Computational Methods for the Identification of Molecular Targets of Toxic Food Additives. Butylated Hydroxytoluene as a Case Study
Abstract
1. Introduction
2. Results
2.1. Target Prediction
2.2. Molecular Docking Studies
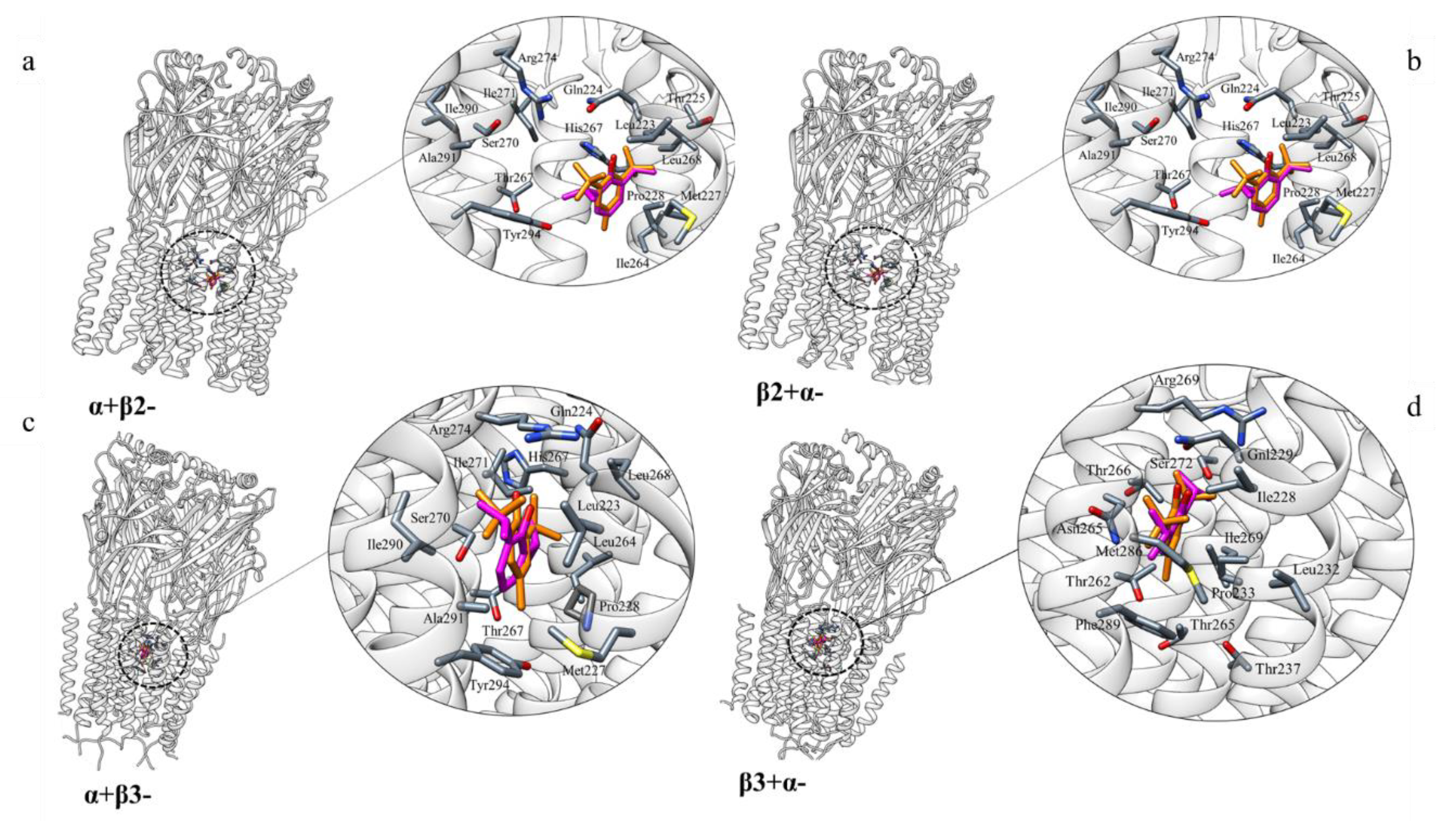
2.2.1. GABA-A
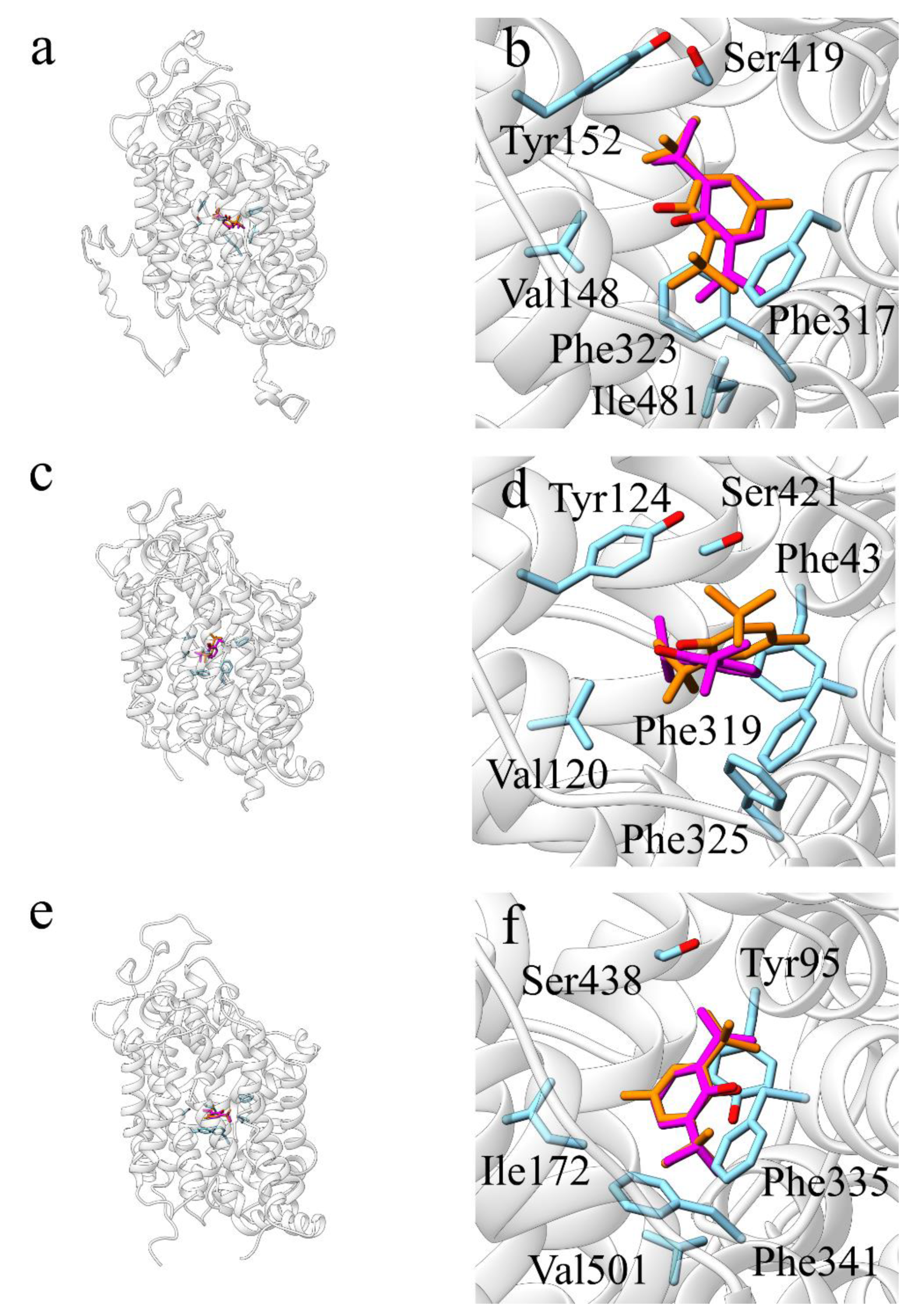
2.2.2. 5-HT2B and 5-HT2C Receptors
2.2.3. Cyclooxygenase 1 (COX-1)
2.2.4. Sodium-Dependent Noradrenaline Transporter (hNET, SLC6A2)
2.3. Virtual Screening against ChEMBL Compounds
3. Discussion
3.1. GABA-A Receptor
3.2. 5-HT2B-2CR
3.3. COX-1
3.4. NET
4. Methods
4.1. SwissTarget Prediction
4.2. Molecular Modeling
4.3. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Silva, M.M.; Lidon, F.C. Food preservatives–An overview on applications and side effects. Emirates J. Food Agric. 2016, 28, 366–373. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 1259/2011 of 2 December 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for dioxins, dioxin-like PCBs and non dioxin-like PCBs in foodstuffs. Off. J. Eur. Union 2011, 320, 18–23. [Google Scholar]
- European Commission. European Parliament and the Concil of the European Union Regulation (EC) No 1333/2008 of the European Parliament ans of the Council of 16 December 2998 on food additives. Off. J. Eur. Union 2008, 51, 16–33. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Food, U.S. Drug Administration Food and Drugs. US Codefed. 1984, 380. Available online: https://www.fda.gov/ (accessed on 6 May 2020).
- Jeong, S.H.; Kim, B.Y.; Kang, H.G.; Ku, H.O.; Cho, J.H. Effects of butylated hydroxyanisole on the development and functions of reproductive system in rats. Toxicology 2005, 208, 49–62. [Google Scholar] [CrossRef]
- Vandghanooni, S.; Forouharmehr, A.; Eskandani, M.; Barzegari, A.; Kafil, V.; Kashanian, S.; Ezzati Nazhad Dolatabadi, J. Cytotoxicity and DNA fragmentation properties of butylated hydroxyanisole. DNA Cell Biol. 2013, 32, 98–103. [Google Scholar] [CrossRef]
- Liu, R.; Mabury, S.A. Synthetic Phenolic Antioxidants and Transformation Products in Human Sera from United States Donors. Environ. Sci. Technol. Lett. 2018, 5, 419–423. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Manzanos, M.J.; Goicoechea, E.; Guillén, M.D. 2,6-Di-Tert-Butyl-Hydroxytoluene and Its Metabolites in Foods. Compr. Rev. Food Sci. Food Saf. 2015, 14, 67–80. [Google Scholar] [CrossRef]
- Liang, X.; Zhao, Y.; Liu, W.; Li, Z.; Souders II, C.L.; Martyniuk, C.J. Butylated hydroxytoluene induces hyperactivity and alters dopamine-related gene expression in larval zebrafish (Danio rerio). Environ. Pollut. 2020, 257, 113624. [Google Scholar] [CrossRef]
- Rani, M.; Shim, W.J.; Han, G.M.; Jang, M.; Song, Y.K.; Hong, S.H. Benzotriazole-type ultraviolet stabilizers and antioxidants in plastic marine debris and their new products. Sci. Total Environ. 2017, 579, 745–754. [Google Scholar] [CrossRef]
- Zhang, R.; Li, C.; Li, Y.; Cui, X.; Ma, L.Q. Determination of 2,6-di-tert-butyl-hydroxytoluene and its transformation products in indoor dust and sediment by gas chromatography–mass spectrometry coupled with precolumn derivatization. Sci. Total Environ. 2018, 619–620, 552–558. [Google Scholar] [CrossRef]
- Wang, W.; Kannan, K. Quantitative identification of and exposure to synthetic phenolic antioxidants, including butylated hydroxytoluene, in urine. Environ. Int. 2019, 128, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Kharkar, P.S.; Warrier, S.; Gaud, R.S. Reverse docking: A powerful tool for drug repositioning and drug rescue. Future Med. Chem. 2014, 6, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Cereto-Massagué, A.; Ojeda, M.J.; Valls, C.; Mulero, M.; Pujadas, G.; Garcia-Vallve, S. Tools for in silico target fishing. Methods 2015, 71, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Lucas, X.; Bendik, I.; Günther, S.; Merfort, I. Target Fishing by Cross-Docking to Explain Polypharmacological Effects. Chem. Med. Chem. 2015, 10, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, G.; Zhou, Y.; Lin, C.; Chen, S.; Lin, Y.; Mai, S.; Huang, Z. Reverse screening methods to search for the protein targets of chemopreventive compounds. Front. Chem. 2018, 6, 138. [Google Scholar] [CrossRef] [PubMed]
- Schuffenhauer, A.; Floersheim, P.; Acklin, P.; Jacoby, E. Similarity metrics for ligands reflecting the similarity of the target proteins. J. Chem. Inf. Comput. Sci. 2003, 43, 391–405. [Google Scholar] [CrossRef]
- Hawkins, P.C.D.; Skillman, A.G.; Nicholls, A. Comparison of shape-matching and docking as virtual screening tools. J. Med. Chem. 2007, 50, 74–82. [Google Scholar] [CrossRef]
- Chen, Z.; Li, H.L.; Zhang, Q.J.; Bao, X.G.; Yu, K.Q.; Luo, X.M.; Zhu, W.L.; Jiang, H.L. Pharmacophore-based virtual screening versus docking-based virtual screening: A benchmark comparison against eight targets. Acta Pharmacol. Sin. 2009, 30, 1694–1708. [Google Scholar] [CrossRef]
- Li, G.B.; Yang, L.L.; Xu, Y.; Wang, W.J.; Li, L.L.; Yang, S.Y. A combined molecular docking-based and pharmacophore-based target prediction strategy with a probabilistic fusion method for target ranking. J. Mol. Graph. Model. 2013, 44, 278–285. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Liljas, A.; Kannan, K.K.; Bergstén, P.C.; Waara, I.; Fridborg, K.; Strandberg, B.; Carlbom, U.; Järup, L.; Lövgren, S.; Petef, M. Crystal structure of human carbonic anhydrase C. Nat. New Biol. 1972, 235, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Şentürk, M.; Gülçin, I.; Daştan, A.; Irfan Küfrevioǧlu, Ö.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg. Med. Chem. 2009, 17, 3207–3211. [Google Scholar] [CrossRef] [PubMed]
- Bormann, J. The “ABC” of GABA receptors. Trends Pharmacol. Sci. 2000, 21, 16–19. [Google Scholar] [CrossRef]
- Whiting, P.J.; Bonnert, T.P.; McKernan, R.M.; Farrar, S.; Le Bourdellès, B.; Heavens, R.P.; Smith, D.W.; Hewson, L.; Rigby, M.R.; Sirinathsinghji, D.J.S.; et al. Molecular and functional diversity of the expanding GABA-A receptor gene family. Ann. N. Y. Acad. Sci. 1999, 868, 645–653. [Google Scholar] [CrossRef]
- Kroeze, W.; Kristiansen, K.; Roth, B. Molecular Biology of Serotonin Receptors - Structure and Function at the Molecular Level. Curr. Top. Med. Chem. 2002, 2, 507–528. [Google Scholar] [CrossRef]
- Bradley, P.B.; Engel, G.; Feniuk, W.; Fozard, J.R.; Humphrey, P.P.A.; Middlemiss, D.N.; Mylecharane, E.J.; Richardson, B.P.; Saxena, P.R. Proposals for the classification and nomenclature of functional receptors for 5-hydroxytryptamine. Neuropharmacology 1986, 25, 563–576. [Google Scholar] [CrossRef]
- Hamon, M.; Gallissot, M.C.; Menard, F.; Gozlan, H.; Bourgoin, S.; Vergé, D. 5-HT3 receptor binding sites are on capsaicin-sensitive fibres in the rat spinal cord. Eur. J. Pharmacol. 1989, 164, 315–322. [Google Scholar] [CrossRef]
- Kesim, M.; Duman, E.N.; Kadioglu, M.; Yaris, E.; Kalyoncu, N.I.; Erciyes, N. The different roles of 5-HT2 and 5-HT3 receptors on antinociceptive effect of paroxetine in chemical stimuli in mice. J. Pharmacol. Sci. 2005, 97, 61–66. [Google Scholar] [CrossRef]
- Roth, B.L.; Ciaranello, R.D.; Meltzer, H.Y. Binding of typical and atypical antipsychotic agents to transiently expressed 5-HT(1C) receptors. J. Pharmacol. Exp. Ther. 1992, 260, 1361–1365. [Google Scholar]
- Fitzpatrick, F. Cyclooxygenase Enzymes: Regulation and Function. Curr. Pharm. Des. 2005, 10, 577–588. [Google Scholar] [CrossRef]
- Nelson, N. The Family of Na+/Cl− Neurotransmitter Transporters. J. Neurochem. 1998, 71, 1785–1803. [Google Scholar] [CrossRef]
- Masson, J.; Sagné, C.; Hamon, M.; El Mestikawy, S. Neurotransmitter transporters in the central nervous system. Pharmacol. Rev. 1999, 51, 439–464. [Google Scholar] [PubMed]
- Homburger, J.A.; Meiler, S.E. Anesthesia drugs, immunity, and long-term outcome. Curr. Opin. Anaesthesiol. 2006, 19, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Green, T.R.; Bennett, S.R.; Nelson, V.M. Specificity and properties of propofol as an antioxidant free radical scavenger. Toxicol. Appl. Pharmacol. 1994, 129, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Di Muzio, E.; Toti, D.; Polticelli, F. DockingApp: A user friendly interface for facilitated docking simulations with AutoDock Vina. J. Comput. Aided Mol. Des. 2017, 31, 213–218. [Google Scholar] [CrossRef]
- Lundström, S.; Twycross, R.; Mihalyo, M.; Wilcock, A. Propofol. J. Pain Symptom Manag. 2010, 40, 466–470. [Google Scholar] [CrossRef]
- Marik, P. Propofol: Therapeutic Indications and Side-Effects. Curr. Pharm. Des. 2004, 10, 3639–3649. [Google Scholar] [CrossRef]
- Yip, G.M.S.; Chen, Z.W.; Edge, C.J.; Smith, E.H.; Dickinson, R.; Hohenester, E.; Townsend, R.R.; Fuchs, K.; Sieghart, W.; Evers, A.S.; et al. A propofol binding site on mammalian GABA A receptors identified by photolabeling. Nat. Chem. Biol. 2013, 9, 715–720. [Google Scholar] [CrossRef]
- Matsunaga, F.; Gao, L.; Huang, X.P.; Saven, J.G.; Roth, B.L.; Liu, R. Molecular interactions between general anesthetics and the 5HT2B receptor. J. Biomol. Struct. Dyn. 2015, 33, 211–218. [Google Scholar] [CrossRef] [PubMed]
- McCorvy, J.D.; Roth, B.L. Structure and function of serotonin G protein-coupled receptors. Pharmacol. Ther. 2015, 150, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.L.; Shoham, M.; Choudhary, M.S.; Khan, N. Identification of conserved aromatic residues essential for agonist binding and second messenger production at 5-hydroxytryptamine2A receptors. Mol. Pharmacol. 1997, 52, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Blobaum, A.L.; Marnett, L.J. Structural and functional basis of cyclooxygenase inhibition. J. Med. Chem. 2007, 50, 1425–1441. [Google Scholar] [CrossRef] [PubMed]
- Gaulton, A.; Bellis, L.J.; Bento, A.P.; Chambers, J.; Davies, M.; Hersey, A.; Light, Y.; McGlinchey, S.; Michalovich, D.; Al-Lazikani, B.; et al. ChEMBL: A large-scale bioactivity database for drug discovery. Nucleic Acids Res. 2012, 40, D1100–D1107. [Google Scholar] [CrossRef]
- Branen, A.L. Toxicology and biochemistry of butylated hydroxyanisole and butylated hydroxytoluene. J. Am. Oil Chem. Soc. 1975, 52, 59. [Google Scholar] [CrossRef]
- Babich, H. Butylated hydroxytoluene (BHT): A review. Environ. Res. 1982, 29, 1–29. [Google Scholar] [CrossRef]
- Malkinson, A.M. Putative mutagens and carcinogens in foods III. Butylated hydroxytoluene (BHT). Environ. Mutagen. 1983, 5, 353–362. [Google Scholar] [CrossRef]
- Kahl, R. Synthetic antioxidants: Biochemical actions and interference with radiation, toxic compounds, chemical mutagens and chemical carcinogens. Toxicology 1984, 33, 185–228. [Google Scholar] [CrossRef]
- Anguilar, F.; Crebelli, R.; Dusemund, B.; Galtier, P.; Gilbert, J.; Gott, D.M.; Gundert-Remy, U.; König, J.; Lambrè, C.; Leblanc, J.-C.; et al. EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) Scientific Opinion on the re-evaluation of butylated hydroxytoluene BHT (E 321) as a food additive. EFSA J. 2012, 10, 2588. [Google Scholar]
- Kelsom, C.; Lu, W. Development and specification of GABAergic cortical interneurons. Cell Biosci. 2013, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Kann, O.; Papageorgiou, I.E.; Draguhn, A. Highly energized inhibitory interneurons are a central element for information processing in cortical networks. J. Cereb. Blood Flow Metab. 2014, 34, 1270–1282. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, A.M.; Moshé, S.L.; Galanopoulou, A.S. Interneuronopathies and their role in early life epilepsies and neurodevelopmental disorders. Epilepsia Open 2017, 2, 284–306. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.Y.; Wong, A.H.C. GABAergic inhibitory neurons as therapeutic targets for cognitive impairment in schizophrenia. Acta Pharmacol. Sin. 2018, 39, 733–753. [Google Scholar] [CrossRef] [PubMed]
- Goetz, T.; Arslan, A.; Wisden, W.; Wulff, P. GABAA receptors: Structure and function in the basal ganglia. Prog. Brain Res. 2007, 160, 21–41. [Google Scholar] [PubMed]
- Bali, M.; Akabas, M.H. Defining the Propofol Binding Site Location on the GABAA Receptor. Mol. Pharmacol. 2004, 65, 68–76. [Google Scholar] [CrossRef]
- Shin, D.J.; Germann, A.L.; Johnson, A.D.; Forman, S.A.; Steinbach, J.H.; Akk, G. Propofol is an allosteric agonist with multiple binding sites on concatemeric ternary GABAA receptors. Mol. Pharmacol. 2018, 93, 178–189. [Google Scholar] [CrossRef]
- Woll, K.A.; Murlidaran, S.; Pinch, B.J.; Hénin, J.; Wang, X.; Salari, R.; Covarrubias, M.; Dailey, W.P.; Brannigan, G.; Garcia, B.A.; et al. A novel bifunctional alkylphenol anesthetic allows characterization of γ-aminobutyric acid, type A (GABAA), receptor subunit binding selectivity in synaptosomes. J. Biol. Chem. 2016, 291, 20473–20486. [Google Scholar] [CrossRef]
- Laverty, D.; Desai, R.; Uchański, T.; Masiulis, S.; Stec, W.J.; Malinauskas, T.; Zivanov, J.; Pardon, E.; Steyaert, J.; Miller, K.W.; et al. Cryo-EM structure of the human α1β3γ2 GABAA receptor in a lipid bilayer. Nature 2019, 565, 516–520. [Google Scholar] [CrossRef]
- Ueno, S.; Wick, M.J.; Ye, Q.; Harrison, N.L.; Harris, R.A. Subunit mutations affect ethanol actions on GABA(A) receptors expressed in Xenopus oocytes. Br. J. Pharmacol. 1999, 127, 377–382. [Google Scholar] [CrossRef][Green Version]
- John Mihic, S.; Ye, Q.; Wick, M.J.; Koltchine, V.V.; Krasowski, M.D.; Finn, S.E.; Mascia, M.P.; Fernando Valenzuela, C.; Hanson, K.K.; Greenblatt, E.P.; et al. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature 1997, 389, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Harrison, N.L. The actions of sevoflurane and desflurane on the γ-aminobutyric acid receptor type A: Effects of TM2 mutations in the α and β subunits. Anesthesiology 2003, 99, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.X.; Engblom, A.C.; Kristiansen, U.; Schousboe, A.; Olsen, R.W. A single glycine residue at the entrance to the first membrane-spanning domain of the γ-aminobutyric acid type a receptor β2 subunit affects allosteric sensitivity to GABA and anesthetics. Mol. Pharmacol. 2000, 57, 474–484. [Google Scholar] [CrossRef]
- Serafini, R.; Bracamontes, J.; Steinbach, J.H. Structural domains of the human GABA(A) receptor β3 subunit involved in the actions of pentobarbital. J. Physiol. 2000, 524, 649–676. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.S.S.; Olcese, R.; Olsen, R.W. A Single M1 Residue in the β2 Subunit Alters Channel Gating of GABAA Receptor in Anesthetic Modulation and Direct Activation. J. Biol. Chem. 2003, 278, 42821–42828. [Google Scholar] [CrossRef] [PubMed]
- Wallner, M.; Lindemeyer, A.K.; Olsen, R.W.; Wallner, M.; Lindemeyer, A.K.; Olsen, R.W. GABAA Receptor Physiology and Pharmacology. In The Oxford Handbook of Neuronal Ion Channels; Bhattacharjee, A., Ed.; Oxford University Press: Oxford, UK, 2018; Available online: https://www.oxfordhandbooks.com/view/10.1093/oxfordhb/9780190669164.001.0001/oxfordhb-9780190669164-e-6 (accessed on 6 May 2020).
- Bockaert, J.; Claeysen, S.; Bécamel, C.; Dumuis, A.; Marin, P. Neuronal 5-HT metabotropic receptors: Fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006, 326, 553–572. [Google Scholar] [CrossRef]
- Barnes, N.M.; Sharp, T. A review of central 5-HT receptors and their function. Neuropharmacology 1999, 38, 1083–1152. [Google Scholar] [CrossRef]
- Darmon, M.; Al Awabdh, S.; Emerit, M.-B.; Masson, J. Insights into Serotonin Receptor Trafficking: Cell Membrane Targeting and Internalization. Prog. Mol. Biol. Transl. Sci. 2015, 132, 97–126. [Google Scholar]
- Filip, M.; Bader, M. Overview on 5-HT receptors and their role in physiology and pathology of the central nervous system. Pharmacol. Rep. 2009, 61, 761–777. [Google Scholar] [CrossRef]
- Giulietti, M.; Vivenzio, V.; Piva, F.; Principato, G.; Bellantuono, C.; Nardi, B. How much do we know about the coupling of G-proteins to serotonin receptors? Mol. Brain 2014, 7, 49. [Google Scholar] [CrossRef]
- Millan, M.J.; Marin, P.; Bockaert, J.; Mannoury la Cour, C. Signaling at G-protein-coupled serotonin receptors: Recent advances and future research directions. Trends Pharmacol. Sci. 2008, 29, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.R.; Mukhin, Y.V.; Gelasco, A.; Turner, J.; Collinsworth, G.; Gettys, T.W.; Grewal, J.S.; Garnovskaya, M.N. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol. Ther. 2001, 92, 179–212. [Google Scholar] [CrossRef]
- Berridge, M. Inositol trisphosphate and calcium signalling. Nature 1993, 361, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Hannon, J.; Hoyer, D. Article in press. Mol. Biol. 2008, 195, 198–213. [Google Scholar]
- Julius, D.; Huang, K.N.; Livelli, T.J.; Axel, R.; Jessell, T.M. The 5HT2 receptor defines a family of structurally distinct but functionally conserved serotonin receptors. Proc. Natl. Acad. Sci. USA 1990, 87, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.A.; Cohen, M.L. 5-Hydroxytryptamine2B receptor signaling in rat stomach fundus: Role of voltage-dependent calcium channels, intracellular calcium release and protein kinase C. J. Pharmacol. Exp. Ther. 1995, 272, 143–150. [Google Scholar]
- De Lucchini, S.; Ori, M.; Nardini, M.; Marracci, S.; Nardi, I. Expression of 5-HT2B and 5-HT2C receptor genes is associated with proliferative regions of Xenopus developing brain and eye. Mol. Brain Res. 2003, 115, 196–201. [Google Scholar] [CrossRef]
- Elangbam, C.S. Drug-induced valvulopathy: An update. Toxicol. Pathol. 2010, 38, 837–848. [Google Scholar] [CrossRef]
- Ellis, E.S.; Byrne, C.; Murphy, O.E.; Tilford, N.S.; Baxter, G.S. Mediation by 5-hydroxytryptamine2B receptors of endothelium-dependent relaxation in rat jugular vein. Br. J. Pharmacol. 1995, 114, 400–404. [Google Scholar] [CrossRef]
- Fiorica-Howells, E.; Maroteaux, L.; Gershon, M.D. Serotonin and the 5-HT(2B) receptor in the development of enteric neurons. J. Neurosci. 2000, 20, 294–305. [Google Scholar] [CrossRef]
- Launay, J.-M.; Hervé, P.; Callebert, J.; Mallat, Z.; Collet, C.; Doly, S.; Belmer, A.; Diaz, S.L.; Hatia, S.; Côté, F.; et al. Serotonin 5-HT2B receptors are required for bone-marrow contribution to pulmonary arterial hypertension. Blood 2012, 119, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Lesurtel, M.; Graf, R.; Aleil, B.; Walther, D.J.; Tian, Y.; Jochum, W.; Gachet, C.; Bader, M.; Clavien, P.-A. Platelet-derived serotonin mediates liver regeneration. Science 2006, 312, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Nebigil, C.G.; Choi, D.S.; Dierich, A.; Hickel, P.; Le Meur, M.; Messaddeq, N.; Launay, J.M.; Maroteaux, L. Serotonin 2B receptor is required for heart development. Proc. Natl. Acad. Sci. USA 2000, 97, 9508–9513. [Google Scholar] [CrossRef] [PubMed]
- Wouters, M.M.; Gibbons, S.J.; Roeder, J.L.; Distad, M.; Ou, Y.; Strege, P.R.; Szurszewski, J.H.; Farrugia, G. Exogenous Serotonin Regulates Proliferation of Interstitial Cells of Cajal in Mouse Jejunum through 5-HT2B Receptors. Gastroenterology 2007, 133, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Devroye, C.; Cathala, A.; Piazza, P.V.; Spampinato, U. The central serotonin2B receptor as a new pharmacological target for the treatment of dopamine-related neuropsychiatric disorders: Rationale and current status of research. Pharmacol. Ther. 2018, 181, 143–155. [Google Scholar] [CrossRef]
- Muller, C.P.; Jacobs, B.L. Handbook of the Behavioral Neurobiology of Serotonin; Elsevier: London, UK, 2010; pp. 3–818. [Google Scholar]
- Leysen, J. 5-HT2 Receptors. Curr. Drug Target CNS Neurol. Disord. 2004, 3, 11–26. [Google Scholar] [CrossRef]
- Minami, K.; Uezono, Y. Gq protein-Coupled Receptors as Targets for Anesthetics. Curr. Pharm. Des. 2006, 12, 1931–1937. [Google Scholar] [CrossRef]
- Minami, K.; Uezono, Y. The recent progress in research on effects of anesthetics and analgesics on G protein-coupled receptors. J. Anesth. 2013, 27, 284–292. [Google Scholar] [CrossRef]
- Drini, M. Peptic ulcer disease and non-steroidal anti-inflammatory drugs. Aust. Prescr. 2017, 40, 91–93. [Google Scholar] [CrossRef]
- Bauer, A.K.; Dwyer-Nield, L.D.; Keil, K.; Koski, K.; Malkinson, A.M. Butylated hydroxytoluene (BHT) induction of pulmonary inflammation: A role in tumor promotion. Exp. Lung Res. 2001, 27, 197–216. [Google Scholar] [CrossRef]
- Inada, T.; Kubo, K.; Kambara, T.; Shingu, K. Propofol inhibits cyclo-oxygenase activity in human monocytic THP-1 cells. Can. J. Anesth. 2009, 56, 222–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shannon, J.R.; Flattem, N.L.; Jordan, J.; Jacob, G.; Black, B.K.; Biaggioni, I.; Blakely, R.D.; Robertson, D. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N. Engl. J. Med. 2000, 342, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Urwin, R.E.; Bennetts, B.; Wilcken, B.; Lampropoulos, B.; Beumont, P.; Clarke, S.; Russell, J.; Tanner, S.; Nunn, K.P. Anorexia nervosa (restrictive subtype) is associated with a polymorphism in the novel norepinephrine transporter gene promoter polymorphic region. Mol. Psychiatry 2002, 7, 652–657. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wong, E.H.F.; Sonders, M.S.; Amara, S.G.; Tinholt, P.M.; Piercey, M.F.P.; Hoffmann, W.P.; Hyslop, D.K.; Franklin, S.; Porsolt, R.D.; Bonsignori, A.; et al. Reboxetine: A pharmacologically potent, selective, and specific norepinephrine reuptake inhibitor. Biol. Psychiatry 2000, 47, 818–829. [Google Scholar] [CrossRef]
- Shahani, S.K.; Lingamaneni, R.; Hemmings, H.C. General anesthetic actions on norepinephrine, dopamine, and γ-aminobutyric acid transporters in stably transfected cells. Anesth. Analg. 2002, 95, 893–899. [Google Scholar] [CrossRef]
- Wang, K.H.; Penmatsa, A.; Gouaux, E. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature 2015, 521, 322–327. [Google Scholar] [CrossRef]
- Coleman, J.A.; Green, E.M.; Gouaux, E. X-ray structures and mechanism of the human serotonin transporter. Nature 2016, 532, 334–339. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, L. Antidepressants modulate the in vitro inhibitory effects of propofol and ketamine on norepinephrine and serotonin transporter function. J. Clin. Neurosci. 2008, 15, 1264–1269. [Google Scholar] [CrossRef]
- Hara, K.; Yanagihara, N.; Minami, K.; Hirano, H.; Sata, T.; Shigematsu, A.; Izumi, F. Dual effects of intravenous anesthetics on the function of norepinephrine transporters. Anesthesiology 2000, 93, 1329–1335. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Armstrong, M.S.; Morris, G.M.; Finn, P.W.; Sharma, R.; Moretti, L.; Cooper, R.I.; Richards, W.G. ElectroShape: Fast molecular similarity calculations incorporating shape, chirality and electrostatics. J. Comput. Aided Mol. Des. 2010, 24, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.S.; Finn, P.W.; Morris, G.M.; Richards, W.G. Improving the accuracy of ultrafast ligand-based screening: Incorporating lipophilicity into ElectroShape as an extra dimension. J. Comput. Aided Mol. Des. 2011, 25, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protoc. 2011, 5, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.; Sali, A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef] [PubMed]
- Fiser, A.; Do, R.; Sali, A. Modeling of loops in protein structures. Protein Sci. 2000, 9, 1753–1773. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. Software news and update AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar]
- Riniker, S.; Landrum, G.A. Better Informed Distance Geometry: Using What We Know to Improve Conformation Generation. J. Chem. Inf. Model. 2015, 55, 2562–2574. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Predicted Target | Probability Value |
|---|---|
| Carbonic anhydrase II | 1.00 |
| GABA-A receptor; α-1/β-2/γ-2 | 0.61 |
| Serotonin 2b receptor | 0.37 |
| GABA-A receptor; α-1/β-3/γ-2 | 0.37 |
| Cyclooxygenase-1 | 0.37 |
| Norepinephrine transporter | 0.37 |
| Serotonin 2c receptor | 0.37 |
| Target. | AutoDock Vina Score | ||
|---|---|---|---|
| BHT | PFL | FLP a | |
| GABA-AR α+β2- b | −5.6 | −5.3 | |
| GABA-AR β2+α- | −6.6 | −6.1 | |
| GABA-AR α+β3- | −5.3 | −6.6 | |
| GABA-AR β3+α- | 1.5 | −2.5 | |
| 5-HT2BR | −6.3 | −6.5 | |
| 5-HT2CR | −7.0 | −6.3 | |
| COX-1 | −6.1 | −8.5 | |
| hNET | −7.4 | −6.8 | |
| dDAT | −6.5 | −5.9 | |
| hSERT | −7.6 | −6.8 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tortosa, V.; Pietropaolo, V.; Brandi, V.; Macari, G.; Pasquadibisceglie, A.; Polticelli, F. Computational Methods for the Identification of Molecular Targets of Toxic Food Additives. Butylated Hydroxytoluene as a Case Study. Molecules 2020, 25, 2229. https://doi.org/10.3390/molecules25092229
Tortosa V, Pietropaolo V, Brandi V, Macari G, Pasquadibisceglie A, Polticelli F. Computational Methods for the Identification of Molecular Targets of Toxic Food Additives. Butylated Hydroxytoluene as a Case Study. Molecules. 2020; 25(9):2229. https://doi.org/10.3390/molecules25092229
Chicago/Turabian StyleTortosa, Valentina, Valentina Pietropaolo, Valentina Brandi, Gabriele Macari, Andrea Pasquadibisceglie, and Fabio Polticelli. 2020. "Computational Methods for the Identification of Molecular Targets of Toxic Food Additives. Butylated Hydroxytoluene as a Case Study" Molecules 25, no. 9: 2229. https://doi.org/10.3390/molecules25092229
APA StyleTortosa, V., Pietropaolo, V., Brandi, V., Macari, G., Pasquadibisceglie, A., & Polticelli, F. (2020). Computational Methods for the Identification of Molecular Targets of Toxic Food Additives. Butylated Hydroxytoluene as a Case Study. Molecules, 25(9), 2229. https://doi.org/10.3390/molecules25092229









