Macrophage Migration Inhibitory Factor (MIF) and Its Homologue D-Dopachrome Tautomerase (DDT) Inversely Correlate with Inflammation in Discoid Lupus Erythematosus
Abstract
1. Introduction
2. Results
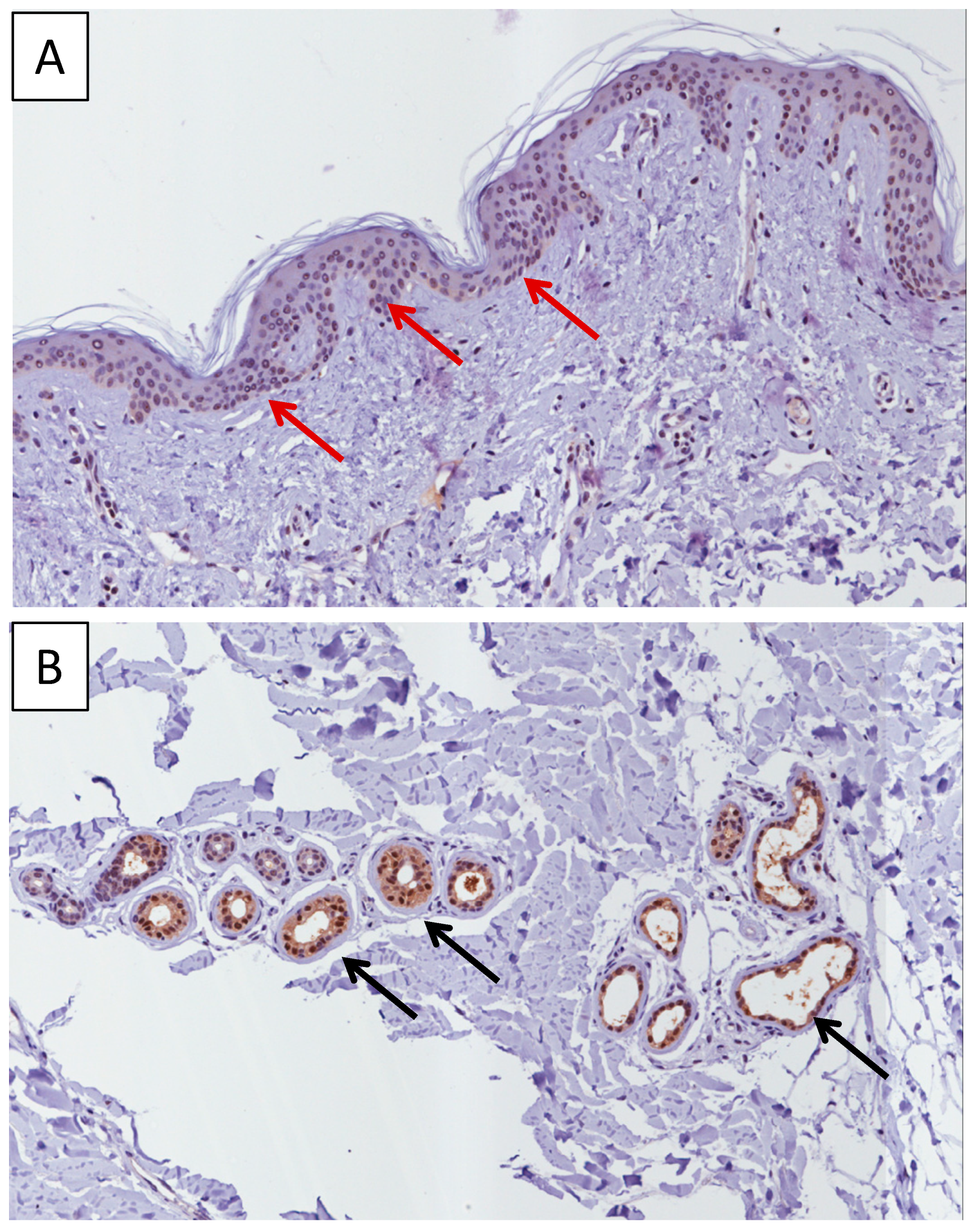
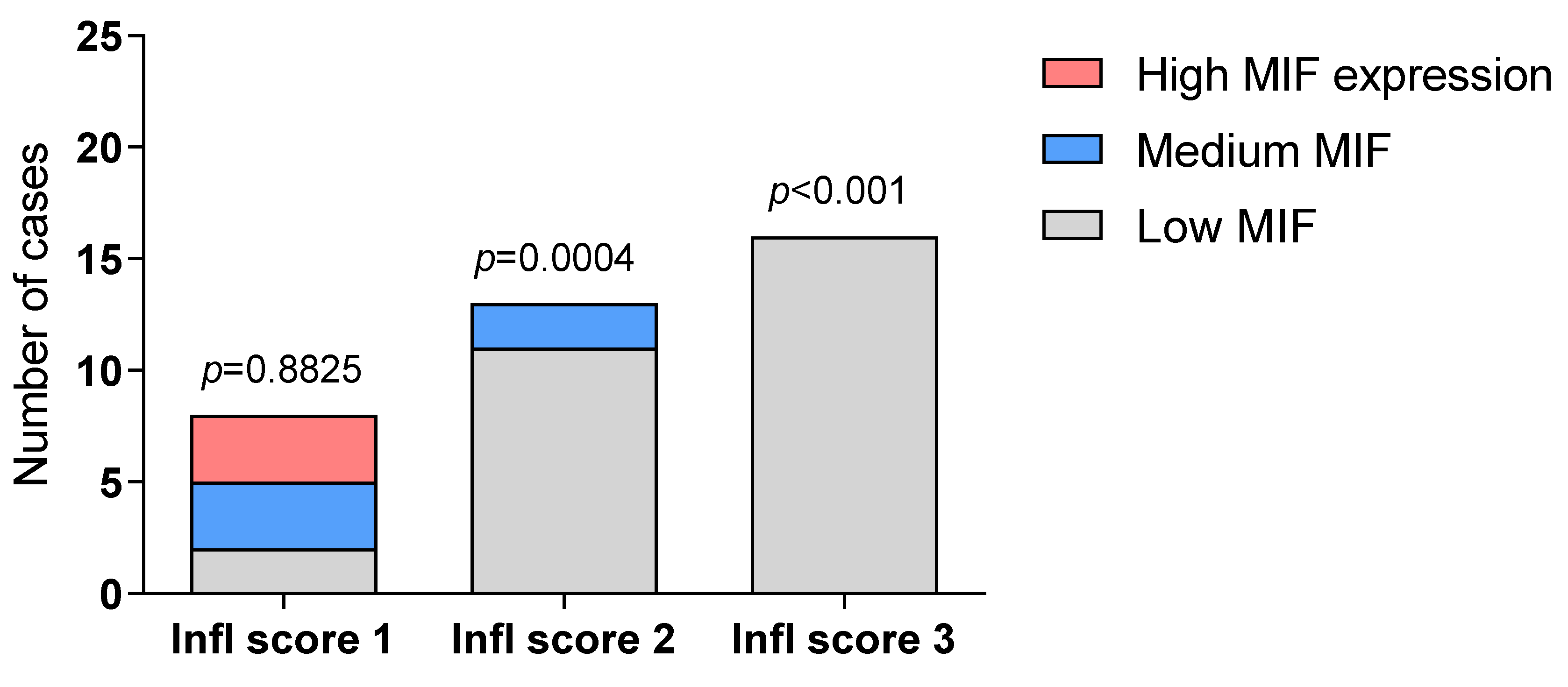
2.1. Immunohistochemistry Analysis of MIF
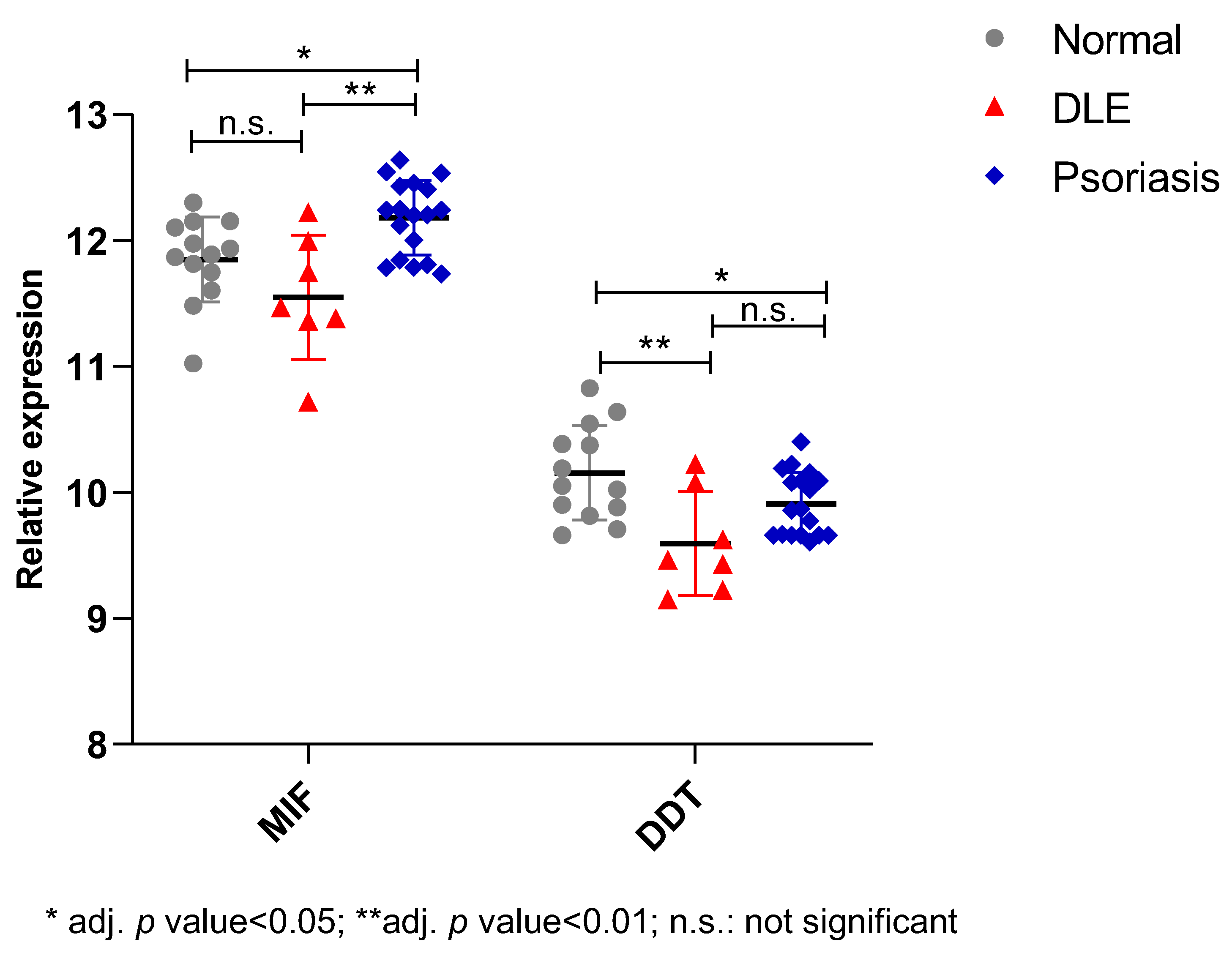
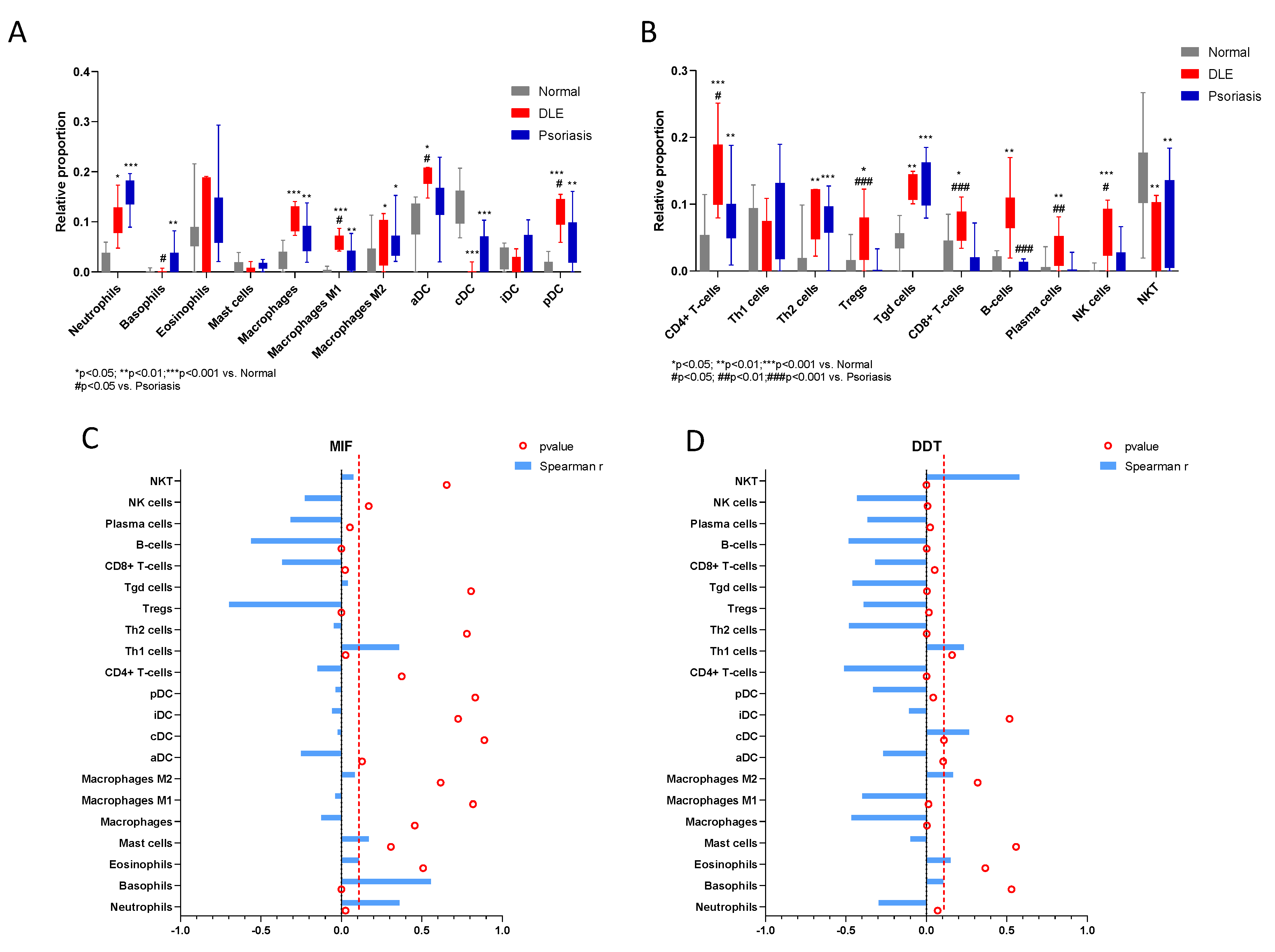
2.2. In Silico Study
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Immunohistochemistry
4.3. Evaluation of Immunohistochemistry
4.4. Microarray Selection and Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, J.; Borucki, R.; Werth, V.P. An update on the pathogenesis of cutaneous lupus erythematosus and its role in clinical practice. Curr. Rheumatol. Rep. 2020, 22, 69. [Google Scholar] [CrossRef] [PubMed]
- McCauliffe, D.P. Cutaneous lupus erythematosus. Semin. Cutan. Med. Surg. 2001, 20, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Jessop, S.; Whitelaw, D.A.; Grainge, M.J.; Jayasekera, P. Drugs for discoid lupus erythematosus. Cochrane Database Syst. Rev. 2017, 2017, CD002954. [Google Scholar] [CrossRef] [PubMed]
- Fairley, J.L.; Oon, S.; Saracino, A.M.; Nikpour, M. Management of cutaneous manifestations of lupus erythematosus: A systematic review. Semin. Arthritis Rheum. 2020, 50, 95–127. [Google Scholar] [CrossRef]
- Stosic-Grujicic, S.; Stojanovic, I.; Nicoletti, F. MIF in autoimmunity and novel therapeutic approaches. Autoimmun. Rev. 2009, 8, 244–249. [Google Scholar] [CrossRef]
- Benedek, G.; Meza-Romero, R.; Jordan, K.; Zhang, Y.; Nguyen, H.; Kent, G.; Li, J.; Siu, E.; Frazer, J.; Piecychna, M.; et al. MIF and D-DT are potential disease severity modifiers in male MS subjects. Proc. Natl. Acad. Sci. USA 2017, 114, E8421–E8429. [Google Scholar] [CrossRef]
- Fagone, P.; Mazzon, E.; Cavalli, E.; Bramanti, A.; Petralia, M.C.; Mangano, K.; Al-Abed, Y.; Bramati, P.; Nicoletti, F. Contribution of the macrophage migration inhibitory factor superfamily of cytokines in the pathogenesis of preclinical and human multiple sclerosis: In silico and in vivo evidences. J. Neuroimmunol. 2018, 322, 46–56. [Google Scholar] [CrossRef]
- Günther, S.; Fagone, P.; Jalce, G.; Atanasov, A.G.; Guignabert, C.; Nicoletti, F. Role of MIF and D-DT in immune-inflammatory, autoimmune, and chronic respiratory diseases: From pathogenic factors to therapeutic targets. Drug Discov. Today 2019, 24, 428–439. [Google Scholar] [CrossRef]
- Mangano, K.; Mazzon, E.; Basile, M.S.; Marco, R.D.; Bramanti, P.; Mammana, S.; Petralia, M.C.; Fagone, P.; Nicoletti, F. Pathogenic role for macrophage migration inhibitory factor in glioblastoma and its targeting with specific inhibitors as novel tailored therapeutic approach. Oncotarget 2018, 9, 17951. [Google Scholar] [CrossRef]
- Harris, J.; VanPatten, S.; Deen, N.S.; Al-Abed, Y.; Morand, E.F. Rediscovering MIF: New tricks for an old cytokine. Trends Immunol. 2019, 40, 447–462. [Google Scholar] [CrossRef]
- Kang, I.; Bucala, R. The immunobiology of MIF: Function, genetics and prospects for precision medicine. Nat. Rev. Rheumatol. 2019, 15, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Jankauskas, S.S.; Wong, D.W.L.; Bucala, R.; Djudjaj, S.; Boor, P. Evolving complexity of MIF signaling. Cell. Signal. 2019, 57, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Lee, J.P.W.; Elgass, K.; Pinar, A.A.; Tate, M.D.; Aitken, E.H.; Fan, H.; Creed, S.J.; Deen, N.S.; Traore, D.A.K.; et al. Macrophage migration inhibitory factor is required for NLRP3 inflammasome activation. Nat. Commun. 2018, 9, 2223. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.S.; Kang, Y.; Wahl, E.R.; Park, H.-J.; Lazova, R.; Leng, L.; Mamula, M.; Krishnaswamy, S.; Bucala, R.; Kang, I. Macrophage migration inhibitory factor regulates U1 small nuclear RNP immune complex-mediated activation of the NLRP3 inflammasome. Arthritis Rheumatol. 2019, 71, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, E.; Mazzon, E.; Basile, M.S.; Mangano, K.; Di Marco, R.; Bramanti, P.; Nicoletti, F.; Fagone, P.; Petralia, M.C. Upregulated expression of macrophage migration inhibitory factor, its analogue D-dopachrome tautomerase, and the CD44 receptor in peripheral CD4 t cells from clinically isolated syndrome patients with rapid conversion to clinical defined multiple sclerosis. Medicina (B Aires) 2019, 55, 667. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, F.; Créange, A.; Orlikowski, D.; Bolgert, F.; Mangano, K.; Metz, C.; Di Marco, R.; Al Abed, Y. Macrophage migration inhibitory factor (MIF) seems crucially involved in Guillain-Barré syndrome and experimental allergic neuritis. J. Neuroimmunol. 2005, 168, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Petralia, M.C.; Mazzon, E.; Fagone, P.; Basile, M.S.; Lenzo, V.; Quattropani, M.C.; Bendtzen, K.; Nicoletti, F. Pathogenic contribution of the Macrophage migration inhibitory factor family to major depressive disorder and emerging tailored therapeutic approaches. J. Affect. Disord. 2020, 263, 15–24. [Google Scholar] [CrossRef]
- Lang, T.; Foote, A.; Lee, J.P.W.; Morand, E.F.; Harris, J. MIF: Implications in the pathoetiology of systemic lupus erythematosus. Front. Immunol. 2015, 6, 577. [Google Scholar] [CrossRef]
- De la Cruz-Mosso, U.; García-Iglesias, T.; Bucala, R.; Estrada-García, I.; González-López, L.; Cerpa-Cruz, S.; Parra-Rojas, I.; Gámez-Nava, J.I.; Pérez-Guerrero, E.E.; Muñoz-Valle, J.F. MIF promotes a differential Th1/Th2/Th17 inflammatory response in human primary cell cultures: Predominance of Th17 cytokine profile in PBMC from healthy subjects and increase of IL-6 and TNF-α in PBMC from active SLE patients. Cell. Immunol. 2018, 324, 42–49. [Google Scholar] [CrossRef]
- Basile, M.S.; Mazzon, E.; Fagone, P.; Longo, A.; Russo, A.; Fallico, M.; Bonfiglio, V.; Nicoletti, F.; Avitabile, T.; Reibaldi, M. Immunobiology of uveal melanoma: State of the art and therapeutic targets. Front. Oncol. 2019, 9, 1145. [Google Scholar] [CrossRef]
- Figueiredo, C.R.; Azevedo, R.A.; Mousdell, S.; Resende-Lara, P.T.; Ireland, L.; Santos, A.; Girola, N.; Cunha, R.L.O.R.; Schmid, M.C.; Polonelli, L.; et al. Blockade of MIF-CD74 signalling on macrophages and dendritic cells restores the antitumour immune response against metastatic melanoma. Front. Immunol. 2018, 9, 1132. [Google Scholar] [CrossRef] [PubMed]
- Soumoy, L.; Kindt, N.; Ghanem, G.; Saussez, S.; Journe, F. Role of macrophage migration inhibitory factor (MIF) in melanoma. Cancers (Basel) 2019, 11, 529. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, E.; Ciurleo, R.; Petralia, M.C.; Fagone, P.; Bella, R.; Mangano, K.; Nicoletti, F.; Bramanti, P.; Basile, M.S. Emerging role of the macrophage migration inhibitory factor family of cytokines in neuroblastoma. Pathogenic effectors and novel therapeutic targets? Molecules 2020, 25, 1194. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Nassri, A.; Kindt, N.; Brown, D.N.; Journe, F.; Saussez, S. Role of macrophage migration inhibitory factor in head and neck cancer and novel therapeutic targets: A systematic review. Head Neck 2017, 39, 2573–2584. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, E.; Mazzon, E.; Mammana, S.; Basile, M.S.; Lombardo, S.D.; Mangano, K.; Bramanti, P.; Nicoletti, F.; Fagone, P.; Petralia, M.C. Overexpression of macrophage migration inhibitory factor and its homologue D-dopachrome tautomerase as negative prognostic factor in neuroblastoma. Brain Sci. 2019, 9, 284. [Google Scholar] [CrossRef] [PubMed]
- Presti, M.; Mazzon, E.; Basile, M.S.; Petralia, M.C.; Bramanti, A.; Colletti, G.; Bramanti, P.; Nicoletti, F.; Fagone, P. Overexpression of macrophage migration inhibitory factor and functionally-related genes, D-DT, CD74, CD44, CXCR2 and CXCR4, in glioblastoma. Oncol. Lett. 2018, 16, 2881–2886. [Google Scholar] [CrossRef]
- Mammana, S.; Bramanti, P.; Mazzon, E.; Cavalli, E.; Basile, M.S.; Fagone, P.; Petralia, M.C.; McCubrey, J.A.; Nicoletti, F.; Mangano, K. Preclinical evaluation of the PI3K/Akt/mTOR pathway in animal models of multiple sclerosis. Oncotarget 2018, 9, 8263–8277. [Google Scholar] [CrossRef]
- Petralia, M.C.; Mazzon, E.; Fagone, P.; Falzone, L.; Bramanti, P.; Nicoletti, F.; Basile, M.S. Retrospective follow-up analysis of the transcriptomic patterns of cytokines, cytokine receptors and chemokines at preconception and during pregnancy, in women with post-partum depression. Exp. Ther. Med. 2019, 18, 2055–2062. [Google Scholar] [CrossRef]
- Petralia, M.C.; Mazzon, E.; Fagone, P.; Russo, A.; Longo, A.; Avitabile, T.; Nicoletti, F.; Reibaldi, M.; Basile, M.S. Characterization of the pathophysiological role of CD47 in uveal melanoma. Molecules 2019, 24, 2450. [Google Scholar] [CrossRef]
- Basile, M.S.; Mazzon, E.; Russo, A.; Mammana, S.; Longo, A.; Bonfiglio, V.; Fallico, M.; Caltabiano, R.; Fagone, P.; Nicoletti, F.; et al. Differential modulation and prognostic values of immune-escape genes in uveal melanoma. PLoS ONE 2019, 14, e0210276. [Google Scholar] [CrossRef]
- Steinhoff, M.; Meinhardt, A.; Steinhoff, A.; Gemsa, D.; Bucala, R.; Bacher, M. Evidence for a role of macrophage migration inhibitory factor in psoriatic skin disease. Br. J. Derm. 1999, 141, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T. Psoriasis and connective tissue diseases. Int. J. Mol. Sci. 2020, 21, 5803. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, A.; Suárez-Fariñas, M.; Fuentes-Duculan, J.; Gonzalez, J.; Cueto, I.; Franks, A.G.; Krueger, J.G. Dominant Th1 and minimal Th17 skewing in discoid lupus revealed by transcriptomic comparison with psoriasis. J. Investig. Derm. 2014, 134, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Gilliver, S.C.; Emmerson, E.; Bernhagen, J.; Hardman, M.J. MIF: A key player in cutaneous biology and wound healing. Exp. Derm. 2011, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Nishihira, J.; Sato, Y.; Kondo, M.; Ogawa, H.; Ohshima, T.; Une, Y.; Todo, S. Involvement of macrophage migration inhibitory factor (MIF) in the mechanism of tumor cell growth. Mol. Med. 1998, 4, 707–714. [Google Scholar] [CrossRef]
- Hudson, J.D.; Shoaibi, M.A.; Maestro, R.; Carnero, A.; Hannon, G.J.; Beach, D.H. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J. Exp. Med. 1999, 190, 1375–1382. [Google Scholar] [CrossRef]
- Farr, L.; Ghosh, S.; Moonah, S. Role of MIF Cytokine/CD74 receptor pathway in protecting against injury and promoting repair. Front. Immunol. 2020, 11, 1273. [Google Scholar] [CrossRef]
- Bacher, M.; Metz, C.N.; Calandra, T.; Mayer, K.; Chesney, J.; Lohoff, M.; Gemsa, D.; Donnelly, T.; Bucala, R. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc. Natl. Acad. Sci. USA 1996, 93, 7849–7854. [Google Scholar] [CrossRef]
- Miller, E.J.; Li, J.; Leng, L.; McDonald, C.; Atsumi, T.; Bucala, R.; Young, L.H. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature 2008, 451, 578–582. [Google Scholar] [CrossRef]
- Lai, Y.C.; Chuang, Y.C.; Chang, C.P.; Yeh, T.M. Macrophage migration inhibitory factor has a permissive role in concanavalin A-induced cell death of human hepatoma cells through autophagy. Cell Death Dis. 2015, 6, e2008. [Google Scholar] [CrossRef]
- Israelson, A.; Ditsworth, D.; Sun, S.; Song, S.W.; Liang, J.; Hruska-Plochan, M.; McAlonis-Downes, M.; Abu-Hamad, S.; Zoltsman, G.; Shani, T.; et al. Macrophage migration inhibitory factor as a chaperone inhibiting accumulation of misfolded SOD1. Neuron 2015, 86, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Shvil, N.; Banerjee, V.; Zoltsman, G.; Shani, T.; Kahn, J.; Abu-Hamad, S.; Papo, N.; Engel, S.; Bernhagen, J.; Israelson, A. MIF inhibits the formation and toxicity of misfolded SOD1 amyloid aggregates: Implications for familial ALS. Cell Death Dis. 2018, 9, 107. [Google Scholar] [CrossRef]
- Leyton-Jaimes, M.F.; Kahn, J.; Israelson, A. AAV2/9-mediated overexpression of MIF inhibits SOD1 misfolding, delays disease onset, and extends survival in mouse models of ALS. Proc. Natl. Acad. Sci. USA 2019, 116, 14755–14760. [Google Scholar] [CrossRef]
- Ruze, A.; Chen, B.D.; Liu, F.; Chen, X.C.; Gai, M.T.; Li, X.M.; Ma, Y.T.; Du, X.J.; Yang, Y.N.; Gao, X.M. Macrophage migration inhibitory factor plays an essential role in ischemic preconditioning-mediated cardioprotection. Clin. Sci. 2019, 133, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Stoppe, C.; Averdunk, L.; Goetzenich, A.; Soppert, J.; Marlier, A.; Kraemer, S.; Vieten, J.; Coburn, M.; Kowark, A.; Kim, B.-S.; et al. The protective role of macrophage migration inhibitory factor in acute kidney injury after cardiac surgery. Sci. Transl. Med. 2018, 10, eaan4886. [Google Scholar] [CrossRef] [PubMed]
- Basile, M.S.; Battaglia, G.; Bruno, V.; Mangano, K.; Fagone, P.; Petralia, M.C.; Nicoletti, F.; Cavalli, E. The dichotomic role of macrophage migration inhibitory factor in neurodegeneration. Int. J. Mol. Sci. 2020, 21, 3023. [Google Scholar] [CrossRef] [PubMed]
- Petralia, M.C.; Battaglia, G.; Bruno, V.; Pennisi, M.; Mangano, K.; Lombardo, S.D.; Fagone, P.; Cavalli, E.; Saraceno, A.; Nicoletti, F.; et al. The role of macrophage migration inhibitory factor in Alzheimer’s disease: Conventionally pathogenetic or unconventionally protective? Molecules 2020, 25, 291. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.J.; Coffey, C.S.; Conwit, R.; Cudkowicz, M.E.; Gleason, T.; Goodman, A.; Klawiter, E.C.; Matsuda, K.; McGovern, M.; Naismith, R.T.; et al. Phase 2 trial of ibudilast in progressive multiple sclerosis. N. Engl. J. Med. 2018, 379, 846–855. [Google Scholar] [CrossRef]
- Sohail, A.; Mushtaq, A.; Iftikhar, A.; Warraich, Z.; Kurtin, S.E.; Tenneti, P.; McBride, A.; Anwer, F. Emerging immune targets for the treatment of multiple myeloma. Immunotherapy 2018, 10, 265–282. [Google Scholar] [CrossRef]
- Healy, Z.R.; Liu, H.; Holtzclaw, W.D.; Talalay, P. Inactivation of tautomerase activity of macrophage migration inhibitory factor by sulforaphane: A potential biomarker for anti-inflammatory intervention. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1516–1523. [Google Scholar] [CrossRef]
- Calonje, E. McKee’s Pathology of the Skin: With Clinical Correlations; Elsevier/Saunders: Philadelphia, PA, USA, 2012; ISBN 9780723437185. [Google Scholar]
- Broggi, G.; Musumeci, G.; Puzzo, L.; Russo, A.; Reibaldi, M.; Ragusa, M.; Longo, A.; Caltabiano, R. Immunohistochemical expression of ABCB5 as a potential prognostic factor in uveal melanoma. Appl. Sci. 2019, 9, 1316. [Google Scholar] [CrossRef]
- Aran, D.; Hu, Z.; Butte, A.J. xCell: Digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017, 18, 220. [Google Scholar] [CrossRef] [PubMed]


| Age (Mean ± S.D.) | ||
| 44.5 ± 14.8 years | ||
| Sex | ||
| Male | 28.60% | |
| Female | 71.40% | |
| BMI (Mean ± S.D.) | 27 ± 2.3 | |
| Response | ||
| Complete remission | 42.80% | |
| Partial remission | 14.30% | |
| Remission with hyperchromic/cicatricial results | 42.90% |
| MIF | AGE | SEX | BMI | RESPONSE | |
|---|---|---|---|---|---|
| Spearman’s test | Correlation Cofficient | −0.272 | 0.036 | 0.202 | 0.610 |
| Sig. (2-taled) | 0.233 | 0.877 | 0.380 | 0.792 |
| MIF | DDT | |||||
|---|---|---|---|---|---|---|
| Spearman R | 95% Confidence Interval | P (Two-Tailed) | Spearman R | 95% Confidence Interval | p (Two-Tailed) | |
| Neutrophils | 0.3605 | 0.03638 to 0.6160 | 0.0262 | −0.2972 | −0.5700 to 0.03468 | 0.07 |
| Basophils | 0.557 | 0.2797 to 0.7485 | 0.0003 | 0.1053 | −0.2312 to 0.4192 | 0.5294 |
| Eosinophils | 0.1106 | −0.2260 to 0.4237 | 0.5084 | 0.1508 | −0.1869 to 0.4566 | 0.3662 |
| Mast cells | 0.1699 | −0.1679 to 0.4720 | 0.3077 | −0.0983 | −0.4134 to 0.2378 | 0.5571 |
| Macrophages | −0.1244 | −0.4351 to 0.2127 | 0.4567 | −0.4653 | −0.6885 to −0.1615 | 0.0032 |
| M1 | −0.03855 | −0.3624 to 0.2936 | 0.8183 | -0.3985 | −0.6428 to −0.08064 | 0.0132 |
| M2 | 0.08375 | −0.2516 to 0.4012 | 0.6172 | 0.1662 | −0.1717 to 0.4690 | 0.3187 |
| aDC | −0.2505 | −0.5349 to 0.08497 | 0.1293 | −0.2675 | −0.5478 to 0.06682 | 0.1045 |
| cDC | −0.02356 | −0.3493 to 0.3073 | 0.8883 | 0.2643 | −0.07027 to 0.5454 | 0.1089 |
| iDC | −0.05881 | −0.3799 to 0.2749 | 0.7258 | −0.1085 | −0.4219 to 0.2280 | 0.5166 |
| pDC | −0.03558 | −0.3598 to 0.2963 | 0.8321 | −0.3312 | −0.5949 to −0.003070 | 0.0422 |
| CD4+ T-cells | −0.148 | −0.4544 to 0.1897 | 0.3752 | −0.5121 | −0.7195 to −0.2208 | 0.001 |
| Th1 cells | 0.3598 | 0.03551 to 0.6155 | 0.0265 | 0.2329 | −0.1035 to 0.5214 | 0.1594 |
| Th2 cells | −0.04677 | −0.3695 to 0.2861 | 0.7804 | −0.4816 | −0.6994 to −0.1819 | 0.0022 |
| Tregs | −0.698 | −0.8350 to −0.4795 | <0.0001 | −0.3901 | −0.6369 to −0.07068 | 0.0155 |
| Tgd cells | 0.04125 | −0.2911 to 0.3648 | 0.8057 | −0.459 | −0.6843 to −0.1537 | 0.0037 |
| CD8+ T-cells | −0.3673 | −0.6208 to −0.04415 | 0.0233 | −0.3184 | −0.5856 to 0.01120 | 0.0514 |
| B-cells | −0.5602 | −0.7506 to −0.2841 | 0.0003 | −0.4842 | −0.7012 to −0.1852 | 0.0021 |
| Plasma cells | −0.316 | −0.5838 to 0.01392 | 0.0533 | −0.3668 | −0.6205 to −0.04356 | 0.0235 |
| NK cells | −0.2272 | −0.5170 to 0.1095 | 0.1702 | −0.4307 | −0.6650 to −0.1190 | 0.007 |
| NKT | 0.07496 | −0.2599 to 0.3937 | 0.6547 | 0.577 | 0.3067 to 0.7612 | 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations All authors have read and agreed to the published version of the manuscript.. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caltabiano, R.; De Pasquale, R.; Piombino, E.; Campo, G.; Nicoletti, F.; Cavalli, E.; Mangano, K.; Fagone, P. Macrophage Migration Inhibitory Factor (MIF) and Its Homologue D-Dopachrome Tautomerase (DDT) Inversely Correlate with Inflammation in Discoid Lupus Erythematosus. Molecules 2021, 26, 184. https://doi.org/10.3390/molecules26010184
Caltabiano R, De Pasquale R, Piombino E, Campo G, Nicoletti F, Cavalli E, Mangano K, Fagone P. Macrophage Migration Inhibitory Factor (MIF) and Its Homologue D-Dopachrome Tautomerase (DDT) Inversely Correlate with Inflammation in Discoid Lupus Erythematosus. Molecules. 2021; 26(1):184. https://doi.org/10.3390/molecules26010184
Chicago/Turabian StyleCaltabiano, Rosario, Rocco De Pasquale, Eliana Piombino, Giorgia Campo, Ferdinando Nicoletti, Eugenio Cavalli, Katia Mangano, and Paolo Fagone. 2021. "Macrophage Migration Inhibitory Factor (MIF) and Its Homologue D-Dopachrome Tautomerase (DDT) Inversely Correlate with Inflammation in Discoid Lupus Erythematosus" Molecules 26, no. 1: 184. https://doi.org/10.3390/molecules26010184
APA StyleCaltabiano, R., De Pasquale, R., Piombino, E., Campo, G., Nicoletti, F., Cavalli, E., Mangano, K., & Fagone, P. (2021). Macrophage Migration Inhibitory Factor (MIF) and Its Homologue D-Dopachrome Tautomerase (DDT) Inversely Correlate with Inflammation in Discoid Lupus Erythematosus. Molecules, 26(1), 184. https://doi.org/10.3390/molecules26010184









