Micelle-in-Liposomes for Sustained Delivery of Anticancer Agents That Promote Potent TRAIL-Induced Cancer Cell Apoptosis
Abstract
1. Introduction
2. Results
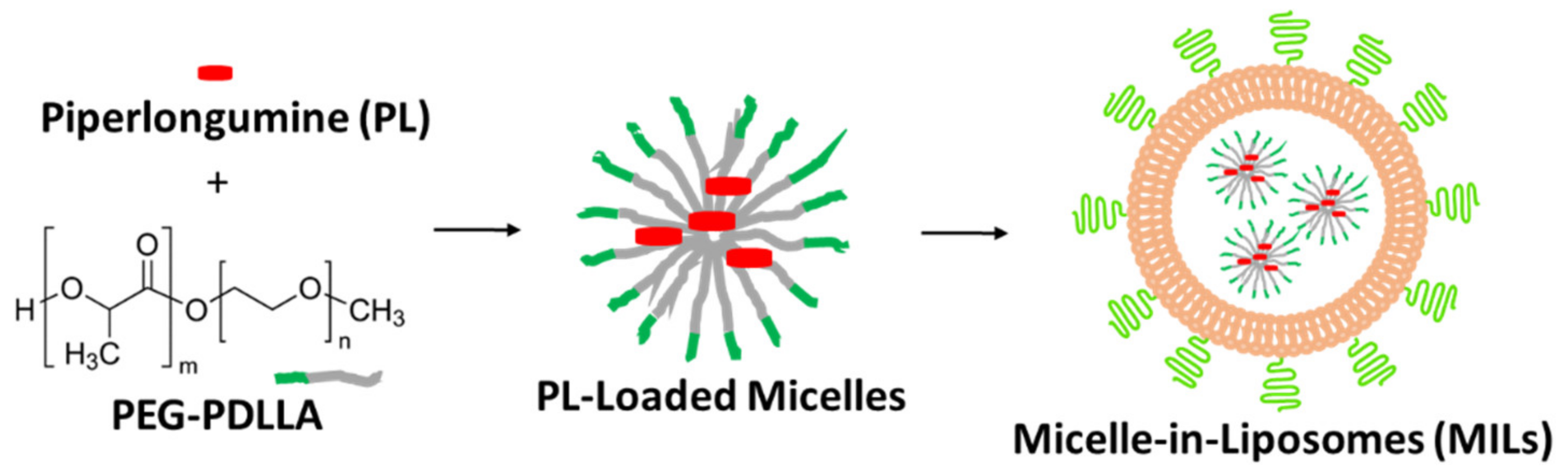
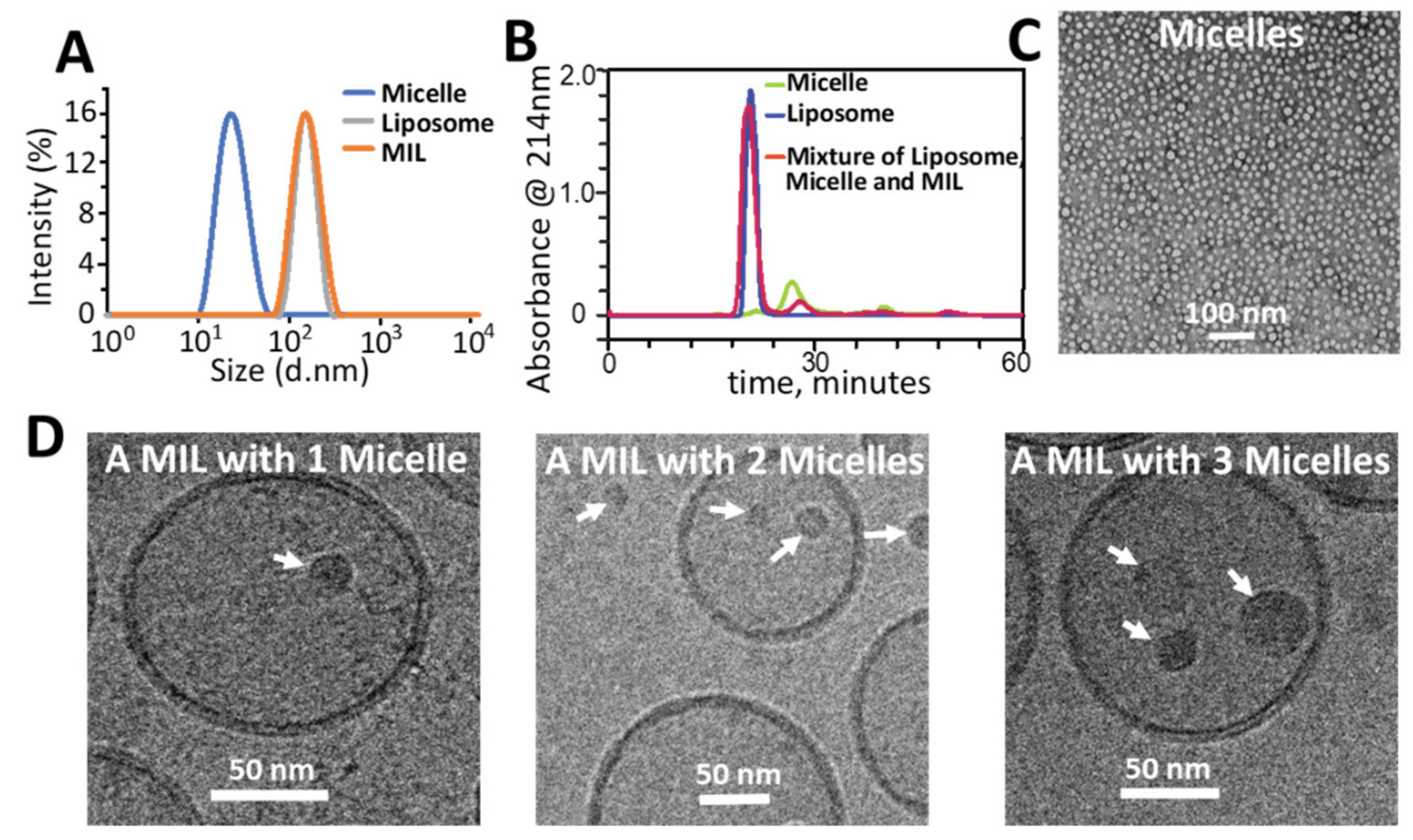
2.1. Preparation and Characterization of MILs
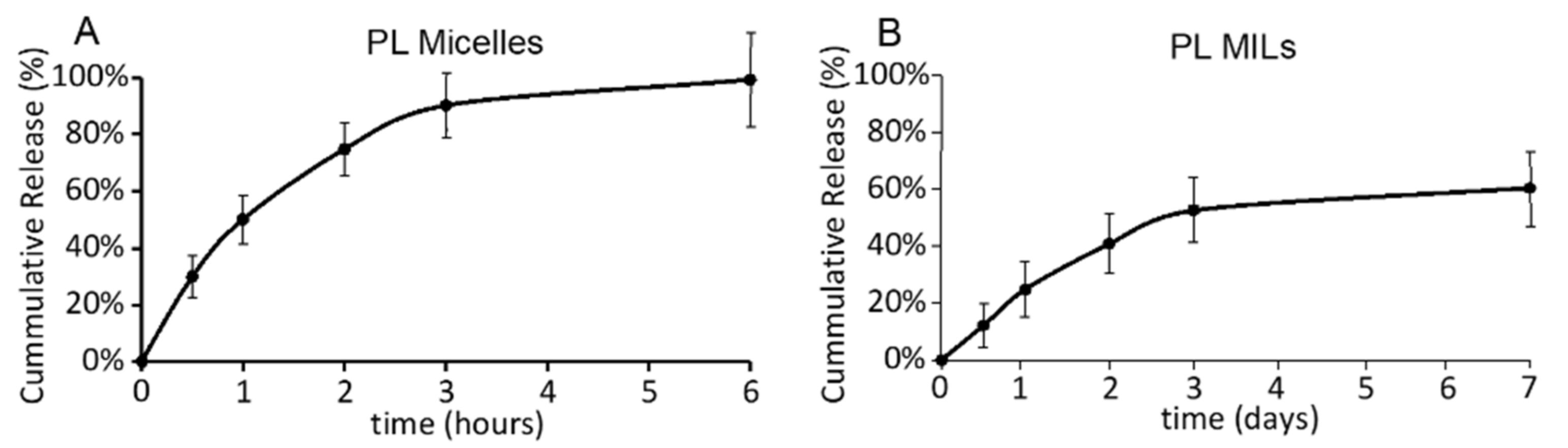
2.2. Piperlongumine Release from PL MILs
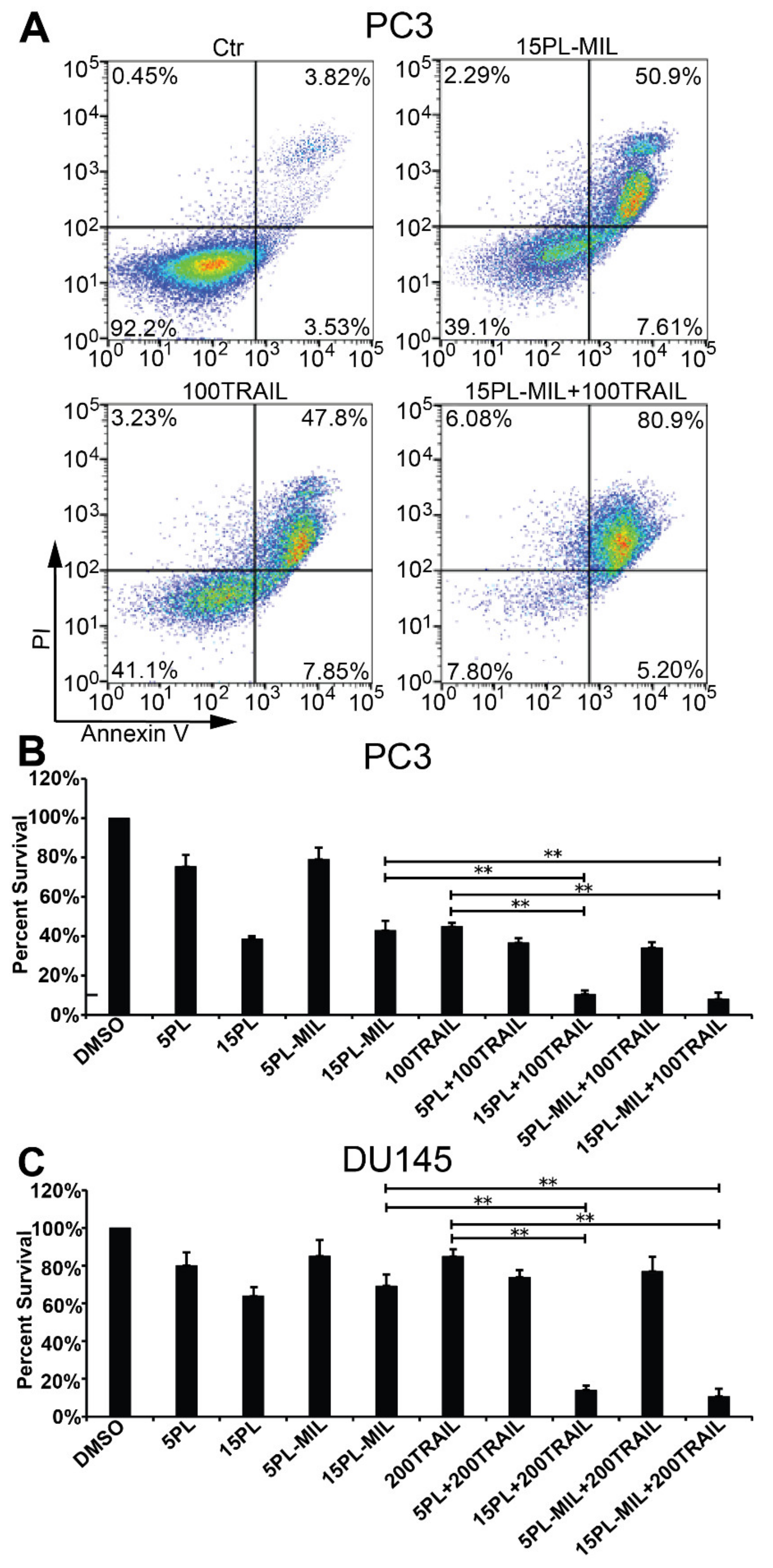
2.3. Combination Cancer Cell Treatment with PL MILs and Liposomal TRAIL
3. Discussion
4. Materials and Methods
4.1. Key Materials
4.2. Preparation of Micelles
4.3. Preparation of Micelle-in-Liposomes (MILs)
4.4. Drug Release Test
4.5. Cell Apoptosis Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bellail, A.C.; Qi, L.; Mulligan, P.; Chhabra, V.; Hao, C. TRAIL agonists on clinical trials for cancer therapy: The promises and the challenges. Rev. Recent Clin. Trials 2009, 4, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Pai, R.C.; Fong, S.; Leung, S.; Lawrence, D.A.; Marsters, S.A.; Blackie, C.; Chang, L.; McMurtrey, A.E.; Hebert, A.; et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Investig. 1999, 104, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Chan, M.F.; Li, J.; King, M.R. Super natural killer cells that target metastases in the tumor draining lymph nodes. Biomaterials 2016, 77, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Jyotsana, N.; Zhang, Z.; Himmel, L.E.; Yu, F.; King, M.R. Minimal dosing of leukocyte targeting TRAIL decreases triple-negative breast cancer metastasis following tumor resection. Sci. Adv. 2019, 5, eaaw4197. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Wayne, E.; Rana, K.; Schaffer, C.B.; King, M.R. TRAIL-coated leukocytes that kill cancer cells in the circulation. Proc. Natl. Acad. Sci. USA 2014, 111, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Wayne, E.C.; Chandrasekaran, S.; Mitchell, M.J.; Chan, M.F.; Lee, R.E.; Schaffer, C.B.; King, M.R. TRAIL-coated leukocytes that prevent the bloodborne metastasis of prostate cancer. J. Control. Release 2016, 223, 215–223. [Google Scholar] [CrossRef]
- Ortiz-Otero, N.; Clinch, A.B.; Hope, J.; Wang, W.; Reinhart-King, C.A.; King, M.R. Cancer associated fibroblasts confer shear resistance to circulating tumor cells during prostate cancer metastatic progression. Oncotarget 2020, 11, 1037–1050. [Google Scholar] [CrossRef]
- Ortiz-Otero, N.; Marshall, J.R.; Lash, B.; King, M.R. Chemotherapy-induced release of circulating-tumor cells into the bloodstream in collective migration units with cancer-associated fibroblasts in metastatic cancer patients. BMC Cancer 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Ortiz-Otero, N.; Marshall, J.R.; Lash, B.W.; King, M.R. Platelet mediated TRAIL delivery for efficiently targeting circulating tumor cells. Nanoscale Adv. 2020. [Google Scholar] [CrossRef]
- Trivedi, R.; Mishra, D.P. Trailing TRAIL Resistance: Novel Targets for TRAIL Sensitization in Cancer Cells. Front. Oncol. 2015, 5, 69. [Google Scholar] [CrossRef]
- De Wilt, L.H.; Kroon, J.; Jansen, G.; De Jong, S.; Peters, G.J.; Kruyt, F.A.E. Bortezomib and TRAIL: A perfect match for apoptotic elimination of tumour cells? Crit. Rev. Oncol. Hematol. 2013, 85, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, J.F.; Mintz, A.; Tian, X.; Dowling, M.L.; Plastaras, J.P.; Dicker, D.T.; Kao, G.D.; El-Deiry, W.S. Tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) and paclitaxel have cooperative in vivo effects against glioblastoma multiforme cells. Mol. Cancer Ther. 2009, 8, 3285–3295. [Google Scholar] [CrossRef] [PubMed]
- Hunter, T.B.; Manimala, N.J.; Luddy, K.A.; Catlin, T.; Antonia, S.J. Paclitaxel and TRAIL synergize to kill paclitaxel-resistant small cell lung cancer cells through a caspase-independent mechanism mediated through AIF. Anticancer Res. 2011, 31, 3193–3204. [Google Scholar] [PubMed]
- Shanker, A.; Brooks, A.D.; Tristan, C.A.; Wine, J.W.; Elliott, P.J.; Yagita, H.; Takeda, K.; Smyth, M.J.; Murphy, W.J.; Sayers, T.J. Treating metastatic solid tumors with bortezomib and a tumor necrosis factor-related apoptosis-inducing ligand receptor agonist antibody. J. Natl. Cancer Inst. 2008, 100, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sharkey, C.C.; King, M.R. Piperlongumine and immune cytokine TRAIL synergize to promote tumor death. Sci. Rep. 2015, 5, 9987. [Google Scholar] [CrossRef]
- Raj, L.; Ide, T.; Gurkar, A.U.; Foley, M.A.; Schenone, M.; Li, X.; Tolliday, N.J.; Golub, T.R.; Carr, S.A.; Shamji, A.F.; et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nat. Cell Biol. 2011, 475, 231–234. [Google Scholar] [CrossRef]
- Sharkey, C.C.; Li, J.; Roy, S.; Wu, Q.; King, M.R. Two-stage nanoparticle delivery of piperlongumine and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) anti-cancer therapy. Technology 2016, 4, 60–69. [Google Scholar] [CrossRef]
- Kwon, G.S.; Okano, T. Polymeric micelles as new drug carriers. Adv. Drug Deliv. Rev. 1996, 21, 107–116. [Google Scholar] [CrossRef]
- Jones, M.-C.; Leroux, J.-C. Polymeric micelles—A new generation of colloidal drug carriers. Eur. J. Pharm. Biopharm. 1999, 48, 101–111. [Google Scholar] [CrossRef]
- Owen, S.C.; Chan, D.P.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Senior, J.; Delgado, C.; Fisher, D.; Tilcock, C.; Gregoriadis, G. Influence of surface hydrophilicity of liposomes on their interaction with plasma protein and clearance from the circulation: Studies with poly(ethylene glycol)-coated vesicles. Biochim. Biophys. Acta Biomembr. 1991, 1062, 77–82. [Google Scholar] [CrossRef]
- Gabizon, A.; Martin, F. Polyethylene Glycol-Coated (Pegylated) Liposomal Doxorubicin. Drugs 1997, 54, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Szoka, F.; Olson, F.; Heath, T.; Vail, W.; Mayhew, E.; Papahadjopoulos, D. Preparation of unilamellar liposomes of intermediate size (0.1–0.2 μm) by a combination of reverse phase evaporation and extrusion through polycarbonate membranes. Biochim. Biophys. Acta Biomembr. 1980, 601, 559–571. [Google Scholar] [CrossRef]
- Aodah, A.; Pavlik, A.; Karlage, K.; Myrdal, P.B. Preformulation Studies on Piperlongumine. PLoS ONE 2016, 11, e0151707. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.-O.; Lee, Y.-H.; Park, J.-A.; Lee, H.-N.; Kim, J.-H.; Kim, J.-Y.; Kim, B.; Hong, S.-E.; Kim, H.-A.; Kim, E.-K.; et al. Piperlongumine induces cell death through ROS-mediated CHOP activation and potentiates TRAIL-induced cell death in breast cancer cells. J.Cancer Res. Clin. Oncol. 2014, 140, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Kim, S.; Li, L.; Wang, S.; Park, K.; Cheng, J.-X. Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Forster resonance energy transfer imaging. Proc. Natl. Acad. Sci. USA 2008, 105, 6596–6601. [Google Scholar] [CrossRef]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.W.; Hennink, W.E. Polymeric Micelles in Anticancer Therapy: Targeting, Imaging and Triggered Release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar] [CrossRef]
- Kim, S.; Shi, Y.; Kim, J.Y.; Park, K.; Cheng, J.-X. Overcoming the barriers in micellar drug delivery: Loading efficiency, in vivostability, and micelle–cell interaction. Expert Opin. Drug Deliv. 2009, 7, 49–62. [Google Scholar] [CrossRef]
- Talelli, M.; Barz, M.; Rijcken, C.J.F.; Kiessling, F.; Hennink, W.E.; Lammers, T. Core-crosslinked polymeric micelles: Principles, preparation, biomedical applications and clinical translation. Nano Today 2015, 10, 93–117. [Google Scholar] [CrossRef]
- Yang, C.; Attia, A.B.E.; Tan, J.P.; Ke, X.; Gao, S.; Hedrick, J.L.; Yang, Y.-Y. The role of non-covalent interactions in anticancer drug loading and kinetic stability of polymeric micelles. Biomaterials 2012, 33, 2971–2979. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Owen, S.C.; Shoichet, M.S. Stability of Self-Assembled Polymeric Micelles in Serum. Macromolecules 2011, 44, 6002–6008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, C.; Jin, Y.; Liu, X.; Wang, Z.; Shaw, J.P.; Baguley, B.C.; Wu, Z.; Liu, J. Multiseed liposomal drug delivery system using micelle gradient as driving force to improve amphiphilic drug retention and its anti-tumor efficacy. Drug Deliv. 2018, 25, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Franzè, S.; Musazzi, U.M.; Minghetti, P.; Cilurzo, F. Drug-in-micelles-in-liposomes (DiMiL) systems as a novel approach to prevent drug leakage from deformable liposomes. Eur. J. Pharm. Sci. 2019, 130, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Romana, B.; Hassan, M.; Sonvico, F.; Pereira, G.G.; Mason, A.F.; Thordarson, P.; Bremmell, K.E.; Barnes, T.J.; Prestidge, C.A. A liposome-micelle-hybrid (LMH) oral delivery system for poorly water-soluble drugs: Enhancing solubilisation and intestinal transport. Eur. J. Pharm. Biopharm. 2020, 154, 338–347. [Google Scholar] [CrossRef]
- Weiner, A.L. Liposomes for Protein Delivery: Selecting Manufacture and Development Processes. Immunomethods 1994, 4, 201–209. [Google Scholar] [CrossRef]
- Xu, Y.; Szoka, F.C. Mechanism of DNA Release from Cationic Liposome/DNA Complexes Used in Cell Transfection. Biochemistry 1996, 35, 5616–5623. [Google Scholar] [CrossRef]
- Colletier, J.-P.; Chaize, B.; Winterhalter, M.; Fournier, D. Protein encapsulation in liposomes: Efficiency depends on interactions between protein and phospholipid bilayer. BMC Biotechnol. 2002, 2, 9. [Google Scholar] [CrossRef]
- Xu, X.; Costa, A.; Burgess, D.J. Protein Encapsulation in Unilamellar Liposomes: High Encapsulation Efficiency and A Novel Technique to Assess Lipid-Protein Interaction. Pharm. Res. 2012, 29, 1919–1931. [Google Scholar] [CrossRef]
- Chaize, B.; Colletier, J.-P.; Winterhalter, M.; Fournier, D. Encapsulation of enzymes in liposomes: High encapsulation efficiency and control of substrate permeability. Artif. Cells Blood Substit. Biotechnol. 2004, 32, 67–75. [Google Scholar] [CrossRef]
- Aliabadi, H.; Elhasi, S.; Mahmud, A.; Gulamhusein, R.; Mahdipoor, P.; Lavasanifar, A. Encapsulation of hydrophobic drugs in polymeric micelles through co-solvent evaporation: The effect of solvent composition on micellar properties and drug loading. Int. J. Pharm. 2007, 329, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Vemuri, S.; Rhodes, C. Preparation and characterization of liposomes as therapeutic delivery systems: A review. Pharm. Acta Helv. 1995, 70, 95–111. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Patel, S.B.; King, M.R. Micelle-in-Liposomes for Sustained Delivery of Anticancer Agents That Promote Potent TRAIL-Induced Cancer Cell Apoptosis. Molecules 2021, 26, 157. https://doi.org/10.3390/molecules26010157
Zhang Z, Patel SB, King MR. Micelle-in-Liposomes for Sustained Delivery of Anticancer Agents That Promote Potent TRAIL-Induced Cancer Cell Apoptosis. Molecules. 2021; 26(1):157. https://doi.org/10.3390/molecules26010157
Chicago/Turabian StyleZhang, Zhenjiang, Sagar B. Patel, and Michael R. King. 2021. "Micelle-in-Liposomes for Sustained Delivery of Anticancer Agents That Promote Potent TRAIL-Induced Cancer Cell Apoptosis" Molecules 26, no. 1: 157. https://doi.org/10.3390/molecules26010157
APA StyleZhang, Z., Patel, S. B., & King, M. R. (2021). Micelle-in-Liposomes for Sustained Delivery of Anticancer Agents That Promote Potent TRAIL-Induced Cancer Cell Apoptosis. Molecules, 26(1), 157. https://doi.org/10.3390/molecules26010157




