Coumarin’s Anti-Quorum Sensing Activity Can Be Enhanced When Combined with Other Plant-Derived Small Molecules
Abstract
1. Introduction
2. Results
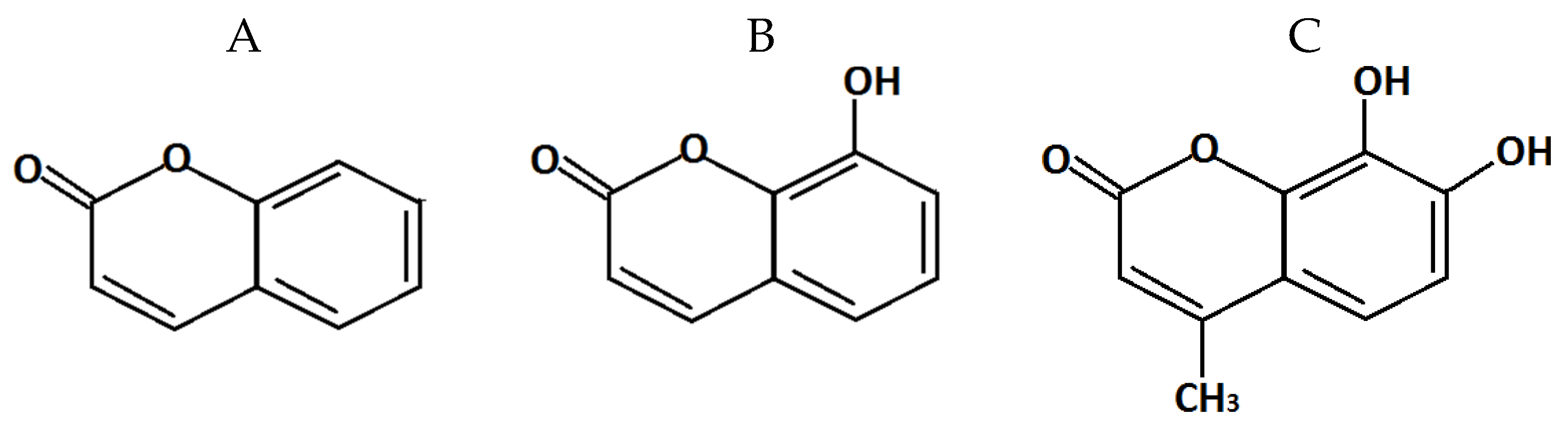
2.1. Effect of Coumarin and Its Derivatives in Chromobacterium violaceum ATCC 31532 Violacein Production Bioassay
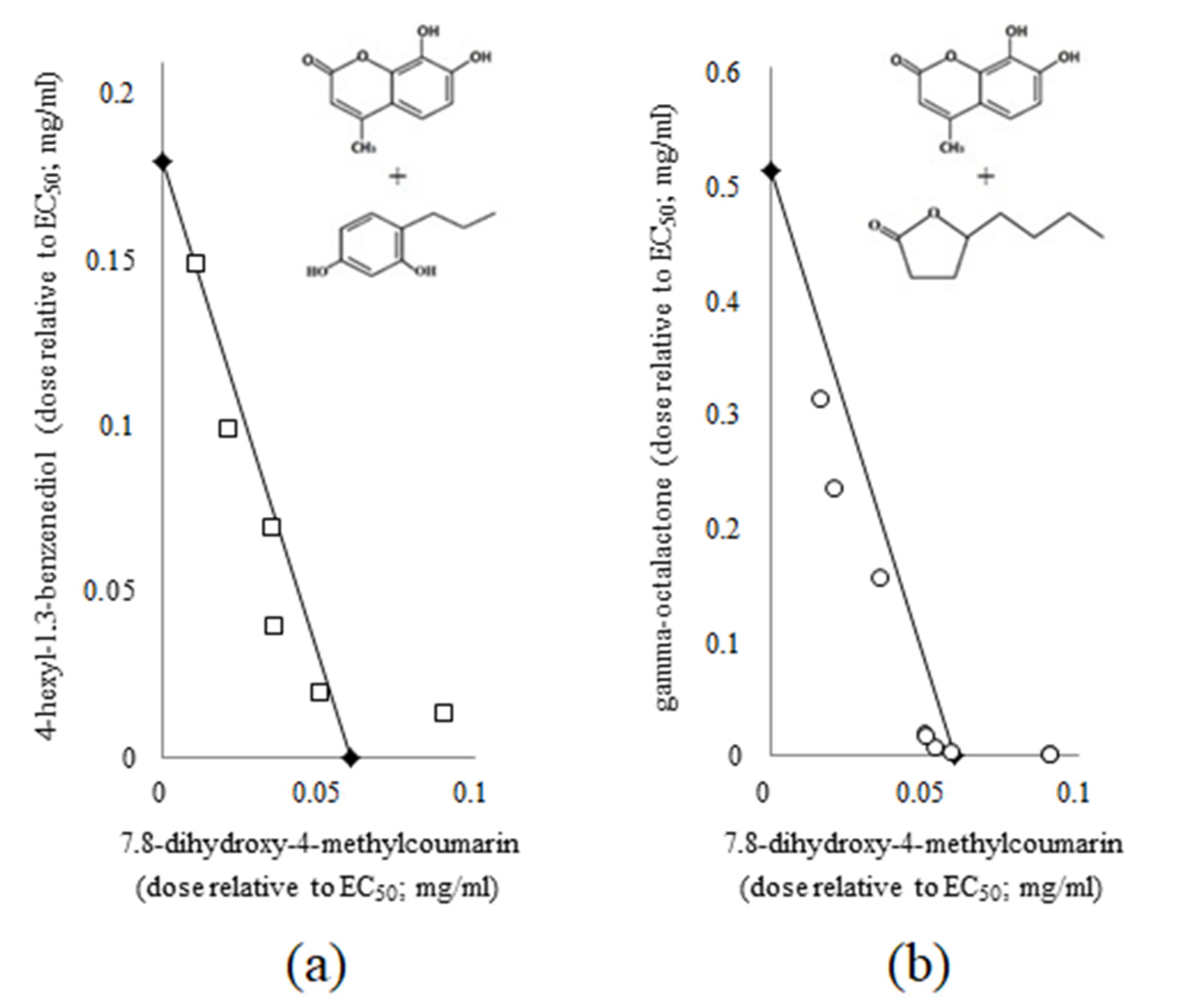
2.2. Analysis of the Combined Use of Coumarin Derivatives (7.8-Dihydroxy-4-methylcumarin) with Other Small Plant-Derived Molecules in C. violaceum ATCC 31532
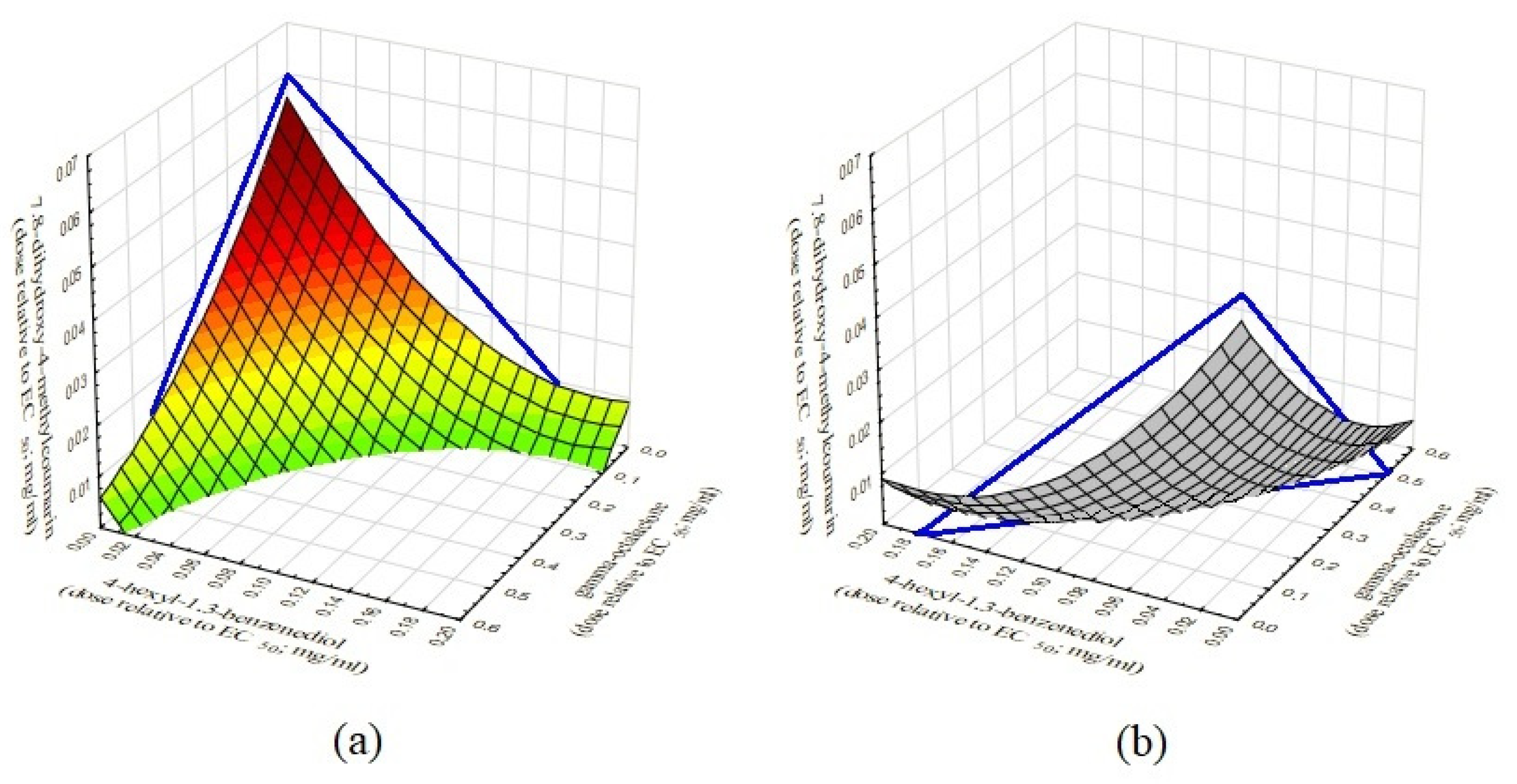
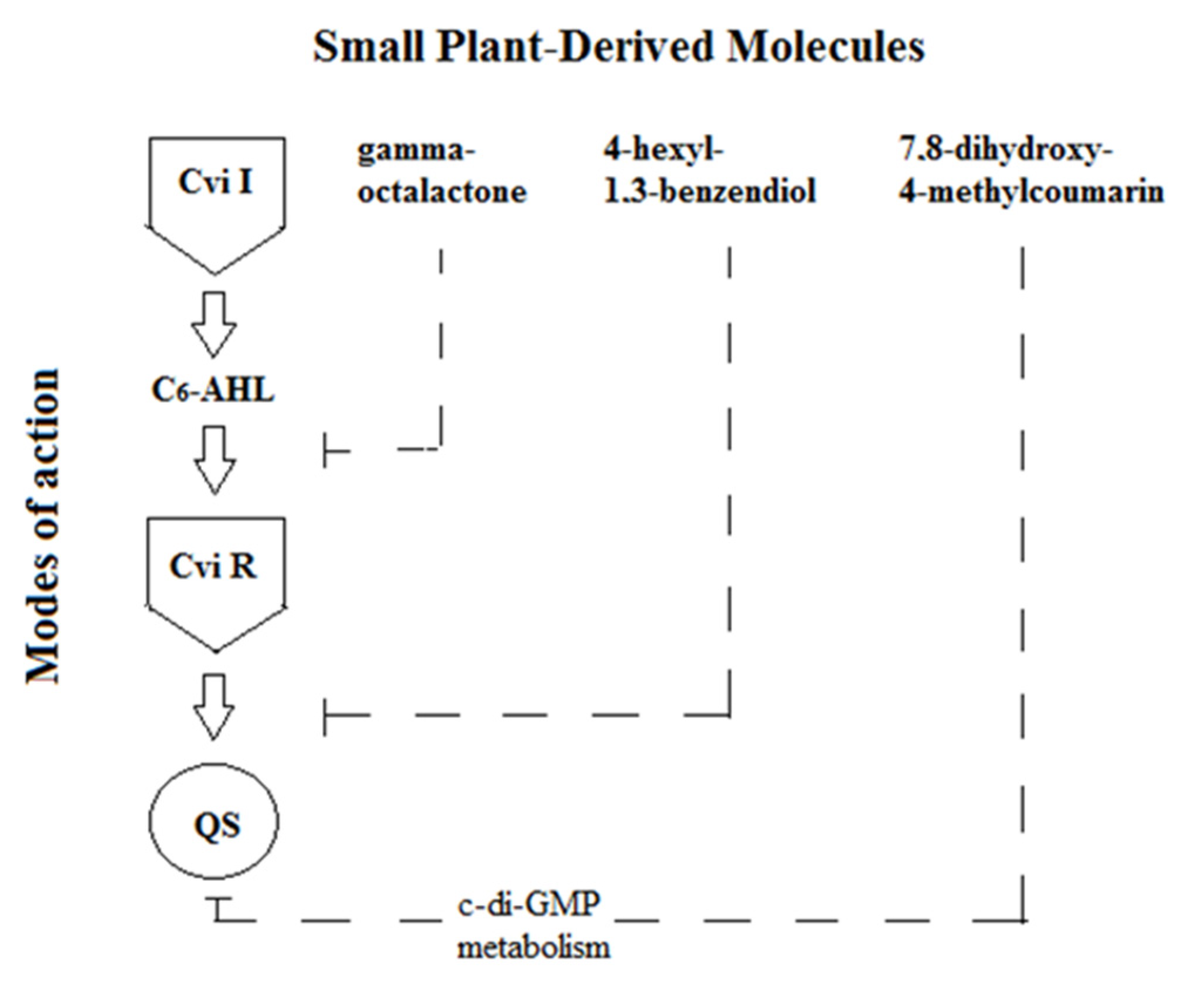
2.3. Evaluation of the Effect of a Three-Component Composition of Small Plant-Derived Molecules on the Quorum Sensing in C. violaceum ATCC 31532
3. Discussion
4. Materials and Methods
4.1. Chemical Compounds
4.2. Bacterial Strain
4.3. Methods for Investigating Anti-QS Activity of Coumarin Derivatives in C. violaceum ATCC 31532 Bioassay
4.4. Evaluation of the Combined Use of Coumarins and Small Plant-Derived Molecules against in C. violaceum ATCC 31532
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on Natural Coumarin Lead Compounds for Their Pharmacological Activity. BioMed Res. Int. 2013, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.L.; Liu, Y.; Li, Y.; Ting, H.J.; Kohli, G.S.; Cai, Z.; Suwanchaikasem, P.; Goh, K.K.K.; Ng, S.P.; Tolker-Nielsen, T. Reduced Intracellular c-di-GMP Content Increases Expression of Quorum Sensing-Regulated Genes in Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 2017, 7, 451. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An Important Class of Phytochemicals. In Phytochemicals-Isolation, Characterisation and Role in Human Health; Rao, A.V., Rao, L.G., Eds.; InTech: Rijeka, Croatia, 2015; ISBN 978-953-51-2170-1. [Google Scholar]
- Reen, F.J.; Gutierrez-Barranquero, J.A.; Parages, M.L.; O Gara, F. Coumarin: A novel player in microbial Quorum sensing and biofilm formation inhibition. Appl. Microbiol. Biotechnol. 2018, 102, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.F.; Figueiredo, G.F.; Pedro, L.P.; Amorin, Y.M.; Andrade, J.T.; Passos, T.F.; Rodrigues, F.F.; Souza, I.L.A.; Gonçalves, T.P.R.; Dos Santos Lima, L.A.R.; et al. Umbelliferone (7-hydroxycoumarin): A non-toxic antidiarrheal and antiulcerogenic coumarin. Biomed. Pharmacother. 2020, 129, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Olanlokun, J.O.; Bodede, O.; Prinsloo, G.; Olorunsogo, O.O. Comparative antimalarial, toxicity and mito-protective effects of Diospyros mespiliformis Hochst. ex A. DC. and Mondia whitei (Hook.f.) Skeels on Plasmodium berghei infection in mice. J. Ethnopharmacol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.J.; Gieling, R.G. Preclinical Evaluation of Ureidosulfamate Carbonic Anhydrase IX/XII Inhibitors in the Treatment of Cancers. Int. J. Mol. Sci. 2019, 23, 6080. [Google Scholar] [CrossRef]
- Akkol, E.K.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and Coumarin-Related Compounds in Pharmacotherapy of Cancer. Cancers 2020, 7, 1959. [Google Scholar]
- Shahzadi, I.; Ali, Z.; Baek, S.H.; Mirza, B.; Ahn, K.S. Assessment of the Antitumor Potential of Umbelliprenin, a Naturally Occurring Sesquiterpene Coumarin. Biomedicines 2020, 5, 126. [Google Scholar] [CrossRef]
- Nasser, M.I.; Zhu, S.; Hu, H.; Huang, H.; Guo, M.; Zhu, P. Effects of imperatorin in the cardiovascular system and cancer. Biomed. Pharmacother. 2019, 120, 109401. [Google Scholar] [CrossRef]
- Duan, J.; Shi, J.; Ma, X.; Xuan, Y.; Li, P.; Wang, H.; Fan, Y.; Gong, H.; Wang, L.; Pang, Y.; et al. Esculetin inhibits proliferation, migration, and invasion of clear cell renal cell carcinoma cells. Biomed. Pharmacother. 2020, 125, 110031. [Google Scholar] [CrossRef]
- Usman, H.; Ullah, M.A.; Jan, H.; Siddiquah, A.; Drouet, S.; Anjum, S.; Giglioli-Guviarc’h, N.; Hano, C.; Abbasi, B.H. Interactive Effects of Wide-Spectrum Monochromatic Lights on Phytochemical Production, Antioxidant and Biological Activities of Solanum Xanthocarpum Callus Cultures. Molecules 2020, 9, 2201. [Google Scholar] [CrossRef] [PubMed]
- Urbagarova, B.M.; Shults, E.E.; Taraskin, V.V.; Radnaeva, L.D.; Petrova, T.N.; Rybalova, T.V.; Frolova, T.S.; Pokrovskii, A.G.; Ganbaatar, J. Chromones and coumarins from Saposhnikovia divaricata (Turcz.) Schischk. Growing in Buryatia and Mongolia and their cytotoxicity. J. Ethnopharmacol. 2020, 261, 112517. [Google Scholar] [CrossRef] [PubMed]
- Bihani, T. Plumeria rubra L.—A review on its ethnopharmacological, morphological, phytochemical, pharmacological and toxicological studies. J. Ethnopharmacol. 2021, 264, 113291. [Google Scholar] [CrossRef] [PubMed]
- Starzak, K.; Świergosz, T.; Matwijczuk, A.; Creaven, B.; Podleśny, J.; Karcz, D. Anti-Hypochlorite, Antioxidant, and Catalytic Activity of Three Polyphenol-Rich Super-Foods Investigated with the Use of Coumarin-Based Sensors. Biomolecules 2020, 10, 723. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Qian, L.; Cao, L.; Tan, H.; Huang, Y.; Xue, X.; Shen, Y.; Zhou, S. Virtual screening for novel quorum sensing inhibitors to eradicate biofilm formation of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2008, 79, 119–126. [Google Scholar] [CrossRef]
- Takomthong, P.; Waiwut, P.; Yenjai, C.; Sripanidkulchai, B.; Reubroycharoen, P.; Lai, R.; Kamau, P.; Boonyarat, C. Structure-Activity Analysis and Molecular Docking Studies of Coumarins from Toddalia asiatica as Multifunctional Agents for Alzheimer’s Disease. Biomedicines 2020, 8, 107. [Google Scholar] [CrossRef]
- Janus, Ł.; Radwan-Pragłowska, J.; Piątkowski, M.; Bogdał, D. Coumarin-Modified CQDs for Biomedical Applications-Two-Step Synthesis and Characterization. Int. J. Mol. Sci. 2020, 21, 8073. [Google Scholar] [CrossRef]
- Lee, E.J.; Kang, M.K.; Kim, Y.H.; Kim, D.Y.; Oh, H.; Kim, S.I.; Oh, S.Y.; Na, W.; Kang, Y.H. Coumarin Ameliorates Impaired Bone Turnover by Inhibiting the Formation of Advanced Glycation End Products in Diabetic Osteoblasts and Osteoclasts. Biomolecules 2020, 10, 1052. [Google Scholar] [CrossRef]
- Koga, H.; Negishi, M.; Kinoshita, M.; Fujii, S.; Mori, S.; Ishigami-Yuasa, M.; Kawachi, E.; Kagechika, H.; Tanatani, A. Development of Androgen-Antagonistic Coumarinamides with a Unique Aromatic Folded Pharmacophore. Int. J. Mol. Sci. 2020, 21, 5584. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, N.; Liang, W.; Han, Q.; Zhang, W.; Li, C. Quorum sensing-disrupting coumarin suppressing virulence phenotypes in Vibrio Splendidus. Appl. Microbiol. Biotechnol. 2016, 101, 3371–3378. [Google Scholar] [CrossRef]
- Gutiérrez-Barranquero, J.A.; Reen, F.J.; McCarthy, R.R.; O’Gara, F. Deciphering the role of coumarin as a novel quorum sensing inhibitor suppressing virulence phenotypes in bacterial pathogens. Appl. Microbiol. Biotechnol. 2015, 99, 3303–3316. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.M.; Jiang, F.; Zhang, G.L.; Wang, J.Y.; Zhu, Y.H.; Liu, X.Y. Inhibition of Hafnia alvei H4 Biofilm Formation by the Food Additive Dihydrocoumarin. J. Food Prot. 2017, 12, 842–847. [Google Scholar] [CrossRef] [PubMed]
- D’Almeida, R.E.; Molina, R.D.I.; Viola, C.M.; Luciardi, M.C.; Nieto Peñalver, C.; Bardón, A.; Arena, M.E. Comparison of seven structurally related coumarins on the inhibition of Quorum sensing of Pseudomonas aeruginosa and Chromobacterium violaceum. Bioorg. Chem. 2017, 73, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Girennavar, B.; Cepeda, M.L.; Soni, K.A.; Vikram, A.; Jesudhasan, P.; Jayaprakasha, G.K.; Pillai, S.D.; Patil, B.S. Grapefruit juice and its furocoumarins inhibits autoinducer signaling and biofilm formation in bacteria. Int. J. Food Microbiol. 2008, 125, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Inchagova, K.S.; Duskaev, G.K.; Deryabin, D.G. The suppression of the “quorum sensing” Chromobacterium violaceum when exposed to combinations of amikacin with activated carbon or small molecules of plant origin (pyrogallol and coumarin). Microbiology 2019, 88, 72–82. [Google Scholar] [CrossRef]
- Deryabin, D.G.; Inchagova, K.S. Inhibitory effect of aminoglycosides and tetracyclines on Quorum sensing in Chromobacterium violaceum. Microbiology 2018, 87, 1–8. [Google Scholar] [CrossRef]
- Abate, A.; Dimartino, V.; Spina, P.; Costa, P.L.; Lombardo, C.; Santini, A.; Del Piano, M.; Alimonti, P. Hymecromone in the treatment of motor disorders of the bileducts: A multicenter, double-blind, placebo-controlled clinical study. Drugs Exp. Clin. Res. 2001, 27, 223–231. [Google Scholar]
- Božič-Mijovski, M. Advances in monitoring anticoagulant therapy. Adv. Clin. Chem. 2019, 90, 197–213. [Google Scholar] [CrossRef]
- Dávila-Fajardo, C.L.; Díaz-Villamarín, X.; Antúnez-Rodríguez, A.; Fernández-Gómez, A.E.; García-Navas, P.; Martínez-González, L.J.; Dávila-Fajardo, J.A.; Barrera, J.C. Pharmacogenetics in the treatment of cardiovascular diseases and its current progres regarding implementation in the clinical routine. Genes 2019, 10, 261. [Google Scholar] [CrossRef]
- Opherk, D.; Schuler, G.; Waas, W.; Dietz, R.; Kubler, W. Intravenous carbochromen: A potent and effective drug for estimation of coronary dilatory capacity. Eur. Heart J. 1990, 11, 342–347. [Google Scholar] [CrossRef]
- Gierlak, W.; Kuch, M. How to use cardiac drugs in everyday practice? In Anticoagulants in Cardiology—Vitamin K Antagonists; Czelej, Lublin 1.; 2010; Chapter 6. [Google Scholar]
- Salvo, F.; Bezin, J.; Bosco-Levy, P.; Letinier, L.; Blin, P.; Pariente, A.; Moore, N. Pharmacological treatments of cardiovascular diseases: Evidence from real-life studies. Pharmacol. Res. 2017, 118, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Patel, R.; Kumari, P.; Patel, N. In vitro antimicrobial assessment of coumarin-based s-triazinyl piperazines. Med. Chem. Res. 2012, 21, 1611–1624. [Google Scholar] [CrossRef]
- López, F.; Deglesne, J.; Arroyo, P.A.; Ranneva, R.; Deprez, E. 4hexylresorcinol-a-new-molecule-for-cosmetic-application. J. Biomol. Res. Ther. 2018, 8, 1000170. [Google Scholar]
- Aguedo, M.; Beney, L.; Waché, Y.; Belin, J.M. Interaction of an odorant lactone with model phospholipid bilayers and its strong fluidizing action in yeast membrane. Int. J. Food Microbiol. 2003, 80, 211–215. [Google Scholar] [CrossRef]
- Inchagova, K.S.; Deryabin, D.G.; Duskaev, G.K.; Karimov, I.F.; Ryazanov, V.A. Plant molecules influence on luminescent Escherichia coli K12 TG1 with1constitutiveexpressed luxCDABE genes. FEBS OpenBio 2019, 9, 303–304. [Google Scholar]
- Zhang, J.; Jiang, C.S. Synthesis and evaluation of coumarin/piperazine hybrids as acetylcholinesterase inhibitors. Med. Chem. Res. 2018, 27, 1717–1727. [Google Scholar] [CrossRef]
- Brackman, G.; Hillaert, U.; Van Calenbergh, S.; Nelis, H.J.; Coenye, T. Use of quorum sensing inhibitors to interfere with biofilm formation and development in Burkholderia multivorans and Burkholderia cenocepacia. Res. Microbiol. 2009, 160, 144–151. [Google Scholar] [CrossRef]
- Lindsay, A.; Ahmer, B.M. Effect of sdiA on biosensors of N-acylhomoserine lactones. J. Bacteriol. 2005, 187, 5054–5058. [Google Scholar] [CrossRef]
- Ding, X.; Yin, B.; Qian, L.; Zeng, Z.; Yang, Z.; Li, H.; Lu, Y.; Zhou, S. Screening for novel quorum-sensing inhibitors to interfere with the formation of Pseudomonas aeruginosa biofilm. J. Med. Microbiol. 2011, 60, 1827–1834. [Google Scholar] [CrossRef]
- Durig, A.; Kouskoumvekaki, I.; Vejborg, R.M.; Klemm, P. Chemoinformatics-assisted development of new anti-biofilm compounds. Appl. Microbiol. Biotechnol. 2010, 87, 309–317. [Google Scholar] [CrossRef]
- Todorov, L.; Traykova, M.; Traykov, T. In Vitro Evaluation of the Antioxidant Effect of Yohimbine, Proceedings of the IV European Congress on Toxicology; EUROTOX: Krakow, Poland, 2005; Poster No. 27. [Google Scholar]
- Tyagi, Y.K.; Kumar, A.; Raj, H.G.; Vohra, P.; Gupta, G.; Kumari, R.; Kumar, P.; Gupta, R.K. Synthesis of novel amino and acetyl amino-4-methylcoumarins and evaluation of their antioxidant activity. Eur. J. Med. Chem. 2005, 40, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ding, W.; Xu, Y.Q.; Wu, D.S.; Li, S.L.; Chen, J.N.; Guo, B. New insights into the antibacterial activity of hydroxycoumarins against Ralstonia Solanacearum. Molecules 2016, 21, 468. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.G.; Cho, H.S.; Ryu, S.Y.; Cho, M.H.; Lee, J. Coumarins reduce biofilm formation and the virulence of Escherichia coli O157:H7. Phytomedicine 2014, 21, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Deryabin, D.G.; Kamayeva, A.A.; Tolmacheva, A.A.; El-Registan, G.I. The effects of alkylhydroxybenzenes on homoserine lactone-induced manifestations of quorum sensing in bacteria. Appl. Biochem. Microbiol. 2014, 50, 353–358. [Google Scholar] [CrossRef]
- Stauff, D.L.; Bassler, B.L. Quorum sensing in Chromobacterium violaceum: DNA recognition and gene regulation by the CviR receptor. J. Bacteriol. 2011, 193, 3871–3878. [Google Scholar] [CrossRef]
- Tallarida, R.J. An overview of drug combination analysis with isobolograms: Perspectives in pharmacology. Pharmacol. Exp. Ther. 2006, 3, 1–7. [Google Scholar] [CrossRef]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A review of antibiotic use in food animals: Perspective, policy, and potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef]
| Tested Compound | Characteristics of Antibacterial Activity, mg/mL | Characteristics of Anti-QS Activity, mg/mL | ||
|---|---|---|---|---|
| MIC100 | MIC50 | EC100 | EC50 | |
| Coumarin | 3.650 | 2.689 | 3.650 | 1.105 |
| 7-hydroxycoumarin | 1.267 | 0.497 | 0.633 | 0.199 |
| 7.8-dihydroxy-4-methylcoumarin | 2.400 | 0.325 | 1.200 | 0.150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deryabin, D.; Inchagova, K.; Rusakova, E.; Duskaev, G. Coumarin’s Anti-Quorum Sensing Activity Can Be Enhanced When Combined with Other Plant-Derived Small Molecules. Molecules 2021, 26, 208. https://doi.org/10.3390/molecules26010208
Deryabin D, Inchagova K, Rusakova E, Duskaev G. Coumarin’s Anti-Quorum Sensing Activity Can Be Enhanced When Combined with Other Plant-Derived Small Molecules. Molecules. 2021; 26(1):208. https://doi.org/10.3390/molecules26010208
Chicago/Turabian StyleDeryabin, Dmitry, Kseniya Inchagova, Elena Rusakova, and Galimzhan Duskaev. 2021. "Coumarin’s Anti-Quorum Sensing Activity Can Be Enhanced When Combined with Other Plant-Derived Small Molecules" Molecules 26, no. 1: 208. https://doi.org/10.3390/molecules26010208
APA StyleDeryabin, D., Inchagova, K., Rusakova, E., & Duskaev, G. (2021). Coumarin’s Anti-Quorum Sensing Activity Can Be Enhanced When Combined with Other Plant-Derived Small Molecules. Molecules, 26(1), 208. https://doi.org/10.3390/molecules26010208






