A Review of Composite Phase Change Materials Based on Porous Silica Nanomaterials for Latent Heat Storage Applications
Abstract
1. Introduction
2. Thermal Energy Storage
2.1. Phase Change Materials
2.2. Porous Silica Matrices
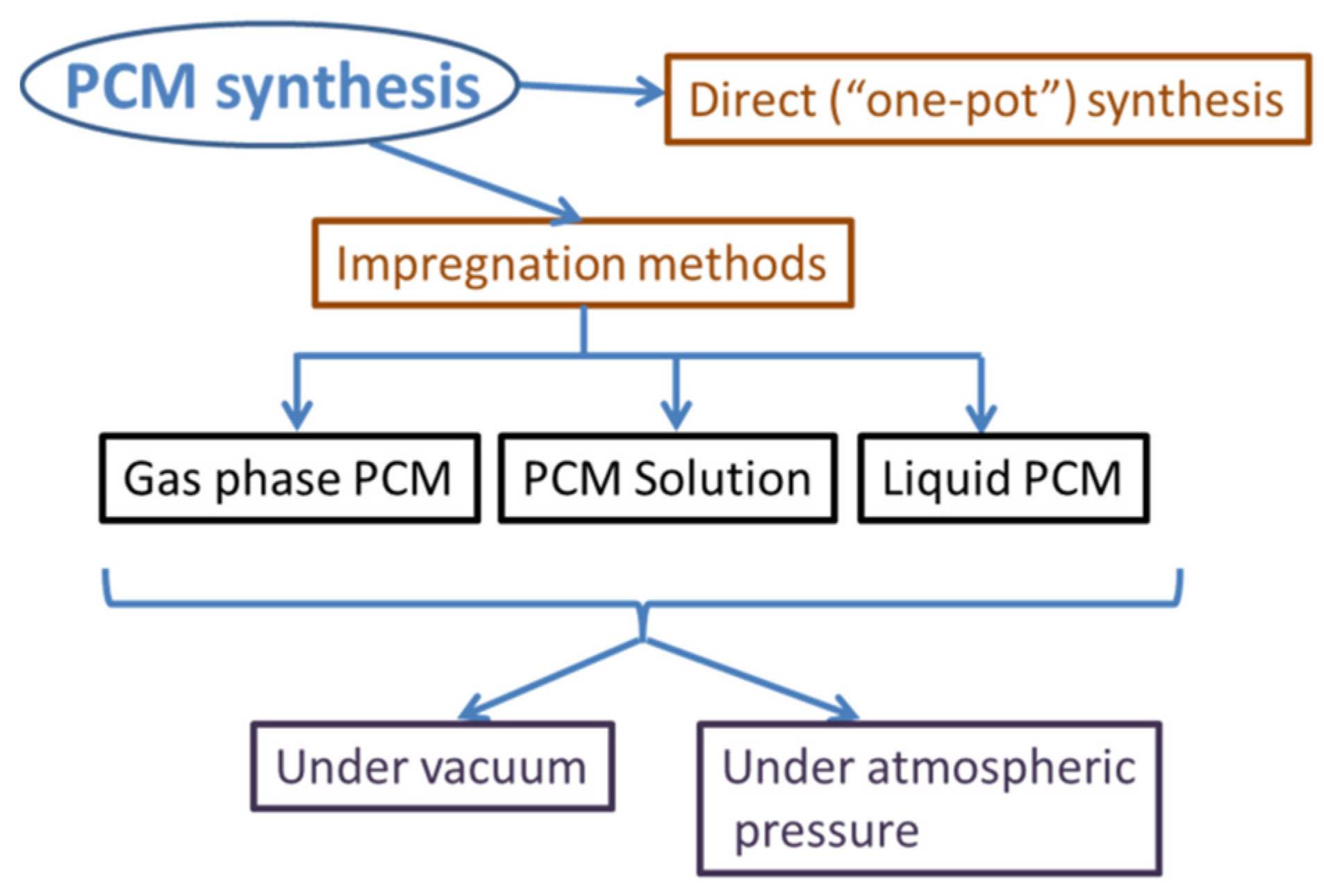
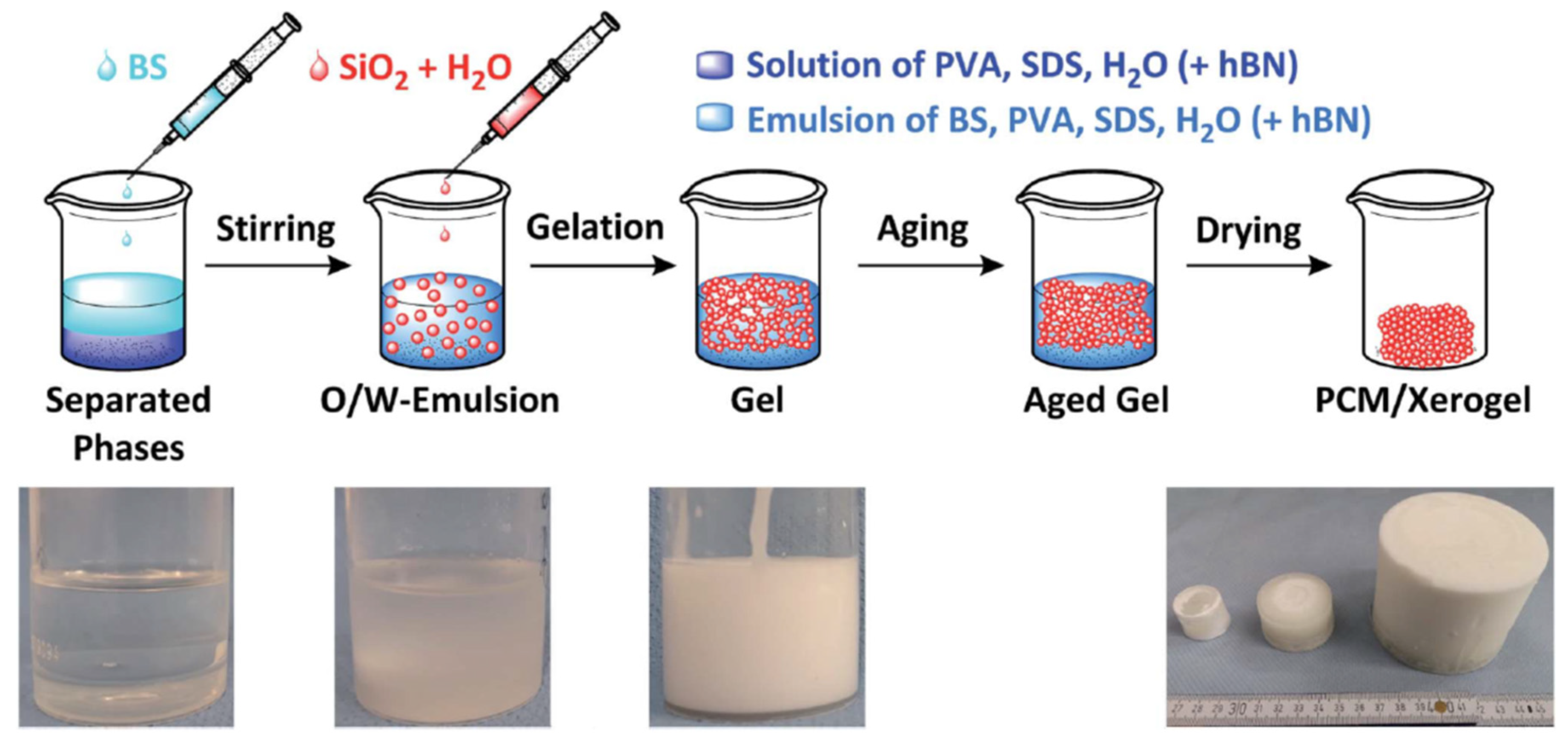
2.3. Porous Silica Nanocomposites: Desired Properties and Synthesis
2.4. Porous Silica Nanocomposites: Structure and Physico-Chemical Properties under Nanoconfinement
3. Phase Change Materials Containing Porous Silica Matrices and Different Heat Storage Compounds
3.1. Paraffins
| PCM | Porous Silica Composite | Ref. | |||||
|---|---|---|---|---|---|---|---|
| Sample | m.p. (°C) | ΔHf (J g−1) | Sample/Synthesis | %PCM (wt.) * | m.p. (°C) | ΔHf (J g−1) | |
| Paraffin wax | 29.0 | 142.0 | Direct synthesis/CTAB + n-pentanol | - | 30.0 | 95.0 | [82] |
| Paraffin | 51.0 | 151.5 | Direct synthesis/TEOS/HCl | 92.1 | 50.2 | 139.6 | [83] |
| Paraffin | 57.0 | - | Aerogel/supercritical EtOH | 75 | - | - | [27] |
| Rubitherm RT 28 | - | - | TEOS/HCl, EG, paraffin | - | 27.7 | 104.4 | [111] |
| n-eicosane | 39.2 | 237.1 | n-C20H42,Fe3O4@SiO2 | 70 | 39.2 | 170.2 | [120] |
| PX25 | ~23 | 96 | 9% hydrophobic fumed silica vs. PCM; 30:70 wt. composite: cement | 27.3 | ~23 | 14.2 | [102] |
| Paraffin | 50.1 | 173.9 | Parafin@SiO2-GO/PVA, Span80, Tween80 | 49 | 49.7 | 87.1 | [118] |
| 25# Paraffin | 25.8 | 107.6 | Vacuum melt impregnation | - | 21.6 | 56.3 | [91] |
| n-eicosane | 37.13 | 249.0 | Aerogel melt impregnation | 84.3 | 36.8 | 198.4 | [101] |
| Paraffin | 57.7 | 161.4 | TEOS/HCl | 60 | 58.2 | 98.0 | [85] |
| Paraffin | 51.9 | 184.1 | 2% EG; 8% 20 nm SiO2; melt addition | 90 | 51.8 | 168.3 | [112] |
| Octadecane | 26.5 | 2300 | Gas transport/12.5 nm SBA-15 | - | 17.4 | 103.3 | [109] |
| Tetradecane | 6.2 | 216.0 | - | −7.4 | 124.2 | ||
| Paraffin | 49.7 | 200.4 | Solvent impregnation/SiO2 NPs | 80 | 52.0 | 156.6 | [100] |
| Paraffin | 28.0 | 168.0 | Melt impregnation/MCM-41 | 60 | 25.5 | 95.0 | [92] |
| Paraffin | 42.2 | 243.0 | Vacuum melt/SiO2-PTFE aerogel | 62.8 | 42.0 | 128.0 | [96] |
| Paraffin | 59.6 | 191.1 | Solution impregnation/CH3-fucntionalized aerogel | 70 | 59.6 | 112.9 | [104] |
| n-Eicosane | 36.9 | 243.3 | Solution impregnation/7% EG/70:30 Eicosane:20 nm SiO2 NP | 65.1 | 35.4 | 135.8 | [114] |
| Paraffin wax | 63.8 | 209.1 | Vacuum melt/CH3/HO-aerogel | 88 | 63.7 | 163.6 | [105] |
| Paraffin wax | 25.7 | 198.0 | Vacuum melt/SiO2-EG 1:1 | 80 | 26.7 | 105.9 | [115] |
| Paraffin | 56.8 | 182.2 | Melt impregnation/aerogel | 75 | 56.3 | 165.2 | [94] |
| Hexadecane | 17.7 | 220 | Vacuum melt/mesoporous silica/300 heat-cool cycles | 45 | 17.1 | 100.1 | [99] |
| Octadecane | 29.9 | 223 | Vacuum melt/mesoporous silica | 45.3 | 28.9 | 84.5 | [81] |
| Octadecane | 28.5 | 212.6 | Direct synthesis | - | 26.3 | 99.3 | [88] |
| Nonadecane | 29.4 | 201.0 | Direct synthesis | - | 26.2 | 80.8 | [89] |
| Octadecane | 28.2 | 232.5 | Vacuum melt/SiO2 NP | 70 | 27.7 | 85.0 | [122] |
3.2. Fatty Acids and Derivatives
| PCM | Porous Silica Composite | Ref. | |||||
|---|---|---|---|---|---|---|---|
| Sample | m.p. (°C) | ΔHf (J g−1) | Sample/Synthesis | %PCM (wt.) | m.p. (°C) | ΔHf (J g−1) | |
| Stearic acid | 55.6 | 176.7 | Direct synthesis/TEOS/HCl | 60 | 54.9 | 109.4 | [85] |
| Dodecanoic acid | 44.6 | 169.0 | Gas transport/12.5 nm SBA-15 | - | 22.3 | 65.9 | [109] |
| Tetradecanol | 36.0 | 198.0 | - | 11.4 | 48.4 | ||
| Decanoic acid | 30.0 | 163.0 | - | 11.1 | 64.3 | ||
| Dodecanol | 24.0 | 196.0 | - | 0.2 | 69.5 | ||
| Quinary eutectic | 12.3 | 134.4 | Melt impregnation/electrospun SiO2 fibers | 80.2 | 13.4 | 107.8 | [132] |
| LA:PA:PAR eutectic | 33.1 | 140.6 | Melt impregnation/SiO2 NPs + HDPE | 75 | 31.5 | 104.4 | [133] |
| Lauric acid | 44.4 | 180.8 | Direct synthesis | 65 | 42.5 | 117.2 | [124] |
| Stearic acid | 59.9 | 177.8 | Solution impregnation/fumed silica | 46 | 58.8 | 82.5 | [137] |
| Octadecanol | - | 235 | Vacuum melt/CH3-aerogel | 86 | - | 153.7 | [144] |
| Lauric acid | 42.7 | 166.0 | Hexane solution/MCF | 83 | 34.0/ 41.2 | 123.7 | [67] |
| CA: LA:PA = 61.9:31.0:7.1 | 15.0 | 120.2 | Melt impregnation/electrospun SiO2 fibers | 81 | 13.7 | 100.9 | [131] |
| Stearic acid | 65.2 | 239.4 | Solution impregnation/Tannic acid templated SiO2 | 70 | 67.1 | 108.8 | [138] |
| CA:PA: SA = 79.3:14.7:6.0 | 18.5 | 139.3 | Melt impregnation/SiO2 NPs | 75 | 17.2 | 99.4 | [134] |
| CA:MA = 72:28 | 21.7 | 139.2 | Direct synthesis | 40 | 21.15 | 55.6 | [126] |
| Stearic acid | - | 221.8 | Solution impregnation/MOS | 70 | - | 108.0 | [140] |
| Myristic acid | 57.7 | 184.3 | Solution impregnation/WMSN | 65 | 54.7 | 92.0 | [142] |
| Stearic acid | 52.5 | 172.7 | Vacuum melt/SiO2 NP | 70 | 52.1 | 77.6 | [122] |
| Octadecanol | 57.2 | 234.5 | 70 | 56.4 | 47.0 | ||
| Lauric acid | 42.7 | 176.1 | Vacuum melt/MCF | 84 | 31.5/41.7 | 128.1 | [146] |
| CA-PA (85:15) | 27.5 | 151.5 | Melt/Fumed silica+5% CNT | 30.4 | 25.2 | 41.2 | [147] |
| Stearic acid | 56.6 | 170.3 | Direct synthesis | 76 | 53.8 | 118.3 | [127] |
| Lauric acid | 44.2 | 165.8 | Direct synthesis; TEOS+MTES | 42.2 | 82.7 | [129] | |
| Stearic acid | 68.4 | 213.6 | Melt impregnation/MCF-COOH | 79 | 58.9/68.8 | 128.3 | [66] |
| Methyl laurate | 4.0 | 210.1 | Direct synthesis, VTES/PVA | 71 | 6.7 | 151.3 | [145] |
| Palmitic acid | 62.8 | 209.7 | Solution impregnation; 5% GNP | 70 | 60.6 | 128.4 | [148] |
| Octadecanol | 57.8 | 237.8 | Melt impregnation/2% GO- SiO2 aerogel | 75 | 53 | 129.6 | [149] |
| 1, 8-Cctanediol | 62.4 | 225.1 | Direct synthesis, TEOS/HCl | 70 | 61.3 | 157.7 | [128] |
| Caprylic acid | 12.0 | 139.9 | Melt impregnation/silica gel | 48 | 13.8 | 46.4 | [136] |
| Palmitic acid | 50.0 | 213.1 | Melt impregnation/C-SiO2 aerogel | 82 | 187.7 | 43.4 | [150] |
3.3. Polyethylene Glycol (PEG)-Based PCMs
| PCM | Porous Silica Composite | Ref. | |||||
|---|---|---|---|---|---|---|---|
| Sample | m.p. (°C) | ΔHf (J g−1) | Synthesis | %PCM (wt.) | m.p. (°C) | ΔHf (J g−1) | |
| PEG 600 | 18.5 | 118.2 | solution impregnation | 62 | 19.6 | 71.6 | [171] |
| PEG 2000 | 52.5 | 153.0 | vacuum impregnation | 60 | 50.8 | 58.76 | [173] |
| PEG 4000 | 53.8 | 202.1 | vacuum impregnation | 80 | 50.8 | 136.6 | [167] |
| PEG 4000 | 59.1 | 183.4 | vacuum impregnation | 70 | 57.8 | 121.7 | [166] |
| PEG 4000 | 54.5 | 192.4 | vacuum impregnation | 70 | 53.0 | 73.8 | [168] |
| PEG 6000 | 61.7 | 178.6 | sol-gel | 97.3 | 60.4 | 164.9 | [172] |
| PEG 6000 distearate | 52.9 | 145.1 | sol-gel | 61.6 | 52.9 | 69.7 | [170] |
| PEG 6000 | 59 | 171 | sol-gel | 44.3 | 56.8 | 91.9 | [160] |
| PEG 6000 | 61.4 | 212.8 | sol-gel | 88.5 | 58.4 | 167.0 | [163] |
| PEG 1000 | 35.1 | 146.7 | sol-gel | 84.5 | 35.2 | 113.8 | [159] |
| PEG 1500 | 41.1 | 164.6 | sol-gel | 38.4 | 80.0 | 132.4 | [158] |
| PEG 2000 + PEG 10,000 | 51.7/62.4 | 180.6/ 170.9 | sol-gel co-crystallization | 36 | 56.5 | 108.6 | [165] |
3.4. Small Organic Compounds
3.5. Hydrated Salts
| PCM | Porous Silica Composite | Ref. | |||||
|---|---|---|---|---|---|---|---|
| Sample | m.p. (°C) | ΔHf (J g−1) | Synthesis | %PCM (wt.) | m.p. (°C) | ΔHf (J g−1) | |
| Na2SO4·10H2O-Na2HPO4·12H2O | 36.7 | 226.9 | Melt impregnation/sol-gel SiO2 + PVP | 70 | 30.1 | 106.2 | [185] |
| Melt impregnation/sol-gel SiO2 | 70 | 28.5 | 67.5 | [186] | |||
| MgCl2.6H2O:Mg(NO3)2·6H2O (41.3:58.7) | 58.8 | 118.5 | Melt impregnation/fumed silica | 85 | 54.3 | 88.1 | [180] |
| Na2SO4·10H2O-Na2HPO4·12H2O | - | 221.4 | - | 70 | - | 64.1 | [191] |
| TBAB:H2O (4:6) | 11.8 | 211.9 | Melt impregnation/hydrophilic fumed silica | 70 | 8.3 | 134.0 | [188] |
| CaCl2·6H2O | 29.4 | 199.9 | Melt impregnation/SiO2 NPs | 75 | 25.1 | 148.2 | [181] |
3.6. Molten Salts
| PCM | Porous Silica Composite | Ref. | |||||
|---|---|---|---|---|---|---|---|
| Sample | m.p. (°C) | ΔHf (J g−1) | Synthesis | %PCM (wt.) | m.p. (°C) | ΔHf (J g−1) | |
| Na2SO4 | 888.7 | 167.1 | Direct sol-gel synthesis | 50 | 886.0 | 82.3 | [192] |
| NaNO3 | 308.0 | 189.0 | Direct sol-gel synthesis | 60 | 302.0 | 108.0 | [193] |
| LiNO3 | 253.8 | 369.9 | Melt impregnation/KCC-1 | 70 | 251.3 | 292.2 | [166] |
| Na2CO3-K2CO3/MgO | 702.9 | 81.4 | Sintering/SiO2 NPs | 90 | 703.6 | 76.2 | [196] |
| NaCl-CaCl2 (1:1 mol) | 499.5 | 208.2 | Reactive melting/8.1 nm MSN | 95 | 499.1 | 60.8 | [197] |
| Na2SO4 | 886.7 | 167.1 | Direct sol-gel synthesis | 60 | 886.9 | 100.8 | [199] |
| LiNO3 | - | - | Direct sol-gel synthesis | 60 | 232.8 | 236.3 | [195] |
| NaNO3:KNO3 (1:1 mol) | 221.8 | 96.9 | Melt impregnation/MCF | 90 | 201.0 221.2 | 78.7 | [74] |
| Na(Cl, Br, MoO4) | 454.5 522.1 | 78.9 137.3 | Melt impregnation/MCM-41 | 80 | 450.4 514.4 | 52.8 111.2 | [198] |
3.7. Metals, Alloys and Elemental PCMs
4. Conclusions
5. Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Minakshi, M.; Mitchell, D.R.G.; Jones, R.T.; Pramanik, N.C.; Jean-Fulcrand, A.; Garnweitner, G. A Hybrid Electrochemical Energy Storage Device Using Sustainable Electrode Materials. ChemistrySelect 2020, 5, 1597–1606. [Google Scholar] [CrossRef]
- Divakaran, A.M.; Hamilton, D.; Manjunatha, K.N.; Minakshi, M. Design, Development and Thermal Analysis of Reusable Li-Ion Battery Module for Future Mobile and Stationary Applications. Energies 2020, 13, 1477. [Google Scholar] [CrossRef]
- Ling, T.-C.; Poon, C.-S. Use of phase change materials for thermal energy storage in concrete: An overview. Constr. Build. Mater. 2013, 46, 55–62. [Google Scholar] [CrossRef]
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef]
- Abhat, A. Low temperature latent heat thermal energy storage: Heat storage materials. Sol. Energy 1983, 30, 313–332. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; de Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Oró, E.; de Gracia, A.; Castell, A.; Farid, M.M.; Cabeza, L.F. Review on phase change materials (PCMs) for cold thermal energy storage applications. Appl. Energy 2012, 99, 513–533. [Google Scholar] [CrossRef]
- Cárdenas, B.; León, N. High temperature latent heat thermal energy storage: Phase change materials, design considerations and performance enhancement techniques. Renew. Sustain. Energy Rev. 2013, 27, 724–737. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, S. Medium- and high-temperature latent heat thermal energy storage: Material database, system review, and corrosivity assessment. Int. J. Energy Res. 2019, 43, 621–661. [Google Scholar] [CrossRef]
- Gerislioglu, B.; Ahmadivand, A.; Karabiyik, M.; Sinha, R.; Pala, N. VO2-Based Reconfigurable Antenna Platform with Addressable Microheater Matrix. Adv. Electron. Mater. 2017, 3, 1700170. [Google Scholar] [CrossRef]
- Gerislioglu, B.; Bakan, G.; Ahuja, R.; Adam, J.; Mishra, Y.K.; Ahmadivand, A. The role of Ge2Sb2Te5 in enhancing the performance of functional plasmonic devices. Mater. Today Phys. 2020, 12, 100178. [Google Scholar] [CrossRef]
- Bakan, G.; Gerislioglu, B.; Dirisaglik, F.; Jurado, Z.; Sullivan, L.; Dana, A.; Lam, C.; Gokirmak, A.; Silva, H. Extracting the temperature distribution on a phase-change memory cell during crystallization. J. Appl. Phys. 2016, 120, 164504. [Google Scholar] [CrossRef]
- Laraib Tariq, S.; Muhammad Ali, H.; Ammar Akram, M.; Mansoor Janjua, M.; Ahmadlouydarab, M. Nanoparticles enhanced Phase Change Materials (NePCMs)-A Recent Review. Appl. Eng. 2020, 176, 115305. [Google Scholar]
- Tauseef, u.R.; Ali, H.M.; Janjua, M.M.; Sajjad, U.; Yan, W.-M. A critical review on heat transfer augmentation of phase change materials embedded with porous materials/foams. Int. J. Heat Mass Transf. 2019, 135, 649–673. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Li, X.; Wu, X. Novel composite phase change materials with enhancement of light-thermal conversion, thermal conductivity and thermal storage capacity. Sol. Energy 2020, 196, 419–426. [Google Scholar] [CrossRef]
- Brown, R.C.; Rasberry, J.D.; Overmann, S.P. Microencapsulated phase-change materials as heat transfer media in gas-fluidized beds. Powder Technol. 1998, 98, 217–222. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Z.; Yang, T.; Qin, D.; Li, S.; Zhang, G.; Haghighat, F.; Joybari, M.M. A review on macro-encapsulated phase change material for building envelope applications. Build. Environ. 2018, 144, 281–294. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, T.; Zhang, D.; Wang, K.; Wang, Y. Paraffin/chitosan composite phase change materials fabricated by piercing-solidifying method for thermal energy storage. AIP Adv. 2020, 10, 035218. [Google Scholar] [CrossRef]
- Tao, Y.B.; He, Y.-L. A review of phase change material and performance enhancement method for latent heat storage system. Renew. Sustain. Energy Rev. 2018, 93, 245–259. [Google Scholar] [CrossRef]
- Hawes, D.W.; Feldman, D. Absorption of phase change materials in concrete. Sol. Energy Mater. Sol. Cells 1992, 27, 91–101. [Google Scholar] [CrossRef]
- Tang, J.; Yang, M.; Dong, W.; Yang, M.; Zhang, H.; Fan, S.; Wang, J.; Tan, L.; Wang, G. Highly porous carbons derived from MOFs for shape-stabilized phase change materials with high storage capacity and thermal conductivity. RSC Adv. 2016, 6, 40106–40114. [Google Scholar] [CrossRef]
- Sarı, A.; Karaipekli, A.; Alkan, C. Preparation, characterization and thermal properties of lauric acid/expanded perlite as novel form-stable composite phase change material. Chem. Eng. J. 2009, 155, 899–904. [Google Scholar] [CrossRef]
- Voronin, D.V.; Ivanov, E.; Gushchin, P.; Fakhrullin, R.; Vinokurov, V. Clay Composites for Thermal Energy Storage: A Review. Molecules 2020, 25, 1504. [Google Scholar] [CrossRef]
- Huang, X.; Xia, W.; Zou, R. Nanoconfinement of phase change materials within carbon aerogels: Phase transition behaviours and photo-to-thermal energy storage. J. Mater. Chem. A 2014, 2, 19963–19968. [Google Scholar] [CrossRef]
- Xiangfa, Z.; Hanning, X.; Jian, F.; Changrui, Z.; Yonggang, J. Pore structure modification of silica matrix infiltrated with paraffin as phase change material. Chem. Eng. Res. Des. 2010, 88, 1013–1017. [Google Scholar] [CrossRef]
- Zhang, F.; Zhong, Y.; Yang, X.; Lin, J.; Zhu, Z. Encapsulation of metal-based phase change materials using ceramic shells prepared by spouted bed CVD method. Sol. Energy Mater. Sol. Cells 2017, 170, 137–142. [Google Scholar] [CrossRef]
- Gupta, R.; Kedia, S.; Saurakhiya, N.; Sharma, A.; Ranjan, A. Composite nanofibrous sheets of fatty acids and polymers as thermo-regulating enclosures. Sol. Energy Mater. Sol. Cells 2016, 157, 676–685. [Google Scholar] [CrossRef]
- Hölderich, W.; Hesse, M.; Näumann, F. Zeolites: Catalysts for Organic Syntheses. Angew. Chem. Int. Ed. Engl. 1988, 27, 226–246. [Google Scholar] [CrossRef]
- Koohsaryan, E.; Anbia, M. Nanosized and hierarchical zeolites: A short review. Chin. J. Catal. 2016, 37, 447–467. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Chen, J.; Ling, Z.; Fang, X.; Zhang, Z. Experimental and numerical investigation of form-stable dodecane/hydrophobic fumed silica composite phase change materials for cold energy storage. Energy Convers. Manag. 2015, 105, 817–825. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Soleimani Dorcheh, A.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Kistler, S.S.; Caldwell, A.G. Thermal Conductivity of Silica Aërogel. Ind. Eng. Chem. 1934, 26, 658–662. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Huo, Q.; Margolese, D.I.; Stucky, G.D. Surfactant Control of Phases in the Synthesis of Mesoporous Silica-Based Materials. Chem. Mater. 1996, 8, 1147–1160. [Google Scholar] [CrossRef]
- Fan, J.; Yu, C.; Gao, F.; Lei, J.; Tian, B.; Wang, L.; Luo, Q.; Tu, B.; Zhou, W.; Zhao, D. Cubic Mesoporous Silica with Large Controllable Entrance Sizes and Advanced Adsorption Properties. Angew. Chem. 2003, 115, 3254–3258. [Google Scholar] [CrossRef]
- Rao, A.P.; Rao, A.V.; Pajonk, G.M. Hydrophobic and Physical Properties of the Two Step Processed Ambient Pressure Dried Silica Aerogels with Various Exchanging Solvents. J. Sol-Gel Sci. Technol. 2005, 36, 285–292. [Google Scholar] [CrossRef]
- Deaconu, M.; Nicu, I.; Tincu, R.; Brezoiu, A.-M.; Mitran, R.-A.; Vasile, E.; Matei, C.; Berger, D. Tailored doxycycline delivery from MCM-41-type silica carriers. Chem. Zvesti 2018, 72, 1869–1880. [Google Scholar] [CrossRef]
- Iswar, S.; Malfait, W.J.; Balog, S.; Winnefeld, F.; Lattuada, M.; Koebel, M.M. Effect of aging on silica aerogel properties. Microporous Mesoporous Mater. 2017, 241, 293–302. [Google Scholar] [CrossRef]
- Ramkumar, R.; Minakshi Sundaram, M. A biopolymer gel-decorated cobalt molybdate nanowafer: Effective graft polymer cross-linked with an organic acid for better energy storage. New J. Chem. 2016, 40, 2863–2877. [Google Scholar] [CrossRef]
- Barmi, M.J.; Minakshi, M. Tuning the Redox Properties of the Nanostructured CoMoO4 Electrode: Effects of Surfactant Content and Synthesis Temperature. ChemPlusChem 2016, 81, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhang, Z.; Zhang, W.; Wu, Y.; Zhang, J.; Imran, Z.; Zhang, D. Synthesis and Applications of Porous Glass. Shanghai Jiaotong Daxue Xuebao 2019, 24, 681–698. [Google Scholar] [CrossRef]
- Wu, H.; Tang, B.; Wu, P. Novel Hollow Mesoporous Silica Spheres/Polymer Hybrid Membrane for Ultrafiltration. J. Phys. Chem. C 2011, 116, 2246–2252. [Google Scholar] [CrossRef]
- Diacon, A.; Rusen, E.; Trifan, A.; Șomoghi, R.; Tutunaru, O.; Crăciun, G.; Busuioc, C.; Voicu, G. Preparation of metal and metal oxide doped silica hollow spheres and the evaluation of their catalytic performance. Colloid Polym. Sci. 2020, 298, 1401–1410. [Google Scholar] [CrossRef]
- Feng, X.; Fryxell, G.E.; Wang, L.-Q.; Kim, A.Y.; Liu, J.; Kemner, K.M. Functionalized Monolayers on Ordered Mesoporous Supports. Science 1997, 276, 923–926. [Google Scholar] [CrossRef]
- Mitran, R.-A.; Nastase, S.; Matei, C.; Berger, D. Tailoring the dissolution rate enhancement of aminoglutethimide by functionalization of MCM-41 silica: A hydrogen bonding propensity approach. RSC Adv. 2015, 5, 2592–2601. [Google Scholar] [CrossRef]
- Kecht, J.; Schlossbauer, A.; Bein, T. Selective Functionalization of the Outer and Inner Surfaces in Mesoporous Silica Nanoparticles. Chem. Mater. 2008, 20, 7207–7214. [Google Scholar] [CrossRef]
- Tao, Z. Mesoporous silica-based nanodevices for biological applications. RSC Adv. 2014, 4, 18961–18980. [Google Scholar] [CrossRef]
- Mitran, R.-A.; Berger, D.; Băjenaru, L.; Năstase, S.; Andronescu, C.; Matei, C. Azobenzene functionalized mesoporous AlMCM-41-type support for drug release applications. Cent. Eur. J. Chem. 2014, 12, 788–795. [Google Scholar] [CrossRef]
- Lin, S.; Shi, L.; Ribeiro Carrott, M.M.L.; Carrott, P.J.M.; Rocha, J.; Li, M.R.; Zou, X.D. Direct synthesis without addition of acid of Al-SBA-15 with controllable porosity and high hydrothermal stability. Microporous Mesoporous Mater. 2011, 142, 526–534. [Google Scholar] [CrossRef]
- Berger, D.; Nastase, S.; Mitran, R.A.; Petrescu, M.; Vasile, E.; Matei, C.; Negreanu-Pirjol, T. Mesostructured silica and aluminosilicate carriers for oxytetracycline delivery systems. Int. J. Pharm. 2016, 510, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Chaikriangkrai, A.; Takeshita, Y.; Shibata, M. Synthesis of iron-containing mesoporous silica aiming to use as a new sunscreen ultraviolet absorber. Chem. Lett. 2011, 40, 693–695. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q. A simple method to directly synthesize Al-SBA-15 mesoporous materials with different Al contents. Solid State Commun. 2008, 148, 529–533. [Google Scholar] [CrossRef]
- Souza, K.C.; Mohallem, N.D.S.; Sousa, E.M.B. Mesoporous silica-magnetite nanocomposite: Facile synthesis route for application in hyperthermia. J. Sol-Gel Sci. Technol. 2009, 53, 418–427. [Google Scholar] [CrossRef]
- Hurley, K.R.; Lin, Y.-S.; Zhang, J.; Egger, S.M.; Haynes, C.L. Effects of Mesoporous Silica Coating and Postsynthetic Treatment on the Transverse Relaxivity of Iron Oxide Nanoparticles. Chem. Mater. 2013, 25, 1968–1978. [Google Scholar] [CrossRef]
- Mitran, R.-A.; Matei, C.; Berger, D.; Băjenaru, L.; Moisescu, M.G. Controlling drug release from mesoporous silica through an amorphous, nanoconfined 1-tetradecanol layer. Eur. J. Pharm. Biopharm. 2018, 127, 318–325. [Google Scholar] [CrossRef]
- Baeza, A.; Colilla, M.; Vallet-Regí, M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery. Expert Opin. Drug Deliv. 2015, 12, 319–337. [Google Scholar] [CrossRef]
- Mitran, R.-A.; Culita, D.C.; Atkinson, I. Thermal stability enhancement of mesoporous SBA-15 silica through nanoconfinement of ceria nanoparticles. Microporous Mesoporous Mater. 2020, 306, 110484. [Google Scholar] [CrossRef]
- Doukeh, R.; Popovici, D.; Trifoi, A.; Bombos, M.; Banu, I. A study on the alkylation of m-cresol with 1-decene over mesoporous silica supported tungstophosphoric acid (HPW). React. Kinet. Mech. Catal. 2020, 131, 793–804. [Google Scholar] [CrossRef]
- Neffati, R.; Judeinstein, P.; Rault, J. Freezing, melting and dynamics of supercooled water confined in porous glass. J. Phys. Condens. Matter 2020, 32, 465101. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tang, Z.; Chang, Y.; Gao, H.; Cheng, P.; Tao, Z.; Lv, J. Toward Tailoring Chemistry of Silica-Based Phase Change Materials for Thermal Energy Storage. iScience 2020, 23, 101606. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, C.; Li, W.; Fan, X.; Wu, Z.; Zheng, J.; Li, X. Novel phase change behavior of n-eicosane in nanoporous silica: Emulsion template preparation and structure characterization using small angle X-ray scattering. Phys. Chem. Chem. Phys. 2013, 15, 14390–14395. [Google Scholar] [CrossRef]
- Matei, C.; Buhǎlţeanu, L.; Berger, D.; Mitran, R.-A. Functionalized mesoporous silica as matrix for shape-stabilized phase change materials. Int. J. Heat Mass Transf. 2019, 144, 118699. [Google Scholar] [CrossRef]
- Mitran, R.A.; Berger, D.; Munteanu, C.; Matei, C. Evaluation of Different Mesoporous Silica Supports for Energy Storage in Shape-Stabilized Phase Change Materials with Dual Thermal Responses. J. Phys. Chem. C 2015, 119, 15177–15184. [Google Scholar] [CrossRef]
- Riikonen, J.; Salonen, J.; Lehto, V.-P. Utilising thermoporometry to obtain new insights into nanostructured materials. J. Anal. Calorim. 2011, 105, 811–821. [Google Scholar] [CrossRef]
- Juras, B.; Martynas, K.; Jan, M.; Georg, V.; Winfried, B.; Venkatesan, U.; Martin, H.; Andreas, P. Broadband dielectric spectroscopy of water confined in MCM-41 molecular sieve materials—low-temperature freezing phenomena. J. Phys. Condens. Matter 2005, 17, 2843. [Google Scholar]
- Petrov, O.V.; Vargas-Florencia, D.; Furó, I. Surface Melting of Octamethylcyclotetrasiloxane Confined in Controlled Pore Glasses: Curvature Effects Observed by 1H NMR. J. Phys. Chem. B 2007, 111, 1574–1581. [Google Scholar] [CrossRef]
- Liu, E.; Dore, J.C.; Webber, J.B.W.; Khushalani, D.; Jähnert, S.; Findenegg, G.H.; Hansen, T. Neutron diffraction and NMR relaxation studies of structural variation and phase transformations for water/ice in SBA-15 silica: I. The over-filled case. J. Phys. Condens. Matter 2006, 18, 10009. [Google Scholar] [CrossRef]
- Wallacher, D.; Knorr, K. Melting and freezing of Ar in nanopores. Phys. Rev. B 2001, 63, 104202. [Google Scholar] [CrossRef]
- Husár, B.; Commereuc, S.; Lukáč, I.; Chmela, Š.; Nedelec, J.M.; Baba, M. Carbon Tetrachloride as a Thermoporometry Liquid Probe To Study the Cross-Linking of Styrene Copolymer Networks. J. Phys. Chem. B 2006, 110, 5315–5320. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mitran, R.-A.; Lincu, D.; Buhǎlţeanu, L.; Berger, D.; Matei, C. Shape-stabilized phase change materials using molten NaNO3–KNO3 eutectic and mesoporous silica matrices. Sol. Energy Mater. Sol. Cells 2020, 215, 110644. [Google Scholar] [CrossRef]
- Mitran, R.A.; Berger, D.; Matei, C. Phase Change Materials Based on Mesoporous Silica. Curr. Org. Chem. 2018, 22, 2644–2663. [Google Scholar] [CrossRef]
- Liu, S.; Ma, G.; Xie, S.; Jia, Y.; Sun, J.; Jing, Y. Diverting the phase transition behaviour of adipic acid via mesoporous silica confinement. RSC Adv. 2016, 6, 111787–111796. [Google Scholar] [CrossRef]
- Sterczyńska, A.; Deryło-Marczewska, A.; Śliwińska-Bartkowiak, M.; Piotrowska, J.Z.; Jarek, M.; Domin, K. Phase transitions of octamethylcyclotetrasiloxane confined inside aluminosilicate and silicate nanoporous matrices. J. Anal. Calorim. 2014, 118, 263–276. [Google Scholar] [CrossRef][Green Version]
- Zhang, D.; Tian, S.; Xiao, D. Experimental study on the phase change behavior of phase change material confined in pores. Sol. Energy 2007, 81, 653–660. [Google Scholar] [CrossRef]
- Majda, D.; Korzeniowska, A.; Makowski, W.; Michalik-Zym, A.; Napruszewska, B.D.; Zimowska, M.; Serwicka, E.M. Thermoporosimetry of n-alkanes for characterization of mesoporous SBA-15 silicas–Refinement of methodology. Microporous Mesoporous Mater. 2016, 222, 33–43. [Google Scholar] [CrossRef]
- Lee, J.A.; Rösner, H.; Corrigan, J.F.; Huang, Y. Phase Transitions of Naphthalene and Its Derivatives Confined in Mesoporous Silicas. J. Phys. Chem. C 2011, 115, 4738–4748. [Google Scholar] [CrossRef]
- Nomura, T.; Zhu, C.; Sheng, N.; Tabuchi, K.; Sagara, A.; Akiyama, T. Shape-stabilized phase change composite by impregnation of octadecane into mesoporous SiO2. Sol. Energy Mater. Sol. Cells 2015, 143, 424–429. [Google Scholar] [CrossRef]
- Miao, C.; Lü, G.; Yao, Y.; Tang, G.; Weng, D. Preparation of shape-stabilized phase change materials as temperature-adjusting powder. Front. Mater. Sci. 2007, 1, 284–287. [Google Scholar] [CrossRef]
- Li, H.; Fang, G.; Liu, X. Synthesis of shape-stabilized paraffin/silicon dioxide composites as phase change material for thermal energy storage. J. Mater. Sci. 2010, 45, 1672–1676. [Google Scholar] [CrossRef]
- Zhang, M.; Hong, Y.; Ding, S.; Hu, J.; Fan, Y.; Voevodin, A.A.; Su, M. Encapsulated nano-heat-sinks for thermal management of heterogeneous chemical reactions. Nanoscale 2010, 2, 2790–2797. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Su, L.; Liu, M.; Li, J.; Zhang, Z.; Li, B. Confinement effect of silica mesopores on thermal behavior of phase change composites. J. Sol-Gel Sci. Technol. 2016, 80, 180–188. [Google Scholar] [CrossRef]
- Hu, M.; Yan, Z.; Peng, L.; Guo, N.; Liu, Z. Optimization of preparation and analysis of Paraffin/SiO2 composite PCMs via sol-gel method. IOP Conf. Ser. Earth Environ. Sci. 2019, 242, 032005. [Google Scholar] [CrossRef]
- Hu, M.; Guo, N.; Wang, L. Preparation and assessment of paraffin/SiO2composite phase change material based on the efficacy coefficient method. Heat Mass Transf. 2020, 56, 1921–1929. [Google Scholar] [CrossRef]
- Liang, S.; Li, Q.; Zhu, Y.; Chen, K.; Tian, C.; Wang, J.; Bai, R. Nanoencapsulation of n-octadecane phase change material with silica shell through interfacial hydrolysis and polycondensation in miniemulsion. Energy 2015, 93, 1684–1692. [Google Scholar] [CrossRef]
- He, F.; Wang, X.; Wu, D. Phase-change characteristics and thermal performance of form-stable n-alkanes/silica composite phase change materials fabricated by sodium silicate precursor. Renew. Energy 2015, 74, 689–698. [Google Scholar] [CrossRef]
- Liu, H.; Niu, J.; Wang, X.; Wu, D. Design and construction of mesoporous silica/n-eicosane phase-change nanocomposites for supercooling depression and heat transfer enhancement. Energy 2019, 188, 116075. [Google Scholar] [CrossRef]
- Kong, X.; Zhong, Y.; Rong, X.; Min, C.; Qi, C. Building energy storage panel based on paraffin/expanded perlite: Preparation and thermal performance study. Materials 2016, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, S.; Zhu, S.; Ma, J.; Sun, Z.; Farid, M. Evaluation of paraffin infiltrated in various porous silica matrices as shape-stabilized phase change materials for thermal energy storage. Energy Convers. Manag. 2018, 171, 361–370. [Google Scholar] [CrossRef]
- Qu, Y.; Chen, J.; Liu, L.; Xu, T.; Wu, H.; Zhou, X. Study on properties of phase change foam concrete block mixed with paraffin / fumed silica composite phase change material. Renew. Energy 2020, 150, 1127–1135. [Google Scholar] [CrossRef]
- Zhou, X.; Xiao, H.; Feng, J.; Zhang, C.; Jiang, Y. Preparation and thermal properties of paraffin/porous silica ceramic composite. Compos. Sci. Technol. 2009, 69, 1246–1249. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Liu, L.; Lu, Z.; Sanjayan, J.G.; Duan, W.H. Development of granular expanded perlite/paraffin phase change material composites and prevention of leakage. Sol. Energy 2016, 137, 179–188. [Google Scholar] [CrossRef]
- Lyu, J.; Li, G.; Liu, M.; Zhang, X. Aerogel-Directed Energy-Storage Films with Thermally Stimulant Multiresponsiveness. Langmuir 2019, 35, 943–949. [Google Scholar] [CrossRef]
- Snehal, K.; Das, B.B.; Kumar, S. Influence of Integration of Phase Change Materials on Hydration and Microstructure Properties of Nanosilica Admixed Cementitious Mortar. J. Mater. Civ. Eng. 2020, 32, 04020108. [Google Scholar] [CrossRef]
- Chung, O.; Jeong, S.G.; Yu, S.; Kim, S. Thermal performance of organic PCMs/micronized silica composite for latent heat thermal energy storage. Energy Build. 2014, 70, 180–185. [Google Scholar] [CrossRef]
- Goitandia, A.M.; Beobide, G.; Aranzabe, E.; Aranzabe, A. Development of content-stable phase change composites by infiltration into inorganic porous supports. Sol. Energy Mater. Sol. Cells 2015, 134, 318–328. [Google Scholar] [CrossRef]
- Belessiotis, G.V.; Papadokostaki, K.G.; Favvas, E.P.; Efthimiadou, E.K.; Karellas, S. Preparation and investigation of distinct and shape stable paraffin/SiO2 composite PCM nanospheres. Energy Convers. Manag. 2018, 168, 382–394. [Google Scholar] [CrossRef]
- Shaid, A.; Wang, L.; Islam, S.; Cai, J.Y.; Padhye, R. Preparation of aerogel-eicosane microparticles for thermoregulatory coating on textile. Appl. Eng. 2016, 107, 602–611. [Google Scholar] [CrossRef]
- Li, H.; Chen, H.; Li, X.; Sanjayan, J.G. Development of thermal energy storage composites and prevention of PCM leakage. Appl. Energy 2014, 135, 225–233. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Li, H.; Liu, L.; Lu, Z.; Zhang, T.; Duan, W.H. Integration of form-stable paraffin/nanosilica phase change material composites into vacuum insulation panels for thermal energy storage. Appl. Energy 2015, 159, 601–609. [Google Scholar] [CrossRef]
- Gao, H.; Bo, L.; Liu, P.; Chen, D.; Li, A.; Ou, Y.; Dong, C.; Wang, J.; Chen, X.; Hou, C.; et al. Ambient pressure dried flexible silica aerogel for construction of monolithic shape-stabilized phase change materials. Sol. Energy Mater. Sol. Cells 2019, 201, 110122. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, J.; Wang, G.; Zhang, R.; Peng, X. Preparation of paraffin/SiO2 aerogel stable-stabilized phase change composites for high-humidity environment. J. Mater. Sci. 2020, 55, 1511–1524. [Google Scholar] [CrossRef]
- Wang, F.; Gao, S.; Pan, J.; Li, X.; Liu, J. Short-chain modified SiO2 with high absorption of organic PCM for thermal protection. Nanomaterials 2019, 9, 657. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Tao, S.; An, Y.; Meng, C.; Hu, T. Phase change in modified hierarchically porous monolith: An extra energy increase. Microporous Mesoporous Mater. 2014, 193, 69–76. [Google Scholar] [CrossRef]
- Wen, X.Y.; Ling, Z.Y.; Fang, X.M.; Zhang, Z.G. Preparation of RT28/ hydrophobic-fumed-silica composite phase change materials and their thermal insulation application in lithium ion battery. J. Chem. Eng. Chin. Univ. 2016, 30, 1178–1183. [Google Scholar]
- Choi, J.; Fujita, H.; Ogura, M.; Sakoda, A. Confinement effect on enthalpy of fusion and melting point of organic phase change materials in cylindrical nanospace of mesoporous silica and carbon. Adsorption 2018, 24, 345–355. [Google Scholar] [CrossRef]
- Choi, J.; Yoshie, K.; Moteki, T.; Ogura, M. Theoretical Evaluation of an Organic Phase Change Material (PCM)-Inserted Dual-Functional Adsorbent for the Recovery of Heat of Adsorption. Ind. Eng. Chem. Res. 2019, 58, 10114–10118. [Google Scholar] [CrossRef]
- Li, M.; Wu, Z.; Tan, J. Properties of form-stable paraffin/silicon dioxide/expanded graphite phase change composites prepared by sol-gel method. Appl. Energy 2012, 92, 456–461. [Google Scholar] [CrossRef]
- Han, X.; Zhao, T.; Gao, X.; Li, H. Preparation and characterization of high-temperature non-flowing SiO2/EG/paraffin composites by high-temperature refining. Colloids Surf. A 2018, 542, 1–7. [Google Scholar] [CrossRef]
- Lv, Y.; Situ, W.; Yang, X.; Zhang, G.; Wang, Z. A novel nanosilica-enhanced phase change material with anti-leakage and anti-volume-changes properties for battery thermal management. Energy Convers. Manag. 2018, 163, 250–259. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, C.; Fang, G. Preparation and thermal properties of n-eicosane/nano-SiO2/expanded graphite composite phase-change material for thermal energy storage. Mater. Chem. Phys. 2020, 240, 122178. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, K.; Kou, Y.; Wang, S.; Shi, Q. A facile strategy of constructing composite form-stable phase change materials with superior high thermal conductivity using silicagel industrial wastes. Sol. Energy 2020, 207, 51–58. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, Y.; Yuan, H.; Feng, D.; Zhang, X.; Wang, G. Thermal properties of C17H36/MCM-41 composite phase change materials. Comput. Mater. Sci. 2015, 109, 300–307. [Google Scholar] [CrossRef]
- Afgan, S.; Khushnood, R.A.; Memon, S.A.; Iqbal, N. Development of structural thermal energy storage concrete using paraffin intruded lightweight aggregate with nano-refined modified encapsulation paste layer. Constr. Build. Mater. 2019, 228, 116768. [Google Scholar] [CrossRef]
- Yuan, K.; Wang, H.; Liu, J.; Fang, X.; Zhang, Z. Novel slurry containing graphene oxide-grafted microencapsulated phase change material with enhanced thermo-physical properties and photo-thermal performance. Sol. Energy Mater. Sol. Cells 2015, 143, 29–37. [Google Scholar] [CrossRef]
- Amaral, C.; Gama, N.V.; Mohseni, F.; Amaral, J.S.; Amaral, V.S.; Marques, P.A.A.P.; Barros-Timmons, A.; Vicente, R. Development of structural layers PVC incorporating phase change materials for thermal energy storage. Appl. Eng. 2020, 179, 115707. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, X.; Wu, D. Design and synthesis of magnetic microcapsules based on n-eicosane core and Fe3O4/SiO2 hybrid shell for dual-functional phase change materials. Appl. Energy 2014, 134, 456–468. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Li, J.; Ma, C.; Guo, L.; Meng, X. Solar-driven phase change microencapsulation with efficient Ti4O7 nanoconverter for latent heat storage. Nano Energy 2018, 53, 579–586. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Min, X.; Fan, B. Integration of Pore Confinement and Hydrogen-Bond Influence on the Crystallization Behavior of C18 PCMs in Mesoporous Silica for Form-Stable Phase Change Materials. ACS Sustain. Chem. Eng. 2018, 6, 897–908. [Google Scholar] [CrossRef]
- Tan, N.; Xie, T.; Hu, P.; Feng, Y.; Li, Q.; Zhao, S.; Zhou, H.-N.; Zeng, W.-B.; Zeng, J.-L. Preparation and characterization of capric-palmitic acids eutectics/silica xerogel/exfoliated graphite nanoplatelets form-stable phase change materials. J. Energy Storage 2020, 102016. [Google Scholar] [CrossRef]
- Fang, G.; Li, H.; Liu, X. Preparation and properties of lauric acid/silicon dioxide composites as form-stable phase change materials for thermal energy storage. Mater. Chem. Phys. 2010, 122, 533–536. [Google Scholar] [CrossRef]
- Tahan Latibari, S.; Mehrali, M.; Mehrali, M.; Indra Mahlia, T.M.; Cornelis Metselaar, H.S. Synthesis, characterization and thermal properties of nanoencapsulated phase change materials via sol–gel method. Energy 2013, 61, 664–672. [Google Scholar] [CrossRef]
- Meng, D.; Zhao, K.; Zhao, W.; Jiang, G. Preparation and characterization of CA-MA eutectic/silicon dioxide nanoscale composite phase change material from water glass via sol-gel method. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2017, 32, 1048–1056. [Google Scholar] [CrossRef]
- Fu, Z.; Dai, L.; Yi, Y.; Luo, J.; Li, B. Structure and thermal properties of stearic acid/silica composites as form-stable phase change materials. J. Sol-Gel Sci. Technol. 2018, 87, 419–426. [Google Scholar] [CrossRef]
- Wang, C.; Cai, Z.; Wang, T.; Chen, K. Preparation and thermal properties of shape-stabilized 1, 8-octanediol /SiO2 composites via sol gel methods. Mater. Chem. Phys. 2020, 250, 123041. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Li, X.; Wu, X. Preparation of hydrophobic lauric acid/SiO2 shape-stabilized phase change materials for thermal energy storage. J. Energy Storage 2019, 21, 611–617. [Google Scholar] [CrossRef]
- Marske, F.; E Silva, J.M.D.S.; Wehrspohn, R.B.; Hahn, T.; Enke, D. Synthesis of monolithic shape-stabilized phase change materials with high mechanical stability: Via a porogen-assisted in situ sol-gel process. RSC Adv. 2020, 10, 3072–3083. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, G.; Liu, M.; Zhang, J.; Wang, Q.; Wei, Q. Fabrication and characterization of capric-lauric-palmitic acid/electrospun SiO2 nanofibers composite as form-stable phase change material for thermal energy storage/retrieval. Sol. Energy 2015, 118, 87–95. [Google Scholar] [CrossRef]
- Zhang, J.; Narh, C.; Lv, P.; Cai, Y.; Zhou, H.; Hou, X.; Wei, Q. Preparation of novel form–stable composite phase change materials with porous silica nanofibrous mats for thermal storage/retrieval. Colloids Surf. A 2019, 570, 1–10. [Google Scholar] [CrossRef]
- Nemati, S.; Pircheraghi, G. Fabrication of a form-stable phase change material with green fatty acid and recycled silica nanoparticles from spent lead-acid battery separators with enhanced thermal conductivity. Acta 2020, 693, 178781. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, H.; Gao, X.; Xu, T.; Fang, Y.; Zhang, Z. Fabrication and characterization of form-stable capric-palmitic-stearic acid ternary eutectic mixture/nano-SiO2 composite phase change material. Energy Build. 2017, 147, 41–46. [Google Scholar] [CrossRef]
- Reddy, V.J.; Yadav, J.S.; Chattopadhyay, S. Phase change material loaded form-stable composites for low temperature thermal buffering application. Mater. Chem. Phys. 2020, 247, 122859. [Google Scholar] [CrossRef]
- Vennapusa, J.R.; Konala, A.; Dixit, P.; Chattopadhyay, S. Caprylic acid based PCM composite with potential for thermal buffering and packaging applications. Mater. Chem. Phys. 2020, 253, 123453. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, T.D.; Zheng, H.; Feng, H.X. Stearic acid/silica fume composite as form-stable phase change material for thermal energy storage. Energy Build. 2011, 43, 2365–2370. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Wang, B.; Lv, M.; Zhu, Y.; Gao, J. Fabrication and characterization of novel shape-stabilized stearic acid composite phase change materials with tannic-acid-templated mesoporous silica nanoparticles for thermal energy storage. RSC Adv. 2017, 7, 15625–15631. [Google Scholar] [CrossRef]
- Fan, S.; Gao, H.; Dong, W.; Tang, J.; Wang, J.; Yang, M.; Wang, G. Shape-Stabilized Phase Change Materials Based on Stearic Acid and Mesoporous Hollow SiO2 Microspheres (SA/SiO2) for Thermal Energy Storage. Eur. J. Inorg. Chem. 2017, 2017, 2138–2143. [Google Scholar] [CrossRef]
- Gao, J.; Lv, M.; Lu, J.; Chen, Y.; Zhang, Z.; Zhang, X.; Zhu, Y. Enhanced Thermal Properties of Novel Latent Heat Thermal Storage Material Through Confinement of Stearic Acid in Meso-Structured Onion-Like Silica. JOM 2017, 69, 2785–2790. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Tang, X.; Liu, Y.; Han, Z.; Chen, Y. Comparison study between mesoporous silica nanoscale microsphere and active carbon used as the matrix of shape-stabilized phase change material. Sci. Rep. 2019, 9, 16056. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, Y.; Guo, X.; Tao, W.; Wang, J.; Gao, S.; Gao, J. Mesoporous silica nanoparticles with wrinkled structure as the matrix of myristic acid for the preparation of a promising new shape-stabilized phase change material via simple method. RSC Adv. 2018, 8, 34224–34231. [Google Scholar] [CrossRef]
- Han, L.; Ma, G.; Xie, S.; Sun, J.; Jia, Y.; Jing, Y. Preparation and characterization of the shape-stabilized phase change material based on sebacic acid and mesoporous MCM-41. J. Anal. Calorim. 2017, 130, 935–941. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Z.; Xia, W.; Zou, R.; Han, R.P.S. Alkylated phase change composites for thermal energy storage based on surface-modified silica aerogels. J. Mater. Chem. A 2015, 3, 1935–1940. [Google Scholar] [CrossRef]
- Wan, X.; Chen, C.; Tian, S.; Guo, B. Thermal characterization of net-like and form-stable ML/SiO2 composite as novel PCM for cold energy storage. J. Energy Storage 2020, 28, 101276. [Google Scholar] [CrossRef]
- Mitran, R.-A.; Berger, D.; Matei, C. Improving thermal properties of shape-stabilized phase change materials containing lauric acid and mesocellular foam silica by assessing thermodynamic properties of the non-melting layer. Acta 2018, 660, 70–76. [Google Scholar] [CrossRef]
- Sarı, A.; Bicer, A.; Al-Ahmed, A.; Al-Sulaiman, F.A.; Zahir, M.H.; Mohamed, S.A. Silica fume/capric acid-palmitic acid composite phase change material doped with CNTs for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 179, 353–361. [Google Scholar] [CrossRef]
- Lin, Y.; Cong, R.; Chen, Y.; Fang, G. Thermal properties and characterization of palmitic acid/nano silicon dioxide/graphene nanoplatelet for thermal energy storage. Int. J. Energy Res. 2020, 44, 5621–5633. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, Q.; Chen, C.; Li, L.; Yuan, W. Developing a heat-insulating composite phase change material with light-to-thermal conversion performance from graphene oxide/silica hybrid aerogel. Appl. Eng. 2020, 174, 115303. [Google Scholar]
- Ding, J.; Wu, X.; Shen, X.; Cui, S.; Chen, X. A promising form-stable phase change material composed of C/SiO2 aerogel and palmitic acid with large latent heat as short-term thermal insulation. Energy 2020, 210, 118478. [Google Scholar] [CrossRef]
- Liu, P.; Gao, H.; Chen, X.; Chen, D.; Lv, J.; Han, M.; Cheng, P.; Wang, G. In situ one-step construction of monolithic silica aerogel-based composite phase change materials for thermal protection. Compos. Part B 2020, 195, 108072. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, S.; Zhai, M.; Sui, J.; Lan, X.; Gao, J. Thermal Properties of Nano-Sized Polyethylene Glycol Confined in Silica Gels for Latent Heat Storage. Acta 2017, 655, 211–218. [Google Scholar] [CrossRef]
- Yang, H.; Feng, L.; Wang, C.; Zhao, W.; Li, X. Confinement Effect of SiO2 Framework on Phase Change of PEG in Shape-Stabilized PEG/SiO2 Composites. Eur. Polym. J. 2012, 48, 803–810. [Google Scholar] [CrossRef]
- Qian, Y.; Wei, P.; Jiang, P.; Li, Z.; Yan, Y.; Ji, K.; Deng, W. Preparation of Shape-Stabilized Co-Crystallized Poly (Ethylene Glycol) Composites as Thermal Energy Storage Materials. Energy Convers. Manag. 2013, 76, 101–108. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, T. Influence of SiO2 Pore Structure on Phase Change Enthalpy of Shape-Stabilized Polyethylene Glycol/Silica Composites. J. Mater. Sci. 2013, 48, 3716–3721. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, H.; Tang, B.; Xu, S.; Shufen, Z. Novel Light–Driven CF/PEG/SiO2 Composite Phase Change Materials with High Thermal Conductivity. Sol. Energy Mater. Sol. Cells 2018, 174, 538–544. [Google Scholar] [CrossRef]
- Weng, Z.; Wu, K.; Luo, F.; Xiao, F.; Zhang, Q.; Wang, S.; Lu, M. Fabrication of High Thermal Conductive Shape-Stabilized Polyethylene Glycol/Silica Phase Change Composite by Two-Step Sol Gel Method. Compos. Part A Appl. Sci. Manuf. 2018, 110, 106–112. [Google Scholar] [CrossRef]
- Sun, K.; Kou, Y.; Zheng, H.; Liu, X.; Tan, Z.; Shi, Q. Using Silicagel Industrial Wastes to Synthesize Polyethylene Glycol/Silica-Hydroxyl Form-Stable Phase Change Materials for Thermal Energy Storage Applications. Sol. Energy Mater. Sol. Cells 2018, 178, 139–145. [Google Scholar] [CrossRef]
- Serrano, A.; Martín del Campo, J.; Peco, N.; Rodriguez, J.F.; Carmona, M. Influence of Gelation Step for Preparing PEG–SiO2 Shape-Stabilized Phase Change Materials by Sol–Gel Method. J. Sol-Gel Sci. Technol. 2019, 89, 731–742. [Google Scholar] [CrossRef]
- Wan, X.; Su, L.; Guo, B. Design and Preparation of Novel Shapeable PEG/SiO2/AA Shape-Stabilized Phase Change Materials Based on Double-Locked Network with Enhanced Heat Storage Capacity for Thermal Energy Regulation and Storage. Powder Technol. 2019, 353, 98–109. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, B.; Zhang, S. Novel Network Structural PEG/PAA/SiO2 Composite Phase Change Materials with Strong Shape Stability for Storing Thermal Energy. Sol. Energy Mater. Sol. Cells 2020, 216, 110678. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.; Shi, X.; Yao, C.; Kuai, C. Use of PEG/SiO2 Phase Change Composite to Control Porous Asphalt Concrete Temperature. Constr. Build. Mater. 2020, 245, 118459. [Google Scholar] [CrossRef]
- Yan, D.; Tang, B.; Zhang, S. Preparation and Performances of Sunlight-Induced Phase Change PEG/SiO2-Dye Composite for Solar Energy Conversion and Storage. Sol. Energy Mater. Sol. Cells 2020, 215, 110657. [Google Scholar] [CrossRef]
- Xu, J.; Yang, T.; Xu, X.; Guo, X.; Cao, J. Processing Solid Wood into a Composite Phase Change Material for Thermal Energy Storage by Introducing Silica-Stabilized Polyethylene Glycol. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106098. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Fang, Y.; Ding, J. Preparation and Performance of Form-Stable Polyethylene Glycol/Silicon Dioxide Composites as Solid-Liquid Phase Change Materials. Appl. Energy 2009, 86, 170–174. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Min, X.; Deng, Y.; Guan, W.; Ning, L. Radial-like Mesoporous Silica Sphere: A Promising New Candidate of Supporting Material for Storage of Low-, Middle-, and High-Temperature Heat. Energy 2016, 112, 1074–1083. [Google Scholar] [CrossRef]
- Gao, J.; Tao, W.; Chen, D.; Guo, X.; Chen, Y.; Jiang, Y. High Performance Shape-Stabilized Phase Change Material with Nanoflower-like Wrinkled Mesoporous Silica Encapsulating Polyethylene Glycol: Preparation and Thermal Properties. Nanomaterials 2018, 8, 385. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, H.; Wang, B.; Shi, Q.; Gao, J.; Cui, Z.; Wan, Y. Dopamine Functionalization for Improving Crystallization Behaviour of Polyethylene Glycol in Shape-Stable Phase Change Material with Silica Fume as the Matrix. J. Clean. Prod. 2019, 208, 951–959. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, J.; Zhang, X.; Shi, Q.; Han, Z.; Chen, Y. Facile Functionalized Mesoporous Silica Using Biomimetic Method as New Matrix for Preparation of Shape-Stabilized Phase-Change Material with Improved Enthalpy. Int. J. Energy Res. 2019, 43, 8649–8659. [Google Scholar] [CrossRef]
- Kumar, A.; Jain, H.; Tripathi, B.P. Synthesis and Nanoencapsulation of Poly(Ethylene Glycol)-Distearates Phase Change Materials for Latent Heat Storage and Release. ACS Appl. Energy Mater. 2020, 3, 5965–5976. [Google Scholar] [CrossRef]
- Abbasi Hattan, H.; Madhkhan, M.; Marani, A. Thermal and Mechanical Properties of Building External Walls Plastered with Cement Mortar Incorporating Shape-Stabilized Phase Change Materials (SSPCMs). Constr. Build. Mater. 2020, 121385. [Google Scholar] [CrossRef]
- Li, B.; Shu, D.; Wang, R.; Zhai, L.; Chai, Y.; Lan, Y.; Cao, H.; Zou, C. Polyethylene Glycol/Silica (PEG@SiO2) Composite Inspired by the Synthesis of Mesoporous Materials as Shape-Stabilized Phase Change Material for Energy Storage. Renew. Energy 2020, 145, 84–92. [Google Scholar] [CrossRef]
- Feng, D.; Feng, Y.; Li, P.; Zang, Y.; Wang, C.; Zhang, X. Modified Mesoporous Silica Filled with PEG as a Shape-Stabilized Phase Change Materials for Improved Thermal Energy Storage Performance. Microporous Mesoporous Mater. 2020, 292, 109756. [Google Scholar] [CrossRef]
- Wang, A.; Chen, C.; Xu, G. Silica/acetamide composite as form-stable phase change material for latent heat thermal energy storage. J. Adv. Microsc. Res. 2012, 7, 286–291. [Google Scholar] [CrossRef]
- Pethurajan, V.; Sivan, S.; Konatt, A.J.; Reddy, A.S. Facile approach to improve solar thermal energy storage efficiency using encapsulated sugar alcohol based phase change material. Sol. Energy Mater. Sol. Cells 2018, 185, 524–535. [Google Scholar] [CrossRef]
- Zhou, X.; Xiao, H.; Feng, J.; Zhang, C.; Jiang, Y. Preparation, properties and thermal control applications of silica aerogel infiltrated with solid-liquid phase change materials. J. Exp. Nanosci. 2012, 7, 17–26. [Google Scholar]
- Toyoda, T.; Narisada, R.; Suzuki, H.; Hidema, R.; Komoda, Y. Fabrication process of silica hard-shell microcapsule (HSMC) containing phase-change materials. Chem. Lett. 2014, 43, 820–821. [Google Scholar] [CrossRef]
- Zhai, M.; Zhang, S.; Sui, J.; Tian, F.; Lan, X.Z. Solid–solid phase transition of tris(hydroxymethyl)aminomethane in nanopores of silica gel and porous glass for thermal energy storage. J. Anal. Calorim. 2017, 129, 957–964. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Sun, R.; Lai, M.; Du, R.; Zhang, Z. The anti-supercooling effect of surface-modified nano-scaled SiO2 in hydrated salts phase transition system. J. Phys. Conf. Ser. 2009, 188, 012046. [Google Scholar] [CrossRef]
- Ling, Z.; Liu, J.; Wang, Q.; Lin, W.; Fang, X.; Zhang, Z. MgCl2·6H2O-Mg(NO3)2·6H2O eutectic/SiO2 composite phase change material with improved thermal reliability and enhanced thermal conductivity. Sol. Energy Mater. Sol. Cells 2017, 172, 195–201. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Q.; Liu, Y.; Wang, D.; Song, W.; Chen, Y.; Liu, J. Calcium chloride hexahydrate/nano-SiO2 composites as form-stable phase change materials for building energy conversation: The influence of pore size of nano-SiO2. Energy Build. 2020, 208, 109672. [Google Scholar] [CrossRef]
- Gao, C.-F.; Wang, L.-P.; Li, Q.-F.; Wang, C.; Nan, Z.-D.; Lan, X.-Z. Tuning thermal properties of latent heat storage material through confinement in porous media: The case of (1-CnH2n+1NH3)2ZnCl4 (n=10 and 12). Sol. Energy Mater. Sol. Cells 2014, 128, 221–230. [Google Scholar] [CrossRef]
- Li, Q.F.; Wang, C.; Lan, X.Z. Solid-solid phase transition of (1-C14H29NH3)2ZnCl4 in nanopores of silica gel for thermal energy storage. Chin. Chem. Lett. 2017, 28, 49–54. [Google Scholar] [CrossRef]
- Garay Ramirez, B.M.L.; Glorieux, C.; San Martin Martinez, E.; Flores Cuautle, J.J.A. Tuning of thermal properties of sodium acetate trihydrate by blending with polymer and silver nanoparticles. Appl. Eng. 2014, 62, 838–844. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, T. Preparation and characterization of hydrated salts/silica composite as shape-stabilized phase change material via sol-gel process. Acta 2014, 591, 10–15. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, T. The dependence of phase change enthalpy on the pore structure and interfacial groups in hydrated salts/silica composites via sol–gel. J. Colloid Interface Sci. 2015, 448, 100–105. [Google Scholar] [CrossRef]
- Peng, S.; Huang, J.; Wang, T.; Zhu, P. Effect of fumed silica additive on supercooling, thermal reliability and thermal stability of Na2HPO4·12H2O as inorganic PCM. Acta 2019, 675, 1–8. [Google Scholar] [CrossRef]
- Zou, T.; Fu, W.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Preparation and performance of form-stable TBAB hydrate/SiO2 composite PCM for cold energy storage. Int. J. Refrig. 2019, 101, 117–124. [Google Scholar] [CrossRef]
- Fang, Y.; Su, J.; Tang, Y.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z. Form-stable Na2SO4·10H2O-Na2HPO4·12H2O eutectic/hydrophilic fumed silica composite phase change material with low supercooling and low thermal conductivity for indoor thermal comfort improvement. Int. J. Energy Res. 2020, 44, 3171–3182. [Google Scholar] [CrossRef]
- Fu, W.; Zou, T.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Preparation and properties of phase change temperature-tuned composite phase change material based on sodium acetate trihydrate–urea/fumed silica for radiant floor heating system. Appl. Eng. 2019, 162, 114253. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, T. Enthalpy of solid−liquid phase change confined in porous materials. Ind. Eng. Chem. Res. 2016, 55, 11536–11541. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, T. Preparation and Characterization of Sodium Sulfate/Silica Composite as a Shape-stabilized Phase Change Material by Sol-gel Method. Chin. J. Chem. Eng. 2014, 22, 360–364. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, T. Study on preparation and thermal properties of sodium nitrate/silica composite as shape-stabilized phase change material. Acta 2015, 613, 66–70. [Google Scholar] [CrossRef]
- Wu, X.; Ding, J.; Kong, Y.; Sun, Z.; Shao, G.; Li, B.; Wu, J.; Zhong, Y.; Shen, X.; Cui, S. Synthesis of a novel three-dimensional Na2SO4@SiO2@Al2O3-SiO2 phase change material doped aerogel composite with high thermal resistance and latent heat. Ceram. Int. 2018, 44, 21855–21865. [Google Scholar] [CrossRef]
- Milián, Y.E.; Reinaga, N.; Grágeda, M.; Ushak, S. Development of new inorganic shape stabilized phase change materials with LiNO3 and LiCl salts by sol-gel method. J. Sol-Gel Sci. Technol. 2020, 94, 22–33. [Google Scholar]
- Deng, Z.F.; Tan, H.; Zhang, G.Q.; Xu, G.Z.; Yang, C.Y.; Chang, L.; Du, Z.L. Effects of SiO2/SiC particles on the thermal stability of carbonate eutectic salt/ceramic composite. IOP Conf. Ser. Mater. Sci. Eng. 2019, 504, 012034. [Google Scholar] [CrossRef]
- Mitran, R.-A.; Petrescu, S.; Şomǎcescu, S.; Mocioiu, O.C.; Buhǎlţeanu, L.; Berger, D.; Matei, C. Nanocomposite phase change materials based on NaCl–CaCl2 and mesoporous silica. J. Anal. Calorim. 2019, 138, 2555–2563. [Google Scholar] [CrossRef]
- Mitran, R.A.; Lincu, D.; Ioniţǎ, S.; Deaconu, M.; Jerca, V.V.; Mocioiu, O.C.; Berger, D.; Matei, C. High temperature shape–Stabilized phase change materials obtained using mesoporous silica and NaCl–NaBr–Na2MoO4 salt eutectic. Sol. Energy Mater. Sol. Cells 2020, 218, 110760. [Google Scholar] [CrossRef]
- Milian, Y.E.; Ushak, S. Design of synthesis route for inorganic shape-stabilized phase change materials. Direct sol–gel process versus vacuum impregnation method. J. Sol-Gel Sci. Technol. 2020, 94, 67–79. [Google Scholar] [CrossRef]
- Shin, S.J.; Guzman, J.; Yuan, C.W.; Liao, C.Y.; Boswell-Koller, C.N.; Stone, P.R.; Dubon, O.D.; Minor, A.M.; Watanabe, M.; Beeman, J.W.; et al. Embedded binary eutectic alloy nanostructures: A new class of phase change materials. Nano Lett. 2010, 10, 2794–2798. [Google Scholar] [CrossRef]
- Wei, H.; Wang, C.; Yang, S.; Han, J.; Yang, M.; Zhang, J.; Lu, Y.; Liu, X. A strategy for designing microencapsulated composite phase change thermal storage materials with tunable melting temperature. Sol. Energy Mater. Sol. Cells 2019, 203, 110166. [Google Scholar] [CrossRef]














| Heat Storage | Sensible Heat | Latent Heat | Chemical Heat | |
|---|---|---|---|---|
| Properties | ||||
| Energy density | <600–800 kJ kg−1 0.8–1.7 J g−1 K−1 [4] | ~100–1800 kJ kg−1 * | 300–3000 kJ kg−1 [4] | |
| Temperature difference needed for storage, ΔT | 25–1200 °C [4] | 0–50 °C * | 100–500 °C [4] | |
| Volume change | ~1% [4] | 10–40% [5] | >1000% (at 1 atm) [4] | |
| Complexity | Very simple | Simple | Complex | |
| Maturity | Industrial scale | Pilot scale | Laboratory scale | |
| Type | Property | Value | Benefits |
|---|---|---|---|
| Thermal properties | Heat of fusion | High | Increased energy storage density |
| Specific heat | High | ||
| Thermal conductivity | High | Increased power density, lower temperature gradients | |
| Melting point | Determines operating temperature | ||
| Physical properties | Volume change on transition | Low | Increases stability, minimizes leakage |
| Vapor pressure | Low | Decreases evaporative loss of material | |
| Crystallization rate | High | Decreases the hysteresis between charging and discharging | |
| Supercooling degree | Low | ||
| Chemical properties | Thermal & chemical stability | High | Increases life cycle |
| Reactivity/corrosiveness | Low | ||
| Non-toxic, non-flammable, non-explosive | High | Increases safety and decreases system complexity | |
| Wettability & surface tension | High | Formation of shape stabilized materials with the silica matrix and higher PCM loading | |
| Economic properties | Cost | Low | Improved economic efficiency and decreased risk |
| Abundance | High | ||
| Environmental impact | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitran, R.-A.; Ioniţǎ, S.; Lincu, D.; Berger, D.; Matei, C. A Review of Composite Phase Change Materials Based on Porous Silica Nanomaterials for Latent Heat Storage Applications. Molecules 2021, 26, 241. https://doi.org/10.3390/molecules26010241
Mitran R-A, Ioniţǎ S, Lincu D, Berger D, Matei C. A Review of Composite Phase Change Materials Based on Porous Silica Nanomaterials for Latent Heat Storage Applications. Molecules. 2021; 26(1):241. https://doi.org/10.3390/molecules26010241
Chicago/Turabian StyleMitran, Raul-Augustin, Simona Ioniţǎ, Daniel Lincu, Daniela Berger, and Cristian Matei. 2021. "A Review of Composite Phase Change Materials Based on Porous Silica Nanomaterials for Latent Heat Storage Applications" Molecules 26, no. 1: 241. https://doi.org/10.3390/molecules26010241
APA StyleMitran, R.-A., Ioniţǎ, S., Lincu, D., Berger, D., & Matei, C. (2021). A Review of Composite Phase Change Materials Based on Porous Silica Nanomaterials for Latent Heat Storage Applications. Molecules, 26(1), 241. https://doi.org/10.3390/molecules26010241









