Effect of Jakyakgamcho-Tang Extracts on H2O2-Induced C2C12 Myoblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of 30% Ethanol and Water Extracts of Jakyakgamcho-Tang
2.2. UPLC-Quadruple Time-of-Flight Mass Spectrometry (UPLC-Q-Tof/MS) Analysis
2.3. Determination of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) in JGT-W and JGT-E
2.4. Scavenging of 2,2-Azino-Bis-3-Ethylbenzothiazoline-6-Sulfonic Acid (ABTS)
2.5. 2,2′-Diphenyl-1-Picrylhydrazyl (DPPH) Free Radical Assay
2.6. Cell Culture
2.7. Cell Viability Assay in H2O2-Induced C2C12 Cells
2.8. Intracellular ROS Measurements in H2O2-Induced C2C12 Cells
2.9. Statistical Analysis
3. Results
3.1. Qualitative and Quantitative Analyses of JGT Components by UPLC-PDA-ESI-QTOF-MS
3.2. Effects of JGT-E and JGT-W on ABTS- and DPPH-Scavenging Activities
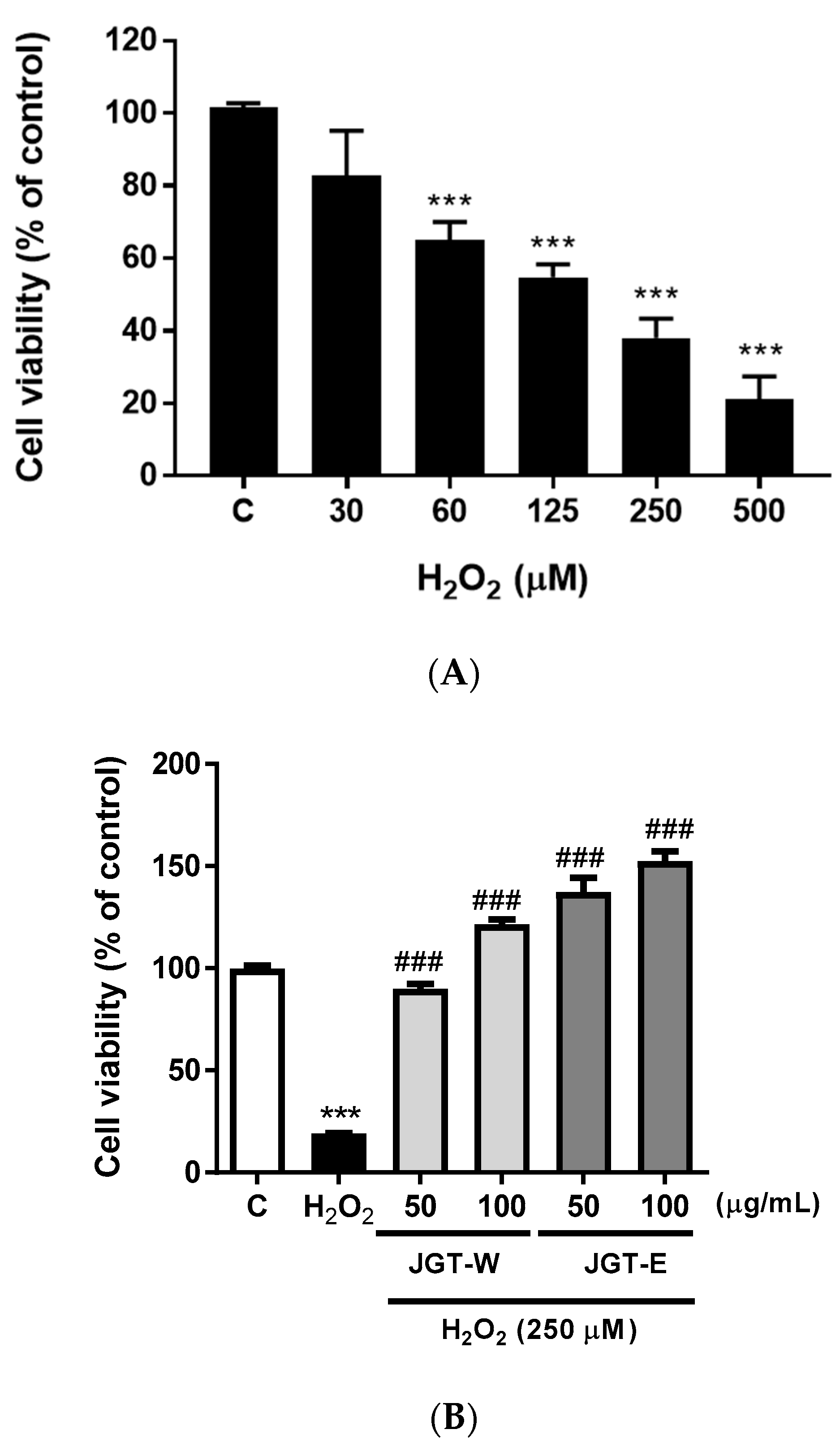
3.3. JGT-E and JGT-W Protect C2C12 Myocytes against H2O2-Induced Decrease in Cell Viability
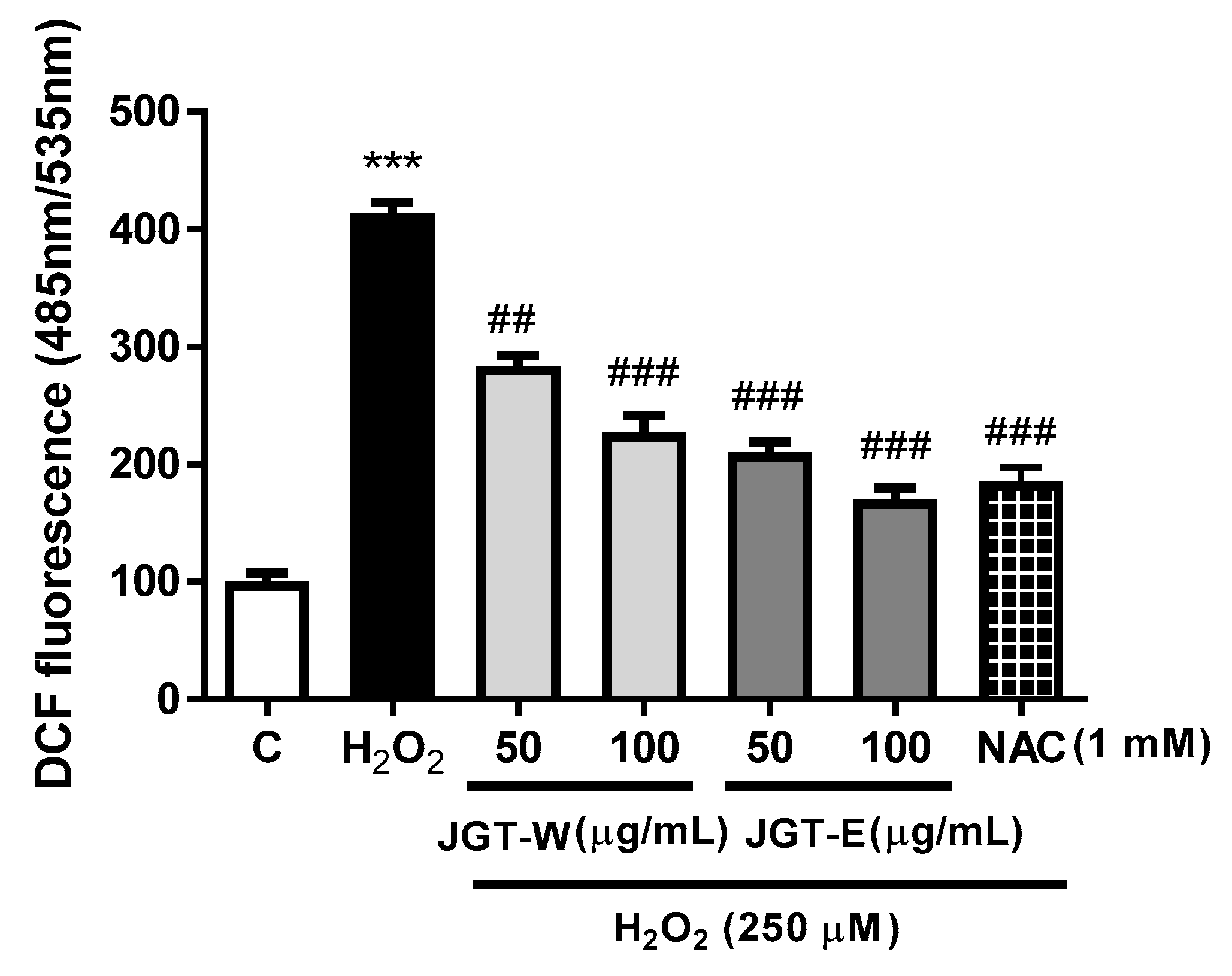
3.4. JGT-E and JGT-W Protect C2C12 Myocytes from H2O2-Induced Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meng, S.J.; Yu, L.J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010, 7, 1509–1526. [Google Scholar] [CrossRef]
- Robinson, S.; Cooper, C.; Sayer, A.A. Nutrition and sarcopenia: A review of the evidence and implications for preventive strategies. J. Aging. Res. 2012, 2012, 510801. [Google Scholar] [CrossRef]
- Scicchitano, B.M.; Pelosi, L.; Sica, G.; Musarò, A. The physiopathologic role of oxidative stress in skeletal muscle. Mech. Aging Dev. 2018, 170, 37–44. [Google Scholar] [CrossRef]
- Farnsworth, N.R.; Akerele, O.; Bingel, A.S.; Soejarto, D.D.; Guo, Z. Medicinal plants in therapy. Bull. World Health Org. 1985, 63, 965–981. [Google Scholar] [CrossRef]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar] [CrossRef]
- Niki, E. Interaction of ascorbate and alpha-tocopherol. Ann. N. Y. Acad. Sci. 1987, 498, 186–199. [Google Scholar] [CrossRef]
- Ryan, M.J.; Dudash, H.J.; Docherty, M.; Geronilla, G.B.; Baker, B.A.; Haff, G.G.; Cutlip, R.G.; Always, S.E. Vitamin E and C Supplementation reduces oxidative stress, improves antioxidant enzymes and positive muscle work in chronically loaded muscles of aged rats. Exp. Gerontol. 2010, 45, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Lee, S.; Elam, M.L.; Johnson, S.A.; Kang, J.; Arjmandi, B.H. Study to find the best extraction solvent for use with guava leaves (Psidium guajava L.) for high antioxidant efficacy. Food Sci Nutr. 2014, 2, 174–180. [Google Scholar] [CrossRef]
- Han, K.; Kwon, O.; Jung, S.-Y.; Park, I.-H.; Hwang, M.-S.; Park, S.-Y.; Hwang, E.-H.; Lee, J.-H. Jakyakgamcho-Tang in the relief of delayed-onset muscle soreness in healthy adults: Study protocol for a randomized, double-blind, placebo-controlled, crossover design clinical trial. Trials 2020, 21, 211. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.K.; Kim, Y.S.; Lee, I.S.; Kim, J.S. Jakyakgamcho-tang and its major component, Paeonia lactiflora, exhibit potent anti-glycation properties. J. Exerc. Nutr. Biochem. 2016, 20, 60–64. [Google Scholar] [CrossRef]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; Elsohly, M.A.; Khan, I.A. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative study. Evid. Based Complement. Alternat. Med. 2014, 2014, 253875. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Jun, I.; Jeong, S.; Shin, H. The stimulation of myoblast differentiation by electrically conductive sub-micron fibers. Biomaterials 2009, 30, 2038–2047. [Google Scholar] [CrossRef]
- Gomes, M.J.; Martinez, P.F.; Pagan, L.U.; Damatto, R.L.; Cezar, M.D.M.; Lima, A.R.R.; Okoshi, K.; Okoshi, M.P. Skeletal muscle aging: Influence of oxidative stress and physical exercise. Oncotarget 2017, 8, 20428–20440. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Hinoshita, F.; Ogura, Y.; Suzuki, Y.; Hara, S.; Yamada, A.; Tanaka, N.; Yamashita, A.; Marumo, F. Effect of orally administered Shao-Yao-Gan-Cao-Tang (Shakuyaku-Kanzo-To) on muscle cramps in maintenance hemodialysis patients: A preliminary study. Am. J. Chin. Med. 2003, 31, 445–453. [Google Scholar] [CrossRef]
- Hidaka, T.; Shima, T.; Nagira, K.; Ieki, M.; Nakamura, T.; Aono, Y.; Kuraishi, Y.; Arai, T.; Saito, S. Herbal Medicine Shakuyaku-Kanzo-To reduces paclitaxel-induced painful peripheral neuropathy in mice. Eur. J. Pain 2009, 13, 22–27. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Najafabad, A.M.; Jamei, R. Free radical scavenging capacity and antioxidant activity of methanol and ethanol extracts of plum (Prunus domestica L.) in both fresh and dried samples. Avicenna J. Phytomed. 2014, 4, 343–353. [Google Scholar]
- Kwak, D.; Lee, C.; Kong, I.; Choi, D.; Na, C. Comparison of the spasmolytic effects of Jakyak-Gamcho decoctions derived via different extractants. Evid. Based Complement. Alternat. Med. 2015, 2015, 270380. [Google Scholar] [CrossRef]
- Kim, J.-H.; Shin, H.-K.; Seo, C.-S. Chemical interaction between Paeonia lactiflora and Glycyrrhiza uralensis, the components of Jakyakgamcho-tang, using a validated high-performance liquid chromatography method: Herbal combination and chemical interaction in a decoction. J. Sep. Sci. 2014, 37, 2704–2715. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Guo, Y.; Hong, W.; Fang, Y.; Han, D.; Zhang, P.; Wang, X.; Körner, H.; Wie, W. The regulatory effects of paeoniflorin and its derivative paeoniflorin-6′-o-benzene sulfonate CP-25 on inflammation and immune diseases. Front. Pharmacol. 2019, 10, 57. [Google Scholar] [CrossRef]
- Su, J.; Zhang, P.; Zhang, J.-J.; Qi, X.-M.; Wu, Y.-G.; Shen, J.-J. Effects of total glucosides of paeony on oxidative stress in the kidney from diabetic rats. Phytomedicine 2010, 17, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Kobe, A.; Kuriya, M.; Hiroki, Y.; Yahagi, T.; Sakakibara, I.; Matsuzaki, K.; Amano, T. Neuroprotective effect of liquiritin as an antioxidant via an increase in glucose-6-phosphate dehydrogenase expression on B65 neuroblastoma cells. Eur. J. Pharmacol. 2017, 815, 381–390. [Google Scholar] [CrossRef]
- Nakashima, H.; Matsui, T.; Yoshida, O.; Isowa, Y.; Kido, Y.; Motoki, Y.; Ito, M.; Shigeta, S.; Mori, T.; Yamamoto, N. A new anti-human immunodeficiency virus substance, glycyrrhizin sulfate; endowment of glycyrrhizin with reverse transcriptase-inhibitory activity by chemical modification. Jpn. J. Cancer Res. 1987, 78, 767–771. [Google Scholar]
- Fu, Y.; Hsieh, T.; Guo, J.; Kunicki, J.; Lee, M.Y.W.T.; Darzynkiewicz, Z.; Wu, J.M. Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells. Biochem. Biophys. Res. Commun. 2004, 322, 263–270. [Google Scholar] [CrossRef]
- Zhang, Q.; Nie, J.; Chen, S.-J.; Li, Q. Protective effects of ethyl gallate and pentagalloylglucose, the active components of Qingwen Baidu decoction, against lipopolysaccharide-induced acute lung injury in rats. Drug Des. Devel. Ther. 2019, 13, 71–77. [Google Scholar] [CrossRef]
- Piao, M.; Kang, K.; Zhang, R.; Ko, D.; Wang, Z.; Lee, K.; Chang, W.; Chae, S.; Jee, Y.; Shin, T.; et al. Antioxidant properties of 1,2,3,4,6-penta-o-galloyl-β-d-glucose from Elaeocarpus sylvestris var. ellipticus. Food Chem. 2009, 115, 412–418. [Google Scholar] [CrossRef]
- Patnaik, S.S.; Simionescu, D.T.; Goergen, C.G.; Hoyt, K.; Sirsi, S.; Finol, E.A. Pentagalloyl glucose and its functional role in vascular health: Biomechanics and drug-delivery characteristics. Ann. Biomed. Eng. 2019, 47, 39–59. [Google Scholar] [CrossRef]
- Kaur, P.; Kaur, S.; Kumar, N.; Singh, B.; Kumar, S. Evaluation of antigenotoxic activity of isoliquiritin apioside from Glycyrrhiza glabra L. Toxicol. In Vitro 2009, 23, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Alrushaid, S.; Davies, N.M.; Martinez, S.E.; Sayre, C.L. Pharmacological characterization of liquiritigenin, a chiral flavonoid in licorice. Res. Pharm. Sci. 2016, 11, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.; Khan, I.; Hussain, A.; Shahat, A.A.; Tawab, A.; Qasim, M.; Adnan, M.; Al-Said, M.S.; Ullah, R.; Khan, S.N. Glycyrrhiza glabra HPLC fractions: Identification of aldehydo isoophiopogonone and liquiritigenin having activity against multidrug resistant bacteria. BMC Complement Altern. Med. 2018, 18, 140. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C. Mechanism of action of glycyrrhizic acid in inhibition of epstein-barr virus replication in vitro. Antiviral Res. 2003, 59, 41–47. [Google Scholar] [CrossRef]
- Li, Z.; Chen, C.; Zhu, X.; Li, Y.; Yu, R.; Xu, W. Glycyrrhizin suppresses RANKL-induced osteoclastogenesis and oxidative stress through inhibiting NF-κB and MAPK and activating AMPK/Nrf2. Calcif Tissue Int. 2018, 103, 324–337. [Google Scholar] [CrossRef]
- Shyh-Chang, N.; Daley, G.Q.; Cantley, L.C. Stem cell metabolism in tissue development and aging. Development 2013, 140, 2535–2547. [Google Scholar] [CrossRef]
- Yu, T.; Dohl, J.; Elenberg, F.; Chen, Y.; Deuster, P. Curcumin induces concentration-dependent alterations in mitochondrial function through ROS in C2C12 mouse myoblasts. J. Cell Physiol. 2019, 234, 6371–6381. [Google Scholar] [CrossRef]
- Cockfield, J.A.; Schafer, Z.T. Antioxidant defenses: A context-specific vulnerability of cancer cells. Cancers 2019, 11, 1208. [Google Scholar] [CrossRef]
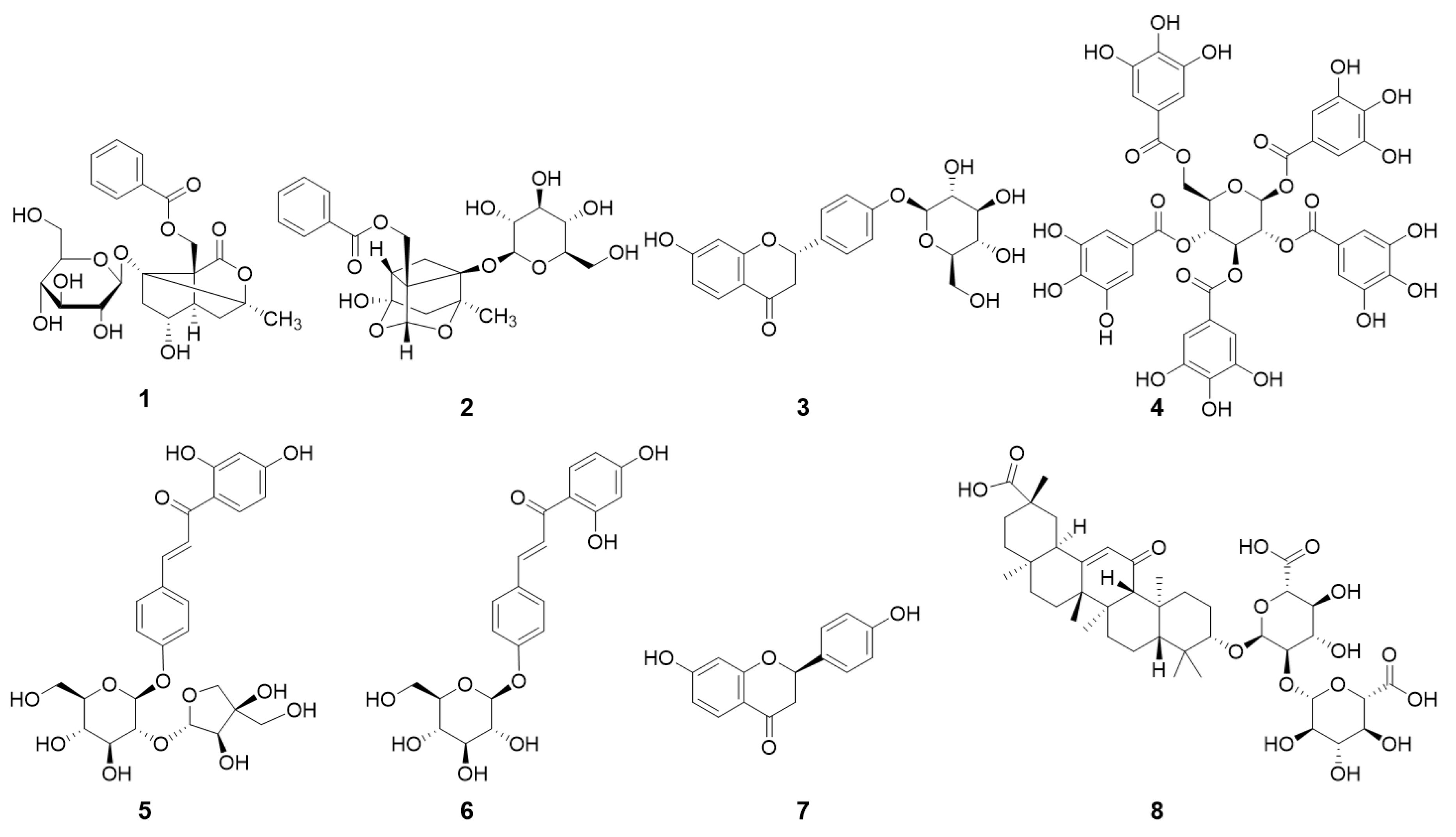
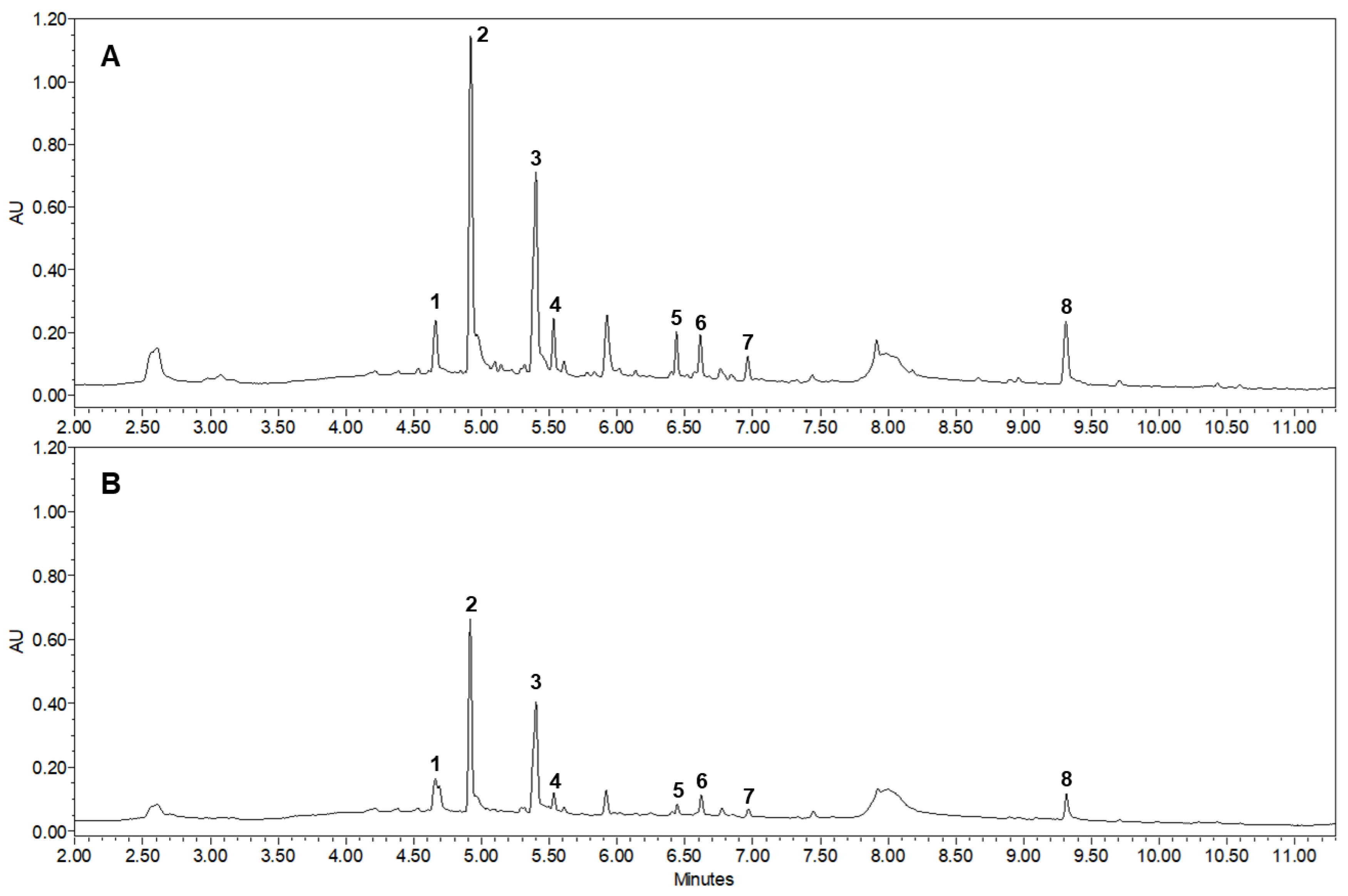
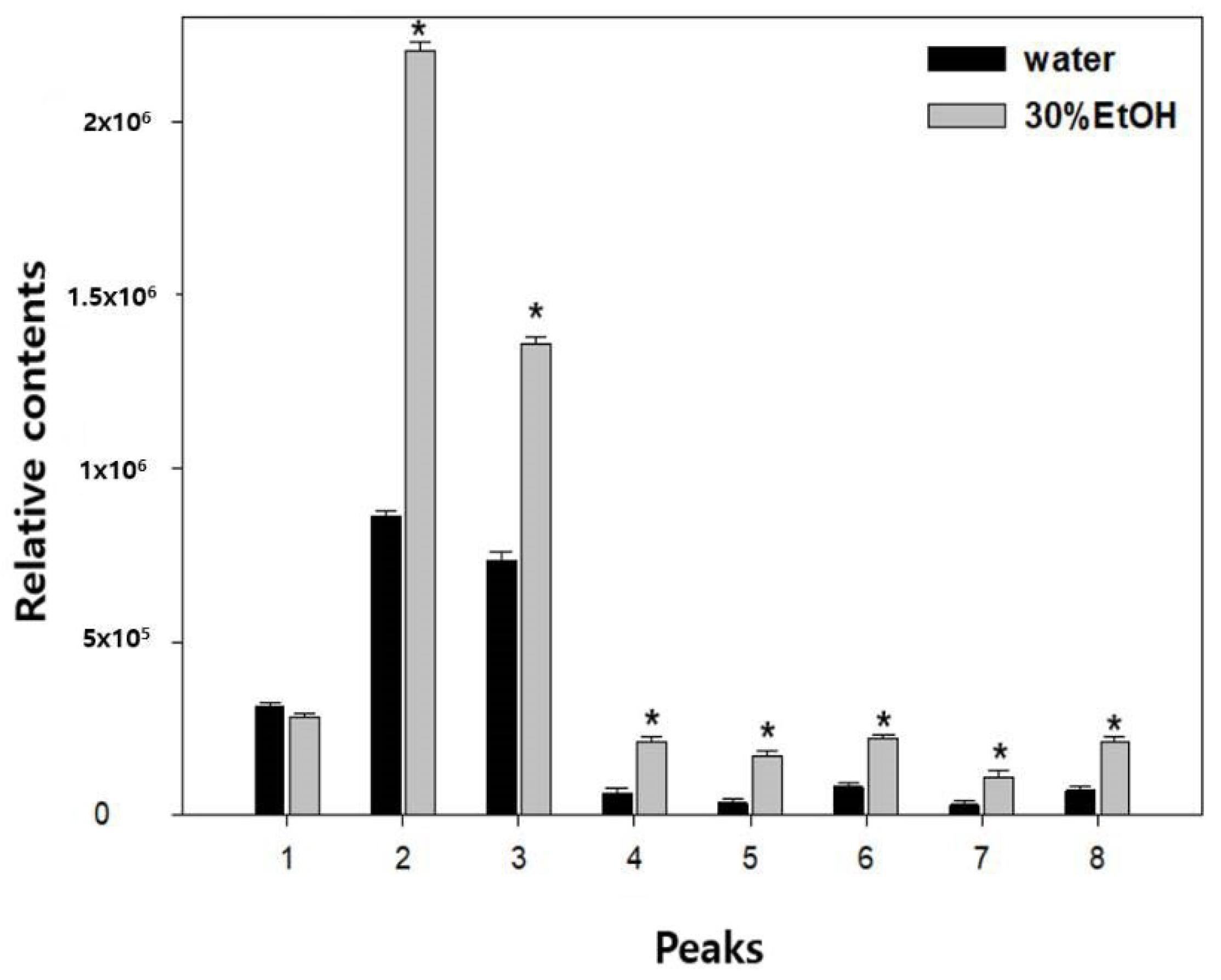
| Sample | TPC (mg GAE g−1) | TFC (mg QE g−1) |
|---|---|---|
| Water extract of P. lactiflora root | 17.17 ± 0.6 | 3.25 ± 0.3 |
| 30% (w/v) ethanol extract of P. lactiflora root | 29.70 ± 1.0 * | 8.11 ± 0.1 * |
| Water extract of G. uralensis root | 18.37 ± 0.1 | 3.72 ± 0.1 |
| 30% (w/v) ethanol extract of G. uralensis root | 23.10 ± 1.6 * | 7.02 ± 0.2 * |
| JGT-W | 25.49 ± 0.8 | 3.94 ± 0.5 |
| JGT-E | 34.01 ± 2.6 ** | 7.32 ± 0.6 ** |
| Samples | ABTS | DPPH | ||
|---|---|---|---|---|
| Inhibition (%) | IC50 (µg mL−1) | Inhibition (%) | IC50 (µg mL−1) | |
| JGT-W | 72.8 ± 1.2 | 60.5 ± 0.9 | 25.1 ± 1.3 | 742.8 ± 2.1 |
| JGT-E | 80.8 ± 1.1 | 34.2 ± 0.4 *** | 38.9 ± 1.1 | 436.1 ± 3.7 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.S.; Yuk, H.J.; Kim, D.S. Effect of Jakyakgamcho-Tang Extracts on H2O2-Induced C2C12 Myoblasts. Molecules 2021, 26, 215. https://doi.org/10.3390/molecules26010215
Kim YS, Yuk HJ, Kim DS. Effect of Jakyakgamcho-Tang Extracts on H2O2-Induced C2C12 Myoblasts. Molecules. 2021; 26(1):215. https://doi.org/10.3390/molecules26010215
Chicago/Turabian StyleKim, Young Sook, Heung Joo Yuk, and Dong Seon Kim. 2021. "Effect of Jakyakgamcho-Tang Extracts on H2O2-Induced C2C12 Myoblasts" Molecules 26, no. 1: 215. https://doi.org/10.3390/molecules26010215
APA StyleKim, Y. S., Yuk, H. J., & Kim, D. S. (2021). Effect of Jakyakgamcho-Tang Extracts on H2O2-Induced C2C12 Myoblasts. Molecules, 26(1), 215. https://doi.org/10.3390/molecules26010215







