Hypergolics in Carbon Nanomaterials Synthesis: New Paradigms and Perspectives
Abstract
1. Introduction
2. Results and Discussion
2.1. Carbon Nanosheets
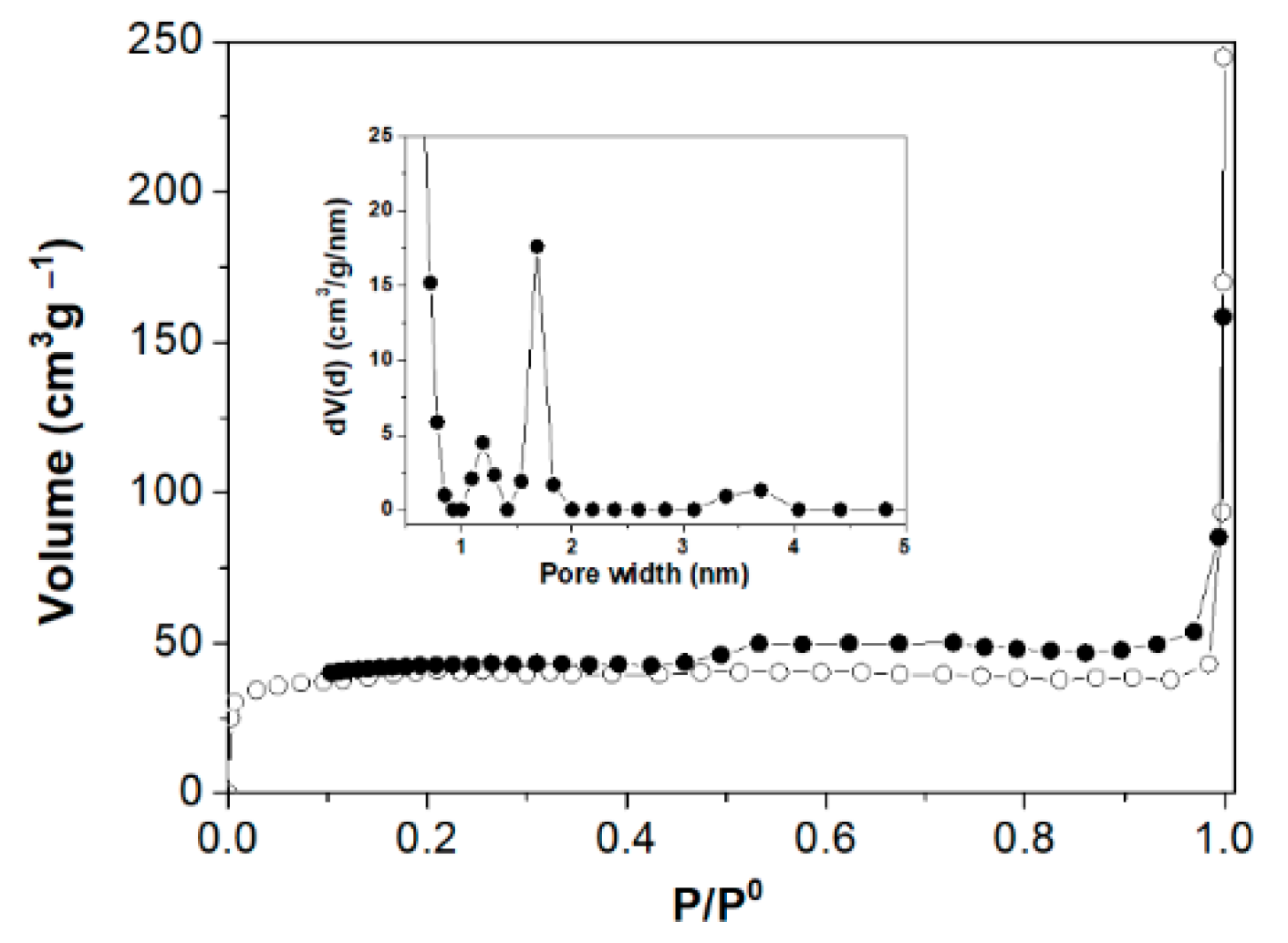
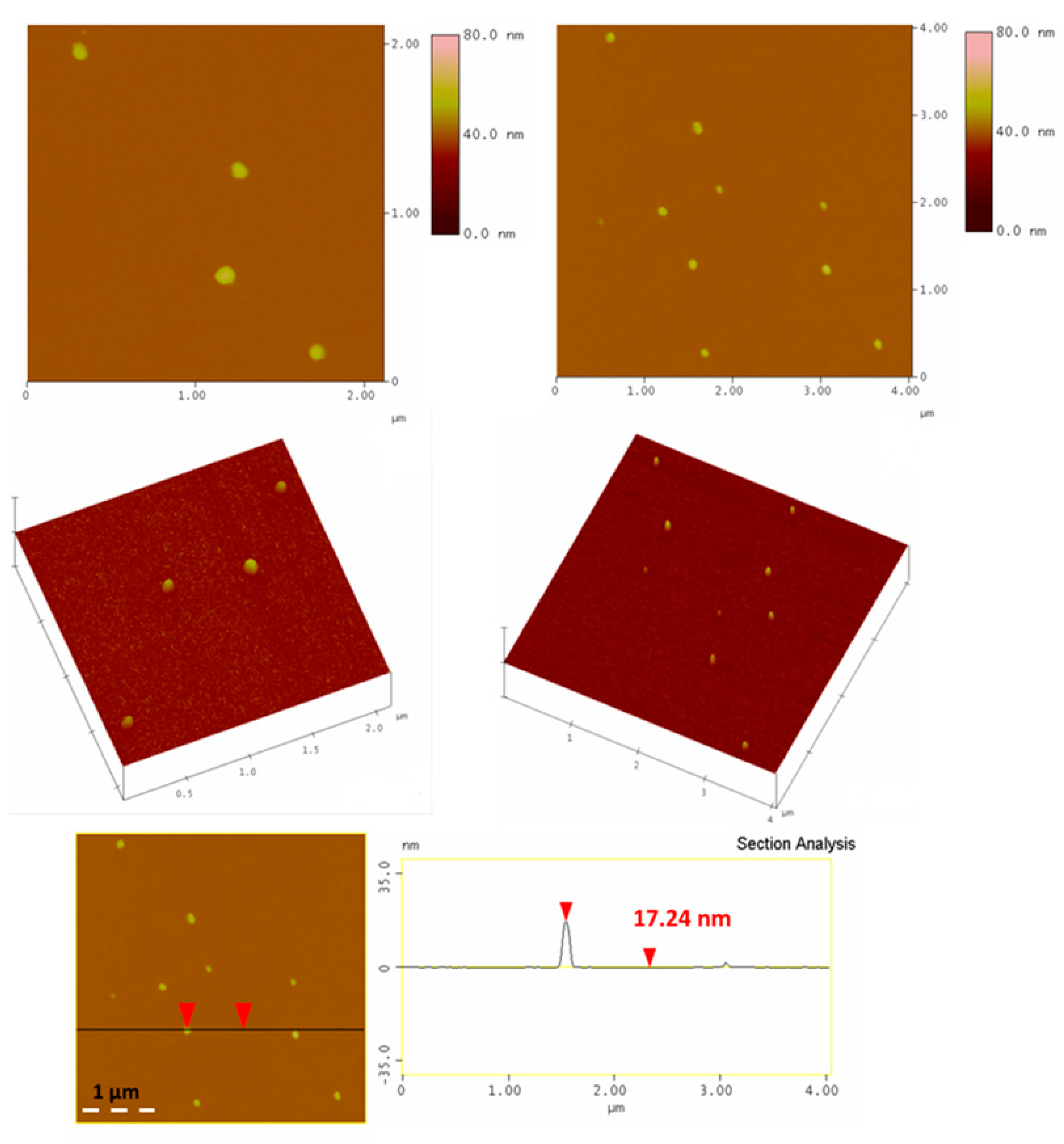
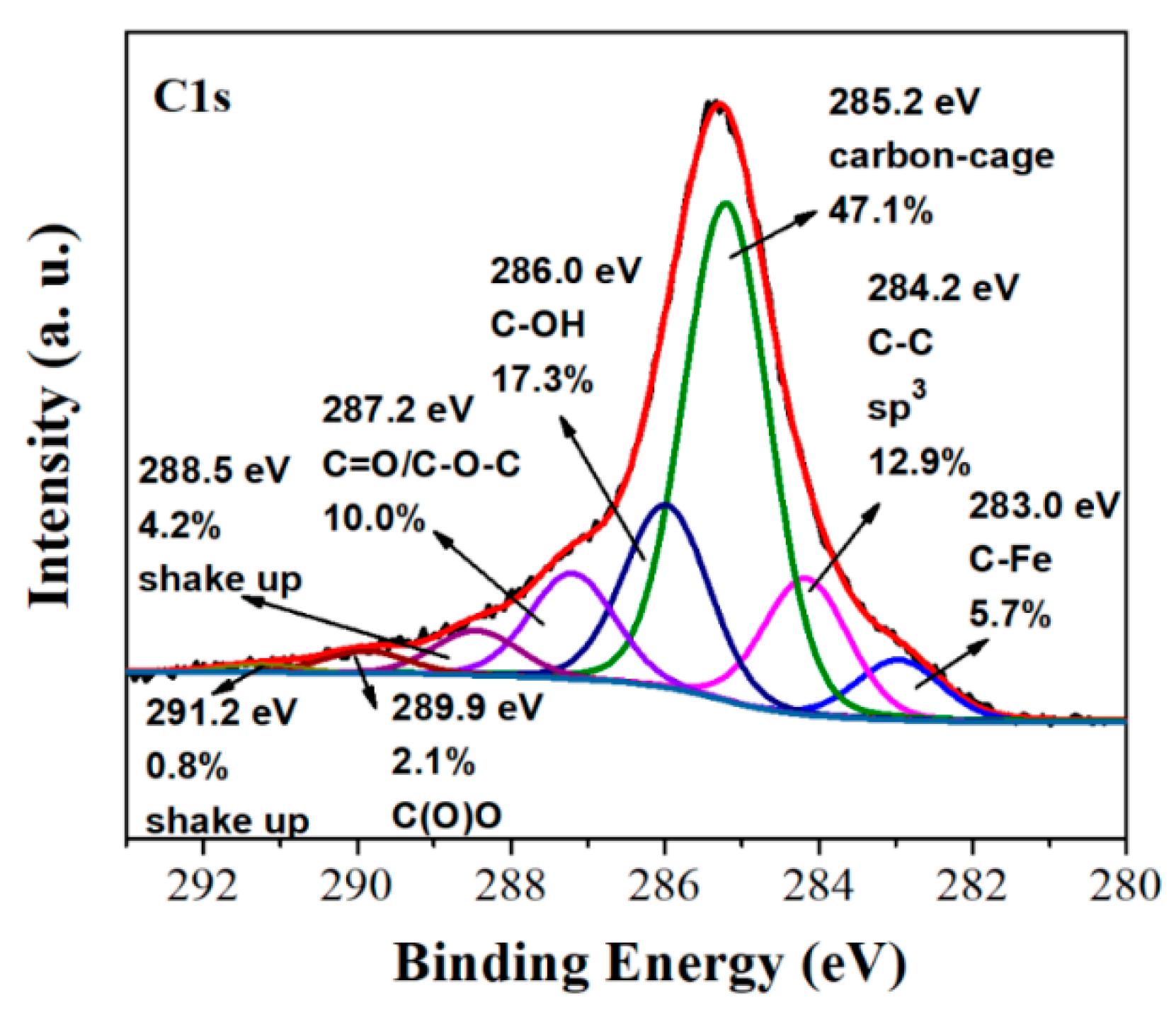
2.2. Fullerols
3. Materials and Methods
3.1. Carbon Nanosheets
3.2. Fullerols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef] [PubMed]
- Hypergolic Propellant. Available online: https://en.wikipedia.org/wiki/Hypergolic_propellant (accessed on 28 December 2019).
- Silva, G.D.; lha, K. Hypergolic Systems: A Review in Patents. J. Aerosp. Technol. Manag. 2012, 4, 407–412. [Google Scholar] [CrossRef][Green Version]
- Schneider, S.; Hawkins, T.; Rosander, M.; Vaghjiani, G.; Chambreau, S.; Drake, G. Ionic Liquids as Hypergolic Fuels. Energy Fuels 2008, 22, 2871–2872. [Google Scholar] [CrossRef]
- Baikousi, M.; Chalmpes, N.; Spyrou, K.; Bourlinos, A.B.; Avgeropoulos, A.; Gournis, D.; Karakassides, M.A. Direct production of carbon nanosheets by self-ignition of pyrophoric lithium dialkylamides in air. Mater. Lett. 2019, 254, 58–61. [Google Scholar] [CrossRef]
- Chalmpes, N.; Spyrou, K.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Synthesis of Highly Crystalline Graphite from Spontaneous Ignition of In Situ Derived Acetylene and Chlorine at Ambient Conditions. Molecules 2020, 25, 297. [Google Scholar] [CrossRef] [PubMed]
- Chalmpes, N.; Asimakopoulos, G.; Spyrou, K.; Vasilopoulos, K.C.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Functional Carbon Materials Derived through Hypergolic Reactions at Ambient Conditions. Nanomaterials 2020, 10, 566. [Google Scholar] [CrossRef]
- Sodium Peroxide + Cornflakes + Milk = Explosion! Available online: https://www.youtube.com/watch?v=M57TOMvsNsI (accessed on 1 February 2018).
- Wen, X.; Liu, H.; Zhang, L.; Zhang, J.; Fu, C.; Shi, X.; Chen, X.; Mijowska, E.; Chen, M.-J.; Wang, D.-Y. Large-scale converting waste coffee grounds into functional carbon materials as high-efficient adsorbent for organic dyes. Bioresour. Technol. 2019, 272, 92–98. [Google Scholar] [CrossRef]
- Gao, G.; Cheong, L.-Z.; Wang, D.; Shen, C. Pyrolytic carbon derived from spent coffee grounds as anode for sodium-ion batteries. Carbon Resour. Convers. 2018, 1, 104–108. [Google Scholar] [CrossRef]
- Fan, H.; Shen, W. Carbon Nanosheets: Synthesis and Application. ChemSusChem 2015, 8, 2004–2027. [Google Scholar] [CrossRef]
- Vileno, B.; Marcoux, P.R.; Lekka, M.; Sienkiewicz, A.; Fehér, T.; Forró, L. Spectroscopic and Photophysical Properties of a Highly Derivatized C60 Fullerol. Adv. Funct. Mater. 2006, 16, 120–128. [Google Scholar] [CrossRef]
- Roh, J.-S. Structural Study of the Activated Carbon Fiber Using Laser Raman Spectroscopy. Carbon Lett. 2008, 9, 127–130. [Google Scholar] [CrossRef]
- Thommes, M. Physical Adsorption Characterization of Nanoporous Materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Karthick Kumar, S.; Suresh, S.; Murugesan, S.; Raj, S.P. CuO thin films made of nanofibers for solar selective absorber applications. Sol. Energy 2013, 94, 299–304. [Google Scholar] [CrossRef]
- Jönsson, G.; Fredriksson, H.; Sellappan, R.; Chakarov, D. Nanostructures for Enhanced Light Absorption in Solar Energy Devices. Int. J. Photoenergy 2011, 2011, 939807. [Google Scholar]
- Coutts, T.J. A review of progress in thermophotovoltaic generation of electricity. Renew. Sustain. Energy Rev. 1999, 3, 77–184. [Google Scholar] [CrossRef]
- Jewur, S.S.; Kuriacose, J.C. Studies on the thermal decomposition of ferric acetate. Thermochim. Acta 1977, 19, 195–200. [Google Scholar] [CrossRef]
- Pinheiro, E.A.; Pereira de Abreu Filho, P.; Galembeck, F.; Correa da Silva, E.; Vargas, H. Magnetite crystal formation from iron(III) hydride acetate. An ESR study. Langmuir 1987, 3, 445–448. [Google Scholar] [CrossRef]
- Yan, Q.-L.; Gozin, M.; Zhao, F.-Q.; Cohen, A.; Pang, S.-P. Highly energetic compositions based on functionalized carbon nanomaterials. Nanoscale 2016, 8, 4799–4851. [Google Scholar] [CrossRef]
- Wang, S.; He, P.; Zhang, J.M.; Jiang, H.; Zhu, S.Z. Novel and Efficient Synthesis of Water-Soluble [60]Fullerenol by Solvent-Free Reaction. Synth. Commun. 2005, 35, 1803–1808. [Google Scholar] [CrossRef]
- Afreen, S.; Kokubo, K.; Muthoosamy, K.; Manickam, S. Hydration or hydroxylation: Direct synthesis of fullerenol from pristine fullerene [C60] via acoustic cavitation in the presence of hydrogen peroxide. RSC Adv. 2017, 7, 31930–31939. [Google Scholar] [CrossRef]
- Meier, M.S.; Kiegiel, J. Preparation and Characterization of the Fullerene Diols 1,2-C60(OH)2, 1,2-C70(OH)2, and 5,6-C70(OH)2. Org. Lett. 2001, 3, 1717–1719. [Google Scholar] [CrossRef] [PubMed]
- Goswami, T.H.; Singh, R.; Alam, S.; Mathur, G.N. One-Pot Synthesis of a Novel Water-Soluble Fullerene-Core Starlike Macromolecule via Successive Michael and Nucleophilic Addition Reaction. Chem. Mater. 2004, 16, 2442–2448. [Google Scholar] [CrossRef]
- Kokubo, K. Water-Soluble Single-Nano Carbon Particles: Fullerenol and Its Derivatives. In The Delivery of Nanoparticles; InTech: London, UK, 2012. [Google Scholar]
- Djordjevic, A.; Srdjenovic, B.; Seke, M.; Petrovic, D.; Injac, R.; Mrđanović, J. Review of Synthesis and Antioxidant Potential of Fullerenol Nanoparticles. J. Nanomater. 2015, 2015, 567073. [Google Scholar] [CrossRef]
- Maxwell, A.J.; Brühwiler, P.A.; Nilsson, A.; Mårtensson, N.; Rudolf, P. Photoemission, autoionization, and x-ray-absorption spectroscopy of ultrathin-film C60 on Au(110). Phys. Rev. B 1994, 49, 10717–10725. [Google Scholar] [CrossRef]
- Enkvist, C.; Lunell, S.; Sjögren, B.; Brühwiler, P.A.; Svensson, S. The C1s shakeup spectra of Buckminsterfullerene, acenaphthylene, and naphthalene, studied by high resolution x-ray photoelectron spectroscopy and quantum mechanical calculations. J. Chem. Phys. 1995, 103, 6333–6342. [Google Scholar] [CrossRef]
- Leiro, J.A.; Heinonen, M.H.; Laiho, T.; Batirev, I.G. Core-level XPS spectra of fullerene, highly oriented pyrolitic graphite, and glassy carbon. J. Electron Spectrosc. Relat. Phenom. 2003, 128, 205–213. [Google Scholar] [CrossRef]
- Felicissimo, M.; Jarzab, D.; Gorgoi, M.; Forster, M.; Scherf, U.; Scharber, M.; Svensson, S.; Rudolf, P.; Loi, M. Determination of vertical phase separation in a polyfluorene copolymer: Fullerene derivative solar cell blend by X-ray photoelectron spectroscopy. J. Mater. Chem. 2009, 19, 4899–4901. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalmpes, N.; Spyrou, K.; Vasilopoulos, K.C.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Gioti, C.; Karakassides, M.A.; Gournis, D. Hypergolics in Carbon Nanomaterials Synthesis: New Paradigms and Perspectives. Molecules 2020, 25, 2207. https://doi.org/10.3390/molecules25092207
Chalmpes N, Spyrou K, Vasilopoulos KC, Bourlinos AB, Moschovas D, Avgeropoulos A, Gioti C, Karakassides MA, Gournis D. Hypergolics in Carbon Nanomaterials Synthesis: New Paradigms and Perspectives. Molecules. 2020; 25(9):2207. https://doi.org/10.3390/molecules25092207
Chicago/Turabian StyleChalmpes, Nikolaos, Konstantinos Spyrou, Konstantinos C. Vasilopoulos, Athanasios B. Bourlinos, Dimitrios Moschovas, Apostolos Avgeropoulos, Christina Gioti, Michael A. Karakassides, and Dimitrios Gournis. 2020. "Hypergolics in Carbon Nanomaterials Synthesis: New Paradigms and Perspectives" Molecules 25, no. 9: 2207. https://doi.org/10.3390/molecules25092207
APA StyleChalmpes, N., Spyrou, K., Vasilopoulos, K. C., Bourlinos, A. B., Moschovas, D., Avgeropoulos, A., Gioti, C., Karakassides, M. A., & Gournis, D. (2020). Hypergolics in Carbon Nanomaterials Synthesis: New Paradigms and Perspectives. Molecules, 25(9), 2207. https://doi.org/10.3390/molecules25092207









