Evaluation of Changes in Protein Quality of High-Pressure Treated Aqueous Aquafaba
Abstract
1. Introduction
2. Results and Discussion
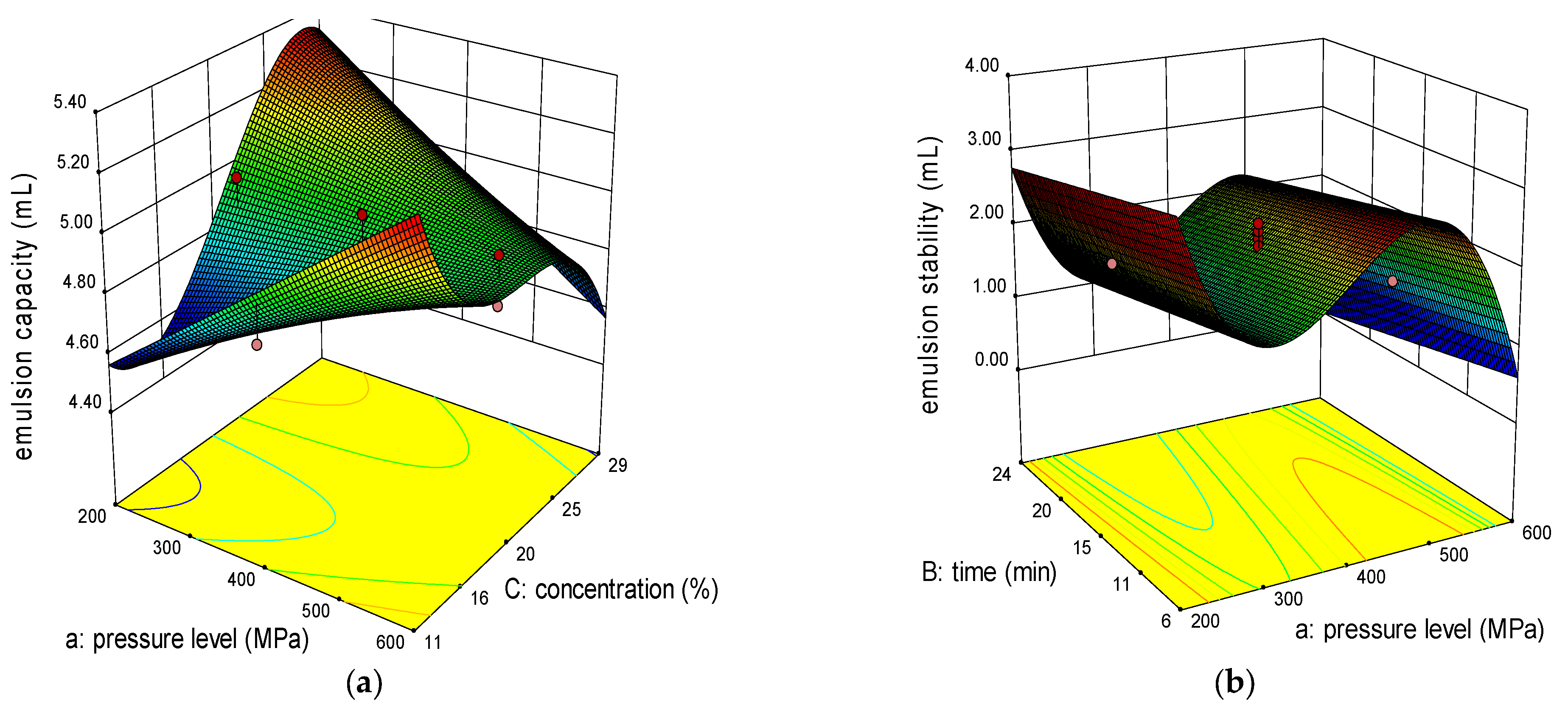
2.1. Effect of Variables on Emulsion Properties
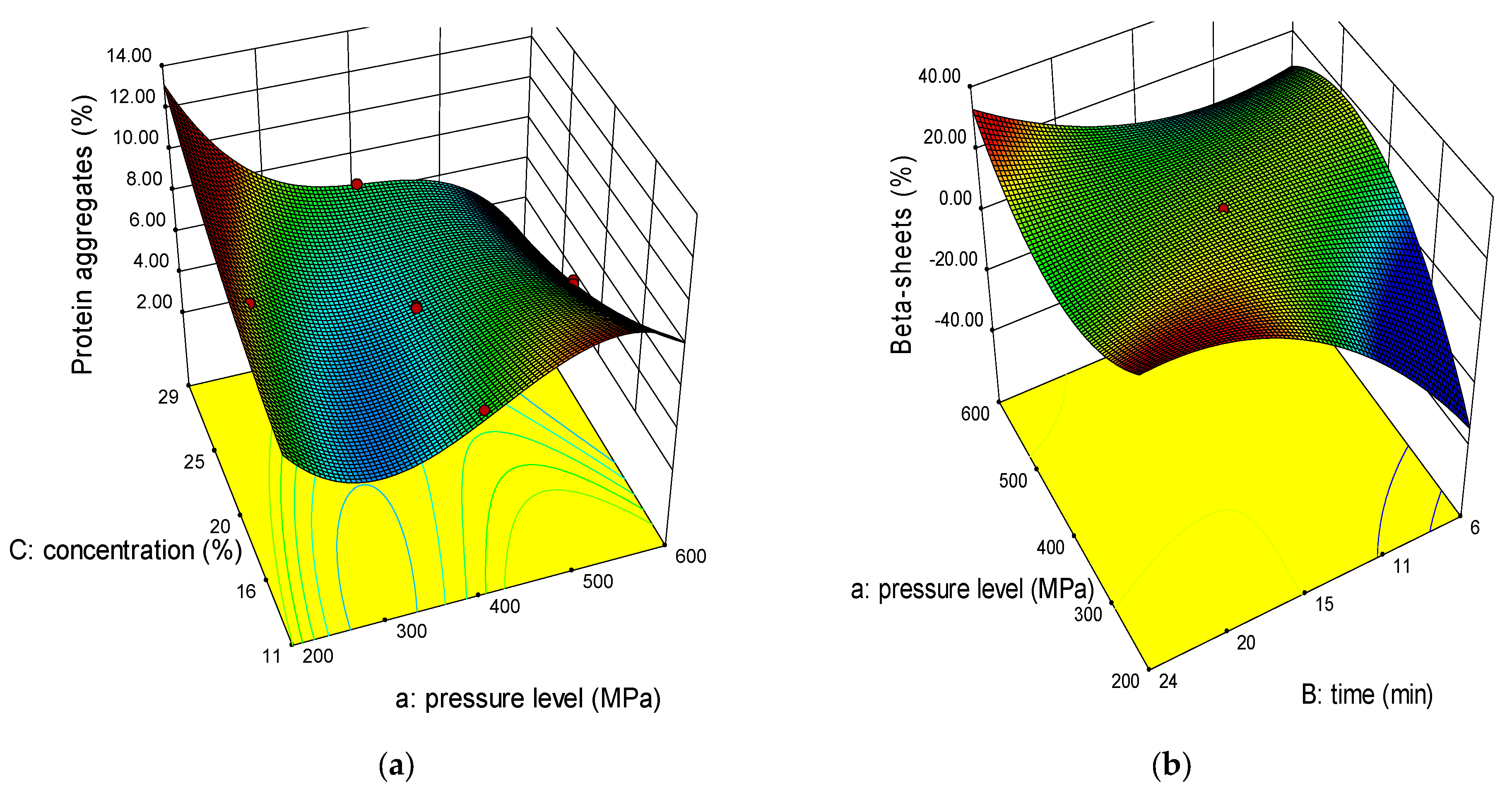
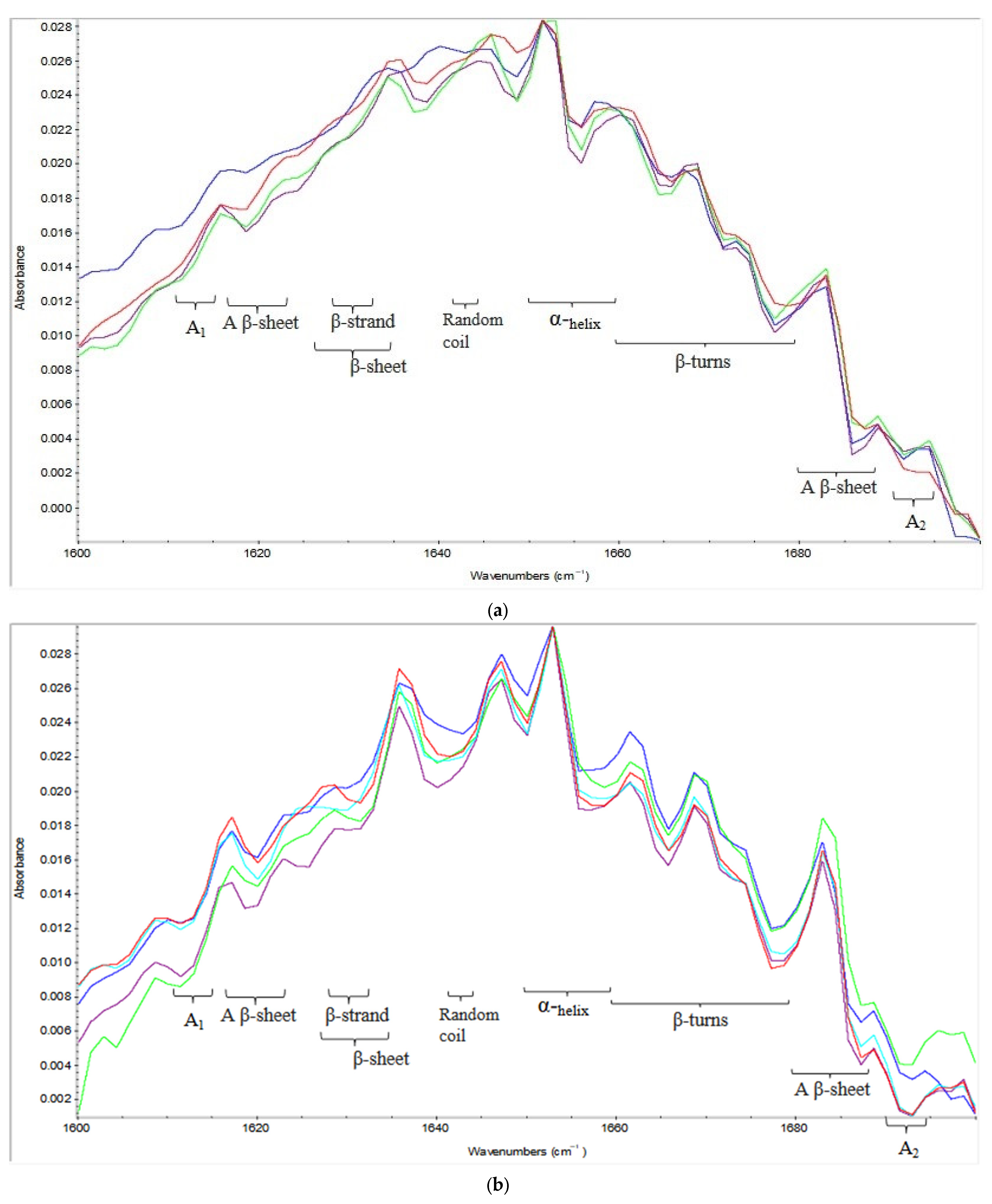
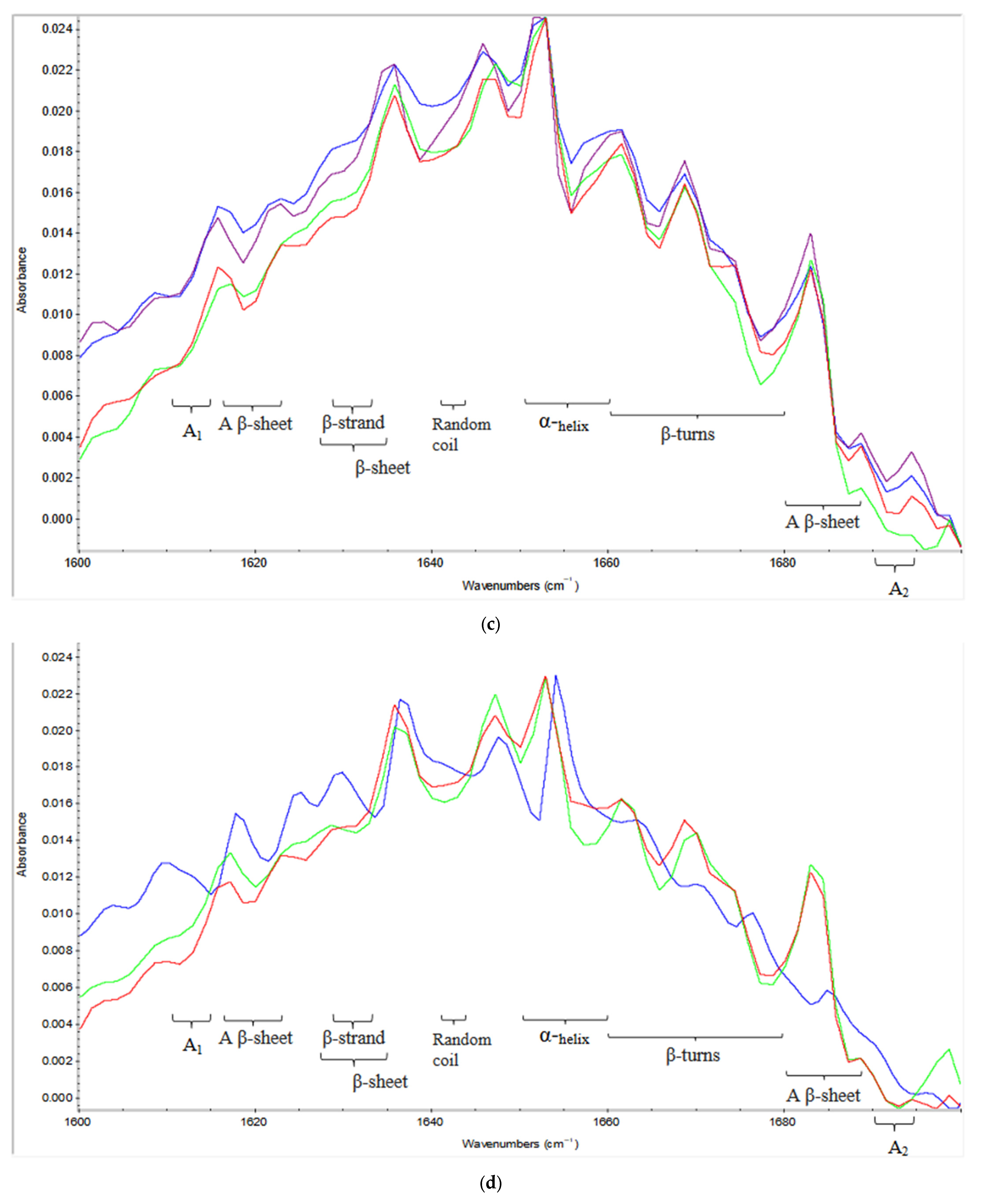
2.2. Effect of Variables on Secondary Structure
2.2.1. Protein Aggregates
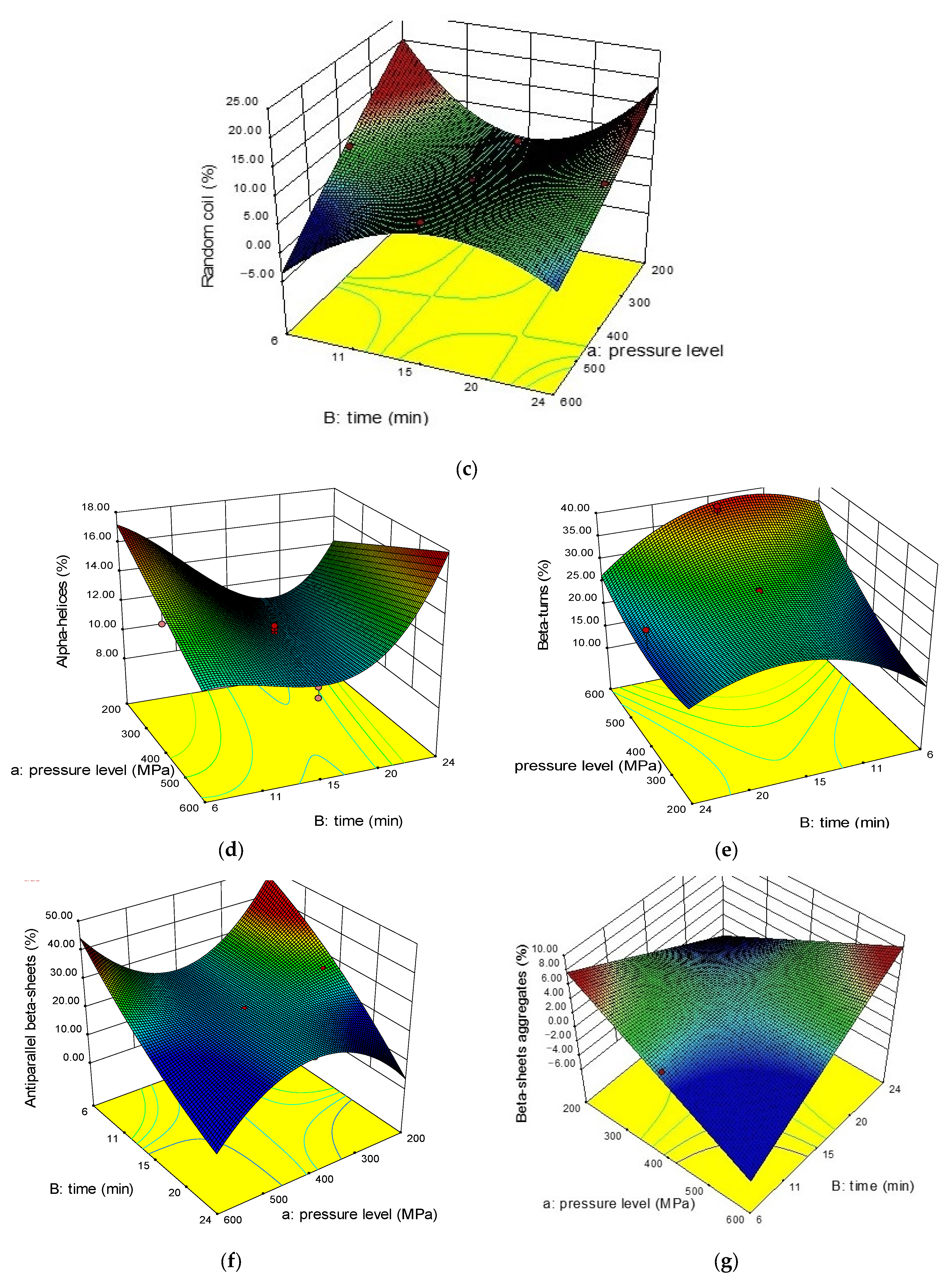
2.2.2. Beta Structures (β-Sheets, β-Turns, Antiparallel β-Sheets)
2.2.3. Alpha (α) Helices and Random Coils
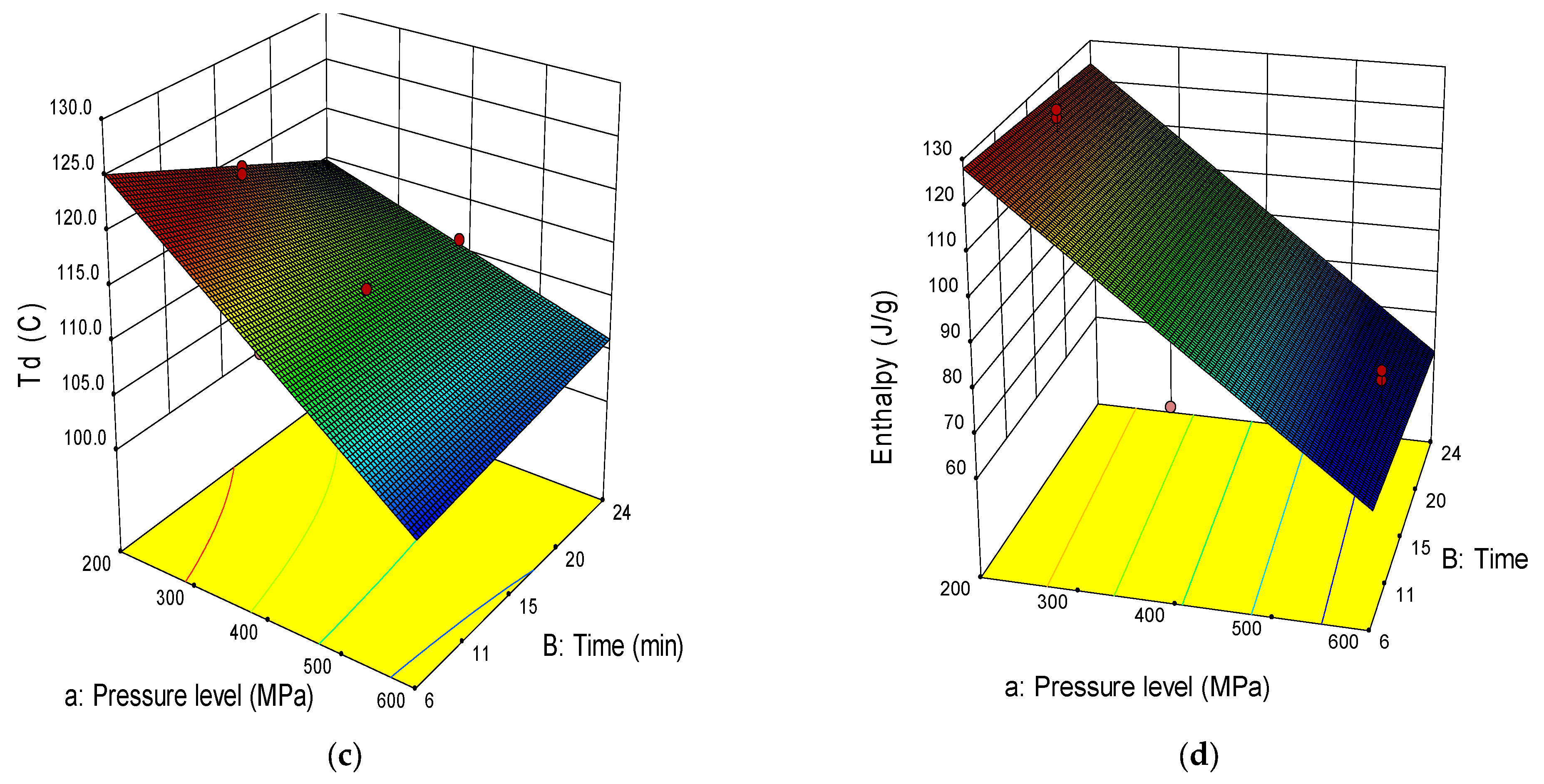
2.3. Thermal Properties
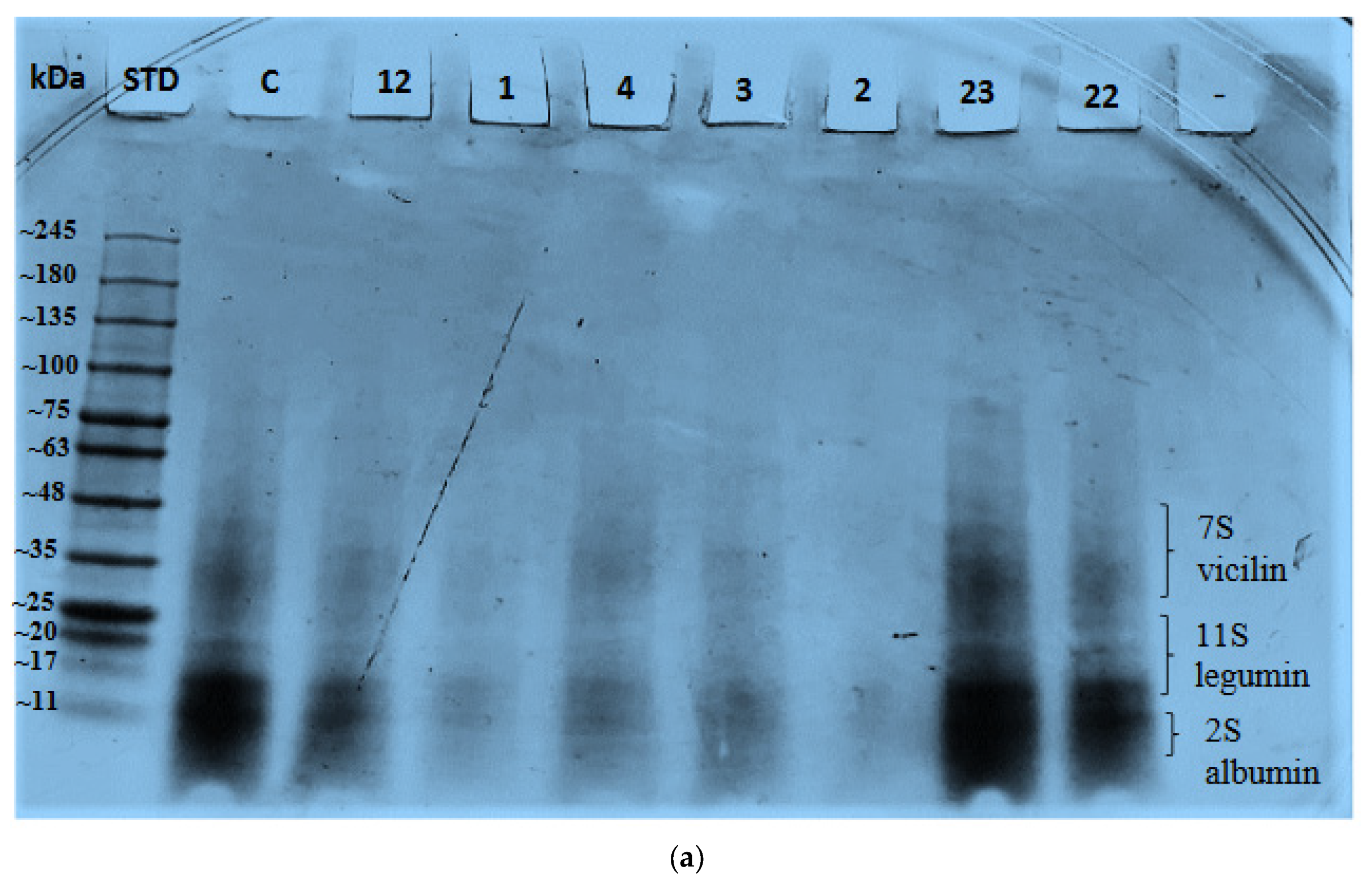
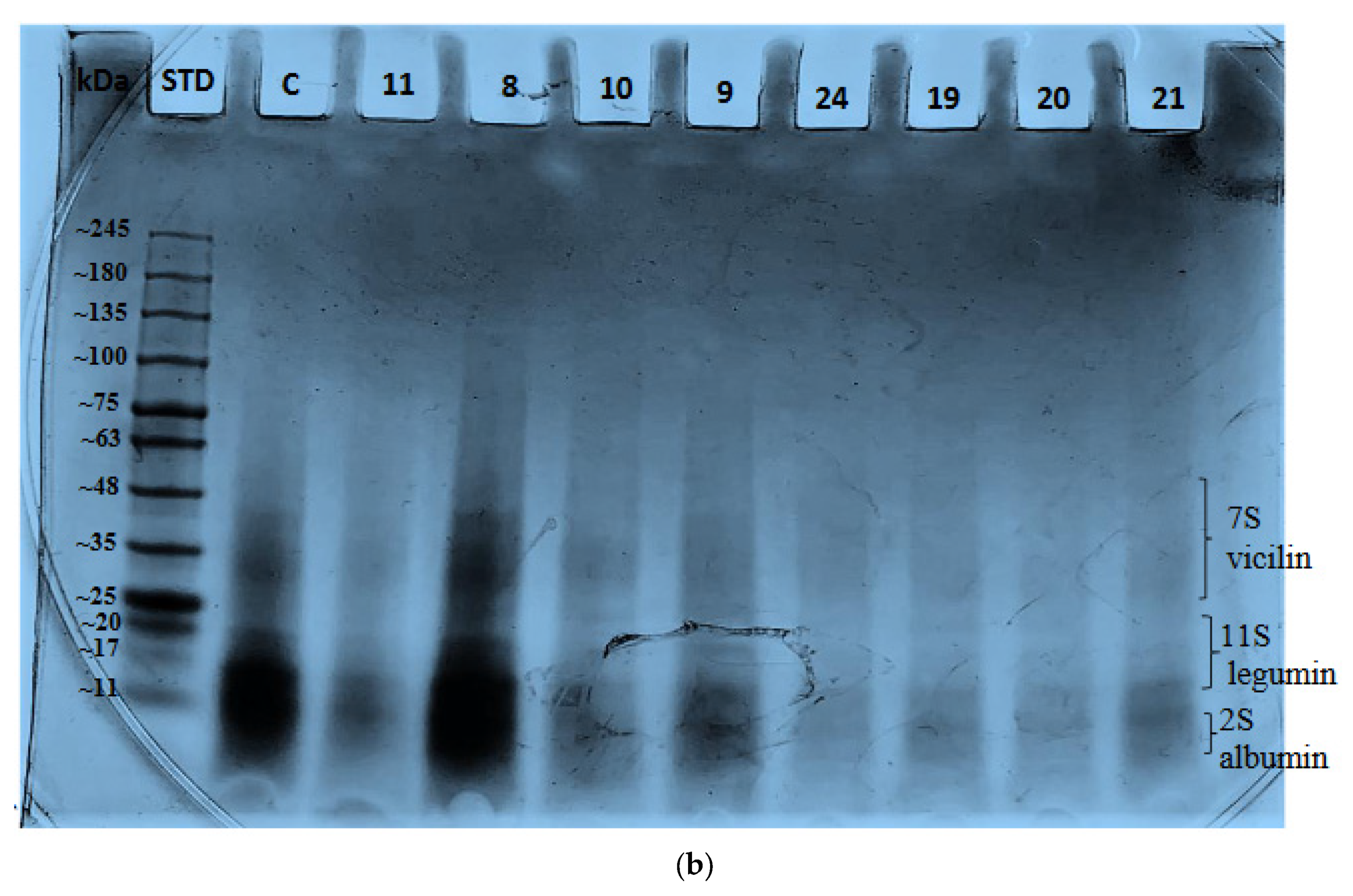
2.4. Effect of Variables on Protein Bands
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. High Pressure (HP) Treatment
3.4. Emulsion Capacity and Stability
3.5. Fourier Transform Infrared (FTIR) Spectroscopy
3.6. Thermal Properties
3.7. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
3.8. Experimental Design
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Almeida Costa, G.E.; Silva Queiroz-Monici, K.; Reis, S.M.P.M.; de Oliveira, A.C. Chemical Composition, Dietary Fibre and Resistant Starch Contents of Raw and Cooked Pea, Common Bean, Chickpea and Lentil Legumes. Food Chem. 2006, 94, 327–330. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Chen, Y.; Kaur, A.; Yu, L. Pulse Proteins: Secondary Structure, Functionality and Applications. J. Food Sci. Technol. 2019, 56, 2787–2798. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Obielodan, M.; Sismour, E.; Arnett, A.; Alzahrani, S.; Zhang, B. Physicochemical, Functional, Thermal and Structural Properties of Isolated Kabuli Chickpea Proteins as Affected by Processing Approaches. Int. J. Food Sci. Technol. 2017, 52, 1147–1154. [Google Scholar] [CrossRef]
- Aguilera, Y.; Esteban, R.M.; Benítez, V.; Molla, E.; Martín-Cabrejas, M.A. Starch, Functional Properties, and Microstructural Characteristics in Chickpea and Lentil as Affected by Thermal Processing. J. Agric. Food Chem. 2009, 57, 10682–10688. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Murillo, C.E.; Veyna-Torres, J.I.; Cavazos-Tamez, L.M.; de la Rosa-Millán, J.; Serna-Saldívar, S.O. Physicochemical Characteristics, ATR-FTIR Molecular Interactions and in Vitro Starch and Protein Digestion of Thermally-Treated Whole Pulse Flours. Food Res. Int. 2018, 105, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Klamczynska, B.; Czuchajowska, Z.; Baik, B.K. Composition, Soaking, Cooking Properties and Thermal Characteristics of Starch of Chickpeas, Wrinkled Peas and Smooth Peas. Int. J. Food Sci. Technol. 2001, 36, 563–572. [Google Scholar] [CrossRef]
- Papathanasiou, M.M.; Reineke, K.; Gogou, E.; Taoukis, P.S.; Knorr, D. Impact of high pressure treatment on the available glucose content of various starch types: A case study on wheat, tapioca, potato, corn, waxy corn and resistant starch (RS3). Innov. Food Sci. Emerg. Technol. 2015, 30, 24–30. [Google Scholar] [CrossRef]
- Lafarga, T.; Villaró, S.; Bobo, G.; Aguiló-Aguayo, I. Optimisation of the PH and Boiling Conditions Needed to Obtain Im-proved Foaming and Emulsifying Properties of Chickpea Aquafaba Using a Response Surface Methodology. Int. J. Gastron. Food Sci. 2019, 18, 100177. [Google Scholar] [CrossRef]
- Shim, Y.Y.; Mustafa, R.; Shen, J.; Ratanapariyanuch, K.; Reaney, M.J.T. Composition and properties of aquafaba: Water re-covered from commercially canned chickpeas. J. Vis. Exp. 2018, 132, e56305. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, R.; He, Y.; Shim, Y.Y.; Reaney, M.J. Aquafaba, Wastewater from Chickpea Canning, Functions as an Egg Replacer in Sponge Cake. Int. J. Food Sci. Technol. 2018, 53, 2247–2255. [Google Scholar] [CrossRef]
- Stantiall, S.E.; Dale, K.J.; Calizo, F.S.; Serventi, L. Application of Pulses Cooking Water as Functional Ingredients: The Foaming and Gelling Abilities. Eur. Food Res. Technol. 2017, 244, 97–104. [Google Scholar] [CrossRef]
- Ahmed, J.; Thomas, L.; Taher, A.; Joseph, A. Impact of High Pressure Treatment on Functional, Rheological, Pasting, and Structural Properties of Lentil Starch Dispersions. Carbohydr. Polym. 2016, 152, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Jin, Y.; Luo, Y.; Zhong, Y.; Yue, J.; Song, X.; Zhao, Y. Impact of continuous or cycle high hydrostatic pressure on the ultrastructure and digestibility of rice starch granules. J. Cereal Sci. 2014, 60, 302–310. [Google Scholar] [CrossRef]
- Famelart, M.-H.; Chapron, L.; Piot, M.; Brulé, G.; Durier, C. High Pressure-Induced Gel Formation of Milk and Whey Con-centrates. J. Food Eng. 1998, 36, 149–164. [Google Scholar] [CrossRef]
- Meurer, M.C.; de Souza, D.; Marczak, L.D.F. Effects of ultrasound on technological properties of chickpea cooking water (aquafaba). J. Food Eng. 2020, 265, 109688. [Google Scholar] [CrossRef]
- Alsalman, F.B.; Tulbek, M.; Nickerson, M.; Ramaswamy, H.S. Evaluation and optimization of functional and antinutritional properties of aquafaba. Legume Sci. 2020, 2, e30. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An Overview of Pickering Emulsions: Sol-id-Particle Materials, Classification, Morphology, and Applications. Front. Pharmacol. 2017, 8, 287. [Google Scholar] [CrossRef]
- Marefati, A.; Wiege, B.; Haase, N.U.; Matos, M.; Rayner, M. Pickering Emulsifiers Based on Hydrophobically Modified Small Granular Starches–Part I: Manufacturing and Physico-Chemical Characterization. Carbohydr. Polym. 2017, 175, 473–483. [Google Scholar] [CrossRef]
- Marefati, A.; Matos, M.; Wiege, B.; Haase, N.U.; Rayner, M. Pickering Emulsifiers Based on Hydrophobically Modified Small Granular Starches Part II–Effects of Modification on Emulsifying Capacity. Carbohydr. Polym. 2018, 201, 416–424. [Google Scholar] [CrossRef]
- Saari, H.; Johansson, D.B.; Knopp, N.; Sjöö, M.; Rayner, M.; Wahlgren, M. Pickering Emulsions Based on CaCl2-Gelatinized Oat Starch. Food Hydrocoll. 2018, 82, 288–295. [Google Scholar] [CrossRef]
- Hebishy, E.; Buffa, M.; Guamis, B.; Blasco-Moreno, A.; Trujillo, A.J. Physical and Oxidative Stability of Whey Protein Oil-in-Water Emulsions Produced by Conventional and Ultra High-Pressure Homogenization: Effects of Pressure and Pro-tein Concentration on Emulsion Characteristics. Innov. Food Sci. Emerg. Technol. 2015, 32, 79–90. [Google Scholar] [CrossRef]
- Villamonte, G.; Jury, V.; De Lamballerie, M. Stabilizing emulsions using high-pressure-treated corn starch. Food Hydrocoll. 2016, 52, 581–589. [Google Scholar] [CrossRef]
- Peng, W.; Kong, X.; Chen, Y.; Zhang, C.; Yang, Y.; Hua, Y. Effects of heat treatment on the emulsifying properties of pea proteins. Food Hydrocoll. 2016, 52, 301–310. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Nucara, A. Relationship between digestibility and secondary structure of raw and thermally treated legume proteins: A Fourier transform infrared (FT-IR) spectroscopic study. Amino Acids 2012, 43, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Mulla, M.Z.; Arfat, Y.A. Particle size, rheological and structural properties of whole wheat flour doughs as treat-ed by high pressure. Int. J. Food Prop. 2017, 20, 1829–1842. [Google Scholar] [CrossRef]
- Ahmed, J.; Varshney, S.K.; Ramaswamy, H.S. Effect of high pressure treatment on thermal and rheological properties of lentil flour slurry. LWT Food Sci. Technol. 2009, 42, 1538–1544. [Google Scholar] [CrossRef]
- Martínez-Velasco, A.; Lobato-Calleros, C.; Hernández-Rodríguez, B.E.; Román-Guerrero, A.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. High intensity ultrasound treatment of faba bean (Vicia faba L.) protein: Effect on surface properties, foaming ability and structural changes. Ultrason. Sonochem. 2018, 44, 97–105. [Google Scholar] [CrossRef]
- Long, G.; Ji, Y.; Pan, H.; Sun, Z.; Li, Y.; Qin, G. Characterization of thermal denaturation structure and morphology of soy glycinin by FTIR and SEM. Int. J. Food Prop. 2015, 18, 763–774. [Google Scholar] [CrossRef]
- Beck, S.M.; Knoerzer, K.; Arcot, J. Effect of low moisture extrusion on a pea protein isolate’s expansion, solubility, molecu-lar weight distribution and secondary structure as determined by Fourier Transform Infrared Spectroscopy (FTIR). J. Food Eng. 2017, 214, 166–174. [Google Scholar] [CrossRef]
- Yu, P. Protein secondary structures (α-helix and β-sheet) at a cellular level and protein fractions in relation to rumen deg-radation behaviours of protein: A new approach. Br. J. Nutr. 2005, 94, 655–665. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Dore, P.; Nucara, A. Application of Fourier transform infrared spectroscopy to legume seed flour analysis. Food Chem. 2008, 108, 361–368. [Google Scholar] [CrossRef]
- Lawrence, M.C.; Izard, T.; Beuchat, M.; Blagrove, R.J.; Colman, P.M. Structure of Phaseolin at 2.2 Å Resolution: Implications for a Common Vicilin/Legumin Structure and the Genetic Engineering of Seed Storage Proteins. J. Mol. Biol. 1994, 238, 748–776. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Ma, C.Y. Effect of high pressure treatment on aggregation and structural properties of soy protein isolate. LWT Food Sci. Technol. 2009, 42, 606–611. [Google Scholar] [CrossRef]
- Alvarez, M.D.; Fuentes, R.; Olivares, M.D.; Canet, W. Effects of high hydrostatic pressure on rheological and thermal prop-erties of chickpea (Cicer arietinum L.) flour slurry and heat-induced paste. Innov. Food Sci. Emerg. Technol. 2014, 21, 12–23. [Google Scholar] [CrossRef]
- Ahmed, J.; Thomas, L.; Mulla, M. Dielectric and microstructural properties of high-pressure treated hummus in the selected packaging materials. LWT Food Sci. Technol. 2019, 118, 108885. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.Z.; Arfat, Y.A.; Kumar, V. Effects of High-Pressure Treatment on Functional, Rheological, Thermal and Structural Properties of Thai Jasmine Rice Flour Dispersion. J. Food Process. Preserv. 2017, 41, e12964. [Google Scholar] [CrossRef]
- Ahmed, J.; Al-Attar, H. Structural properties of high-pressure-treated chestnut flour dispersions. Int. J. Food Prop. 2017, 20, S766–S778. [Google Scholar] [CrossRef]
- Floury, J.; Desrumaux, A.; Legrand, J. Effect of ultra-high-pressure homogenization on structure and on rheological proper-ties of soy protein-stabilized emulsions. J. Food Sci. 2002, 67, 3388–3395. [Google Scholar] [CrossRef]
- Molina, E.; Papadopoulou, A.; Ledward, D.A. Emulsifying properties of high pressure treated soy protein isolate and 7S and 11S globulins. Food Hydrocoll. 2001, 15, 263–269. [Google Scholar] [CrossRef]
- Ahmed, J.; Ramaswamy, H.S.; Ayad, A.; Alli, I.; Alvarez, P. Effect of high-pressure treatment on rheological, thermal and structural changes in Basmati rice flour slurry. J. Cereal Sci. 2007, 46, 148–156. [Google Scholar] [CrossRef]
- Buhl, T.F.; Christensen, C.H.; Hammershøj, M. Aquafaba as an egg white substitute in food foams and emulsions: Protein composition and functional behavior. Food Hydrocoll. 2019, 96, 354–364. [Google Scholar] [CrossRef]
- Peyrano, F.; Speroni, F.; Avanza, M.V. Physicochemical and functional properties of cowpea protein isolates treated with temperature or high hydrostatic pressure. Innov. Food Sci. Emerg. Technol. 2016, 33, 38–46. [Google Scholar] [CrossRef]
- Van der Plancken, I.; Van Loey, A.; Hendrickx, M.E. Kinetic study on the combined effect of high pressure and temperature on the physico-chemical properties of egg white proteins. J. Food Eng. 2007, 78, 206–216. [Google Scholar] [CrossRef]
- Kawai, K.; Fukami, K.; Yamamoto, K. State diagram of potato starch–water mixtures treated with high hydrostatic pressure. Carbohydr. Polym. 2007, 67, 530–535. [Google Scholar] [CrossRef]
- Speroni, F.; Añón, M.C.; De Lamballerie, M. Effects of calcium and high pressure on soybean proteins: A calorimetric study. Food Res. Int. 2010, 43, 1347–1355. [Google Scholar] [CrossRef]
- Condés, M.C.; Speroni, F.; Mauri, A.; Añón, M.C. Physicochemical and structural properties of amaranth protein isolates treated with high pressure. Innov. Food Sci. Emerg. Technol. 2012, 14, 11–17. [Google Scholar] [CrossRef]
- Meng, G.T.; Ching, K.M.; Ma, C.Y. Thermal aggregation of globulin from an indigenous Chinese legume, Phaseolus angularis (red bean). Food Chem. 2002, 79, 93–103. [Google Scholar] [CrossRef]
- Cappa, C.; Lucisano, M.; Barbosa-Cánovas, G.V.; Mariotti, M. Physical and structural changes induced by high pressure on corn starch, rice flour and waxy rice flour. Food Res. Int. 2016, 85, 95–103. [Google Scholar] [CrossRef]
- Al-Ruwaih, N.; Ahmed, J.; Mulla, M.F.; Arfat, Y.A. High-pressure assisted enzymatic proteolysis of kidney beans protein isolates and characterization of hydrolysates by functional, structural, rheological and antioxidant properties. LWT Food Sci. Technol. 2019, 100, 231–236. [Google Scholar] [CrossRef]
- Ribeiro, I.C.; Leclercq, C.C.; Simões, N.; Toureiro, A.; Duarte, I.; Freire, J.B.; Chaves, M.M.; Renaut, J.; Pinheiro, C. Identifica-tion of chickpea seed proteins resistant to simulated in vitro human digestion. J. Proteom. 2017, 169, 143–152. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Alhamad, M.N.; Ereifej, K.; Gammoh, S.; Kubow, S.; Tawalbeh, D. Preparation of mayonnaise from extracted plant protein isolates of chickpea, broad bean and lupin flour: Chemical, physiochemical, nutritional and therapeutic properties. J. Food Sci. Technol. 2017, 54, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vioque, R.; Clemente, A.; Vioque, J.; Bautista, J.; Millán, F. Protein isolates from chickpea (Cicer arietinum L.): Chemical composition, functional properties and protein characterization. Food Chem. 1999, 64, 237–243. [Google Scholar] [CrossRef]
- Ahmed, J.; Al-Ruwaih, N.; Mulla, M.; Rahman, M.H. Effect of high pressure treatment on functional, rheological and struc-tural properties of kidney bean protein isolate. LWT Food Sci. Technol. 2018, 91, 191–197. [Google Scholar] [CrossRef]
- Rasekh, J.; Metz, A. Acid precipitated fish protein isolate exhibits good functional properties. Food Prod. Dev. 1973, 7, 18–24. [Google Scholar]
- Cepeda, E.; Villaran, M.C.; Aranguiz, N. Functional properties of faba bean (Vicia faba) protein flour dried by spray drying and freeze drying. J. Food Eng. 1998, 36, 303–310. [Google Scholar] [CrossRef]
- Beck, S.M.; Knoerzer, K.; Sellahewa, J.; Emin, M.A.; Arcot, J. Effect of different heat-treatment times and applied shear on secondary structure, molecular weight distribution, solubility and rheological properties of pea protein isolate as investi-gated by capillary rheometry. J. Food Eng. 2017, 208, 66–76. [Google Scholar] [CrossRef]
- Gorinstein, S.; Zemser, M.; Paredes-López, O. Structural Stability of Globulins. J. Agric. Food Chem. 1996, 44, 100–105. [Google Scholar] [CrossRef]
| Run | A: Pressure Level | B: Time | C: Concentration | Emulsion Capacity | Emulsion Stability | Td | Enthalpy |
|---|---|---|---|---|---|---|---|
| MPa | Min | % | mL | mL | °C | J/g | |
| 1 | 300 | 10 | 15 | 4.83 ± 0.24 | 1.80 ± 0.25 | 118.0 ± 3.0 | 125.4 ± 2.7 |
| 2 | 300 | 20 | 25 | 5.33 ± 0.12 | 1.30 ± 0.24 | 114.9 ± 3.4 | 125.6 ±0.6 |
| 3 | 300 | 10 | 25 | 5.27 ± 0.21 | 1.50 ± 0.21 | 120.5 ± 0.4 | 119.6 ± 2.9 |
| 4 | 300 | 20 | 15 | 4.73 ± 0.21 | 0.90 ± 0.12 | 114.9 ± 2.3 | 111.9 ± 3.1 |
| 5 | 400 | 15 | 20 | 4.90 ± 0.08 | 2.00 ± 0.21 | 112.1 ± 4.6 | 87.7 ± 1.1 |
| 6 | 400 | 15 | 20 | 5.00 ± 0.07 | 2.30 ± 0.21 | 110.5 ± 4.5 | 87.1 ± 1.3 |
| 7 | 400 | 15 | 20 | 4.80 ± 0.08 | 1.80 ± 0.20 | 111.9 ± 4.7 | 88.0 ± 1.3 |
| 8 | 500 | 20 | 15 | 5.10 ± 0.08 | 2.43 ± 0.05 | 114.0 ± 0.7 | 80.4 ± 4.8 |
| 9 | 500 | 20 | 25 | 5.10 ± 0.08 | 2.40 ± 0.43 | 112.8 ± 4.3 | 83.8 ± 2.2 |
| 10 | 500 | 10 | 25 | 5.00 ± 0.08 | 2.53 ± 0.21 | 113.1 ± 2.0 | 86.4 ± 2.0 |
| 11 | 500 | 10 | 15 | 4.83 ± 0.24 | 2.67 ± 0.24 | 115.2 ± 1.1 | 87.9 ± 0.2 |
| 12 | 227 | 15 | 20 | 4.70 ± 0.29 | 2.10 ± 0.46 | 119.9 ± 0.4 | 127.0 ± 1.8 |
| 13 | 227 | 15 | 20 | 5.00 ± 0.30 | 2.30 ± 0.45 | 120.6 ± 0.3 | 125.2 ± 1.7 |
| 14 | 400 | 15 | 20 | 4.90 ± 0.08 | 2.30 ± 0.29 | 110.8 ± 4.0 | 88.4 ± 0.9 |
| 15 | 400 | 15 | 20 | 4.80 ± 0.07 | 2.20 ± 0.29 | 108.2 ± 4.3 | 87.5 ± 1.1 |
| 16 | 400 | 15 | 20 | 5.00 ± 0.08 | 2.00 ± 0.30 | 112.9 ± 4.6 | 88.9 ± 1.0 |
| 17 | 400 | 15 | 20 | 4.90 ± 0.08 | 1.60 ± 0.29 | 109.7 ± 4.4 | 87.1 ± 1.1 |
| 18 | 400 | 15 | 20 | 4.80 ± 0.09 | 2.10 ± 0.31 | 110.3 ± 4.3 | 89.4 ± 0.9 |
| 19 | 400 | 15 | 20 | 5.00 ± 0.07 | 2.20 ± 0.28 | 108.3 ± 4.5 | 88.3 ± 1.0 |
| 20 | 400 | 24 | 20 | 4.60 ± 0.08 | 1.40 ± 0.12 | 111.4 ± 2.4 | 82.4 ± 3.1 |
| 21 | 400 | 15 | 29 | 4.83 ± 0.12 | 2.00 ± 0.12 | 112.0 ± 0.4 | 91.7 ± 3.4 |
| 22 | 400 | 06 | 20 | 4.93 ± 0.09 | 1.90 ± 0.43 | 113.8 ± 1.9 | 80.6 ± 3.0 |
| 23 | 400 | 15 | 11 | 4.80 ± 0.08 | 1.10 ± 0.12 | 112.5 ± 4.8 | 86.0 ± 0.6 |
| 24 | 573 | 15 | 20 | 4.83 ± 0.24 | 1.20 ± 0.08 | 105.0 ± 3.5 | 71.8 ± 2.6 |
| 25 | 573 | 15 | 20 | 5.00 ± 0.23 | 1.30 ± 0.09 | 103.5 ± 3.6 | 74.1 ± 2.4 |
| Control | -- | -- | 20 | 5.00 ± 0.06 | 0.60 ± 0.17 | 115.7 ± 0.3 | 145.2 ± 4.2 |
| Run | A: Pressure Level | B: Time | C: Concentration | Protein Aggregates | Beta-Sheets | Random Coil | Alpha-Helices | Beta- Turns | Antiparallel Beta- Sheets | Beta-Sheets Aggregates |
|---|---|---|---|---|---|---|---|---|---|---|
| MPa | min | % | % | % | % | % | % | % | % | |
| 1 | 300 | 10 | 15 | 5.22 ± 0.22 | 2.73 ± 0.03 | 2.73 ± 0.20 | 16.52 ± 073 | 26.21 ± 2.10 | 39.20 ± 0.30 | 5.71 ± 1.54 |
| 2 | 300 | 20 | 25 | 8.47 ± 0.20 | 15.71 ± 0.08 | 9.05 ± 0.25 | 14.12 ± 0.79 | 22.09 ± 1.83 | 26.07 ± 0.43 | 4.49 ± 1.55 |
| 3 | 300 | 10 | 25 | 6.22 ± 0.21 | 16.22 ± 0.12 | 14.40 ± 0.32 | 14.26 ± 0.85 | 22.08 ± 1.86 | 19.96 ± 0.95 | 5.55 ± 0.92 |
| 4 | 300 | 20 | 15 | 6.62 ± 0.09 | 26.65 ± 0.25 | 7.47 ± 0.75 | 11.34 ± 0.90 | 23.25 ± 1.32 | 20.89 ± 0.99 | 3.78 ± 0.95 |
| 5 | 400 | 15 | 20 | 5.93 ± 0.93 | 18.62 ± 0.84 | 6.99 ± 0.94 | 11.63 ± 0.82 | 28.77 ± 0.72 | 23.80 ± 1.95 | 1.46 ± 0.09 |
| 6 | 400 | 15 | 20 | 6.00 ± 0.95 | 18.87 ± 0.89 | 7.00 ± 0.90 | 11.85 ± 0.80 | 28.68 ± 0.70 | 23.87 ± 1.90 | 1.52 ± 0.10 |
| 7 | 400 | 15 | 20 | 5.87 ± 0.90 | 18.94 ± 0.90 | 6.89 ± 0.91 | 11.70 ± 0.79 | 28.96 ± 0.74 | 23.97 ± 1.83 | 1.46 ± 0.13 |
| 8 | 500 | 20 | 15 | 7.55 ± 0.19 | 15.26 ± 0.84 | 4.94 ± 1.94 | 13.68 ± 0.51 | 27.14 ± 0.80 | 21.50 ± 0.93 | 6.95 ± 0.92 |
| 9 | 500 | 20 | 25 | 8.52 ± 0.29 | 16.35 ± 0.80 | 10.17 ± 1.38 | 14.04 ± 0.48 | 22.74 ± 0.95 | 20.54 ± 0.95 | 6.41 ± 0.32 |
| 10 | 500 | 10 | 25 | 4.62 ± 0.39 | 20.75 ± 0.87 | 3.40 ± 1.29 | 11.10 ± 0.72 | 31.28 ± 0.52 | 28.85 ± 0.23 | 0.00 ± 0.64 |
| 11 | 500 | 10 | 15 | 9.31 ± 0.91 | 11.43 ± 0.90 | 7.37 ± 1.82 | 16.93 ± 0.57 | 29.55 ± 0.92 | 23.03 ± 0.21 | 2.37 ± 0.52 |
| 12 | 227 | 15 | 20 | 8.02 ± 1.24 | 18.45 ± 1.45 | 5.62 ± 0.64 | 10.81 ± 1.92 | 24.56 ± 0.92 | 28.57 ± 0.29 | 0.79 ± 0.14 |
| 13 | 227 | 15 | 20 | 8.00 ± 1.29 | 18.75 ± 1.50 | 5.97 ± 0.61 | 11.02 ± 1.90 | 24.85 ± 0.85 | 28.21 ± 0.28 | 0.87 ± 0.19 |
| 14 | 400 | 15 | 20 | 5.93 ± 1.03 | 18.62 ± 0.90 | 6.99 ± 0.90 | 11.63 ± 0.89 | 28.77 ± 0.70 | 23.80 ± 1.92 | 1.46 ± 0.11 |
| 15 | 400 | 15 | 20 | 6.00 ± 0.92 | 18.53 ± 0.85 | 7.00 ± 0.91 | 11.69 ± 0.82 | 28.97 ± 0.77 | 23.98 ± 1.94 | 1.50 ± 0.09 |
| 16 | 400 | 15 | 20 | 6.10 ± 0.94 | 17.93 ± 0.91 | 6.90 ± 0.92 | 11.36 ± 0.85 | 28.64 ± 0.93 | 23.85 ± 1.91 | 1.48 ± 0.12 |
| 17 | 400 | 15 | 20 | 5.93 ± 0.96 | 18.09 ± 0.87 | 6.73 ± 0.90 | 11.63 ± 0.80 | 28.76 ± 0.84 | 23.95 ± 1.90 | 1.46 ± 0.09 |
| 18 | 400 | 15 | 20 | 5.87 ± 0.90 | 18.69 ± 0.88 | 7.12 ± 0.92 | 11.85 ± 0.89 | 28.32 ± 0.72 | 24.00 ± 1.89 | 1.45 ± 0.12 |
| 19 | 400 | 15 | 20 | 5.90 ± 0.92 | 18.42 ± 0.90 | 7.04 ± 0.92 | 11.73 ± 0.90 | 29.00 ±0.73 | 23.99 ± 1.90 | 1.49 ± 0.12 |
| 20 | 400 | 24 | 20 | 8.13 ± 1.43 | 13.47 ± 1.68 | 10.66 ± 1.39 | 14.19 ± 1.83 | 25.14 ± 1.29 | 23.42 ± 1.53 | 1.08 ± 0.81 |
| 21 | 400 | 15 | 29 | 6.81 ± 1.49 | 14.17 ±1.49 | 6.60 ± 1.92 | 12.89 ± 1.72 | 23.26 ± 1.82 | 25.46 ± 1.82 | 1.57 ± 0.98 |
| 22 | 400 | 06 | 20 | 8.79 ± 1.40 | 14.71 ± 0.74 | 9.39 ± 0.91 | 13.05 ± 1.74 | 19.09 ± 1.56 | 22.82 ± 1.73 | 1.26 ± 1.43 |
| 23 | 400 | 15 | 11 | 7.01 ± 1.00 | 14.29 ± 0.86 | 7.92 ± 1.47 | 9.51 ± 0.98 | 29.09 ± 0.98 | 22.95 ± 1.92 | 0.00 ± 1.92 |
| 24 | 573 | 15 | 20 | 5.41 ± 0.83 | 11.32 ± 1.23 | 8.14 ± 0.92 | 10.28 ± 1.02 | 38.78 ± 0.12 | 20.95 ± 0.82 | 0.00 ± 0.91 |
| 25 | 573 | 15 | 20 | 5.60 ± 0.82 | 11.45 ±1.20 | 8.87 ± 0.90 | 9.57 ± 1.00 | 37.94 ± 0.10 | 21.23 ± 0.80 | 0.00 ± 0.82 |
| Control | -- | -- | 20 | 14.04 ± 0.42 | 21.69 ± 0.28 | 9.93 ± 0.27 | 21.23 ± 0.43 | 7.99 ± 0.4 | 6.68 ± 0.32 | 0.74 ± 0.69 |
| Response | Model | p-Value (Whole-Plot) * | p-Value (Sub-Plot) ** | R2 | Std. Dev. |
|---|---|---|---|---|---|
| Emulsion capacity | Reduced Cubic | 0.8482 | 0.0146 | 0.49 | 0.1669 |
| Emulsion stability | Reduced Cubic | 0.0214 | 0.0145 | 0.84 | 0.4857 |
| Protein aggregates | Reduced Cubic | 0.0004 | <0.0001 | 0.91 | 1.2701 |
| Beta-sheets | Cubic | 0.0116 | <0.0001 | 1.00 | 4.3758 |
| Random coil | Cubic | 0.0361 | <0.0001 | 0.99 | 2.3127 |
| Alpha-helices | Reduced Cubic | 0.5848 | <0.0001 | 0.99 | 1.8828 |
| Beta-turns | Quadratic | 0.0020 | 0.0177 | 0.85 | 4.4858 |
| Antiparallel beta-sheets | Cubic | 0.0081 | <0.0001 | 1.00 | 3.8812 |
| Beta-sheets aggregates | Reduced Quadratic | 0.6486 | <0.0001 | 0.85 | 2.0609 |
| Temperature of Denaturation (Td) | 2FI | 0.0069 | 0.0413 | 0.91 | 4.2 |
| Enthalpy (∆H) | Linear | 0.0014 | 0.2259 | 0.95 | 17.1 |
| Response | Equation |
|---|---|
| Emulsion capacity | +4.91 + 0.0074.47 × a + 0.22 × C − 0.11 × aC + 0.015 × C2 − 0.070 × C3 |
| Emulsion stability | +1.99 + 0.99 × a − 0.17 × B − 0.14 × C + −0.076 × a2 + −0.068 × C2 − 0.42 × a3 + 0.13 × C3 |
| Protein aggregates | +5.95 + 1.01 × a + 1.18 × B − 0.087 × C − 0.82 × aC + 0.81 × BC + 0.24 × a2 + 0.77 × B2 + 0.26 × C2 − 0.58 × a3 − 0.46 × B3 |
| Beta-sheets | +18.52 − 2.08 × a − 0.36× B − 0.036 × C − 3.00 × aB + 0.98 × aC − 4.08 × BC − 1.11 × a2 − 1.21 × B2 − 1.16 × C2 + 2.03× aBC + 3.21 × a2B + 1.66 × a2C + 2.39 × aB2 |
| Random coil | +6.96 + 0.78 × a + 0.37 × B − 0.38 × C + 0.62 × aB − 1.50 × aC − 0.11 × BC + 0.021× a2 + 0.87 × B2 − 0.058 × C2 + 2.41× aBC + 0.098 × a2B + 2.19 × a2C + −1.75 × aB2 |
| Alpha-helices | +11.31 − 0.23 × a − 1.22 × B − 1.42 × C + 0.62 × aB − 0.75 × aC + 1.40 × BC + 1.23 × B2 + 0.42 × C2 + 0.52 × B3 + 0.80 × C3 |
| Beta-turns | +28.76 + 3.23 × a − 0.24× B − 1.29 × C − 1.00 × aB + 0.33 × aC − 0.39 × BC + 0.81 × a2 − 2.44 × B2 − 1.09 × C2 |
| Antiparallel beta-sheets | +23.91 − 2.11× a + 0.17 × B + 0.73 × C + 0.29× aB + 2.37 × aC + 2.21 × BC + 0.33 × a2 − 0.047 × B2 + 0.32 × C2 − 3.90 × aBC − 2.93 × a2B − 1.88× a2C + 0.58 × aB2 |
| Beta-sheets aggregates | +1.90 + −0.30 × a + 0.55 × B + 1.75 × aB |
| Temperature of denaturation (Td) | +112.43 − 3.85 × a − 1.02 × B − 0.13 × C + 0.90 × aB − 0.73 × aC − 0.19 × BC |
| Enthalpy (∆H) | +94.18 − 16.03 × a − 1.03 × B + 1.41 × C |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsalman, F.B.; Ramaswamy, H.S. Evaluation of Changes in Protein Quality of High-Pressure Treated Aqueous Aquafaba. Molecules 2021, 26, 234. https://doi.org/10.3390/molecules26010234
Alsalman FB, Ramaswamy HS. Evaluation of Changes in Protein Quality of High-Pressure Treated Aqueous Aquafaba. Molecules. 2021; 26(1):234. https://doi.org/10.3390/molecules26010234
Chicago/Turabian StyleAlsalman, Fatemah B., and Hosahalli S. Ramaswamy. 2021. "Evaluation of Changes in Protein Quality of High-Pressure Treated Aqueous Aquafaba" Molecules 26, no. 1: 234. https://doi.org/10.3390/molecules26010234
APA StyleAlsalman, F. B., & Ramaswamy, H. S. (2021). Evaluation of Changes in Protein Quality of High-Pressure Treated Aqueous Aquafaba. Molecules, 26(1), 234. https://doi.org/10.3390/molecules26010234







