Immobilization of Eversa® Transform via CLEA Technology Converts It in a Suitable Biocatalyst for Biolubricant Production Using Waste Cooking Oil
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization of the Utilized Oils
2.2. Performance of Liquid and Immobilized Eversa
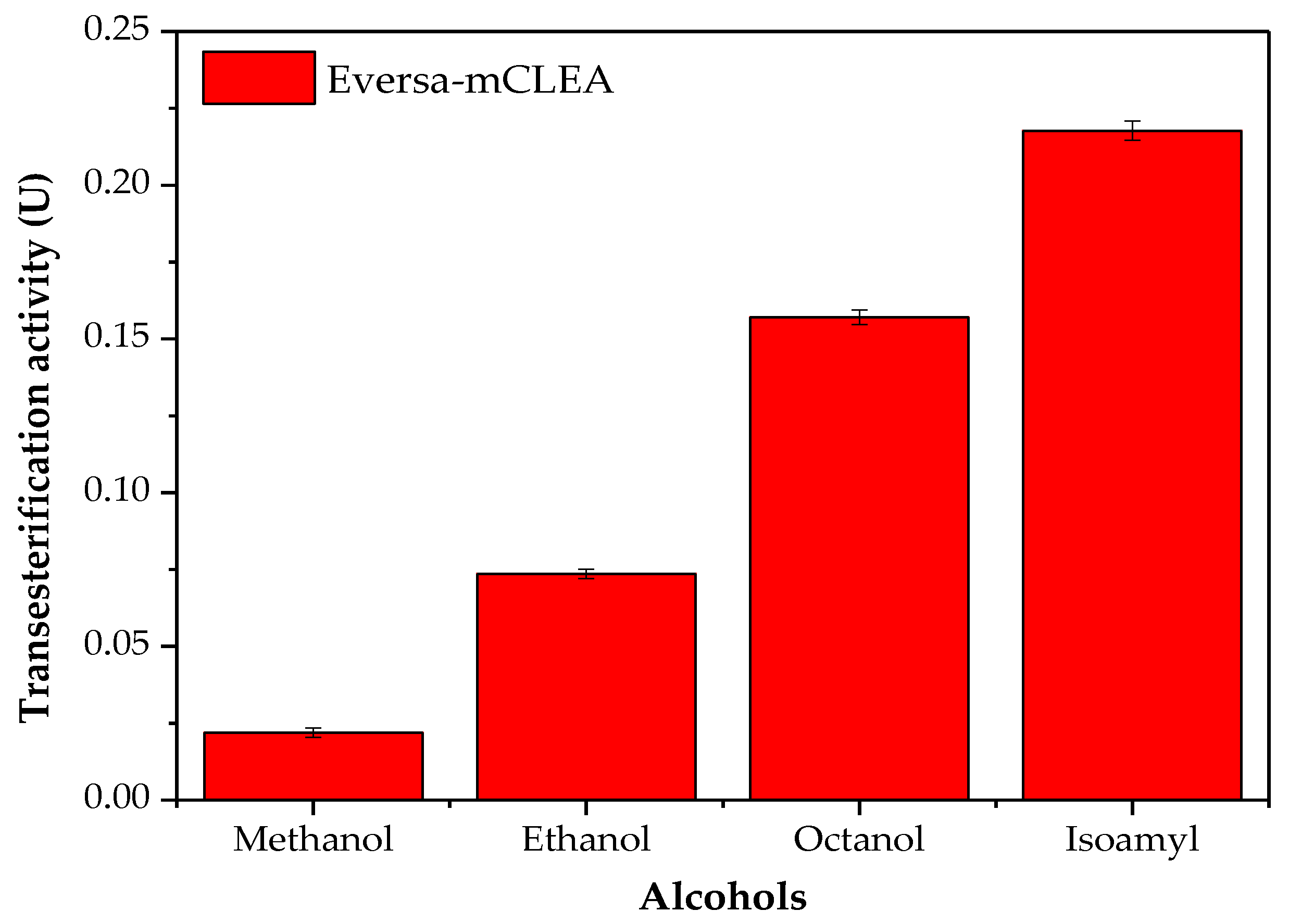
2.3. Effect of the Alcohol Chain on the Transesterification Activity of Immobilized Eversa
2.4. Selection of Oil for Isoamyl Ester Synthesis
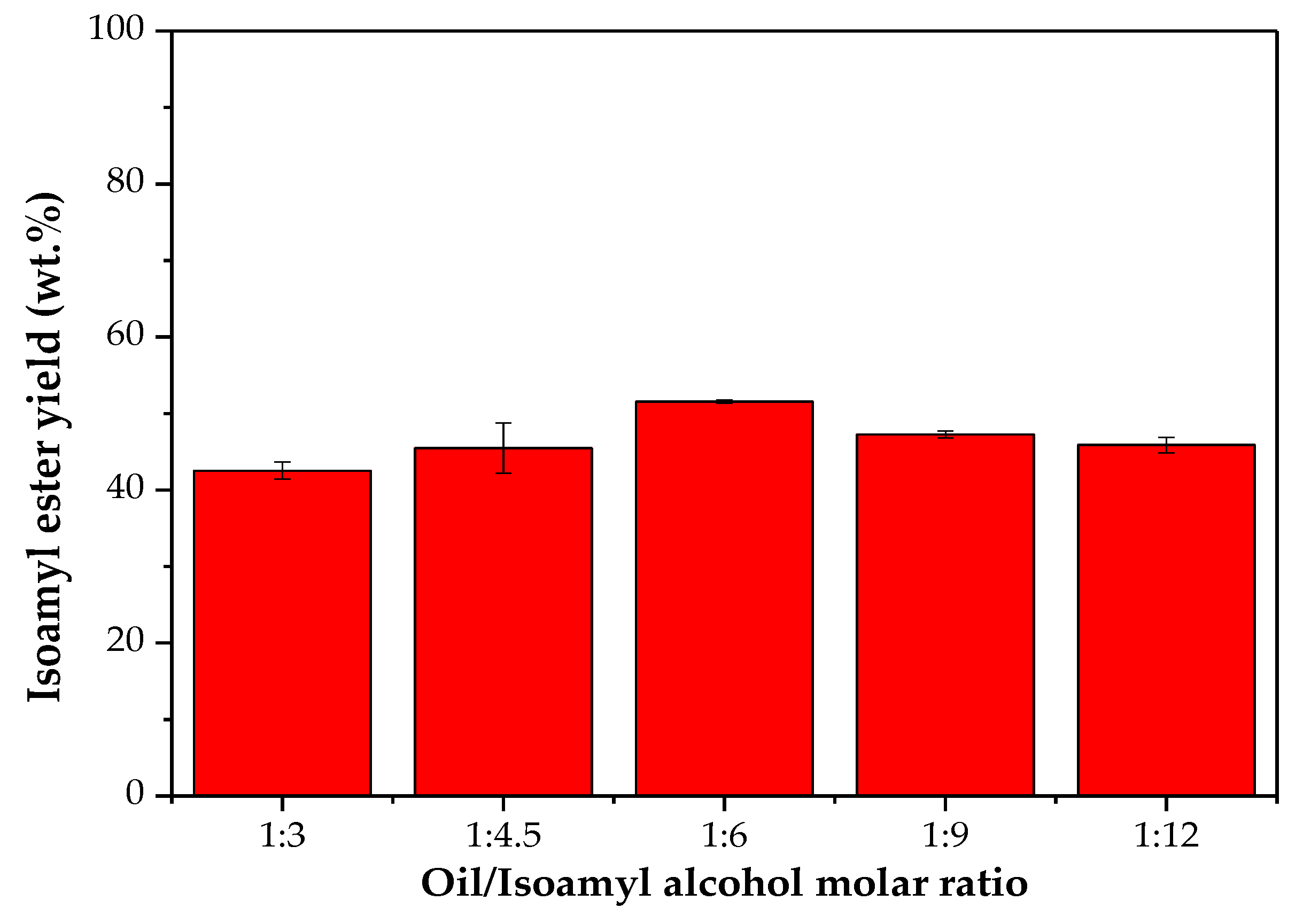
2.5. Effect of the Oil/Alcohol Molar Ratio on the Ester Yield
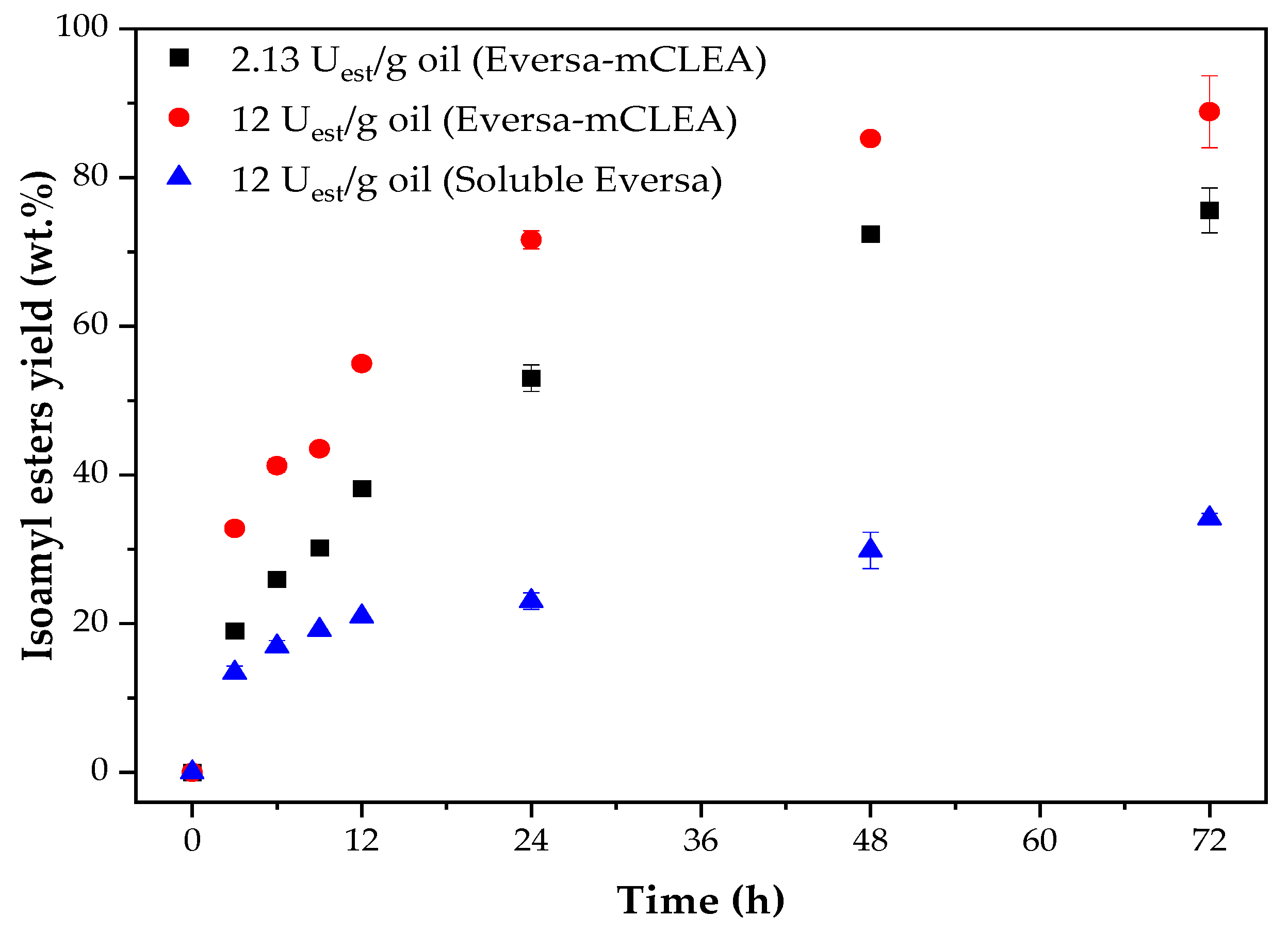
2.6. Effect of Enzyme Load on the Transesterification of WCO and Isoamyl Alcohol
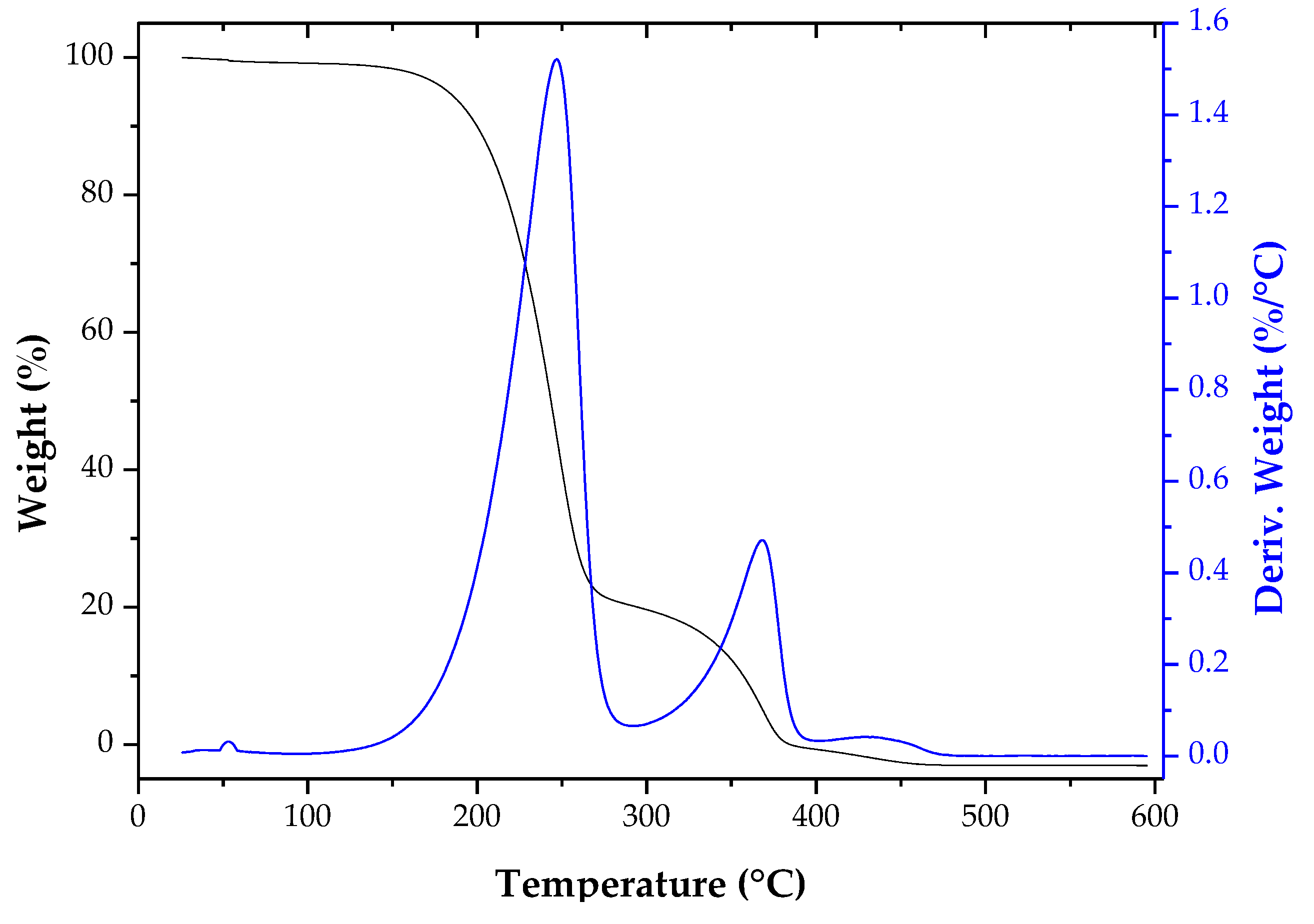
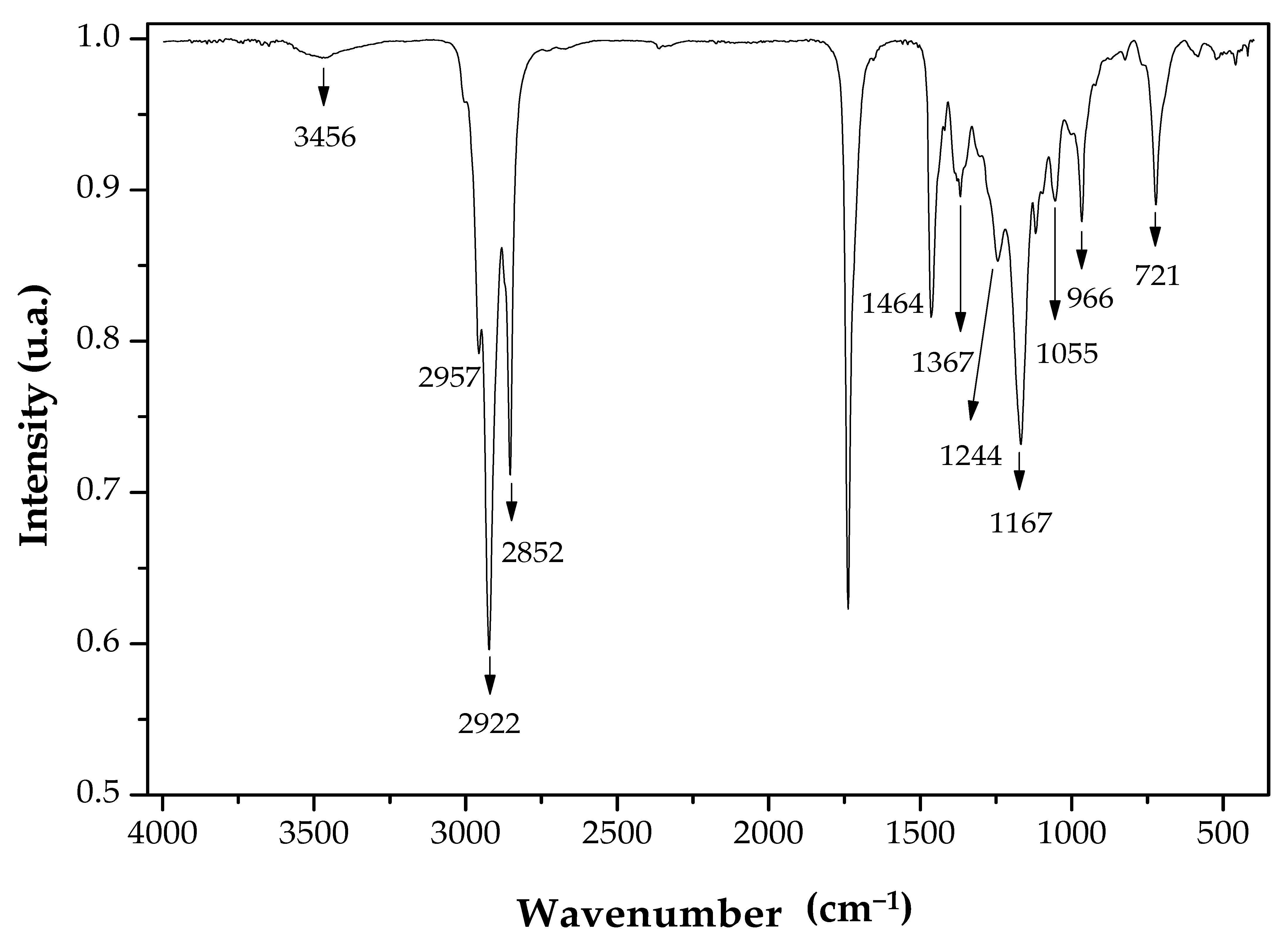
2.7. Characterization of the Fatty Acid Isoamyl Esters
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Characterization of the Oils Used in Synthesis of the Biolubricant
3.2.2. Preparation of Eversa-mCLEA
3.2.3. Enzyme Activity Assays
3.2.4. Transesterification/Esterification Reaction Using Liquid and Immobilized Eversa
3.2.5. Characterization of the Biolubricant
Gas and Liquid Chromatography Analysis
ATR-FTIR and TGA Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Avisha, C.; Debarati, M.; Dipa, B. Biolubricant synthesis from waste cooking oil via enzymatic hydrolysis followed by chemical esterification. J. Chem. Technol. Biotechnol. 2013, 88, 139–144. [Google Scholar]
- Chowdhury, A.; Mitra, D. A kinetic study on the Novozyme 435-catalyzed esterification of free fatty acids with octanol to produce octyl esters. Biotechnol. Prog. 2015, 31, 1494–1499. [Google Scholar] [PubMed]
- Lage, F.A.P.; Bassi, J.J.; Corradini, M.C.C.; Todero, L.M.; Luiz, J.H.H.; Mendes, A.A. Preparation of a biocatalyst via physical adsorption of lipase from Thermomyces lanuginosus on hydrophobic support to catalyze biolubricant synthesis by esterification reaction in a solvent-free system. Enzym. Microb. Technol. 2016, 84, 56–67. [Google Scholar]
- Silva, A.P.T.; Bredda, E.H.; Castro, H.F.; Rós, P.C.M. Enzymatic catalysis: An environmentally friendly method to enhance the transesterification of microalgal oil with fusel oil for production of fatty acid esters with potential application as biolubricants. Fuel 2020, 273, 117786. [Google Scholar]
- Cerón, A.A.; Vilas Boas, R.N.; Biaggio, F.C.; de Castro, H.F. Synthesis of biolubricant by transesterification of palm kernel oil with simulated fusel oil: Batch and continuous processes. Biomass Bioenergy 2018, 119, 166–172. [Google Scholar]
- Encinar, J.M.; Nogales-Delgado, S.; Sánchez, N.; González, J.F. Biolubricants from rapeseed and castor oil transesterification by using Titanium isopropoxide as a catalyst: Production and characterization. Catalysts 2020, 10, 366. [Google Scholar]
- Kania, D.; Yunus, R.; Omar, R.; Rashid, S.A.; Jan, B.M. A review of biolubricants in drilling fluids: Recent research, performance, and applications. J. Pet. Sci. Eng. 2015, 135, 177–184. [Google Scholar]
- Sarno, M.; Iuliano, M.; Cirillo, C. Optimized procedure for the preparation of an enzymatic nanocatalyst to produce a bio-lubricant from waste cooking oil. Chem. Eng. J. 2019, 377, 120273. [Google Scholar]
- Zulkifli, N.W.M.; Azman, S.S.N.; Kalam, M.A.; Masjuki, H.H.; Yunus, R.; Gulzar, M. Lubricity of bio-based lubricant derived from different chemically modified fatty acid methyl ester. Tribol. Int. 2016, 93, 555–562. [Google Scholar]
- Happe, M.; Kouadio, M.; Treanor, C.; Sawall, J.P.; Fornage, A.; Sugnaux, M.; Fischer, F. Size selectivity in lipase catalysed tetrol acylation. J. Mol. Catal. B Enzym. 2014, 109, 40–46. [Google Scholar]
- Idrus, N.F.; Yunus, R.; Abidin, Z.Z.; Rashid, U.; Rahman, N.A. High oleic pentaerythritol tetraester formation via transesterification: Effect of reaction conditions. Indones. J. Chem. 2020, 20, 887–898. [Google Scholar]
- Akerman, C.O.; Gaber, Y.; Ghani, N.A.; Lämsä, M.; Hatti-Kaul, R. Clean synthesis of biolubricants for low temperature applications using heterogeneous catalysts. J. Mol. Catal. B Enzym. 2011, 72, 263–269. [Google Scholar]
- Abdelmoez, W.; Mustafa, A. Oleochemical industry future through biotechnology. J. Oleo Sci. 2014, 63, 545–554. [Google Scholar] [PubMed]
- Zhang, W.; Ji, H.; Song, Y.; Ma, S.; Xiong, W.; Chen, C.; Chen, B.; Zhang, X. Green preparation of branched biolubricant by chemically modifying waste cooking oil with lipase and ionic liquid. J. Clean. Prod. 2020, 274, 122918. [Google Scholar]
- Nagendramma, P.; Kaul, S. Development of ecofriendly/biodegradable lubricants: An overview. Renew. Sustain. Energy Rev. 2012, 16, 764–774. [Google Scholar]
- Bassi, J.J.; Todero, L.M.; Lage, F.A.P.; Khedy, G.I.; Ducas, J.D.; Custódio, A.P.; Pinto, M.A.; Mendes, A.A. Interfacial activation of lipases on hydrophobic support and application in the synthesis of a lubricant ester. Int. J. Biol. Macromol. 2016, 92, 900–909. [Google Scholar]
- Kim, H.; Choi, N.; Kim, Y.; Kim, H.-R.; Lee, J.; Kim, I.-H. Immobilized lipase-catalyzed esterification for synthesis of trimethylolpropane triester as a biolubricant. Renew. Energy 2019, 130, 489–494. [Google Scholar]
- Kleinaitė, E.; Jaška, V.; Tvaska, B.; Matijošytė, I. A cleaner approach for biolubricant production using biodiesel as a starting material. J. Clean. Prod. 2014, 75, 40–44. [Google Scholar]
- Hajar, M.; Vahabzadeh, F. Biolubricant production from castor oil in a magnetically stabilized fluidized bed reactor using lipase immobilized on Fe3O4 nanoparticles. Ind. Crops Prod. 2016, 94, 544–556. [Google Scholar]
- Borges, M.E.; Díaz, L. Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: A review. Renew. Sustain. Energy Rev. 2012, 16, 2839–2849. [Google Scholar]
- Lotti, M.; Pleiss, J.; Valero, F.; Ferrer, P. Enzymatic production of biodiesel: Strategies to overcome methanol inactivation. Biotechnol. J. 2018, 13, 1700155. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.M.; Rancke-Madsen, A.; Holm, H.C.; Burton, R. Production of biodiesel using liquid lipase formulations. J. Am. Oil Chem. Soc. 2016, 93, 905–910. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Arana-Pena, S.; Rocha, T.N.; Miranda, L.P.; Berenguer-Murcia, A.; Tardioli, P.W.; Santos, J.C.S.; Fernandez-Lafuente, R. Liquid lipase preparations designed for industrial production of biodiesel. Is it really an optimal solution? Renew. Energy 2021, 164, 1566–1587. [Google Scholar] [CrossRef]
- Remonatto, D.; de Oliveira, J.V.; Guisan, J.M.; de Oliveira, D.; Ninow, J.; Fernandez-Lorente, G. Production of FAME and FAEE via alcoholysis of sunflower oil by Eversa lipases immobilized on hydrophobic supports. Appl. Biochem. Biotechnol. 2018, 185, 705–716. [Google Scholar] [CrossRef]
- Miranda, L.P.; Guimarães, J.R.; Giordano, R.C.; Fernandez-Lafuente, R.; Tardioli, P.W. Composites of crosslinked aggregates of Eversa® transform and magnetic nanoparticles. Performance in the ethanolysis of soybean oil. Catalysts 2020, 10, 817. [Google Scholar] [CrossRef]
- Bresolin, D.; Hawerroth, B.; de Oliveira Romera, C.; Sayer, C.; de Araújo, P.H.H.; de Oliveira, D. Immobilization of lipase Eversa Transform 2.0 on poly(urea–urethane) nanoparticles obtained using a biopolyol from enzymatic glycerolysis. Bioprocess. Biosyst. Eng. 2020, 43, 1279–1286. [Google Scholar] [CrossRef]
- Su, C.; Nguyen, H.C.; Nguyen, M.L.; Tran, P.T.; Wang, F.; Guan, Y. Liquid lipase-catalyzed hydrolysis of gac oil for fatty acid production: Optimization using response surface methodology. Biotechnol. Prog. 2018, 34, 1129–1136. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Kodali, S.; Balle, T.; Chen, B.; Guo, Z. Liquid lipases for enzymatic concentration of n-3 polyunsaturated fatty acids in monoacylglycerols via ethanolysis: Catalytic specificity and parameterization. Bioresour. Technol. 2017, 224, 445–456. [Google Scholar] [CrossRef]
- Gutierrez-Lazaro, A.; Velasco, D.; Boldrini, D.E.; Yustos, P.; Esteban, J.; Ladero, M. Effect of operating variables and kinetics of the lipase catalyzed transesterification of ethylene carbonate and glycerol. Fermentation 2018, 4, 75. [Google Scholar] [CrossRef]
- Martínez-Sanchez, J.A.; Arana-Peña, S.; Carballares, D.; Yates, M.; Otero, C.; Fernandez-Lafuente, R. Immobilized biocatalysts of Eversa® Transform 2.0 and lipase from Thermomyces lanuginosus: Comparison of some properties and performance in biodiesel production. Catalysts 2020, 10, 738. [Google Scholar]
- Bilal, M.; Asgher, M.; Shah, S.Z.H.; Iqbal, H.M.N. Engineering enzyme-coupled hybrid nanoflowers: The quest for optimum performance to meet biocatalytic challenges and opportunities. Int. J. Biol. Macromol. 2019, 135, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Wahab, R.A.; Elias, N.; Abdullah, F.; Ghoshal, S.K. On the taught new tricks of enzymes immobilization: An all-inclusive overview. React. Funct. Polym. 2020, 152, 104613. [Google Scholar] [CrossRef]
- Dal Magro, L.; Kornecki, J.F.; Klein, M.P.; Rodrigues, R.C.; Fernandez-Lafuente, R. Pectin lyase immobilization using the glutaraldehyde chemistry increases the enzyme operation range. Enzym. Microb. Technol. 2020, 132, 109397. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Monti, R.; Pessela, B.C.C.; Fuentes, M.; Torres, R.; Guisan, J.M.; Fernandez-Lafuente, R. Immobilization of lactase from Kluyveromyces lactis greatly reduces the inhibition promoted by glucose. Full hydrolysis of lactose in milk. Biotechnol. Prog. 2004, 20, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Fernandez–Lafuente, R.; Guisan, J.M.; Ali, S.; Cowan, D. Immobilization of functionally unstable catechol-2,3-dioxygenase greatly improves operational stability. Enzym. Microb. Technol. 2000, 26, 568–573. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization–purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef]
- Silveira, E.A.; Moreno-Perez, S.; Basso, A.; Serban, S.; Pestana-Mamede, R.; Tardioli, P.W.; Farinas, C.S.; Castejon, N.; Fernandez-Lorente, G.; Rocha-Martin, J.; et al. Biocatalyst engineering of Thermomyces lanuginosus lipase adsorbed on hydrophobic supports: Modulation of enzyme properties for ethanolysis of oil in solvent-free systems. J. Biotechnol. 2019, 289, 126–134. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Torrestiana-Sánchez, B.; Dal Magro, L.; Virgen-Ortíz, J.J.; Suárez-Ruíz, F.J.; Rodrigues, R.C.; Fernandez-Lafuente, R. Comparison of acid, basic and enzymatic catalysis on the production of biodiesel after RSM optimization. Renew. Energy 2019, 135, 1–9. [Google Scholar] [CrossRef]
- Honaiser, T.C.; Ficanha, A.M.M.; Dallago, R.M.; Oliveira, D.; Oliveira, J.V.; Paroul, N.; Mignoni, M.L. Immobilization of lipase NS-40116 (Thermomyces lanuginosus) by sol-gel technique using polyethyleneglycol as additive. Ind. Biotechnol. 2019, 15, 35–40. [Google Scholar] [CrossRef]
- Facin, B.R.; Valério, A.; Bresolin, D.; Centenaro, G.; de Oliveira, D.; Oliveira, J.V. Improving reuse cycles of Thermomyces lanuginosus lipase (NS-40116) by immobilization in flexible polyurethane. Biocatal. Biotransformation 2018, 36, 372–380. [Google Scholar] [CrossRef]
- Dantas, A.; Valério, A.; Ninow, J.L.; Oliveira, J.V.; Oliveira, D. Potential application of Thermomyces lanuginosus lipase (TLL) immobilized on nonporous polystyrene particles. Env. Prog. Sustain. Energy 2019, 38, 608–613. [Google Scholar] [CrossRef]
- Bresolin, D.; Estrella, A.S.; da Silva, J.R.P.; Valério, A.; Sayer, C.; de Araújo, P.H.H.; de Oliveira, D. Synthesis of a green polyurethane foam from a biopolyol obtained by enzymatic glycerolysis and its use for immobilization of lipase NS-40116. Bioprocess. Biosyst. Eng. 2019, 42, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Schoevaart, R.; Van Langen, L.M. Cross-linked enzyme aggregates (CLEAs): A novel and versatile method for enzyme immobilization (a review). Biocatal. Biotransformation 2005, 23, 141–147. [Google Scholar] [CrossRef]
- Cao, L.; van Rantwijk, F.; Sheldon, R.A. Cross-linked enzyme aggregates: A simple and effective method for the immobilization of penicillin acylase. Org. Lett. 2000, 2, 1361–1364. [Google Scholar] [CrossRef]
- Sheldon, R. CLEAs, combi-CLEAs and ‘smart’ magnetic CLEAs: Biocatalysis in a bio-based economy. Catalysts 2019, 9, 261. [Google Scholar] [CrossRef]
- Sheldon, R.A. Cross-linked enzyme aggregates as industrial biocatalysts. Org. Process. Res. Dev. 2011, 15, 213–223. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Wang, M.; Jia, C.; Qi, W.; Yu, Q.; Peng, X.; Su, R.; He, Z. Porous-CLEAs of papain: Application to enzymatic hydrolysis of macromolecules. Bioresour. Technol. 2011, 102, 3541–3545. [Google Scholar] [CrossRef]
- Talekar, S.; Shah, V.; Patil, S.; Nimbalkar, M. Porous cross linked enzyme aggregates (p-CLEAs) of Saccharomyces cerevisiae invertase. Catal. Sci. Technol. 2012, 2, 1575–1579. [Google Scholar] [CrossRef]
- Khorshidi, K.J.; Lenjannezhadian, H.; Jamlan, M.; Zeinali, M. Preparation and characterization of nanomagnetic cross-linked cellulase aggregates for cellulose bioconversion. J. Chem. Technol. Biotechnol. 2016, 91, 539–546. [Google Scholar] [CrossRef]
- Kopp, W.; Da Costa, T.P.; Pereira, S.C.; Jafelicci, M.; Giordano, R.C.; Marques, R.F.C.; Araújo-Moreira, F.M.; Giordano, R.L.C. Easily handling penicillin G acylase magnetic cross-linked enzymes aggregates: Catalytic and morphological studies. Process. Biochem. 2014, 49, 38–46. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rathod, V.K. Magnetic macromolecular cross linked enzyme aggregates (CLEAs) of glucoamylase. Enzym. Microb. Technol. 2016, 83, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Pletschke, B.I. Magnetic cross-linked enzyme aggregates (CLEAs): A novel concept towards carrier free immobilization of lignocellulolytic enzymes. Enzym. Microb. Technol. 2014, 61–62, 17–27. [Google Scholar] [CrossRef]
- Zhang, W.-W.; Yang, X.-L.; Jia, J.-Q.; Wang, N.; Hu, C.-L.; Yu, X.-Q. Surfactant-activated magnetic cross-linked enzyme aggregates (magnetic CLEAs) of Thermomyces lanuginosus lipase for biodiesel production. J. Mol. Catal. B Enzym. 2015, 115, 83–89. [Google Scholar] [CrossRef]
- Cruz-Izquierdo, Á.; Picó, E.A.; López, C.; Serra, J.L.; Llama, M.J. Magnetic cross-linked enzyme aggregates (mCLEAs) of Candida antarctica lipase: An efficient and stable biocatalyst for biodiesel synthesis. PLoS ONE 2014, 9, e115202. [Google Scholar] [CrossRef]
- Cui, J.D.; Cui, L.L.; Zhang, S.P.; Zhang, Y.F.; Su, Z.G.; Ma, G.H. Hybrid magnetic cross-linked enzyme aggregates of phenylalanine ammonia lyase from rhodotorula glutinis. PLoS ONE 2014, 9, e97221. [Google Scholar] [CrossRef]
- Loizides, M.; Loizidou, X.; Orthodoxou, D.; Petsa, D. Circular bioeconomy in action: Collection and recycling of domestic used cooking oil through a social, reverse logistics system. Recycling 2019, 4, 16. [Google Scholar] [CrossRef]
- Manley, J.B.; Anastas, P.T.; Cue, B.W. Frontiers in green chemistry: Meeting the grand challenges for sustainability in R&D and manufacturing. J. Clean. Prod. 2008, 16, 743–750. [Google Scholar]
- Viornery-Portillo, E.A.; Bravo-Díaz, B.; Mena-Cervantes, V.Y. Life cycle assessment and emission analysis of waste cooking oil biodiesel blend and fossil diesel used in a power generator. Fuel 2020, 281, 118739. [Google Scholar] [CrossRef]
- Cabral, N.M.; Lorenti, J.P.; Plass, W.; Gallo, J.M.R. Solid acid resin Amberlyst 45 as a catalyst for the transesterification of vegetable oil. Front. Chem. 2020, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mansir, N.; Taufiq-Yap, Y.H.; Rashid, U.; Lokman, I.M. Investigation of heterogeneous solid acid catalyst performance on low grade feedstocks for biodiesel production: A review. Energy Convers. Manag. 2017, 141, 171–182. [Google Scholar] [CrossRef]
- FAO. Codex Alimentarius. CXS 210-1999 Standard for Named Vegetable Oils. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B210-1999%252FCXS_210e.pdf (accessed on 1 January 2021).
- Dörmő, N.; Bélafi-Bakó, K.; Bartha, L.; Ehrenstein, U.; Gubicza, L. Manufacture of an environmental-safe biolubricant from fusel oil by enzymatic esterification in solvent-free system. Biochem. Eng. J. 2004, 21, 229–234. [Google Scholar] [CrossRef]
- Su, E.; Wei, D. Production of fatty acid butyl esters using the low cost naturally immobilized Carica papaya lipase. J. Agric. Food Chem. 2014, 62, 6375–6381. [Google Scholar] [CrossRef]
- Trivedi, J.; Aila, M.; Sharma, C.D.; Gupta, P.; Kaul, S. Clean synthesis of biolubricant range esters using novel liquid lipase enzyme in solvent free medium. Springerplus 2015, 4, 165. [Google Scholar] [CrossRef]
- Raheem, I.; Mohiddin, M.N.B.; Tan, Y.H.; Kansedo, J.; Mubarak, N.M.; Abdullah, M.O.; Ibrahim, M.L. A review on influence of reactor technologies and kinetic studies for biodiesel application. J. Ind. Eng. Chem. 2020, 91, 54–68. [Google Scholar] [CrossRef]
- Díaz-Pérez, M.A.; Serrano-Ruiz, J.C. Catalytic production of jet fuels from biomass. Molecules 2020, 25, 802. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Syazmi, Z.A.H.S.; Mofijur, M.; Abas, A.E.P.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent landscape review on biodiesel production: Technology updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- Yesiloglu, Y. Immobilized lipase-catalyzed ethanolysis of sunflower oil. J. Am. Oil Chem. Soc. 2004, 81, 157–160. [Google Scholar] [CrossRef]
- Hazarika, S.; Goswami, P.; Dutta, N.N.; Hazarika, A.K. Ethyl oleate synthesis by porcine pancreatic lipase in organic solvents. Chem. Eng. J. 2002, 85, 61–68. [Google Scholar] [CrossRef]
- Bonet-Ragel, K.; Canet, A.; Benaiges, M.D.; Valero, F. Effect of acyl-acceptor stepwise addition strategy using alperujo oil as a substrate in enzymatic biodiesel synthesis. J. Chem. Technol. Biotechnol. 2018, 93, 541–547. [Google Scholar] [CrossRef]
- Chowdhury, A.; Sarkar, D.; Mitra, D. Esterification of free fatty acids derived from waste cooking oil with octanol: Process optimization and kinetic modeling. Chem. Eng. Technol. 2016, 39, 730–740. [Google Scholar] [CrossRef]
- Remonatto, D.; Santin, C.M.T.; de Oliveira, D.; Di Luccio, M.; de Oliveira, J.V. FAME production from waste oils through commercial soluble lipase Eversa® catalysis. Ind. Biotechnol. 2016, 12, 254–262. [Google Scholar] [CrossRef]
- Mibielli, G.M.; Fagundes, A.P.; Bender, J.P.; Oliveira, J.V. Lab and pilot plant FAME production through enzyme-catalyzed reaction of low-cost feedstocks. Bioresour. Technol. Rep. 2019, 5, 150–156. [Google Scholar] [CrossRef]
- Fernandes, K.V.; Papadaki, A.; Silva, J.A.C.; Fernandez-Lafuente, R.; Koutinas, A.A.; Freire, D.M.G. Enzymatic esterification of palm fatty-acid distillate for the production of polyol esters with biolubricant properties. Ind. Crops Prod. 2018, 116, 90–96. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Fraga-García, P.; Eigenfeld, M.; Becker, T.M.; Berensmeier, S. Magnetic separation in bioprocessing beyond the analytical scale: From biotechnology to the food industry. Front. Bioeng. Biotechnol. 2019, 7, 233. [Google Scholar] [CrossRef]
- Brechmann, N.A.; Eriksson, P.O.; Eriksson, K.; Oscarsson, S.; Buijs, J.; Shokri, A.; Hjälm, G.; Chotteau, V. Pilot-scale process for magnetic bead purification of antibodies directly from non-clarified CHO cell culture. Biotechnol. Prog. 2019, 35, 1–10. [Google Scholar] [CrossRef]
- Brown, G.N.; Müller, C.; Theodosiou, E.; Franzreb, M.; Thomas, O.R.T. Multi-cycle recovery of lactoferrin and lactoperoxidase from crude whey using fimbriated high-capacity magnetic cation exchangers and a novel “rotor–stator” high-gradient magnetic separator. Biotechnol. Bioeng. 2013, 110, 1714–1725. [Google Scholar] [CrossRef]
- Ebeler, M.; Pilgram, F.; Wolz, K.; Grim, G.; Franzreb, M. Magnetic separation on a new level: Characterization and performance prediction of a cGMP compliant “rotor-stator” high-gradient magnetic separator. Biotechnol. J. 2018, 13, 1700448. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.S.; Kriz, G.S. Introduction to Spectroscopy: A Guide for Students of Organic Chemistry, 2nd ed.; Saunders College Publishing: Philadelphia, PA, USA, 1996. [Google Scholar]
- AOCS Official Method Ca 5a-40. Free Fatty Acids in Crude and Refined Fats and Oils. Official Methods and Recommended Practices of the AOCS, 4th ed.; AOCS Press: Champaign, IL, USA, 1990. [Google Scholar]
- AOCS Official Method Cd 3-25. Saponification Value of Fats and Oils. Official Methods and Recommended Practices of the AOCS, 4th ed.; AOCS Press: Champaign, IL, USA, 1990. [Google Scholar]
- Margreth, M.; Schlink, R.; Steinbach, A. Water determination by Karl Fischer titration. In Pharmaceutical Sciences Encyclopedia; Gard, S.C., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Paula, A.V.; Moreira, A.B.R.; Braga, L.P.; Castro HF, d.e.; Bruno, L.M. Comparação do desempenho da lipase de Candida rugosa imobilizada em suporte híbrido de polissiloxano-polivinilálcool empregando diferentes metodologias. Quim. Nova 2008, 31, 35–40. [Google Scholar] [CrossRef]
- Rukunudin, I.H.; White, P.J.; Bern, C.J.; Bailey, T.B. A modified method for determining free fatty acids from small soybean oil sample sizes. J. Am. Oil Chem. Soc. 1998, 75, 563–568. [Google Scholar] [CrossRef]
- Duvekot, C. Determination of Total FAME and Linolenic Acid Methyl Esters in Biodiesel According to EN-14103; Agilent Technologies, Inc.: Santa Clara, CA, USA, 2020. [Google Scholar]
- Holčapek, M.; Jandera, P.; Fischer, J.; Prokeš, B. Analytical monitoring of the production of biodiesel by high-performance liquid chromatography with various detection methods. J. Chromatogr. A 1999, 858, 13–31. [Google Scholar] [CrossRef]
- Solmaz, H. Combustion, performance and emission characteristics of fusel oil in a spark ignition engine. Fuel Process. Technol. 2015, 133, 20–28. [Google Scholar] [CrossRef]
- Ardebili, S.M.S.; Solmaz, H.; İpci, D.; Calam, A.; Mostafaei, M. A review on higher alcohol of fusel oil as a renewable fuel for internal combustion engines: Applications, challenges, and global potential. Fuel 2020, 279, 118516. [Google Scholar] [CrossRef]
| Parameter | Oleic Acid | Refined Oil | Waste Cooking Oil |
|---|---|---|---|
| Acidity index (mgKOH/g) | ≥99.0 a | 0.12 ± 0.0048 | 3.93 ± 0.08 |
| Saponification index (mgKOH/g) | 206.53 ± 4.97 | 195.31 ± 1.53 | 189.77 ± 8.4 |
| Water content (%) | 0.15 ± 0.0011 | 0.056 ± 0.0029 | 0.081 ± 0.0034 |
| Alcohol | Ester Yield (wt.%) | |
|---|---|---|
| Liquid Eversa | Eversa-mCLEA | |
| Octanol | 34.97 ± 0.96 | 51.18 ± 1.18 |
| Isoamyl | 36.54 ± 0.89 | 52.71 ± 0.72 |
| Acyl Donors | Ester Yield (wt.%) * |
|---|---|
| Refined soybean oil | 52.60 a |
| Waste cooking oil | 51.55 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guimarães, J.R.; Miranda, L.P.; Fernandez-Lafuente, R.; Tardioli, P.W. Immobilization of Eversa® Transform via CLEA Technology Converts It in a Suitable Biocatalyst for Biolubricant Production Using Waste Cooking Oil. Molecules 2021, 26, 193. https://doi.org/10.3390/molecules26010193
Guimarães JR, Miranda LP, Fernandez-Lafuente R, Tardioli PW. Immobilization of Eversa® Transform via CLEA Technology Converts It in a Suitable Biocatalyst for Biolubricant Production Using Waste Cooking Oil. Molecules. 2021; 26(1):193. https://doi.org/10.3390/molecules26010193
Chicago/Turabian StyleGuimarães, José Renato, Letícia Passos Miranda, Roberto Fernandez-Lafuente, and Paulo Waldir Tardioli. 2021. "Immobilization of Eversa® Transform via CLEA Technology Converts It in a Suitable Biocatalyst for Biolubricant Production Using Waste Cooking Oil" Molecules 26, no. 1: 193. https://doi.org/10.3390/molecules26010193
APA StyleGuimarães, J. R., Miranda, L. P., Fernandez-Lafuente, R., & Tardioli, P. W. (2021). Immobilization of Eversa® Transform via CLEA Technology Converts It in a Suitable Biocatalyst for Biolubricant Production Using Waste Cooking Oil. Molecules, 26(1), 193. https://doi.org/10.3390/molecules26010193







