Concept Design, Development and Preliminary Physical and Chemical Characterization of Tamoxifen-Guided-Mesoporous Silica Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
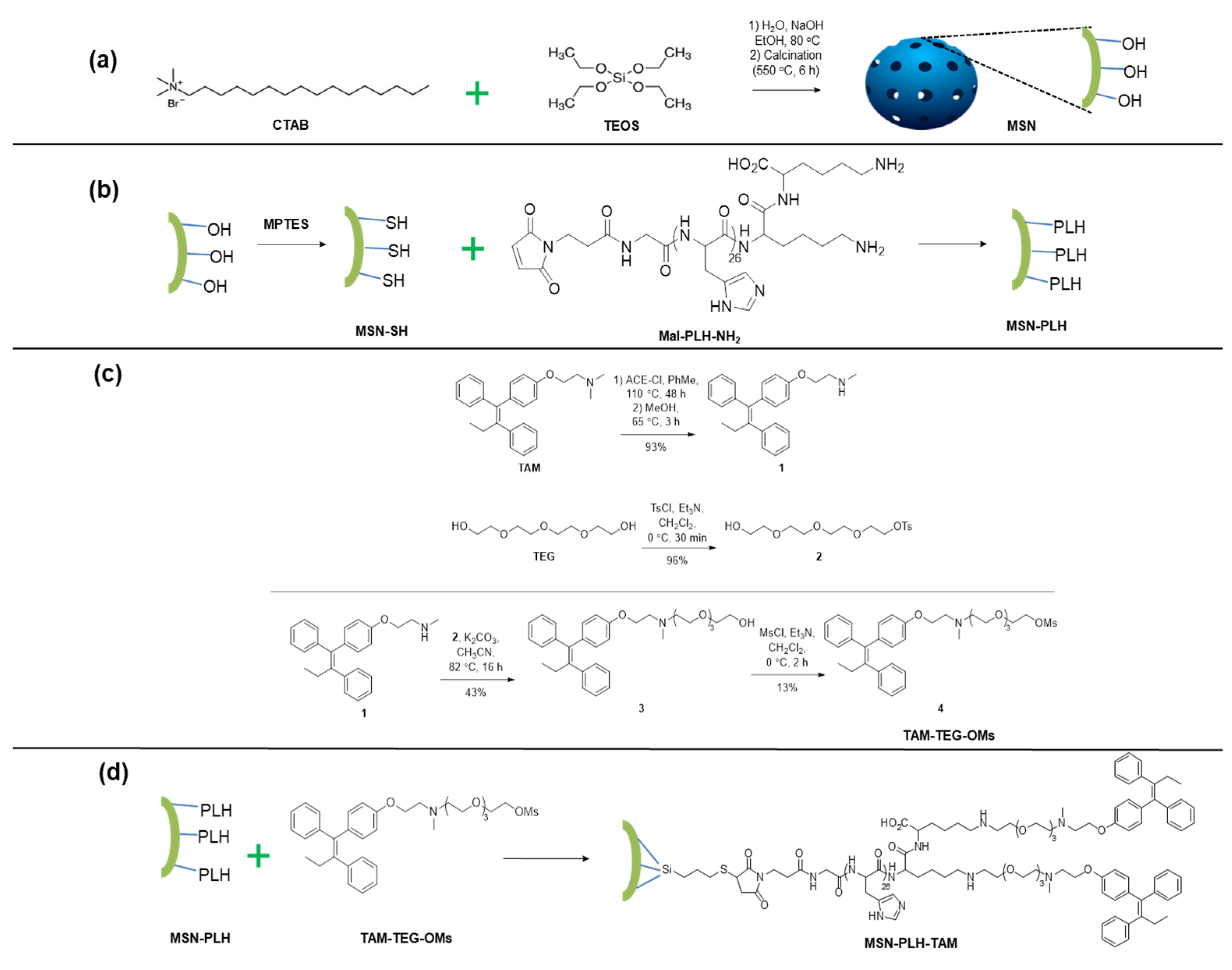
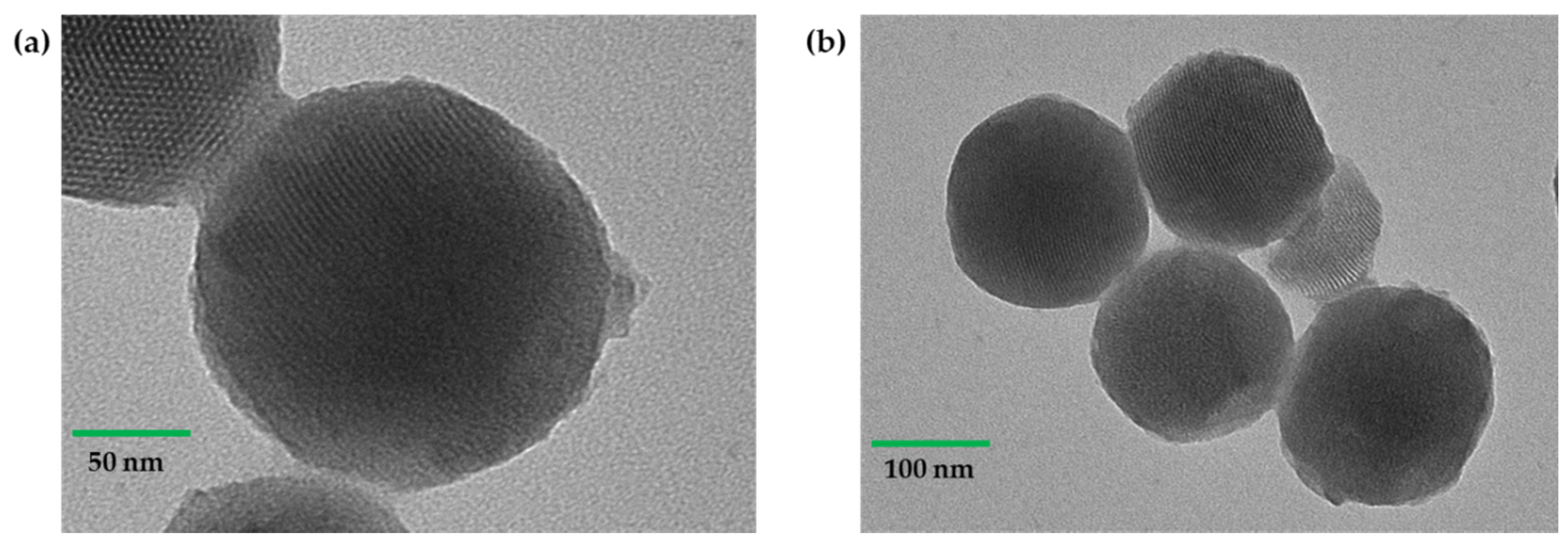
2.1. Preparation of MSN System
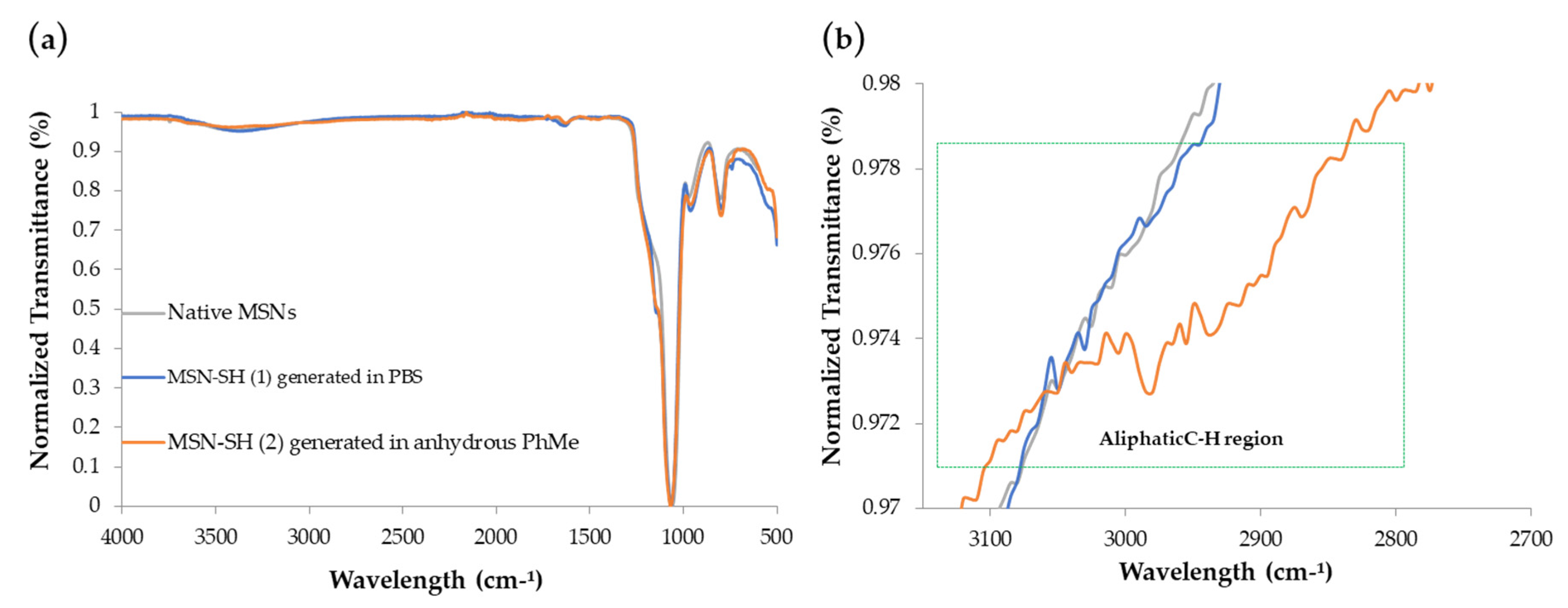
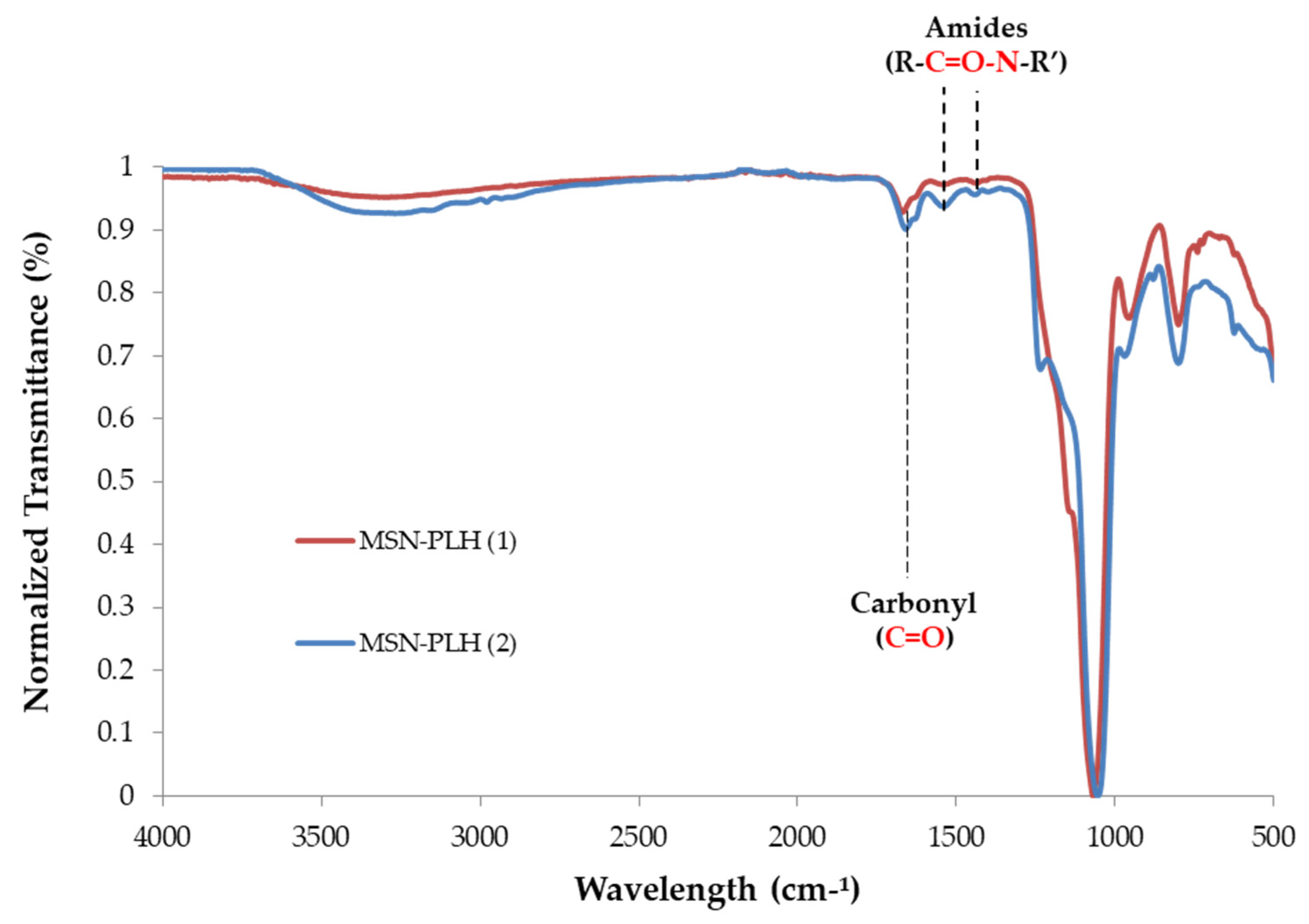
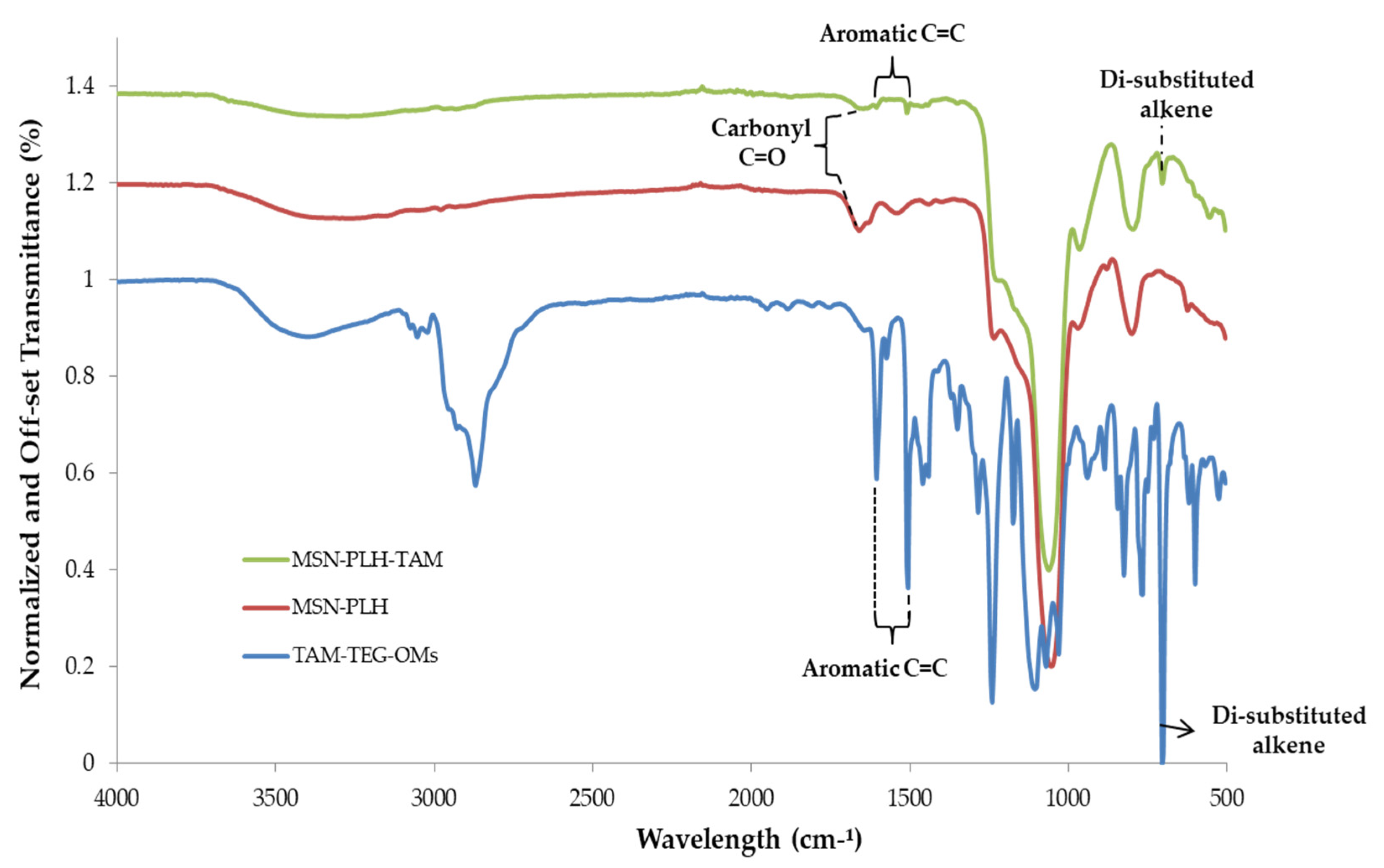
2.2. Synthesis of MSN-PLH
2.3. Synthesis of TAM-TEG-OMs
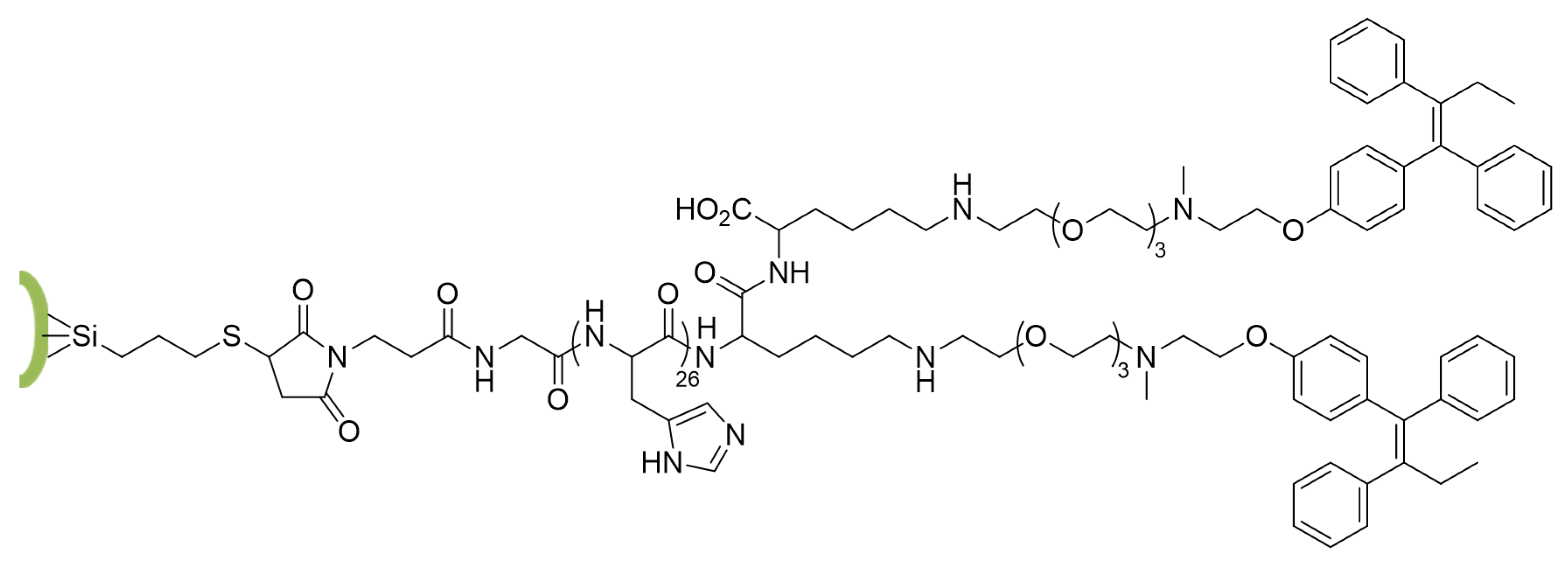
2.4. Synthesis of MSN-PLH-TAM
3. Materials and Methods
3.1. General Methods
3.2. Synthesis of Native MSNs
3.3. Synthesis of MSN-PLH
3.4. Synthesis of (Z)-2-(4-(1,2-Diphenylbut-1-en-1-yl)Phenoxy)-N-Methylethan-1-Amine (NMDT) 1
3.5. Synthesis of 2-(2-(2-(2-Hydroxyethoxy)Ethoxy)Ethoxy)Ethyl 4-Methylbenzenesulfonate 2
3.6. Synthesis of (Z)-1-(4-(1,2-Diphenylbut-1-en-1-yl)Phenoxy)-3-Methyl-6,9,12-Trioxa-3-Azatetradecan-14-ol 3
3.7. Synthesis of (Z)-1-(4-(1,2-Diphenylbut-1-en-1-yl)Phenoxy)-3-Methyl-6,9,12-Trioxa-3-Azatetradecan-14-yl Methanesulfonate 4
3.8. Synthesis of MSN-PLH-TAM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tyczynski, J.; Plesko, I.; Aareleid, T.; Primic-Zakelj, M.; Dalmas, M.; Kurtinaitis, J.; Stengrevics, A.; Parkin, D.M. Breast cancer mortality patterns and time trends in 10 new EU member states: Mortality declining in young women, but still increasing in the elderly. Int. J. Cancer 2004, 112, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer in Australia Statistics. Available online: www.canceraustralia.gov.au/affected-cancer/cancer-types/breast-cancer/statistics (accessed on 1 October 2020).
- Sugerman, D.T. Chemotherapy. JAMA 2013, 310, 218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tierney, A.J.; Leonard, R.C.; Taylor, J.; Closs, S.J.; Chetty, U.; Rodger, A. Side effects expected and experienced by women receiving chemotherapy for breast cancer. BMJ 1991, 302, 272. [Google Scholar] [CrossRef][Green Version]
- Breast cancer targeted therapy: Successes and challenges. Lancet 2017, 389, 2350. [CrossRef]
- Higgins, M.J.; Baselga, J. Targeted therapies for breast cancer. J. Clin. Investig. 2011, 121, 3797–3803. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in nanomedicine: Approved and investigational nanodrugs. Phram. Ther. 2017, 42, 742–755. [Google Scholar]
- Sharma, A.; Jain, N.; Sareen, R. Nanocarriers for Diagnosis and Targeting of Breast Cancer. BioMed Res. Int. 2013, 2013, 960821. [Google Scholar] [CrossRef]
- Dai, Y.; Xu, C.; Sun, X.; Chen, X. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem. Soc. Rev. 2017, 46, 3830–3852. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef]
- Chircov, C.; Spoială, A.; Păun, C.; Crăciun, L.; Ficai, D.; Ficai, A.; Andronescu, E.; Turculeƫ, Ș.C. Mesoporous Silica Platforms with Potential Applications in Release and Adsorption of Active Agents. Molecules 2020, 25, 3814. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Mamaeva, V.; Sahlgren, C.; Lindén, M. Nanoparticles in targeted cancer therapy: Mesoporous silica nanoparticles entering preclinical development stage. Nanomedicine 2012, 7, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Loryuenyong, V.; Muanghom, T.; Apinyanukul, T.; Rutthongjan, P. Synthesis of templated mesoporous silica nanoparticles under base catalysis. Adv. Appl. Ceram. 2011, 110, 335–339. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, X.; Zhang, G.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.-Y. Interaction of Mesoporous Silica Nanoparticles with Human Red Blood Cell Membranes: Size and Surface Effects. ACS Nano 2011, 5, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Wu, C.-W.; Vivero-Escoto, J.L.; Lin, V.S.-Y. Mesoporous Silica Nanoparticles for Reducing Hemolytic Activity towards Mammalian Red Blood Cells. Small 2009, 5, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Watermann, A.; Brieger, J. Mesoporous Silica Nanoparticles as Drug Delivery Vehicles in Cancer. Nanomaterials 2017, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Durfee, P.N.; Lin, Y.-S.; Dunphy, D.R.; Muñiz, A.J.; Butler, K.S.; Humphrey, K.R.; Lokke, A.J.; Agola, J.O.; Chou, S.S.; Chen, I.-M.; et al. Mesoporous Silica Nanoparticle-Supported Lipid Bilayers (Protocells) for Active Targeting and Delivery to Individual Leukemia Cells. ACS Nano 2016, 10, 8325–8345. [Google Scholar] [CrossRef]
- Babaei, M.; Abnous, K.; Taghdisi, S.M.; Farzad, S.A.; Peivandi, M.T.; Ramezani, M.; Alibolandi, M. Synthesis of theranostic epithelial cell adhesion molecule targeted mesoporous silica nanoparticle with gold gatekeeper for hepatocellular carcinoma. Nanomedicine 2017, 12, 1261–1279. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, D.; Yin, X.; Jin, X.; Zhang, X.; Zheng, S.; Wang, C.; Liu, Y. Intracellular pH-responsive and rituximab-conjugated mesoporous silica nanoparticles for targeted drug delivery to lymphoma B cells. J. Exp. Clin. Cancer Res. 2017, 36, 24. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, W.; Wang, B.; Gao, Y.; Song, Z.; Zheng, Q. Ligand-based targeted therapy: A novel strategy for hepatocellular carcinoma. Int. J. Nanomed. 2016, 11, 5645–5669. [Google Scholar] [CrossRef]
- Paterni, I.; Bertini, S.; Granchi, C.; Macchia, M.; Minutolo, F. Estrogen receptor ligands: A patent review update. Expert Opin. Ther. Patents 2013, 23, 1247–1271. [Google Scholar] [CrossRef]
- Yip, C.-H.; Rhodes, A. Estrogen and progesterone receptors in breast cancer. Future Oncol. 2014, 10, 2293–2301. [Google Scholar] [CrossRef] [PubMed]
- Gadag, S.; Sinha, S.; Nayak, Y.; Garg, S.; Nayak, U.Y. Combination Therapy and Nanoparticulate Systems: Smart Approaches for the Effective Treatment of Breast Cancer. Pharmaceutics 2020, 12, 524. [Google Scholar] [CrossRef] [PubMed]
- Elledge, R.M.; Green, S.; Pugh, R.; Allred, D.C.; Clark, G.M.; Hill, J.; Ravdin, P.; Martino, S.; Osborne, C.K. Estrogen receptor (ER) and progesterone receptor (PgR), by ligand-binding assay compared with ER, PgR and pS2, by immuno-histochemistry in predicting response to tamoxifen in metastatic breast cancer: A Southwest Oncology Group Study. Int. J. Cancer 2000, 89, 111–117. [Google Scholar] [CrossRef]
- Wiseman, H. Tamoxifen: Molecular basis of use in cancer treatment and prevention. Gen. Pharmacol. 1996, 5, 923. [Google Scholar]
- Day, C.M.; Hickey, S.M.; Song, Y.; Plush, S.E.; Garg, S. Novel Tamoxifen Nanoformulations for Improving Breast Cancer Treatment: Old Wine in New Bottles. Molecules 2020, 25, 1182. [Google Scholar] [CrossRef]
- Jain, A.S.; Goel, P.N.; Shah, S.M.; Dhawan, V.V.; Nikam, Y.; Gude, R.P.; Nagarsenker, M.S. Tamoxifen guided liposomes for targeting encapsulated anticancer agent to estrogen receptor positive breast cancer cells: In vitro and in vivo evaluation. Biomed. Pharmacother. 2014, 68, 429–438. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Mwakwari, S.C.; Sodji, Q.H.; Oyelere, A.K.; El-Sayed, M.A. Tamoxifen−Poly(ethylene glycol)−Thiol Gold Nanoparticle Conjugates: Enhanced Potency and Selective Delivery for Breast Cancer Treatment. Bioconjug. Chem. 2009, 20, 2247–2253. [Google Scholar] [CrossRef]
- Paris, J.L.; Cabañas, M.V.; Manzano, M.; Vallet-Regí, M. Polymer-Grafted Mesoporous Silica Nanoparticles as Ultrasound-Responsive Drug Carriers. ACS Nano 2015, 9, 11023–11033. [Google Scholar] [CrossRef]
- Climent, E.; Biyikal, M.; Gröninger, D.; Weller, M.G.; Martínez-Máñez, R.; Rurack, K. Multiplexed Detection of Analytes on Single Test Strips with Antibody-Gated Indicator-Releasing Mesoporous Nanoparticles. Angew. Chem. Int. Ed. 2020, 59, 2–10. [Google Scholar] [CrossRef]
- Vivo-Llorca, G.; Candela-Noguera, V.; Alfonso, M.; García-Fernández, A.; Orzáez, M.; Sancenón, F.; Martínez-Máñez, R. MUC1 Aptamer-Capped Mesoporous Silica Nanoparticles for Navitoclax Resistance Overcoming in Triple-Negative Breast Cancer. Chem.-A Eur. J. 2020, 26, 16318–16327. [Google Scholar] [CrossRef]
- Poyatos-Racionero, E.; Gonzalez-Alvarez, I.; González-Álvarez, M.; Martínez-Máñez, R.; Marcos, M.D.; Bernardos, A.; Aznar, E. Surfactant-Triggered Molecular Gate Tested on Different Mesoporous Silica Supports for Gastrointestinal Controlled Delivery. Nanomaterials 2020, 10, 1290. [Google Scholar] [CrossRef] [PubMed]
- Añón, E.; Costero, A.M.; Amorós, P.; El Haskouri, J.; Martínez-Mánez, R.; Parra, M.; Gil, S.; Gaviña, P.; Terencio, M.C.; Alfonso, M. Peptide-Capped Mesoporous Nanoparticles: Toward a more Efficient Internalization of Alendronat. ChemistrySelect 2020, 5, 3618. [Google Scholar] [CrossRef]
- Midoux, P.; Pichon, C.; Yaouanc, J.-J.; Jaffrès, P.-A. Chemical vectors for gene delivery: A current review on polymers, peptides and lipids containing histidine or imidazole as nucleic acids carriers. Br. J. Pharmacol. 2009, 157, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.F.; Tseng, S.J.; Kempson, I.M.; Peng, S.F.; Wu, W.T.; Liu, J.R. Controlled delivery of recombinant adeno-associated virus serotype 2 using pH-sensitive poly(ethylene glycol)-poly-L-histidine hydrogels. Biomaterials 2012, 33, 9239–9245. [Google Scholar] [CrossRef]
- Mu, S.; Liu, Y.; Wang, T.; Zhang, J.; Jiang, D.; Yu, X.; Zhang, N. Unsaturated nitrogen-rich polymer poly(l-histidine) gated reversibly switchable mesoporous silica nanoparticles using “graft to” strategy for drug controlled release. Acta Biomater. 2017, 63, 150–162. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, S.; Ma, Z.; Ji, P.; Ma, X.; Wu, Z.; Qi, X. Genetic recombination of poly(l-lysine) functionalized apoferritin nanocages that resemble viral capsid nanometer-sized platforms for gene therapy. Biomater. Sci. 2020, 8, 1759–1770. [Google Scholar] [CrossRef]
- Al-Lohedan, H.; Bunton, C.A. Ion binding and micellar effects upon reactions of carboxylic anhydrides and carbonate esters. J. Org. Chem. 1982, 47, 1160–1166. [Google Scholar] [CrossRef]
- Miricioiu, M.G.; Iacob, C.; Nechifor, G.; Niculescu, V.-C. High Selective Mixed Membranes Based on Mesoporous MCM-41 and MCM-41-NH2 Particles in a Polysulfone Matrix. Front. Chem. 2019, 7, 332. [Google Scholar] [CrossRef]
- Zhou, X.; Feng, W.; Qiu, K.; Chen, L.; Wang, W.; Nie, W.; Mo, X.; He, C. BMP-2 Derived Peptide and Dexamethasone Incorporated Mesoporous Silica Nanoparticles for Enhanced Osteogenic Differentiation of Bone Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2015, 7, 15777–15789. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, L.; Huang, N.; Cui, W.; Liu, Z.; Xiao, K.; Zhou, Z. Efficient Preparation of Enantiopure D-Phenylalanine through Asymmetric Resolution Using Immobilized Phenylalanine Ammonia-Lyase from Rhodotorula glutinis JN-1 in a Recirculating Packed-Bed Reactor. PLoS ONE 2014, 9, e108586. [Google Scholar] [CrossRef]
- Zhai, Q.; Zhou, C.; Zhao, S.; Peng, C.; Han, Y. Kinetic Study of Alkoxysilane Hydrolysis under Acidic Conditions by Fourier Transform Near Infrared Spectroscopy Combined with Partial Least-Squares Model. Ind. Eng. Chem. Res. 2014, 53, 13598–13609. [Google Scholar] [CrossRef]
- D’Angelo, J. FT-IR determination of aliphatic and aromatic C-H contents of fossil leaf compressions. Part 2: Applications. ALDEQ 2004, 8, 34–38. [Google Scholar]
- Bagiyan, G.A.; Koroleva, I.K.; Soroka, N.V.; Ufimtsev, A.V. Oxidation of thiol compounds by molecular oxygen in aqueous solutions. Russ. Chem. Bull. 2003, 52, 1135–1141. [Google Scholar] [CrossRef]
- Brewer, C.F.; Riehm, J.P. Evidence for possible nonspecific reactions between N-ethylmaleimide and proteins. Anal. Biochem. 1967, 18, 248–255. [Google Scholar] [CrossRef]
- Fowles, J.; Banton, M.; Klapacz, J.; Shen, H. A toxicological review of the ethylene glycol series: Commonalities and differences in toxicity and modes of action. Toxicol. Lett. 2017, 278, 66–83. [Google Scholar] [CrossRef]
- Ho, L.A.; Thomas, E.; McLaughlin, R.A.; Flematti, G.R.; Fuller, R.O. A new selective fluorescent probe based on tamoxifen. Bioorg. Med. Chem. Lett. 2016, 26, 4879–4883. [Google Scholar] [CrossRef]
- Zhang, F.; Song, M.-R.; Yuan, G.-K.; Ye, H.-N.; Tian, Y.; Huang, M.; Xue, J.-P.; Zhang, Z.-H.; Liu, J. A Molecular Combination of Zinc(II) Phthalocyanine and Tamoxifen Derivative for Dual Targeting Photodynamic Therapy and Hormone Therapy. J. Med. Chem. 2017, 60, 6693–6703. [Google Scholar] [CrossRef]
- Smith, B. Hydrocarbons. In Infrared Spectral Interpretation: A Systematic Approach, 1st ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 30–66. [Google Scholar]
- Shchukarev, A.; Korolkov, D. XPS Study of group IA carbonates. Open Chem. 2004, 2, 347–362. [Google Scholar] [CrossRef]
- Nohira, H.; Tsai, W.; Besling, W.F.A.; Young, E.; Petry, J.; Conard, T.; Vandervorst, W.; De Gendt, S.; Heyns, M.M.; Maes, J.; et al. Characterization of ALCVD-Al2O3 and ZrO2 layer using X-ray photoelectron spectroscopy. J. Non-Cryst. Solids 2002, 303, 83–87. [Google Scholar] [CrossRef]
- Wang, R.; Chu, C.; Hu, T.; Dong, Y.; Guo, C.; Sheng, X.; Lin, P.; Chung, C.; Chu, P. Surface XPS characterization of NiTi shape memory alloy after advanced oxidation processes in UV/H2O2 photocatalytic system. Appl. Surf. Sci. 2007, 253, 8507–8512. [Google Scholar] [CrossRef]
- Green, S.M.; Grant, D.M.; Wood, J.V. XPS characterisation of surface modified Ni-Ti shape memory alloy. Mater. Sci. Eng. A 1997, 224, 21–26. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef] [PubMed]
- BeckShon, H.P.; Brooke, S.K.; Bregman, H.; Cee, V.J.; Chakka, N.; Cushing, T.D.; Epstein, O.; Fox, B.M.; Geuns-Meyer, S.; Hao, X.; et al. WO2015129926A1: Pyrazole Amide Derivative. WIPO 2015. 89. Available online: https://patents.google.com/patent/WO2015129926A1 (accessed on 14 April 2019).
- Xie, M.; Shi, H.; Li, Z.; Shen, H.; Ma, K.; Li, B.; Shen, S.; Jin, P.Y. A multifunctional mesoporous silica nanocomposite for targeted delivery, controlled release of doxorubicin and bioimaging. Colloids Surfaces B Biointerfaces 2013, 110, 138–147. [Google Scholar] [CrossRef] [PubMed]

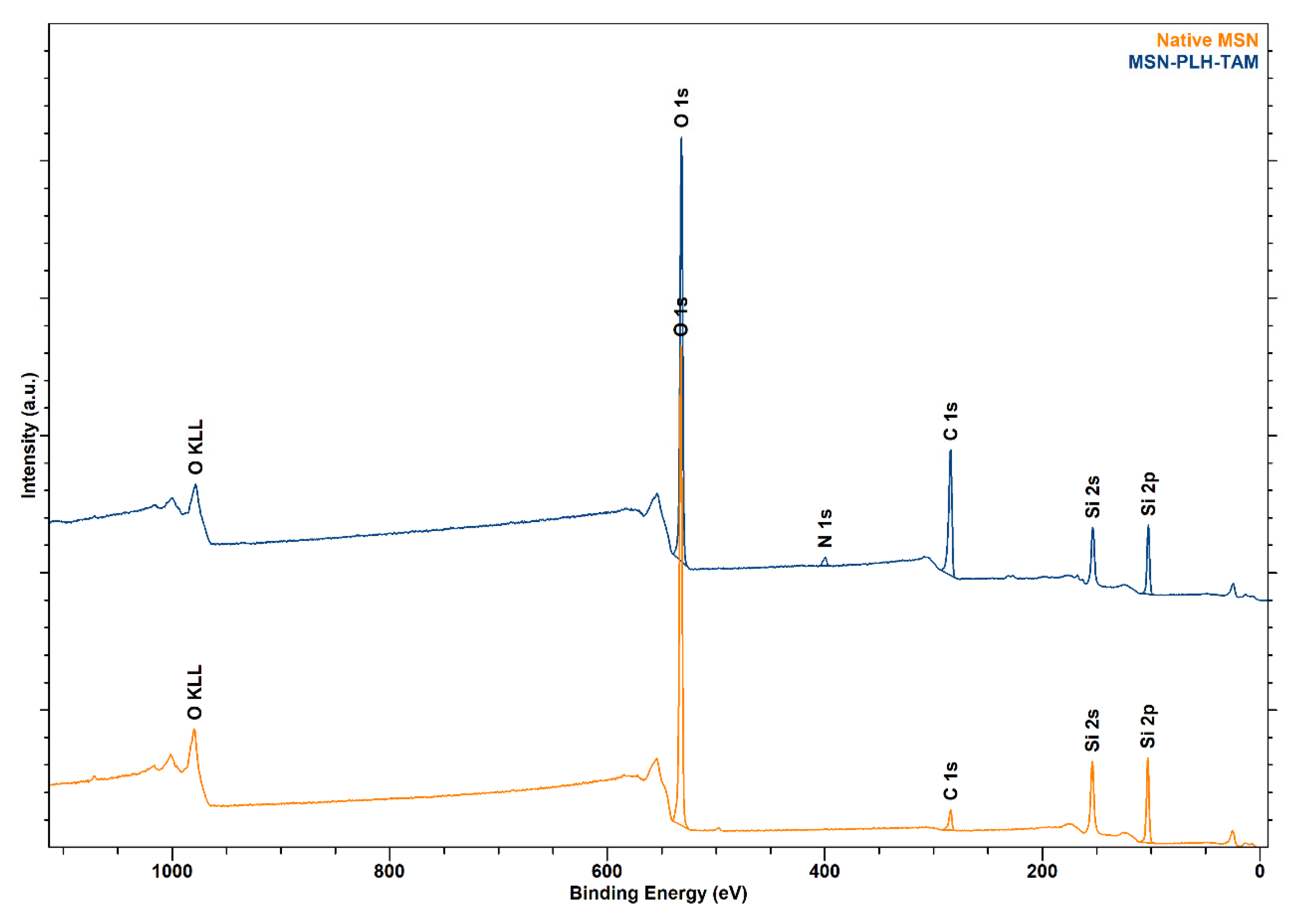
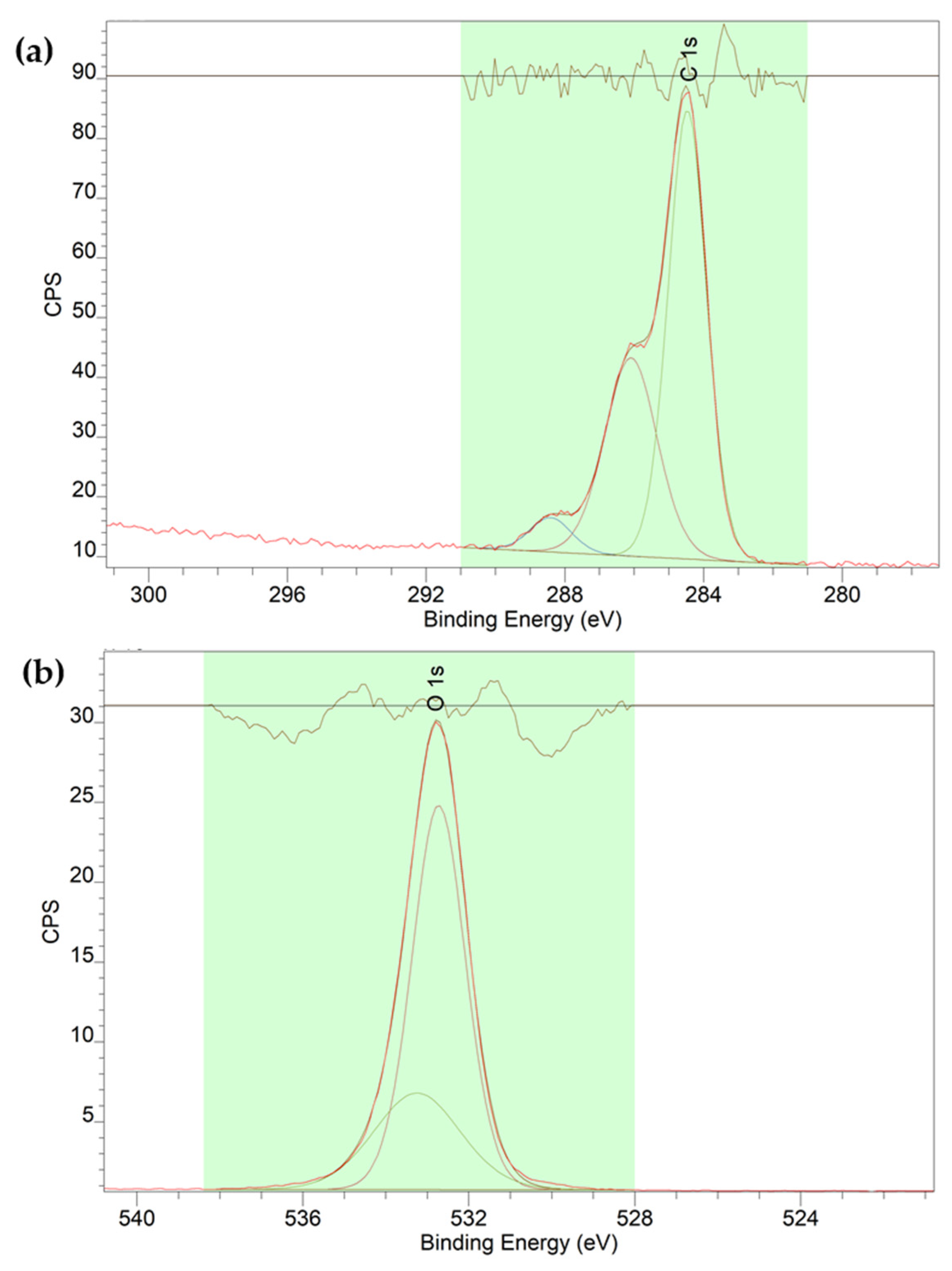
| Sample | SE | At% |
|---|---|---|
| Native MSNs (unfunctionalized) | O | 66.12 |
| C | 8.44 | |
| Si | 25.44 | |
| MSN-PLH-TAM | O | 38.83 |
| C | 44.77 | |
| N | 2.20 | |
| Si | 14.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Day, C.M.; Sweetman, M.J.; Hickey, S.M.; Song, Y.; Liu, Y.; Zhang, N.; Plush, S.E.; Garg, S. Concept Design, Development and Preliminary Physical and Chemical Characterization of Tamoxifen-Guided-Mesoporous Silica Nanoparticles. Molecules 2021, 26, 219. https://doi.org/10.3390/molecules26010219
Day CM, Sweetman MJ, Hickey SM, Song Y, Liu Y, Zhang N, Plush SE, Garg S. Concept Design, Development and Preliminary Physical and Chemical Characterization of Tamoxifen-Guided-Mesoporous Silica Nanoparticles. Molecules. 2021; 26(1):219. https://doi.org/10.3390/molecules26010219
Chicago/Turabian StyleDay, Candace M., Martin J. Sweetman, Shane M. Hickey, Yunmei Song, Yongjun Liu, Na Zhang, Sally E. Plush, and Sanjay Garg. 2021. "Concept Design, Development and Preliminary Physical and Chemical Characterization of Tamoxifen-Guided-Mesoporous Silica Nanoparticles" Molecules 26, no. 1: 219. https://doi.org/10.3390/molecules26010219
APA StyleDay, C. M., Sweetman, M. J., Hickey, S. M., Song, Y., Liu, Y., Zhang, N., Plush, S. E., & Garg, S. (2021). Concept Design, Development and Preliminary Physical and Chemical Characterization of Tamoxifen-Guided-Mesoporous Silica Nanoparticles. Molecules, 26(1), 219. https://doi.org/10.3390/molecules26010219










