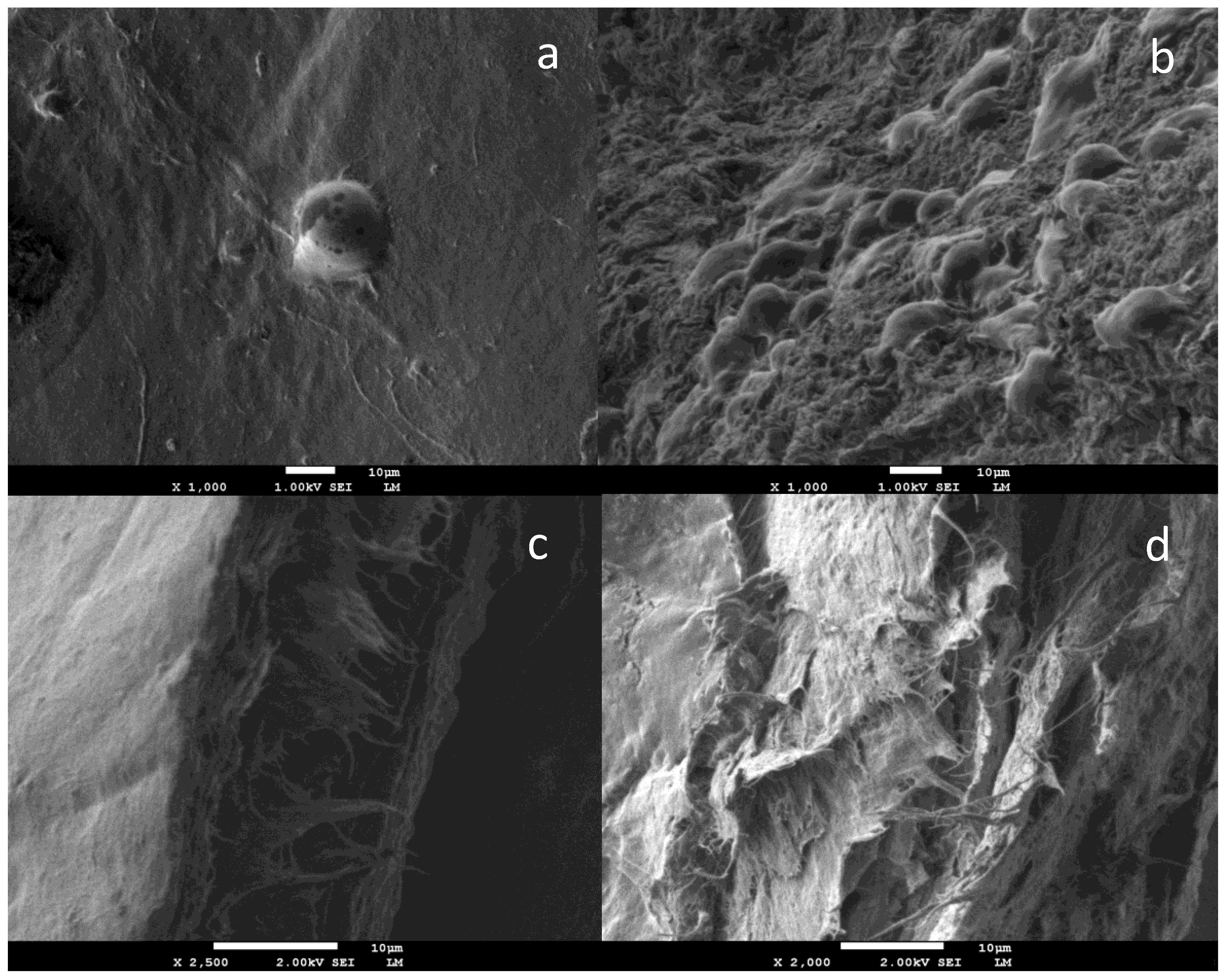
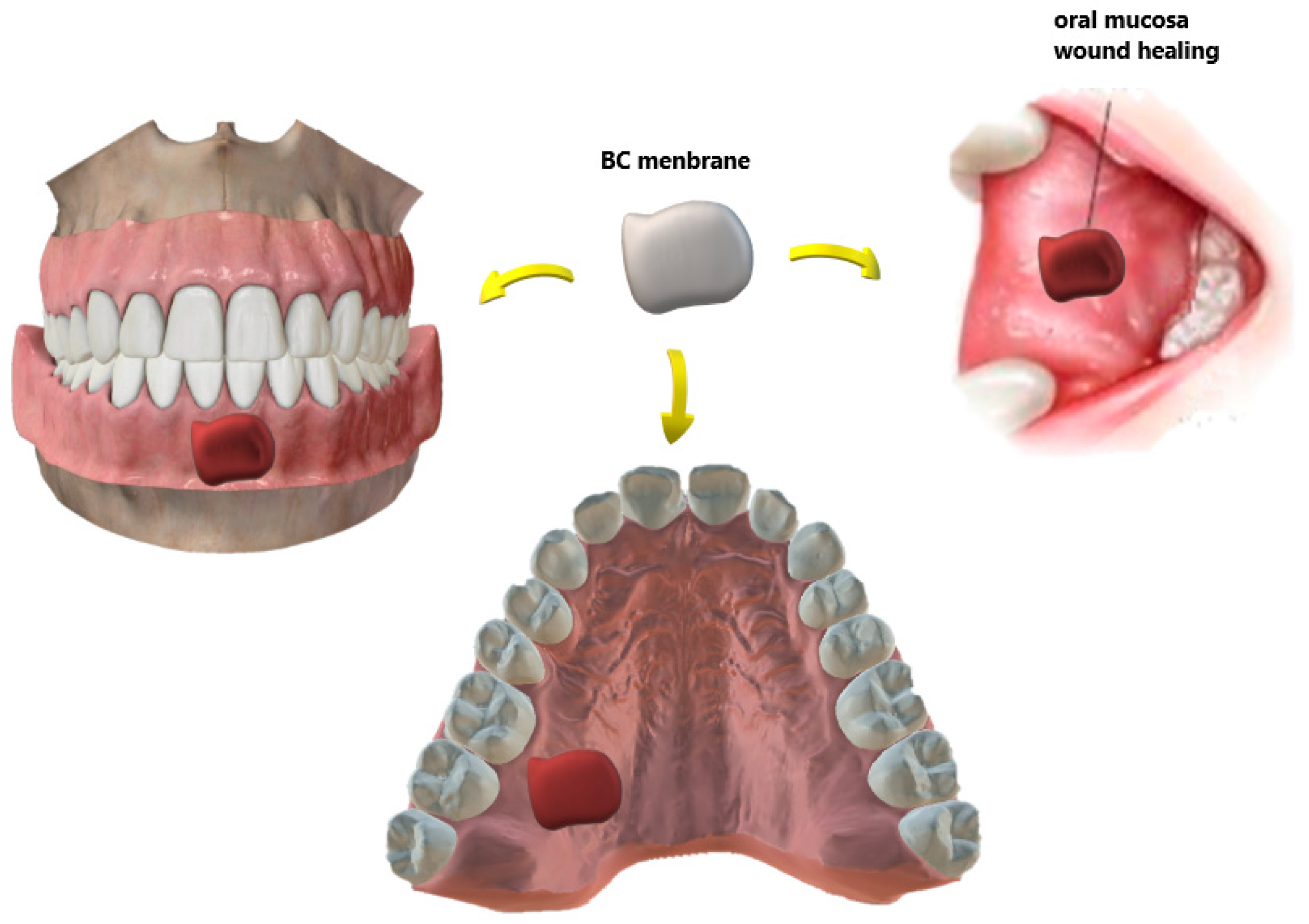
4. BC in Dentistry
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in Drug Delivery and Tissue Engineering: From Discovery to Applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Biondi, M.; Ungaro, F.; Quaglia, F.; Netti, P.A. Controlled drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 229–242. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Chiu, L.L.; Weisel, R.D.; Li, R.-K.; Radisic, M. Defining conditions for covalent immobilization of angiogenic growth factors onto scaffolds for tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, 69–84. [Google Scholar] [CrossRef]
- Cunha, C.; Panseri, S.; Antonini, S. Emerging nanotechnology approaches in tissue engineering for peripheral nerve regeneration. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 50–59. [Google Scholar] [CrossRef]
- Kong, X.; Cui, F.; Wang, X.; Zhang, M.; Zhang, W. Silk fibroin regulated mineralization of hydroxyapatite nanocrystals. J. Cryst. Growth 2004, 270, 197–202. [Google Scholar] [CrossRef]
- Horue, M.; Cacicedo, M.L.; Castro, G.R. New insights into bacterial cellulose materials: Production and modification strategies. Int. J. Adv. Med. Biotechnol. 2018, 1, 44–59. [Google Scholar] [CrossRef]
- Rajwade, J.M.; Paknikar, K.M.; Kumbhar, J.V. Applications of bacterial cellulose and its composites in biomedicine. Appl. Microbiol. Biotechnol. 2015, 99, 2491–2511. [Google Scholar] [CrossRef]
- Da Silva, M.L.A.; Crawford, A.; Mundy, J.; Correlo, V.; Sol, P.; Bhattacharya, M.; Hatton, P.; Reis, R.; Neves, N. Chitosan/polyester-based scaffolds for cartilage tissue engineering: Assessment of extracellular matrix formation. Acta Biomater. 2010, 6, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J. XLIII.—On an acetic ferment which forms cellulose. J. Chem. Soc. Trans. 1886, 49, 432–439. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khan, T.; Park, J.K. Water holding and release properties of bacterial cellulose obtained by in situ and ex situ modification. Carbohydr. Polym. 2012, 88, 596–603. [Google Scholar] [CrossRef]
- Barud, H.S.; Regiani, T.; Marques, R.F.C.; Lustri, W.R.; Messaddeq, Y.; Ribeiro, S.J.L. Antimicrobial Bacterial Cellulose-Silver Nanoparticles Composite Membranes. J. Nanomater. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Klemm, D.O.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Capadona, J.R.; Shanmuganathan, K.; Trittschuh, S.; Seidel, S.; Rowan, S.J.; Weder, C. Polymer Nanocomposites with Nanowhiskers Isolated from Microcrystalline Cellulose. Biomacromolecules 2009, 10, 712–716. [Google Scholar] [CrossRef]
- Li, Y.; Lin, M.; Davenport, J.W. Ab Initio Studies of Cellulose I: Crystal Structure, Intermolecular Forces, and Interactions with Water. J. Phys. Chem. C 2011, 115, 11533–11539. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, S.; Kim, S.H.; Kim, J.H.; Yu, H.; Kim, H.J.; Yang, Y.-H.; Kan, E.; Kim, Y.H.; Lee, S.H. Biocompatible cellulose nanocrystals as supports to immobilize lipase. J. Mol. Catal. B Enzym. 2015, 122, 170–178. [Google Scholar] [CrossRef]
- Weyell, P.; Beekmann, U.; Küpper, C.; Dederichs, M.; Thamm, J.; Fischer, D.; Kralisch, D. Tailor-made material characteristics of bacterial cellulose for drug delivery applications in dentistry. Carbohydr. Polym. 2019, 207, 1–10. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.; Chen, S.; Li, Z.; Sun, X.; Feng, C.; Wang, H.; Xu, Y. In vitro degradability of bacterial cellulose in simulated body fluids and compatibility in vivo. Cellulose 2016, 23, 3187–3198. [Google Scholar] [CrossRef]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The Future Prospects of Microbial Cellulose in Biomedical Applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Falcão, S.C.; Neto, J.E.; Coelho, A.R.D.B. Incorporation by host tissue of two biomaterials used as repair of defects produced in abdominal wall of rats. Acta Cir. Bras. 2008, 23, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Helenius, G.; Bäckdahl, H.; Bodin, A.; Nannmark, U.; Gatenholm, P.; Risberg, B. In vivo biocompatibility of bacterial cellulose. J. Biomed. Mater. Res. Part A 2006, 76, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Autier, L.; Clavreul, A.; Cacicedo, M.L.; Franconi, F.; Sindji, L.; Rousseau, A.; Perrot, R.; Montero-Menei, C.N.; Castro, G.R.; Menei, P. A new glioblastoma cell trap for implantation after surgical resection. Acta Biomater. 2019, 84, 268–279. [Google Scholar] [CrossRef]
- Cacicedo, M.L.; Islan, G.A.; León, I.E.; Alvarez, V.A.; Chourpa, I.; Allard-Vannier, E.; García-Aranda, N.; Díaz-Riascos, Z.V.; Fernández, Y.; Schwartz, S., Jr.; et al. Bacterial cellulose hydrogel loaded with lipid nanoparticles for localized cancer treatment. Colloids Surf. B Biointerfaces 2018, 170, 596–608. [Google Scholar] [CrossRef]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose—Artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Wippermann, J.; Schumann, D.; Klemm, D.; Kosmehl, H.; Salehi-Gelani, S.; Wahlers, T. Preliminary Results of Small Arterial Substitute Performed with a New Cylindrical Biomaterial Composed of Bacterial Cellulose. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 592–596. [Google Scholar] [CrossRef]
- Salgado, A.; Sousa, N.; Silva, N.A.; Neves, N.M.; Reis, R.L. Hydrogels for spinal cord injury regeneration. Nat.-Based Polym. Biomed. Appl. 2008. [Google Scholar] [CrossRef]
- Bäckdahl, H.; Helenius, G.; Bodin, A.; Nannmark, U.; Johansson, B.R.; Risberg, B.; Gatenholm, P. Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials 2006, 27, 2141–2149. [Google Scholar] [CrossRef]
- Rambo, C.R.; Recouvreux, D.D.O.S.; Carminatti, C.; Pitlovanciv, A.; Antonio, R.; Porto, L. Template assisted synthesis of porous nanofibrous cellulose membranes for tissue engineering. Mater. Sci. Eng. C 2008, 28, 549–554. [Google Scholar] [CrossRef]
- Fraser, S.A.; Ting, Y.; Mallon, K.S.; Wendt, A.E.; Murphy, C.J.; Nealey, P.F. Sub-micron and nanoscale feature depth modulates alignment of stromal fibroblasts and corneal epithelial cells in serum-rich and serum-free media. J. Biomed. Mater. Res. Part A 2007, 86, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Ul-Islam, M.; Khan, T.; Park, J.K. Nanoreinforced bacterial cellulose–montmorillonite composites for biomedical applications. Carbohydr. Polym. 2012, 89, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhou, P.; Zhang, S.; Yang, G. Evaluation of bacterial nanocellulose-based uniform wound dressing for large area skin transplantation. Mater. Sci. Eng. C 2013, 33, 2995–3000. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhang, Y.; Li, C.; Wu, Z.; Zhuo, Q.; Huang, X.; Qiu, G.; Zhou, P.; Yang, G. Skin tissue repair materials from bacterial cellulose by a multilayer fermentation method. J. Mater. Chem. 2012, 22, 12349–12357. [Google Scholar] [CrossRef]
- Klemm, D.O.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.G.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Shah, N.; Ul-Islam, M.; Khattak, W.A.; Park, J.K. Overview of bacterial cellulose composites: A multipurpose advanced material. Carbohydr. Polym. 2013, 98, 1585–1598. [Google Scholar] [CrossRef]
- Kucinska-Lipka, J.; Gubanska, I.; Janik, H. Bacterial cellulose in the field of wound healing and regenerative medicine of skin: Recent trends and future prospectives. Polym. Bull. 2015, 72, 2399–2419. [Google Scholar] [CrossRef]
- Barud, H.G.D.O.; Da Silva, R.R.; Barud, H.S.; Tercjak, A.; Gutierrez, J.; Lustri, W.R.; De Oliveira, O.B.; Ribeiro, S.J. A multipurpose natural and renewable polymer in medical applications: Bacterial cellulose. Carbohydr. Polym. 2016, 153, 406–420. [Google Scholar] [CrossRef]
- Barud, H.S.; Cavicchioli, M.; Amaral, T.S.D.; Junior, O.B.D.O.; Santos, D.M.; Petersen, A.L.D.O.A.; Celes, F.S.; Borges, V.D.M.; De Oliveira, C.I.; De Oliveira, P.F.; et al. Preparation and characterization of a bacterial cellulose/silk fibroin sponge scaffold for tissue regeneration. Carbohydr. Polym. 2015, 128, 41–51. [Google Scholar] [CrossRef]
- Lustri, W.R.; Barud, H.G.D.O.; Barud, H.D.S.; Peres, M.F.S.; Gutierrez, J.; Tercjak, A.; De Oliveira, O.B.; Ribeiro, S.J.L. Microbial Cellulose—Biosynthesis Mechanisms and Medical Applications. Cellul. Fundam. Asp. Curr. Trends 2015. [Google Scholar] [CrossRef]
- Cañas-Gutiérrez, A.; Osorio, M.; Molina-Ramírez, C.; Arboleda-Toro, D.; Castro-Herazo, C. Bacterial cellulose: A biomaterial with high potential in dental and oral applications. Cellulose 2020, 27, 1–18. [Google Scholar] [CrossRef]
- Fontana, J.D.; De Souza, A.M.; Fontana, C.K.; Torriani, I.L.; Moreschi, J.C.; Gallotti, B.J.; De Souza, S.J.; Narcisco, G.P.; Bichara, J.A.; Farah, L.F.X. Acetobacter cellulose pellicle as a temporary skin substitute. Appl. Biochem. Biotechnol. 1990, 24–25, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Ludwicka, K.; Cala, J.; Grobelski, B.; Sygut, D.; Jesionek-Kupnicka, D.; Kolodziejczyk, M.; Bielecki, S.; Pasieka, Z. New methods Modified bacterial cellulose tubes for regeneration of damaged peripheral nerves. Arch. Med. Sci. 2013, 3, 527–534. [Google Scholar] [CrossRef]
- Farah, L.F.X. Process for the Preparation of Cellulose Film, Cellulose Film Produced Thereby, Artificial Skin Graft and its Use. 1990. Available online: https://patents.google.com/patent/US4912049A/en (accessed on 13 December 2020).
- Wouk, A.F.P.D.F.; Diniz, J.M.; Cirio, S.M.; Dos Santos, H.; Baltazar, E.L.; Acco, A. Membrana biológica (biofill)—Estudo comparativo com outros agentes promotores da cicatrização da pele em suínos: Aspectos clínicos, histopatológicos e morfométricos. Arch. Veter. Sci. 1998, 3. [Google Scholar] [CrossRef]
- Portal, O.; Clark, W.A.; Levinson, D.J. Microbial cellulose wound dressing in the treatment of nonhealing lower extremity ulcers. Wounds 2009, 21, 1–3. [Google Scholar]
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brown, R.M. Microbial cellulose—The natural power to heal wounds. Biomaterials 2006, 27, 145–151. [Google Scholar] [CrossRef]
- Saska, S.; Teixeira, L.N.; De Oliveira, P.T.; Gaspar, A.M.M.; Ribeiro, S.J.L.; Messaddeq, Y.; Marchetto, R. Bacterial cellulose-collagen nanocomposite for bone tissue engineering. J. Mater. Chem. 2012, 22, 22102–22112. [Google Scholar] [CrossRef]
- Zhijiang, C.; Guang, Y. Bacterial cellulose/collagen composite: Characterization and first evaluation of cytocompatibility. J. Appl. Polym. Sci. 2011, 120, 2938–2944. [Google Scholar] [CrossRef]
- Kaya, M.G.A.; Vuluga, Z.; Panaitescu, D.M.; Vuluga, D.M.; Căşărică, A.; Ghiurea, M. Morphology and thermal stability of bacterial cellulose/collagen composites. Open Chem. 2014, 12, 968–975. [Google Scholar] [CrossRef]
- Moraes, P.R.F.D.S.; Saska, S.; Barud, H.; De Lima, L.R.; Martins, V.D.C.A.; Plepis, A.M.D.G.; Ribeiro, S.J.L.; Gaspar, A.M.M. Bacterial Cellulose/Collagen Hydrogel for Wound Healing. Mater. Res. 2016, 19, 106–116. [Google Scholar] [CrossRef]
- Nakayama, A.; Kakugo, A.; Gong, J.P.; Osada, Y.; Takai, M.; Erata, T.; Kawano, S. High Mechanical Strength Double-Network Hydrogel with Bacterial Cellulose. Adv. Funct. Mater. 2004, 14, 1124–1128. [Google Scholar] [CrossRef]
- Wang, J.; Wan, Y.; Luo, H.; Gao, C.; Huang, Y. Immobilization of gelatin on bacterial cellulose nanofibers surface via crosslinking technique. Mater. Sci. Eng. C 2012, 32, 536–541. [Google Scholar] [CrossRef]
- Kim, J.-H.; Cai, Z.; Chen, Y. Biocompatible Bacterial Cellulose Composites for Biomedical Application. J. Nanotechnol. Eng. Med. 2009, 1, 011006. [Google Scholar] [CrossRef]
- Saibuatong, O.-A.; Phisalaphong, M. Novo aloe vera–bacterial cellulose composite film from biosynthesis. Carbohydr. Polym. 2010, 79, 455–460. [Google Scholar] [CrossRef]
- Piaia, L.; Paes, C.Q.; Porto, L.M. Viability of human dermal fibroblasts cultured on bacterial cellulose and Aloe vera composites. BMC Proc. 2014, 8, P61. [Google Scholar] [CrossRef]
- Lin, W.-C.; Lien, C.-C.; Yeh, H.-J.; Yu, C.-M.; Hsu, S.-H. Bacterial cellulose and bacterial cellulose–chitosan membranes for wound dressing applications. Carbohydr. Polym. 2013, 94, 603–611. [Google Scholar] [CrossRef]
- Cai, Z.; Kim, J. Bacterial cellulose/poly(ethylene glycol) composite: Characterization and first evaluation of biocompatibility. Cellulose 2010, 17, 83–91. [Google Scholar] [CrossRef]
- Figueiredo, A.G.P.R.; Figueiredo, A.R.P.; Alonso-Varona, A.; Fernandes, S.C.M.; Palomares, T.; Rubio-Azpeitia, E.; Barros-Timmons, A.; Silvestre, A.J.D.; Neto, C.; Freire, C.S. Biocompatible Bacterial Cellulose-Poly(2-hydroxyethyl methacrylate) Nanocomposite Films. BioMed Res. Int. 2013, 2013, 1–14. [Google Scholar] [CrossRef][Green Version]
- Maria, L.C.D.S.; Santos, A.L.; Oliveira, P.C.; Barud, H.S.; Messaddeq, Y.; Ribeiro, S.J. Synthesis and characterization of silver nanoparticles impregnated into bacterial cellulose. Mater. Lett. 2009, 63, 797–799. [Google Scholar] [CrossRef]
- Barud, H.S.; Barrios, C.; Regiani, T.; Marques, R.F.; Verelst, M.; Dexpert-Ghys, J.; Messaddeq, Y.; Ribeiro, S.J. Self-supported silver nanoparticles containing bacterial cellulose membranes. Mater. Sci. Eng. C 2008, 28, 515–518. [Google Scholar] [CrossRef]
- Barud, H.D.S.; Júnior, A.M.D.A.; Saska, S.; Mestieri, L.B.; Campos, J.A.D.B.; De Freitas, R.M.; Ferreira, N.U.; Nascimento, A.P.; Miguel, F.G.; Vaz, M.M.D.O.L.L.; et al. Antimicrobial Brazilian Propolis (EPP-AF) Containing Biocellulose Membranes as Promising Biomaterial for Skin Wound Healing. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stoica-Guzun, A.; Stroescu, M.; Tache, F.; Zaharescu, T.; Grosu, E. Effect of electron beam irradiation on bacterial cellulose membranes used as transdermal drug delivery systems. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2007, 265, 434–438. [Google Scholar] [CrossRef]
- Wei, B.; Yang, G.; Hong, F. Preparation and evaluation of a kind of bacterial cellulose dry films with antibacterial properties. Carbohydr. Polym. 2011, 84, 533–538. [Google Scholar] [CrossRef]
- Trovatti, E.; Silva, N.H.C.S.; Duarte, I.F.; Rosado, C.F.; Almeida, I.F.; Costa, P.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Biocellulose Membranes as Supports for Dermal Release of Lidocaine. Biomacromolecules 2011, 12, 4162–4168. [Google Scholar] [CrossRef] [PubMed]
- Trovatti, E.; Freire, C.S.R.; Pinto, P.C.; Almeida, I.F.; Costa, P.; Silvestre, A.J.D.; Neto, C.P.; Rosado, C. Bacterial cellulose membranes applied in topical and transdermal delivery of lidocaine hydrochloride and ibuprofen: In vitro diffusion studies. Int. J. Pharm. 2012, 435, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Ni, Z.; Hessler, N.; Wesarg, F.; Müller, F.A.; Kralisch, D.; Fischer, D. The Biopolymer Bacterial Nanocellulose as Drug Delivery System: Investigation of Drug Loading and Release using the Model Protein Albumin. J. Pharm. Sci. 2013, 102, 579–592. [Google Scholar] [CrossRef]
- Bodin, A.; Bäckdahl, H.; Fink, H.; Gustafsson, L.; Risberg, B.; Gatenholm, P. Influence of cultivation conditions on mechanical and morphological properties of bacterial cellulose tubes. Biotechnol. Bioeng. 2007, 97, 425–434. [Google Scholar] [CrossRef]
- Nimeskern, L.; Ávila, H.M.; Sundberg, J.; Gatenholm, P.; Müller, R.; Stok, K.S. Mechanical evaluation of bacterial nanocellulose as an implant material for ear cartilage replacement. J. Mech. Behav. Biomed. Mater. 2013, 22, 12–21. [Google Scholar] [CrossRef]
- Cavicchioli, M.; Corso, C.T.; Coelho, F.; Mendes, L.; Saska, S.; Soares, C.P.; Souza, F.O.; Franchi, L.P.; Capote, T.S.O.; Scarel-Caminaga, R.M.; et al. Characterization and cytotoxic, genotoxic and mutagenic evaluations of bacterial cellulose membranes incorporated with ciprofloxacin: A potential material for use as therapeutic contact lens. World J. Pharm. Pharm. Sci. 2015, 4, 1626–1647. [Google Scholar]
- Pértile, R.A.N.; Moreira, S.M.G.; Andrade, F.K.; Domingues, L.; Gama, M. Bacterial cellulose modified using recombinant proteins to improve neuronal and mesenchymal cell adhesion. Biotechnol. Prog. 2012, 28, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ma, X.; Chen, S.-W.; Tao, M.; Yuan, L.; Jing, Y. Bacterial Cellulose Membranes Used as Artificial Substitutes for Dural Defection in Rabbits. Int. J. Mol. Sci. 2014, 15, 10855–10867. [Google Scholar] [CrossRef] [PubMed]
- Mello, L.R.; Feltrin, L.T.; Neto, P.T.F.; Ferraz, F.A.P. Duraplasty with biosynthetic cellulose: An experimental study. J. Neurosurg. 1997, 86, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Bodin, A.; Bharadwaj, S.; Wu, S.; Gatenholm, P.; Atala, A.; Zhang, Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials 2010, 31, 8889–8901. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-W.; Lv, X.-G.; Li, Z.; Song, L.-J.; Feng, C.; Xie, M.-K.; Li, C.; Li, H.-B.; Wang, J.-H.; Zhu, W.-D.; et al. Urethral reconstruction with a 3D porous bacterial cellulose scaffold seeded with lingual keratinocytes in a rabbit model. Biomed. Mater. 2015, 10, 055005. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.W.; Park, S.; Lim, K.-T.; Seonwoo, H.; Kim, Y.; Hong, B.H.; Choung, Y.-H.; Chung, J.H. Bacterial Cellulose Nanofibrillar Patch as a Wound Healing Platform of Tympanic Membrane Perforation. Adv. Healthc. Mater. 2013, 2, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Silveira, F.C.A.; Pinto, F.C.M.; Neto, S.D.S.C.; Leal, M.D.C.; Cesário, J.; Aguiar, J.L.D.A. Treatment of tympanic membrane perforation using bacterial cellulose: A randomized controlled trial. Braz. J. Otorhinolaryngol. 2016, 82, 203–208. [Google Scholar] [CrossRef]
- De Souza, F.C.; Olival-Costa, H.; Da Silva, L.; Pontes, P.A.; Lancellotti, C.L.P. Bacterial Cellulose as Laryngeal Medialization Material: An Experimental Study. J. Voice 2011, 25, 765–769. [Google Scholar] [CrossRef]
- Needleman, I.G.; Worthington, H.V.; Giedrys-Leeper, E.; Tucker, R.J. Guided tissue regeneration for periodontal infra-bony defects. Cochrane Database Syst. Rev. 2006, CD001724. [Google Scholar] [CrossRef]
- Verma, P.K.; Srivastava, R.; Gupta, K.K.; Chaturvedi, T.P. Treatment strategy for guided tissue regeneration in various class II furcation defect: Case series. Dent. Res. J. 2013, 10, 689–694. [Google Scholar]
- Gottlow, J.; Nyman, S.; Karring, T.; Lindhe, J. New attachment formation as the result of controlled tissue regeneration. J. Clin. Periodontol. 1984, 11, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Villar, C.C.; Cochran, D.L. Regeneration of Periodontal Tissues: Guided Tissue Regeneration. Dent. Clin. N. Am. 2010, 54, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Novaes, A.B., Jr.; Moraes, N.H.; Novaes, A.B. The use of BioFill as biological membrane in the treatment of furcation lesion with and without the utilization of porous hydroxyapatite. Rev. Bras. Odontol. 1990, 47, 29–32. [Google Scholar]
- Novaes, A.B., Jr.; Novaes, A.B.; Grisi, M.F.; Soares, U.N.; Gabarra, F.R. Gengiflex, an Alkali-Cellulose Membrane for GTR. Braz. Dent. J. 1994, 4, 65–73. [Google Scholar]
- Novaes, A.B. IMZ implants placed into extraction sockets in association with membrane therapy (Gengiflex) and porous hydroxyapatite: A case report. Int. J. Oral Maxillofac. Implant. 1992, 7, 536–540. [Google Scholar]
- Novaes, A.B. Bone formation over a TiAl6 V4 (IMZ) implant placed into an extraction socket in association with membrane therapy (Gengiflex). Clin. Oral Implant. Res. 1993, 4, 106–110. [Google Scholar] [CrossRef]
- An, S.-J.; Lee, S.-H.; Huh, J.-B.; Jeong, S.-I.; Park, J.-S.; Gwon, H.-J.; Kang, E.-S.; Jeong, C.-M.; Lim, Y.-M. Preparation and Characterization of Resorbable Bacterial Cellulose Membranes Treated by Electron Beam Irradiation for Guided Bone Regeneration. Int. J. Mol. Sci. 2017, 18, 2236. [Google Scholar] [CrossRef]
- Chiaoprakobkij, N.; Sanchavanakit, N.; Subbalekha, K.; Pavasant, P.; Phisalaphong, M. Characterization and biocompatibility of bacterial cellulose/alginate composite sponges with human keratinocytes and gingival fibroblasts. Carbohydr. Polym. 2011, 85, 548–553. [Google Scholar] [CrossRef]
- Rogers, G.F.; Greene, A.K. Autogenous Bone Graft. J. Craniofacial Surg. 2012, 23, 323–327. [Google Scholar] [CrossRef]
- Block, M.S.; Kent, J.N. Sinus augmentation for dental implants: The use of autogenous bone. J. Oral Maxillofac. Surg. 1997, 55, 1281–1286. [Google Scholar] [CrossRef]
- Gerressen, M.; Hermanns-Sachweh, B.; Riediger, D.; Hilgers, R.-D.; Spiekermann, H.; Ghassemi, A. Purely cancellous vs. corticocancellous bone in sinus floor augmentation with autogenous iliac crest: A prospective clinical trial. Clin. Oral Implant. Res. 2009, 20, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Ayub, L.G.; Ramos, U.D.; Reino, D.M.; Grisi, M.F.; Taba, M.; Souza, S.L.S.; Palioto, D.B.; Novaes, A.B.; Taba, M. A Modified Surgical Technique for Root Coverage with an Allograft: A 12-Month Randomized Clinical Trial. J. Periodontol. 2014, 85, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Varanini, P.; Orlando, B.; Tonelli, P.; Covani, U. Deep-Frozen Allogeneic Onlay Bone Grafts for Reconstruction of Atrophic Maxillary Alveolar Ridges: A Preliminary Study. J. Oral Maxillofac. Surg. 2009, 67, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, A.; Motamedian, S.R.; Khojaste, M. Success rate of implants placed in autogenous bone blocks versus allogenic bone blocks: A systematic literature review. Ann. Maxillofac. Surg. 2016, 6, 78–90. [Google Scholar] [CrossRef]
- Hatano, N.; Shimizu, Y.; Ooya, K. A clinical long-term radiographic evaluation of graft height changes after maxillary sinus floor augmentation with a 2:1 autogenous bone/xenograft mixture and simultaneous placement of dental implants. Clin. Oral Implant. Res. 2004, 15, 339–345. [Google Scholar] [CrossRef]
- Xu, C.; Su, P.; Chen, X.; Meng, Y.; Yu, W.; Xiang, A.P.; Wang, Y. Biocompatibility and osteogenesis of biomimetic Bioglass-Collagen-Phosphatidylserine composite scaffolds for bone tissue engineering. Biomaterials 2011, 32, 1051–1058. [Google Scholar] [CrossRef]
- de Souza, S.L.S.; Martins, S.H.L.; Bezerra, F.J.B.; Shibli, J.A.; Mantovani, R.V.; da Costa, L.F.A.; Júnior, J.A.B.G. Alveolar ridge preservation and regeneration with biomaterials. In 50 Years Osseointegration Reflections Perspect; Rossetti, P.H.O., Bonachela, W.C., Eds.; VM Cultural: São Paulo, Brazil, 2015; pp. 145–179. [Google Scholar]
- Barrere, F.; Mahmood, T.; De Groot, K.; Van Blitterswijk, C.; Van Blitterswijk, C.A. Advanced biomaterials for skeletal tissue regeneration: Instructive and smart functions. Mater. Sci. Eng. R Rep. 2008, 59, 38–71. [Google Scholar] [CrossRef]
- Stoica-Guzun, A.; Stroescu, M.; Jinga, S.; Jipa, I.; Dobre, T.; Dobre, L. Ultrasound influence upon calcium carbonate precipitation on bacterial cellulose membranes. Ultrason. Sonochem. 2012, 19, 909–915. [Google Scholar] [CrossRef]
- Yin, N.; Chen, S.; Ouyang, Y.; Tang, L.; Yang, J.-X.; Wang, H. Biomimetic mineralization synthesis of hydroxyapatite bacterial cellulose nanocomposites. Prog. Nat. Sci. 2011, 21, 472–477. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Gao, C.; Luo, H.L.; He, F.; Liang, H.; Li, X.L.; Wang, Y.L. Early growth of nano-sized calcium phosphate on phosphorylated bacterial cellulose nanofibers. J. Nanosci. Nanotechnol. 2009, 9, 6494–6500. [Google Scholar] [CrossRef]
- Shi, S.; Chen, S.; Zhang, X.; Shen, W.; Li, X.; Hu, W.; Wang, H. Biomimetic mineralization synthesis of calcium-deficient carbonate-containing hydroxyapatite in a three-dimensional network of bacterial cellulose. J. Chem. Technol. Biotechnol. 2008, 84, 285–290. [Google Scholar] [CrossRef]
- Zhang, S.; Xiong, G.; He, F.; Huang, Y.; Wang, Y.; Wan, Y. Characterisation of Hydroxyapatite/Bacterial Cellulose Nanocomposites. Polym. Polym. Compos. 2009, 17, 353–358. [Google Scholar] [CrossRef]
- Grande, C.J.; Torres, F.G.; Gomez, C.M.; Bañó, M.C. Nanocomposites of bacterial cellulose/hydroxyapatite for biomedical applications. Acta Biomater. 2009, 5, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Boyne, P.J.; James, R.A. Grafting of the maxillary sinus floor with autogenous marrow and bone. J. Oral Surg. 1980, 38, 613–616. [Google Scholar] [PubMed]
- Koike, T.; Sha, J.; Bai, Y.; Matsuda, Y.; Hideshima, K.; Yamada, T.; Kanno, T. Efficacy of Bacterial Cellulose as a Carrier of BMP-2 for Bone Regeneration in a Rabbit Frontal Sinus Model. Materials 2019, 12, 2489. [Google Scholar] [CrossRef] [PubMed]
- Saska, S.; Barud, H.S.; Gaspar, A.M.M.; Marchetto, R.; Ribeiro, S.J.L.; Messaddeq, Y. Bacterial Cellulose-Hydroxyapatite Nanocomposites for Bone Regeneration. Int. J. Biomater. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Tazi, N.; Zhang, Z.; Messaddeq, Y.; Almeida-Lopes, L.; Zanardi, L.M.; Levinson, D.J.; Rouabhia, M. Hydroxyapatite bioactivated bacterial cellulose promotes osteoblast growth and the formation of bone nodules. AMB Express 2012, 2, 61. [Google Scholar] [CrossRef]
- Coelho, F.; Cavicchioli, M.; Specian, S.S.; Scarel-Caminaga, R.M.; Penteado, L.D.A.; De Medeiros, A.I.; Ribeiro, S.J.D.L.; Capote, T.S.D.O. Bacterial cellulose membrane functionalized with hydroxiapatite and anti-bone morphogenetic protein 2: A promising material for bone regeneration. PLoS ONE 2019, 14, e0221286. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, T.; Zhao, Z.; Ren, H.; Zhang, Q.; Yan, Y.; Lv, G. Preparation and characterization of bacterial cellulose microfiber/goat bone apatite composites for bone repair. J. Appl. Polym. Sci. 2013, 129, 595–603. [Google Scholar] [CrossRef]
- Pigossi, S.C.; De Oliveira, G.J.P.L.; Finoti, L.S.; Nepomuceno, R.; Spolidorio, L.C.; Rossa, C.; Ribeiro, S.J.; Saska, S.; Scarel-Caminaga, R.M. Bacterial cellulose-hydroxyapatite composites with osteogenic growth peptide (OGP) or pentapeptide OGP on bone regeneration in critical-size calvarial defect model. J. Biomed. Mater. Res. Part A 2015, 103, 3397–3406. [Google Scholar] [CrossRef]
- Lee, J.M.; Kim, J.H.; Lee, O.J.; Park, C.H. The Fixation Effect of a Silk Fibroin–Bacterial Cellulose Composite Plate in Segmental Defects of the Zygomatic ArchAn Experimental StudySilk Fibroin–Bacterial Cellulose Composite Plate. JAMA Otolaryngol. Neck Surg. 2013, 139, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, A.; Tabuchi, M.; Uo, M.; Tatsumi, H.; Hideshima, K.; Kondo, S.; Sekine, J. Applicability of bacterial cellulose as an alternative to paper points in endodontic treatment. Acta Biomater. 2013, 9, 6116–6122. [Google Scholar] [CrossRef]
- Jinga, S.I.; Voicu, G.; Stoica-Guzun, A.; Stroescu, M.; Grumezescu, A.M.; Bleotu, C. Biocellulose nanowhiskers cement composites for endodontic use. Dig. J. Nanomater. Biostruct. 2014, 9, 543–550. [Google Scholar]
- Costa, L.M.M.; De Olyveira, G.M.; Basmaji, P.; Valido, D.P.; Gois, P.B.P.; Júnior, R.L.A.C.; Filho, L.X. Novel Otoliths/Bacterial Cellulose Nanocomposites as a Potential Natural Product for Direct Dental Pulp Capping. J. Biomater. Tissue Eng. 2012, 2, 48–53. [Google Scholar] [CrossRef]
- Voicu, G.; Jinga, S.-I.; Drosu, B.-G.; Busuioc, C. Improvement of silicate cement properties with bacterial cellulose powder addition for applications in dentistry. Carbohydr. Polym. 2017, 174, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Williams, G.R.; Wu, J.; Wu, J.; Niu, S.; Li, H.; Wang, H.; Zhu, L.-M. Regenerated chitin fibers reinforced with bacterial cellulose nanocrystals as suture biomaterials. Carbohydr. Polym. 2018, 180, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.P.F.; Silva, A.C.Q.; Bastos, V.; Oliveira, H.; Pinto, R.J.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S. Nanocellulose-Based Patches Loaded with Hyaluronic Acid and Diclofenac towards Aphthous Stomatitis Treatment. Nanomaterials 2020, 10, 628. [Google Scholar] [CrossRef]
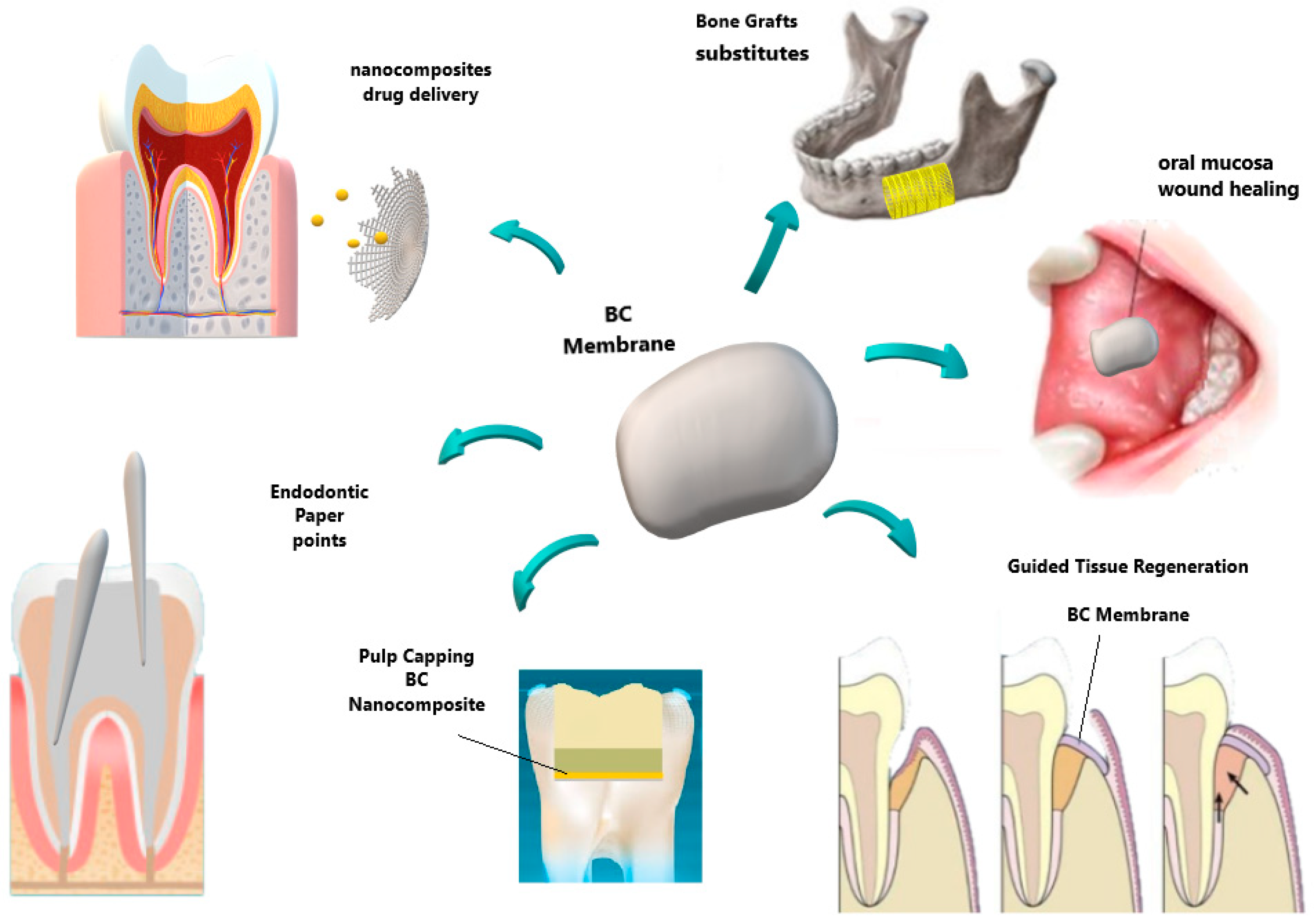
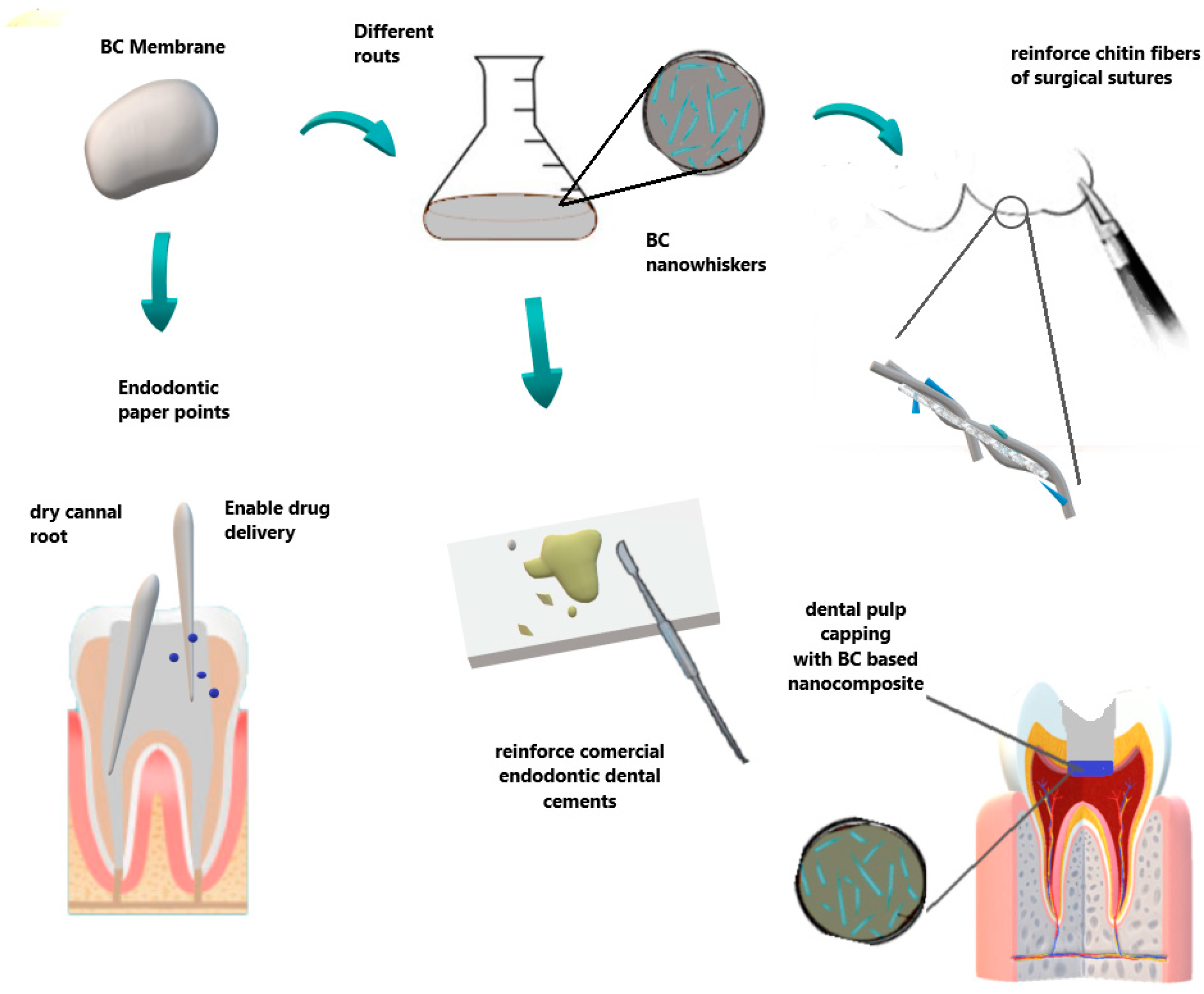
| Dental and Oral Treatments | Potential Use | BC Advantages |
|---|---|---|
| Periodontal treatment | Barrier membrane in GTR technique Novaes Jr et al., [84,85] Novaes [86,87] An et al., [88] | Allowed cell attachment and proliferation Aesthetics importance restoration of oral function Reduction in surgical steps Biocompatibility, presenting no chronic inflammatory reaction |
| Wound dressing/patches | Surgical Wounds, flaps and RAS ulcers Chiaoprakobkij et al., [89] Carvalho et al., [119] | Biocompatible Physical barrier Allow drug delivery |
| Dental pulp tissue treatment | Root canal sealers Jinga et al., [115] Scaffold for regenerative Endodontic treatment Manzine Costa et al., [116] Voeicu et al., [117] | Accelerates the hardening processes of cement Reinforce dental cements Mimics extracellular matrix. Induces mineralized barrier and apical closure |
| Dental surgery | Surgical suture Wu et al., [118] | Improve reinforcement and mechanical properties |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira Barud, H.G.; da Silva, R.R.; Borges, M.A.C.; Castro, G.R.; Ribeiro, S.J.L.; da Silva Barud, H. Bacterial Nanocellulose in Dentistry: Perspectives and Challenges. Molecules 2021, 26, 49. https://doi.org/10.3390/molecules26010049
de Oliveira Barud HG, da Silva RR, Borges MAC, Castro GR, Ribeiro SJL, da Silva Barud H. Bacterial Nanocellulose in Dentistry: Perspectives and Challenges. Molecules. 2021; 26(1):49. https://doi.org/10.3390/molecules26010049
Chicago/Turabian Stylede Oliveira Barud, Hélida Gomes, Robson Rosa da Silva, Marco Antonio Costa Borges, Guillermo Raul Castro, Sidney José Lima Ribeiro, and Hernane da Silva Barud. 2021. "Bacterial Nanocellulose in Dentistry: Perspectives and Challenges" Molecules 26, no. 1: 49. https://doi.org/10.3390/molecules26010049
APA Stylede Oliveira Barud, H. G., da Silva, R. R., Borges, M. A. C., Castro, G. R., Ribeiro, S. J. L., & da Silva Barud, H. (2021). Bacterial Nanocellulose in Dentistry: Perspectives and Challenges. Molecules, 26(1), 49. https://doi.org/10.3390/molecules26010049







