Investigation of the Dynamism of Nanosized SOA Particle Formation in Indoor Air by a Scanning Mobility Particle Sizer and Proton-Transfer-Reaction Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
2.1. Experiments with Gaseous Standard Mixtures
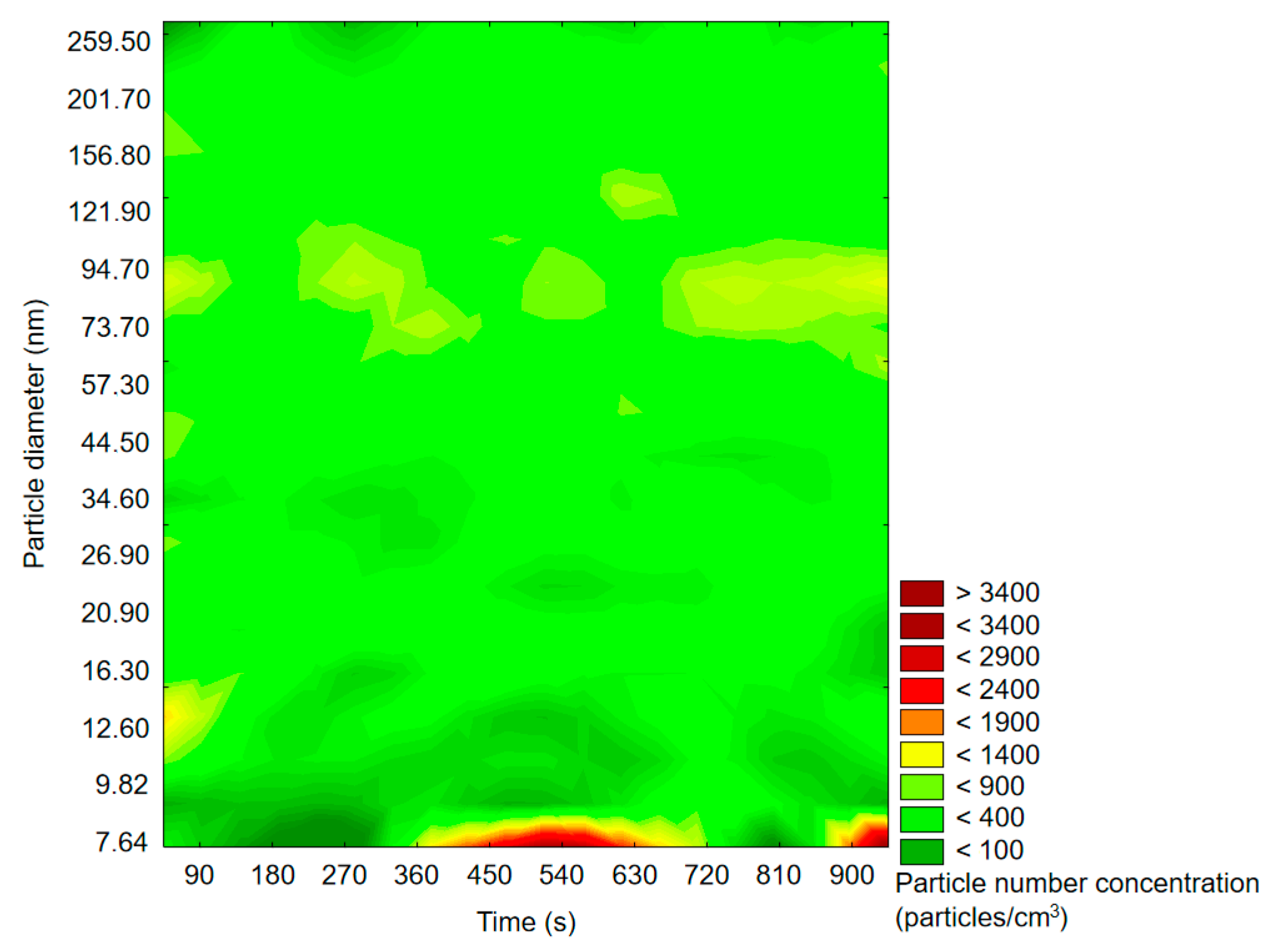
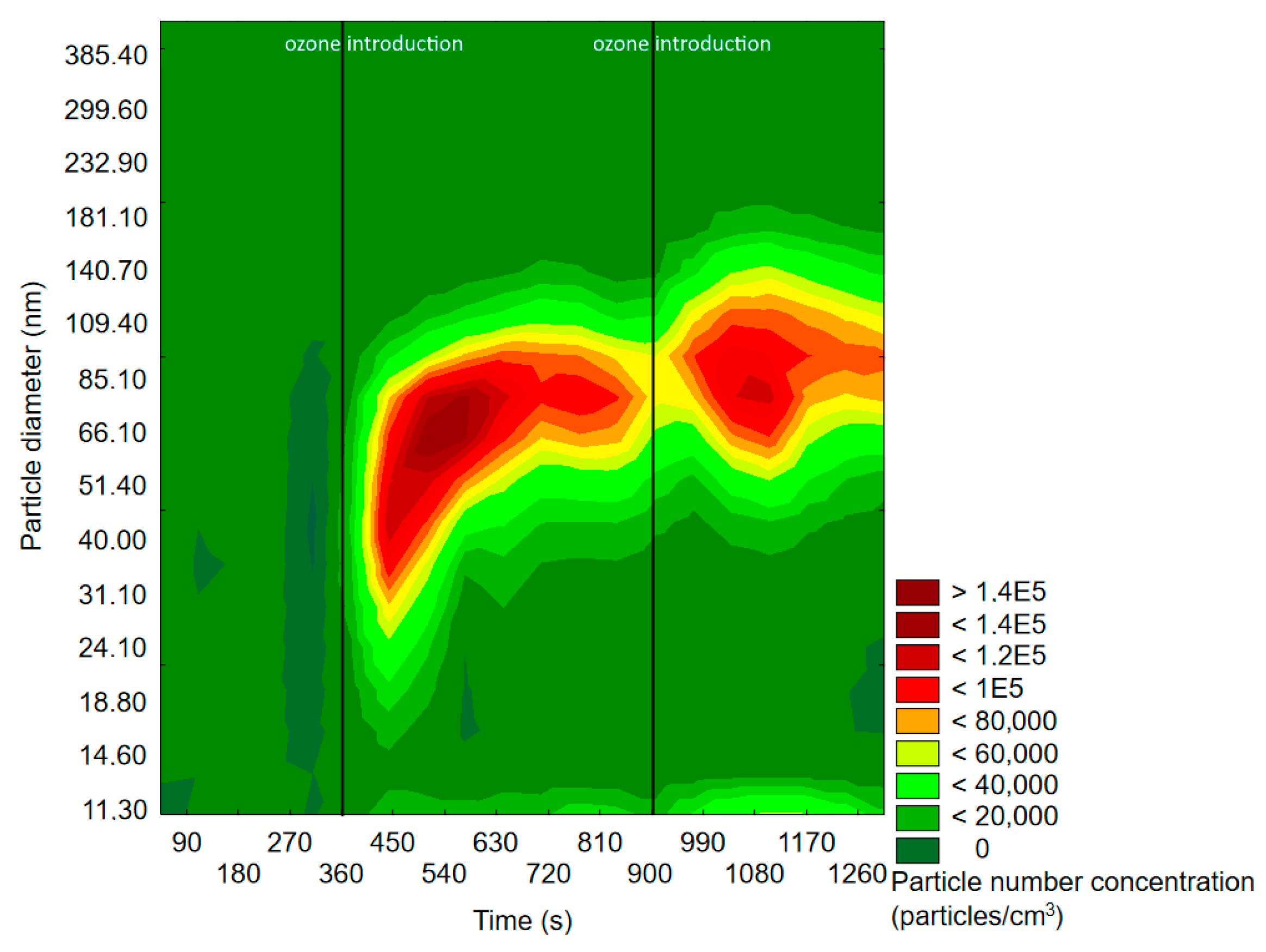
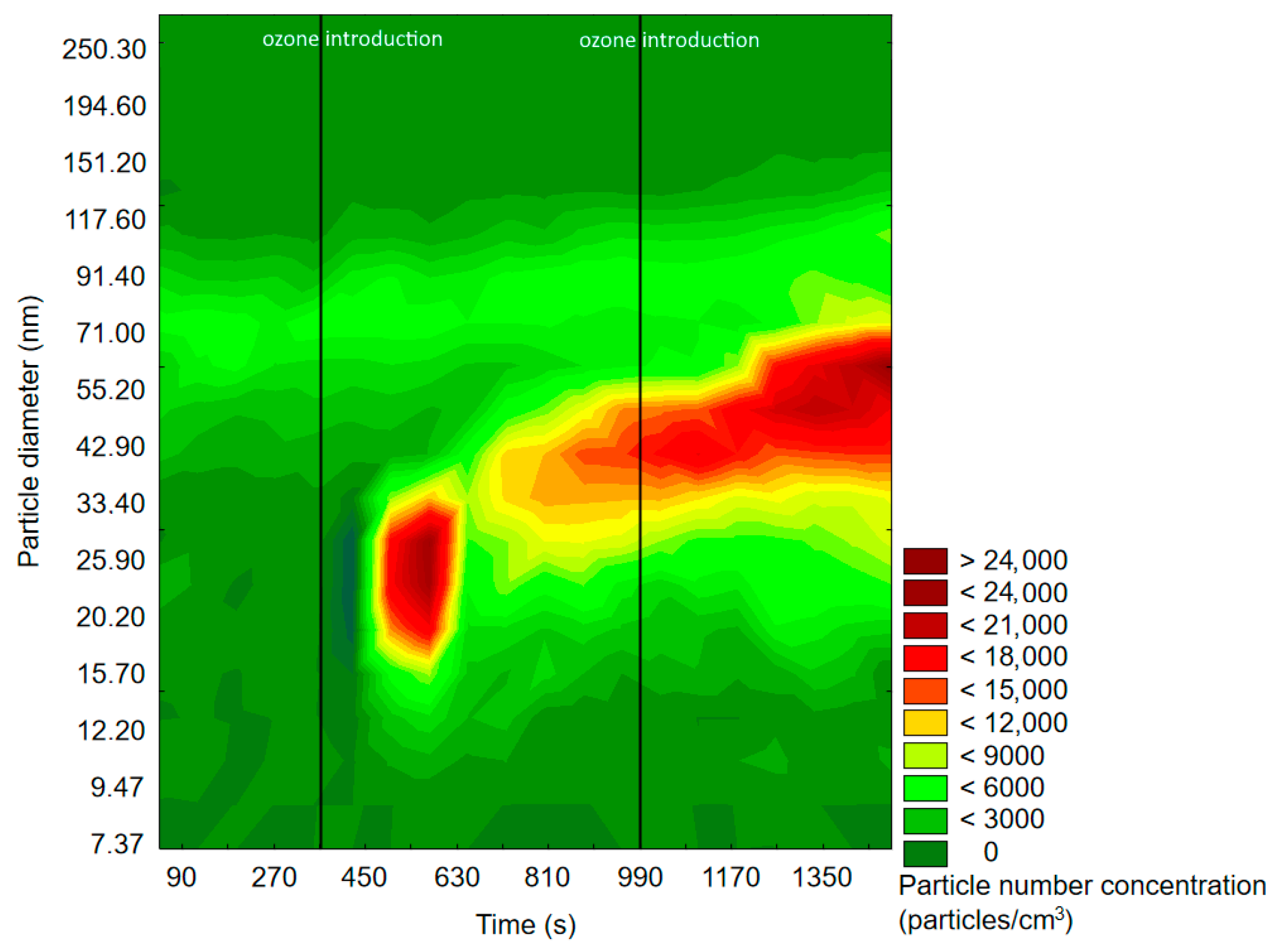
2.1.1. SMPS Measurements
2.1.2. PTR-TOF-MS Experiments
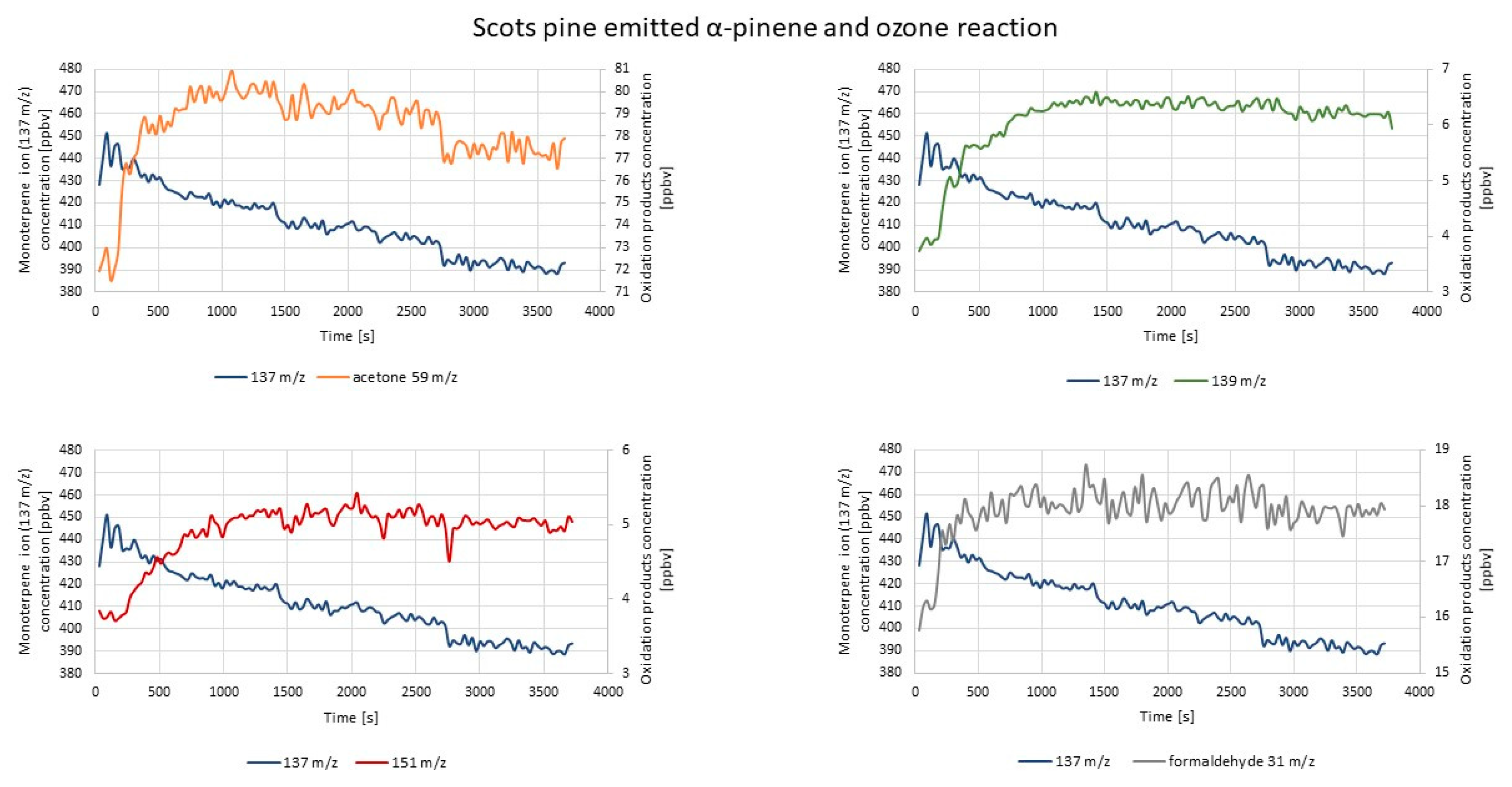
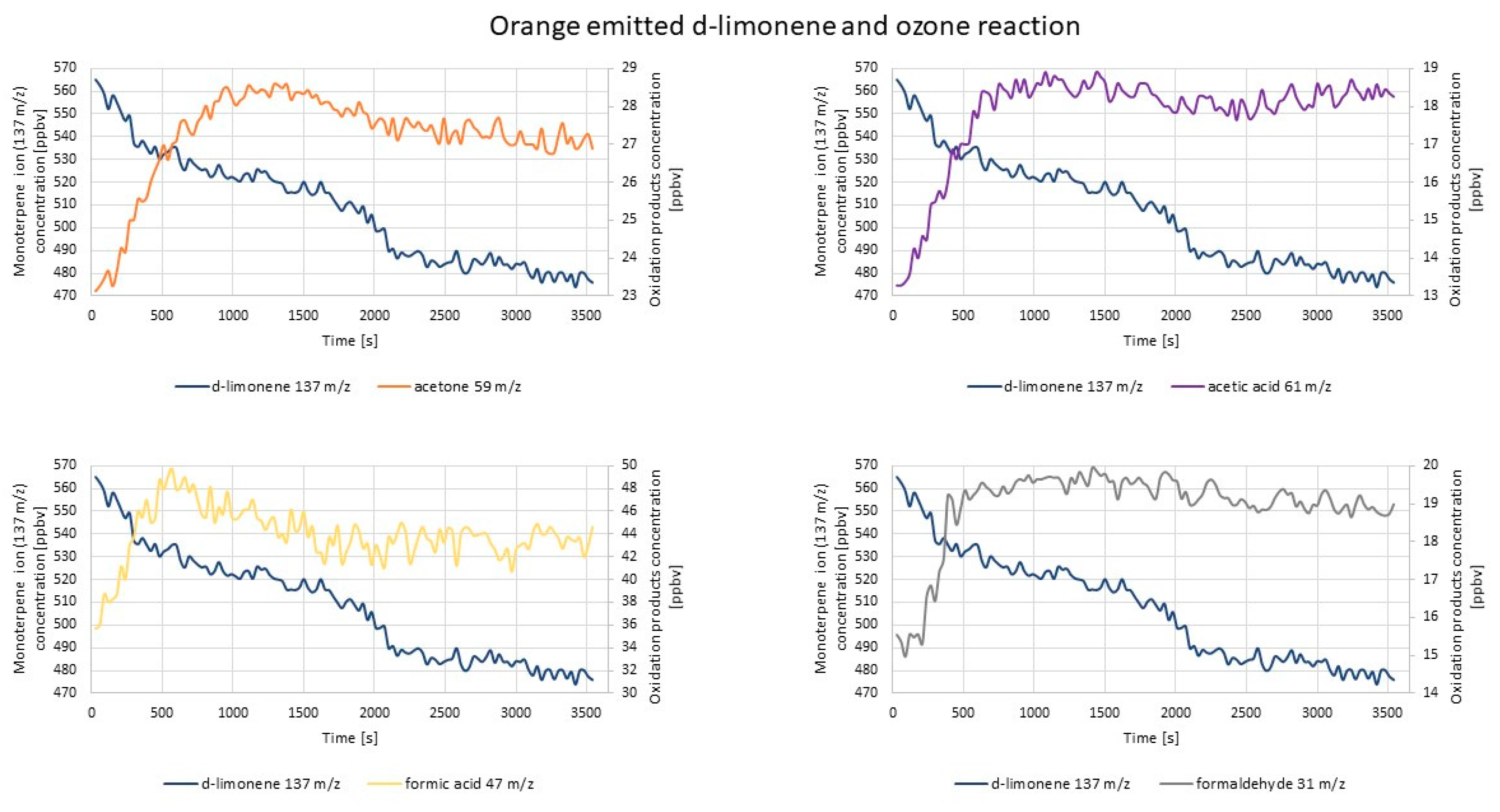
2.2. Experiments with Real Samples
2.2.1. SMPS Measurements
2.2.2. PTR-TOF-MS Measurements
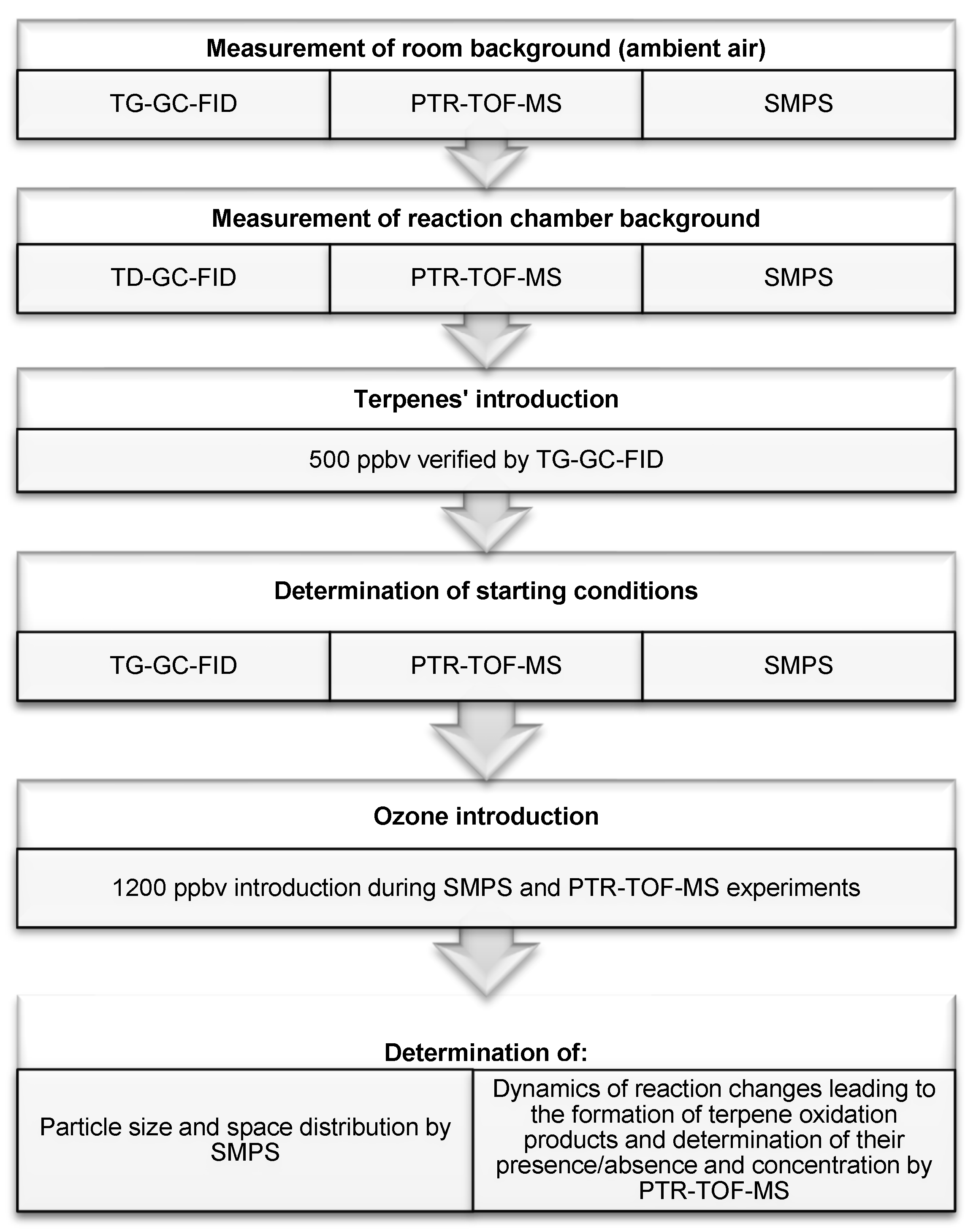
3. Materials and Methods
- (R)-(+)-limonene 97% (Sigma Aldrich, St. Louis, Missouri, USA);
- (+)-α-pinene 98% (Sigma Aldrich, St. Louis, Missouri, USA);
- 1-butanol, EMSURE ACS (Merck, Darmstadt, Germany);
- Ozone was obtained from ozone generator GO 4-100 No 01, power supply voltage 220 V, power 50 VA.
- Orange fruit (peel);
- Scots pine (branch);
3.1. SMPS Measurements
3.2. PTR-TOF-MS Measurements
- α-pinene oxidation products: acetone (59 m/z) [30]; formaldehyde (31 m/z) [68]; nopinone (139 m/z, 140 m/z, 122 m/z, 121 m/z, 93 m/z, 83 m/z); pinonaldehyde (151 m/z, 170 m/z, 169 m/z, 152 m/z, 123 m/z, 109 m/z, 108 m/z, 107 m/z, 99 m/z, 72 m/z, 71 m/z, 43 m/z) [30]; pinonic acid (186 m/z); norpinonaldehyde (155 m/z) [68]; 10-OH-pinoninc acid (201 m/z) [68];
- d-limonene oxidation products: acetone (59 m/z); formaldehyde (31 m/z); formic acid (47 m/z); C3H6O2 (75 m/z); limonaketone (139 m/z); C9H14O2 (155 m/z); limononaldehyde (169 m/z); acetic acid (61 m/z); acetaldehyde (45 m/z) [66].
3.3. TD-GC-FID Measurements
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leech, J.A.; Nelson, W.C.; Burnett, R.T.; Aaron, S.; Raizenne, M.E. It’s about time: A comparison of Canadian and American time-activity patterns. J. Expo. Anal. Environ. Epidemiol. 2002, 12, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Brasche, S.; Bischof, W. Daily time spent indoors in German homes - Baseline data for the assessment of indoor exposure of German occupants. Int. J. Hyg. Environ. Health 2005, 208, 247–253. [Google Scholar] [CrossRef]
- Hussein, T.; Paasonen, P.; Kulmala, M. Activity pattern of a selected group of school occupants and their family members in Helsinki—Finland. Sci. Total Environ. 2012, 425, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Missia, D.A.; Demetriou, E.; Michael, N.; Tolis, E.I.; Bartzis, J.G. Indoor exposure from building materials: A field study. Atmos. Environ. 2010, 44, 4388–4395. [Google Scholar] [CrossRef]
- Wolkoff, P.; Clausen, P.; Wilkins, C.; Nielsen, G. Formation of strong airway irritants in terpene/ozone mixtures. Indoor Air 2000, 10, 82–91. [Google Scholar] [CrossRef]
- Tsigonia, A.; Lagoudi, A.; Chandrinou, S.; Linos, A.; Evlogias, N.; Alexopoulos, E.C. Indoor air in beauty salons and occupational health exposure of cosmetologists to chemical substances. Int. J. Environ. Res. Public Health 2010, 7, 314–324. [Google Scholar] [CrossRef]
- Wolkoff, P.; Larsen, S.T.; Hammer, M.; Kofoed-Sørensen, V.; Clausen, P.A.; Nielsen, G.D. Human reference values for acute airway effects of five common ozone-initiated terpene reaction products in indoor air. Toxicol. Lett. 2013, 216, 54–64. [Google Scholar] [CrossRef]
- Criegee, R. Mechanism of Ozonolysis. Angew. Chem. Int. Ed. Engl. 1975, 14, 745–752. [Google Scholar] [CrossRef]
- Atkinson, R. Gas-Phase Tropospheric Chemistry of Volatile Organic Compounds: 1. Alkanes and Alkenes. J. Phys. Chem. Ref. Data 1997, 26, 215–290. [Google Scholar] [CrossRef]
- Berndt, T.; Böge, O.; Stratmann, F. Gas-phase ozonolysis of α-pinene: Gaseous products and particle formation. Atmos. Environ. 2003, 37, 3933–3945. [Google Scholar] [CrossRef]
- Atkinson, R.; Arey, J. Gas-phase tropospheric chemistry of biogenic volatile organic compounds: A review. Atmos. Environ. 2003, 37, 197–219. [Google Scholar] [CrossRef]
- Hanson, J.R. The Ozonolysis of Terpenoids, a Pandora’s Box of by-Products. J. Chem. Res. 2017, 41, 557–563. [Google Scholar] [CrossRef]
- Weschler, C.J. Chemistry in indoor environments: 20 years of research. Indoor Air 2011, 21, 205–218. [Google Scholar] [CrossRef]
- Sarwar, G.; Corsi, R.; Allen, D.; Weschler, C. The significance of secondary organic aerosol formation and growth in buildings: Experimental and computational evidence. Atmos. Environ. 2003, 37, 1365–1381. [Google Scholar] [CrossRef]
- Yeh, H.C.; Cuddihy, R.G.; Phalen, R.F.; Chang, I.Y. Comparisons of Calculated Respiratory Tract Deposition of Particles Based on the Proposed NCRP Model and the New ICRP66 Model. Aerosol Sci. Technol. 1996, 25, 134–140. [Google Scholar] [CrossRef]
- Dockery, D.W.; Pope, A.C.; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G.; Speizer, F.E. An Association between Air Pollution and Mortality in Six U.S. Cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Spengler, J.D.; Koutrakis, P.; Dockery, D.W.; Raizenne, M.; Speizer, F.E. Health Effects of Acid Aerosols on North American Children: Air Pollution Exposures. Environ. Health Perspect. 1996, 104, 492–499. [Google Scholar] [CrossRef]
- Pope, C.A.; Dockery, D.W. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef]
- Ito, K.; Harashima, H. Coupled CFD analysis of size distributions on indoor secondary organic aerosol derived from ozone/limonene reactions. Build. Environ. 2011, 46, 711–718. [Google Scholar] [CrossRef]
- Borduas, N.; Lin, V.S. Research highlights: Laboratory studies of the formation and transformation of atmospheric organic aerosols. Environ. Sci. Process. Impacts 2016, 18, 425–428. [Google Scholar] [CrossRef]
- Yang, G.; Sau, C.; Lai, W.; Cichon, J.; Li, W. Toxicological analysis of limonene reaction products using an in vitro exposure system. Toxicol. In Vitro 2015, 344, 1173–1178. [Google Scholar]
- Clausen, P.A.; Wilkins, C.K.; Wolkoff, P.; Nielsen, G.D. Chemical and biological evaluation of a reaction mixture of R-(+)-limonene/ozone. Environ. Int. 2001, 26, 511–522. [Google Scholar] [CrossRef]
- Sunil, V.R.; Laumbach, R.J.; Patel, K.J.; Turpin, B.J.; Lim, H.J.; Kipen, H.M.; Laskin, J.D.; Laskin, D.L. Pulmonary effects of inhaled limonene ozone reaction products in elderly rats. Toxicol. Appl. Pharmacol. 2007, 222, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Wolkoff, P.; Clausen, P.A.; Larsen, K.; Hammer, M.; Larsen, S.T.; Nielsen, G.D. Acute airway effects of ozone-initiated d-limonene chemistry: Importance of gaseous products. Toxicol. Lett. 2008, 181, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Wolkoff, P.; Clausen, P.A.; Larsen, S.T.; Hammer, M.; Nielsen, G.D. Airway effects of repeated exposures to ozone-initiated limonene oxidation products as model of indoor air mixtures. Toxicol. Lett. 2012, 209, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Klenø, J.; Wolkoff, P. Changes in eye blink frequency as a measure of trigeminal stimulation by exposure to limonene oxidation products, isoprene oxidation products and nitrate radicals. Int. Arch. Occup. Environ. Health 2004, 77, 235–243. [Google Scholar] [CrossRef]
- Nøjgaard, J.K.; Christensen, K.B.; Wolkoff, P. The effect on human eye blink frequency of exposure to limonene oxidation products and methacrolein. Toxicol. Lett. 2005, 156, 241–251. [Google Scholar] [CrossRef]
- Wilkins, C.K.; Wolkoff, P.; Clausen, P.A.; Hammer, M.; Nielsen, G.D. Upper airway irritation of terpene/ozone oxidation products (TOPS). Dependence on reaction time, relative humidity and initial ozone concentration. Toxicol. Lett. 2003, 143, 109–114. [Google Scholar] [CrossRef]
- Wolkoff, P.; Kjærgaard, S.K. The dichotomy of relative humidity on indoor air quality. Environ. Int. 2007, 33, 850–857. [Google Scholar] [CrossRef]
- Wisthaler, A.; Jensen, N.R.; Winterhalter, R.; Lindinger, W.; Hjorth, J. Measurements of acetone and other gas phase product yields from the OH-initiated oxidation of terpenes by proton-transfer-reaction mass spectrometry (PTR-MS). Atmos. Environ. 2001, 35, 6181–6191. [Google Scholar] [CrossRef]
- Peeters, J.; Müller, J.F.; Stavrakou, T.; Nguyen, V.S. Hydroxyl radical recycling in isoprene oxidation driven by hydrogen bonding and hydrogen tunneling: The upgraded LIM1 mechanism. J. Phys. Chem. A 2014, 118, 8625–8643. [Google Scholar] [CrossRef]
- Sun, T.; Wang, Y.; Zhang, C.; Sun, X.; Wang, W. The chemical mechanism of the limonene ozonolysis reaction in the SOA formation: A quantum chemistry and direct dynamic study. Atmos. Environ. 2011, 45, 1725–1731. [Google Scholar] [CrossRef]
- Leungsakul, S.; Jaoui, M.; Kamens, R.M. Kinetic mechanism for predicting secondary organic aerosol formation from the reaction of d-limonene with ozone. Environ. Sci. Technol. 2005, 39, 9583–9594. [Google Scholar] [CrossRef]
- Glasius, M.; Duane, M.; Larsen, B.R. Determination of polar terpene oxidation products in aerosols by liquid chromatography–ion trap mass spectrometry. J. Chromatogr. 1999, 833, 121–135. [Google Scholar] [CrossRef]
- Koch, S.; Winterhalter, R.; Uherek, E.; Kolloff, A.; Neeb, P.; Moortgat, G.K. Formation of new particles in the gas-phase ozonolysis of monoterpenes. Atmos. Environ. 2000, 34, 4031–4042. [Google Scholar] [CrossRef]
- Jenkin, M.E. Modelling the formation and composition of secondary organic aerosol from α- and β-pinene ozonolysis using MCM v3. Atmos. Chem. Phys. Discuss. 2004, 4, 2905–2948. [Google Scholar] [CrossRef]
- Leungsakul, S.; Jeffries, H.E.; Kamens, R.M. A kinetic mechanism for predicting secondary aerosol formation from the reactions of d-limonene in the presence of oxides of nitrogen and natural sunlight. Atmos. Environ. 2005, 39, 7063–7082. [Google Scholar] [CrossRef]
- Ng, N.L.; Kwan, A.J.; Surratt, J.D.; Chan, A.W.H.; Chhabra, P.S.; Sorooshian, A.; Pye, H.O.T.; Crounse, J.D.; Wennberg, P.O.; Flagan, R.C.; et al. Secondary organic aerosol (SOA) formation from reaction of isoprene with nitrate radicals (NO3). Atmos. Chem. Phys. 2008, 8, 4117–4140. [Google Scholar] [CrossRef]
- Tamás, G.; Weschler, C.J.; Toftum, J.; Fanger, P.O. Influence of ozone-limonene reactions on perceived air quality. Indoor Air 2006, 16, 168–178. [Google Scholar] [CrossRef]
- Weschler, C.J.; Carslaw, N. Indoor Chemistry. Environ. Sci. Technol. 2018, 52, 2419–2428. [Google Scholar] [CrossRef]
- Singer, B.C.; Coleman, B.K.; Destaillats, H.; Hodgson, A.T.; Lunden, M.; Weschler, C.; Nazaroff, W.W. Indoor secondary pollutants from cleaning product and air freshener use in the presence of ozone. Atmos. Environ. 2006, 40, 6696–6710. [Google Scholar] [CrossRef]
- Carslaw, N. A mechanistic study of limonene oxidation products and pathways following cleaning activities. Atmos. Environ. 2013, 80, 507–513. [Google Scholar] [CrossRef]
- Kennedy, A.J.; Diamond, S.; Stanley, J.K.; Coleman, J.; Steevens, J.A.; Chappell, M.A.; Laird, J.; Bednar, A. Nanomaterials Ecotoxicology: A Case Study with Nanosilver. In Nanotechnology Environmental Health and Safety; Elsevier: Amsterdam, The Netherlands, 2014; pp. 117–151. [Google Scholar]
- Brown, D.M.; Wilson, M.R.; Macnee, W.; Stone, V.; Donaldson, K. Size-Dependent Proinflammatory Effects of Ultrafine Polystyrene Particles: A Role for Surface Area and Oxidative Stress in the Enhanced Activity of Ultrafines Size-Dependent Proinflammatory Effects of Ultrafine Polysty-rene Particles: A Role for Surface Area and Oxidative Stress in the Enhanced Activity of Ultrafines. Toxicol. Appl. Pharmacol. 2001, 175, 191–199. [Google Scholar]
- Wittmaack, K. In Search of the Most Relevant Parameter for Quantifying Lung Inflammatory Response to Nanoparticle Exposure: Particle Number, Surface Area, or What? Environ. Health Perspect. 2007, 115, 187–194. [Google Scholar] [CrossRef]
- Phalen, R.F.; Mendez, L.B.; Oldham, M.J. New developments in aerosol dosimetry. Inhal. Toxicol. 2010, 22, 6–14. [Google Scholar] [CrossRef]
- Abbatt, J.P.D.; Chen, W. The atmospheric chemistry of indoor environments. Environ. Sci. Process. Impacts 2020, 22, 25–48. [Google Scholar]
- Vartiainen, E.; Kulmala, M.; Ruuskanen, T.M.; Taipale, R.; Rinne, J.; Vehkamäki, H. Formation and growth of indoor air aerosol particles as a result of d-limonene oxidation. Atmos. Environ. 2006, 40, 7882–7892. [Google Scholar] [CrossRef]
- Riipinen, I.; Yli-Juuti, T.; Pierce, J.R.; Petaja, T.; Worsnop, D.R.; Kulmala, M.; Donahue, N.M. The contribution of organics to atmospheric nanoparticle growth. Nat. Geosci. 2012, 5, 453–458. [Google Scholar] [CrossRef]
- Weschler, C.J.; Shields, H.C. Indoor ozone/terpene reactions as a source of indoor particles. Atmos. Environ. 1999, 33, 2301–2312. [Google Scholar] [CrossRef]
- Huang, H.L.; Tsai, T.J.; Hsu, N.Y.; Lee, C.C.; Wu, P.C.; Su, H.J. Effects of essential oils on the formation of formaldehyde and secondary organic aerosols in an aromatherapy environment. Build. Environ. 2012, 57, 120–125. [Google Scholar] [CrossRef]
- Rösch, C.; Wissenbach, D.K.; von Bergen, M.; Franck, U.; Wendisch, M.; Schlink, U. The lasting effect of limonene-induced particle formation on air quality in a genuine indoor environment. Environ. Sci. Pollut. Res. 2015, 22, 14209–14219. [Google Scholar] [CrossRef]
- Weschler, C.J. Shields Experiments Probing the Influence of Air Exchange Rates on Particles Generated by Indoor Chemistry. Indoor Air 2002, 500–505. [Google Scholar]
- Hsu, D.-J.; Huang, H.-L.; Sheu, S.-C. Characteristics of Air Pollutants and Assessment of Potential Exposure in Spa Centers During Aromatherapy. Environ. Eng. Sci. 2012, 29, 79–85. [Google Scholar] [CrossRef]
- Wierzbicka, A.; Bohgard, M.; Pagels, J.H.; Dahl, A.; Löndahl, J.; Hussein, T.; Swietlicki, E.; Gudmundsson, A. Quantification of differences between occupancy and total monitoring periods for better assessment of exposure to particles in indoor environments. Atmos. Environ. 2015, 106, 419–428. [Google Scholar] [CrossRef]
- Cappellin, L.; Karl, T.; Probst, M.; Ismailova, O.; Winkler, P.M.; Soukoulis, C.; Aprea, E.; Märk, T.D.; Gasperi, F.; Biasioli, F. On quantitative determination of volatile organic compound concentrations using proton transfer reaction time-of-flight mass spectrometry. Environ. Sci. Technol. 2012, 46, 2283–2290. [Google Scholar] [CrossRef]
- Lindinger, W.; Hansel, A.; Jordan, A. On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS) medical applications, food control and environmental research. Int. J. Mass Spectrom. Ion Process. 1998, 173, 191–241. [Google Scholar] [CrossRef]
- Tani, A.; Hayward, S.; Hewitt, C.N. Measurement of monoterpenes and related compounds by proton transfer reaction-mass spectrometry (PTR-MS). Int. J. Mass Spectrom. 2003, 223–224, 561–578. [Google Scholar] [CrossRef]
- Tani, A.; Hayward, S.; Hansel, A.; Hewitt, C.N. Effect of water vapour pressure on monoterpene measurements using proton transfer reaction-mass spectrometry (PTR-MS). Int. J. Mass Spectrom. 2004, 239, 161–169. [Google Scholar] [CrossRef]
- Tani, A. Fragmentation and reaction rate constants of terpenoids determined by proton transfer reaction-mass spectrometry. Environ. Control Biol. 2013, 51, 23–29. [Google Scholar] [CrossRef]
- Lee, A.; Goldstein, A.H.; Kroll, J.H.; Ng, N.L.; Varutbangkul, V.; Flagan, R.C.; Seinfeld, J.H. Gas-phase products and secondary aerosol yields from the photooxidation of 16 different terpenes. J. Geophys. Res. Atmos. 2006, 111, 1–25. [Google Scholar] [CrossRef]
- Bianchi, F.; Kurtén, T.; Riva, M.; Mohr, C.; Rissanen, M.P.; Roldin, P.; Berndt, T.; Crounse, J.D.; Wennberg, P.O.; Mentel, T.F.; et al. Highly Oxygenated Organic Molecules (HOM) from Gas-Phase Autoxidation Involving Peroxy Radicals: A Key Contributor to Atmospheric Aerosol. Chem. Rev. 2019, 119, 3472–3509. [Google Scholar] [CrossRef]
- Breitenlechner, M.; Fisher, L.; Hainer, M.; Heinritzi, M.; Curtius, J.; Hansel, A. PTR3: An Instrument for Studying the Lifecycle of Reactive Organic Carbon in the Atmosphere. Anal. Chem. 2017, 89, 5824–5831. [Google Scholar] [CrossRef]
- Bernhammer, A.-K.; Fisher, L.; Mentler, B.; Heinritzi, M.; Simon, M.; Hansel, A. Production of highly oxygenated organic molecules (HOMs) from trace contaminants during isoprene oxidation. Atmos. Meas. Tech. 2018, 11, 4763–4773. [Google Scholar] [CrossRef]
- Bonn, B.; Schuster, G.; Moortgat, G.K. Influence of water vapor on the process of new particle formation during monoterpene ozonolysis. J. Phys. Chem. A 2002, 106, 2869–2881. [Google Scholar] [CrossRef]
- Gallimore, P.J.; Mahon, B.M.; Wragg, F.P.H.; Fuller, S.J.; Giorio, C.; Kourtchev, I.; Kalberer, M. Multiphase composition changes and reactive oxygen species formation during limonene oxidation in the new Cambridge Atmospheric Simulation Chamber (CASC). Atmos. Chem. Phys. 2017, 17, 9853–9868. [Google Scholar] [CrossRef]
- Jonsson, Å.M.; Hallquist, M.; Ljungström, E. Impact of humidity on the ozone initiated oxidation of limonene, Δ3-carene, and α-pinene. Environ. Sci. Technol. 2006, 40, 188–194. [Google Scholar] [CrossRef]
- Ishizuka, Y.; Tokumura, M.; Mizukoshi, A.; Noguchi, M. Measurement of Secondary Products During Oxidation Reactions of Terpenes and Ozone Based on the PTR-MS Analysis: Effects of Coexistent Carbonyl Compounds. Int. J. Environ. Res. Public Health 2010, 7, 3853–3870. [Google Scholar] [CrossRef]
- Schrader, W.; Geiger, J.; Godejohann, M. Studies of complex reactions using modern hyphenated methods: A-Pinene ozonolysis as a model reaction. J. Chromatogr. A 2005, 1075, 185–196. [Google Scholar] [CrossRef]
- Grosjean, D.; Williams, E.L.; Seinfeld, J.H. Atmospheric oxidation of selected terpenes and related carbonyls: Gas-phase carbonyl products. Environ. Sci. Technol. 1992, 26, 1526–1533. [Google Scholar] [CrossRef]
- Jaoui, M.; Kamens, R.M. Gaseous and particulate oxidation products analysis of a mixture of a-pinene + b-pinene/O3/air in the absence of light and a-pinene + b-pinene/NOx/air in the presence of natural sunlight. Sect. Title Air Pollut. Ind. Hyg. 2003, 44, 259–297. [Google Scholar]
- Librando, V.; Tringali, G. Atmospheric fate of OH initiated oxidation of terpenes. Reaction mechanism of α-pinene degradation and secondary organic aerosol formation. J. Environ. Manag. 2005, 75, 275–282. [Google Scholar] [CrossRef]
- Larsen, B.O.R.; Bella, D.D.I.; Glasius, M.; Winterhalter, R.; Jensen, N.R.; Hjorth, J. Gas-Phase OH Oxidation of Monoterpenes: Gaseous and Particulate Products. J. Atmos. Chem. 2001, 38, 231–276. [Google Scholar] [CrossRef]
- Schroeder, L.M.; Weslien, J. Reduced offspring production in bark beetleTomicus piniperda in pine bolts baited with ethanol and α-pinene, which attract antagonistic insects. J. Chem. Ecol. 1994, 20, 1429–1444. [Google Scholar] [CrossRef]
- Rodríguez, A.; Andrés, V.S.; Cervera, M.; Redondo, A.; Alquézar, B.; Shimada, T.; Gadea, J.; Rodrigo, M.; Zacarías, L.; Palou, L.; et al. The monoterpene limonene in orange peels attracts pests and microorganisms. Plant Signal. Behav. 2011, 6, 1820–1823. [Google Scholar] [CrossRef]
- Bäck, J.; Aalto, J.; Henriksson, M.; Hakola, H.; He, Q.; Boy, M. Chemodiversity of a Scots pine stand and implications for terpene air concentrations. Biogeosciences 2012, 9, 689–702. [Google Scholar] [CrossRef]
- Tarvainen, V.; Hakola, H.; Hellén, H.; Bäck, J.; Hari, P.; Kulmala, M. Temperature and light dependence of the VOC emissions of Scots pine. Atmos. Chem. Phys. 2005, 5, 989–998. [Google Scholar] [CrossRef]
- Janson, R.W. Monoterpene emissions from Scots pine and Norwegian spruce. J. Geophys. Res. Atmos. 1993, 98, 2839–2850. [Google Scholar] [CrossRef]
- Hiltunen, R.; Laakso, I. Gas chromatographic analysis and biogenetic relationships of monoterpene enantiomers in Scots Pine and juniper needle oils. Flavour Fragr. J. 1995, 10, 203–210. [Google Scholar] [CrossRef]
- Haypek, E.; Silva, L.H.; Batista, E.; Marques, D.S.; Meireles, M.A.A.; Meirelles, A.J.A. Recovery of aroma compounds from orange essential oil. Braz. J. Chem. Eng. 2000, 17, 705–712. [Google Scholar] [CrossRef]
- Sarwar, G.; Corsi, R. The effects of ozone/limonene reactions on indoor secondary organic aerosols. Atmos. Environ. 2007, 41, 959–973. [Google Scholar] [CrossRef]
- Hansela, A.; Jordana, A.; Holzingera, R.; Prazellera, P.; Vogelb, W.; Lindingera, W. Proton transfer reaction mass spectrometry: On-line trace gas analysis at the ppb level. Int. J. Mass Spectrom. Ion Process. 1995, 149, 609–619. [Google Scholar] [CrossRef]
- Jordan, A.; Haidacher, S.; Hanel, G.; Hartungen, E.; Märk, L.; Seehauser, H.; Schottkowsky, R.; Sulzer, P.; Märk, T.D. A high resolution and high sensitivity proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS). Int. J. Mass Spectrom. 2009, 286, 122–128. [Google Scholar] [CrossRef]
- SIS Tenax® TA Breakthrough Volume Data. Available online: https://www.sisweb.com/index/referenc/tenaxta.htm (accessed on 2 February 2020).
- Marć, M.; Namieśnik, J.; Zabiegała, B. Small-scale passive emission chamber for screening studies on monoterpene emission flux from the surface of wood-based indoor elements. Sci. Total Environ. 2014, 481, 35–46. [Google Scholar] [CrossRef]
- Salthammer, T. Formaldehyde sources, formaldehyde concentrations and air exchange rates in European housings. Build. Environ. 2019, 150, 219–232. [Google Scholar] [CrossRef]
- Rovira, J.; Roig, N.; Nadal, M.; Schuhmacher, M.; Domingo, J.L. Human health risks of formaldehyde indoor levels: An issue of concern. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2016, 51, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Salthammer, T.; Schulz, N.; Stolte, R.; Uhde, E. Human sensory response to acetone/air mixtures. Indoor Air 2016, 26, 796–805. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds studied in this research are available from the authors. |
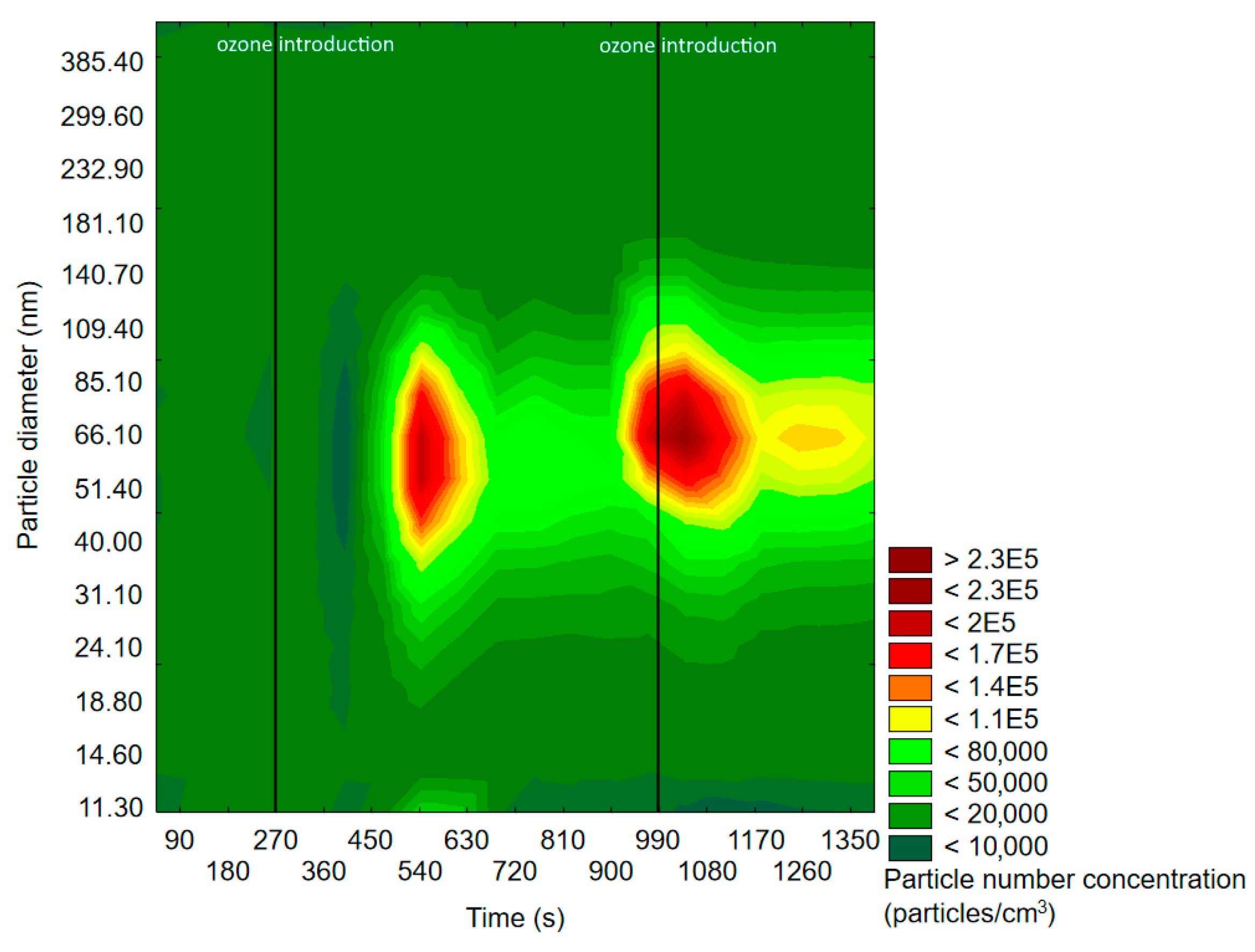
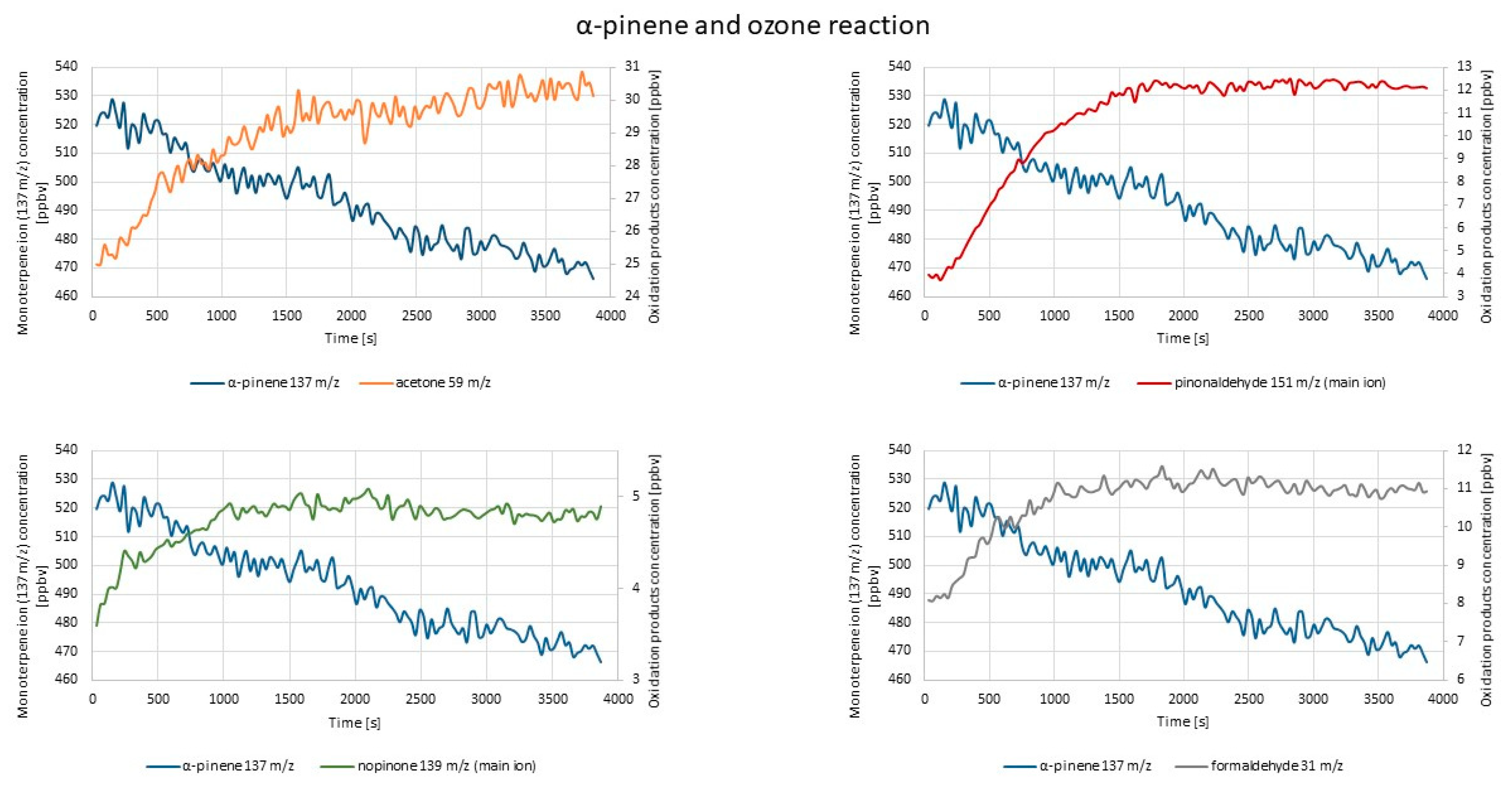
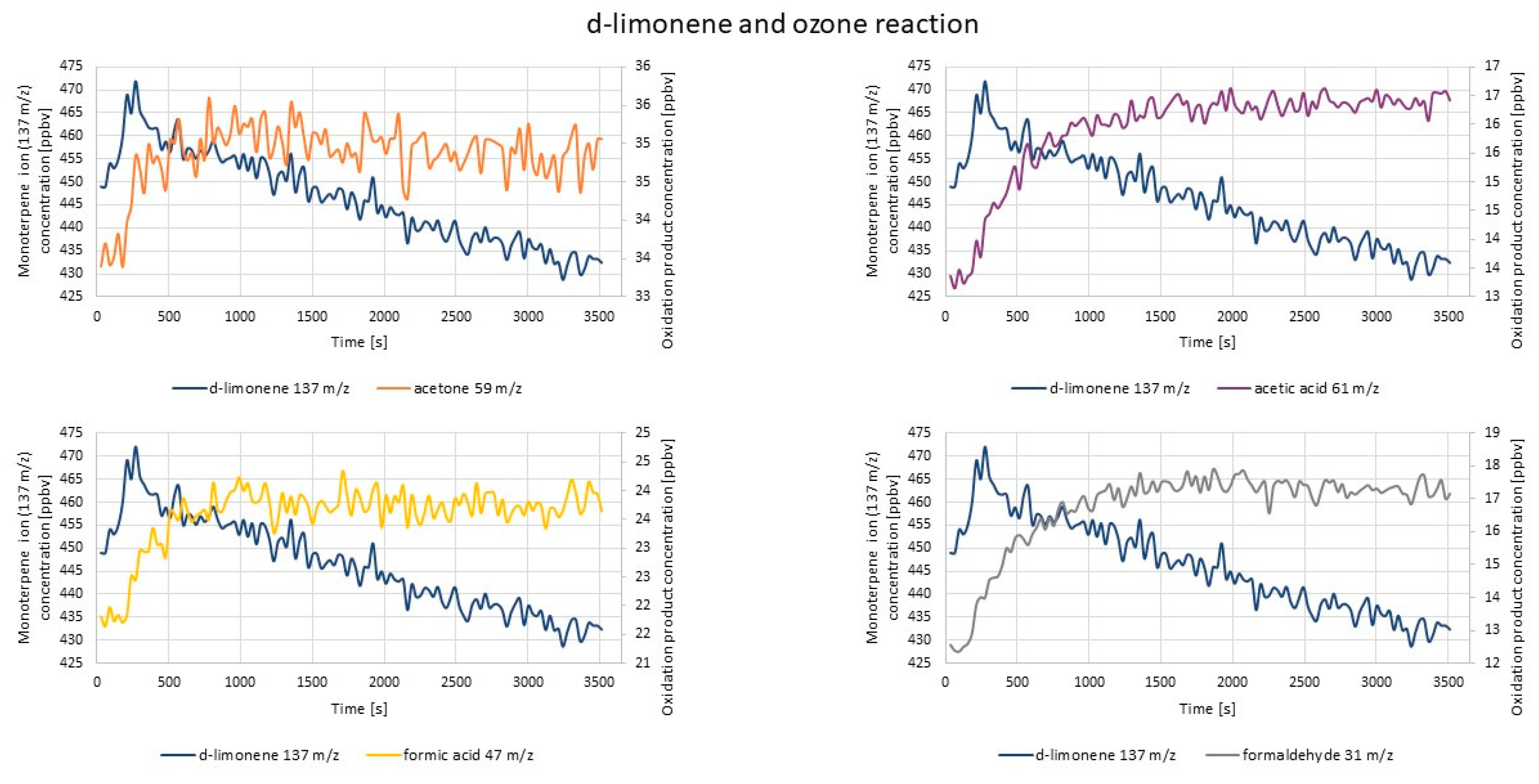


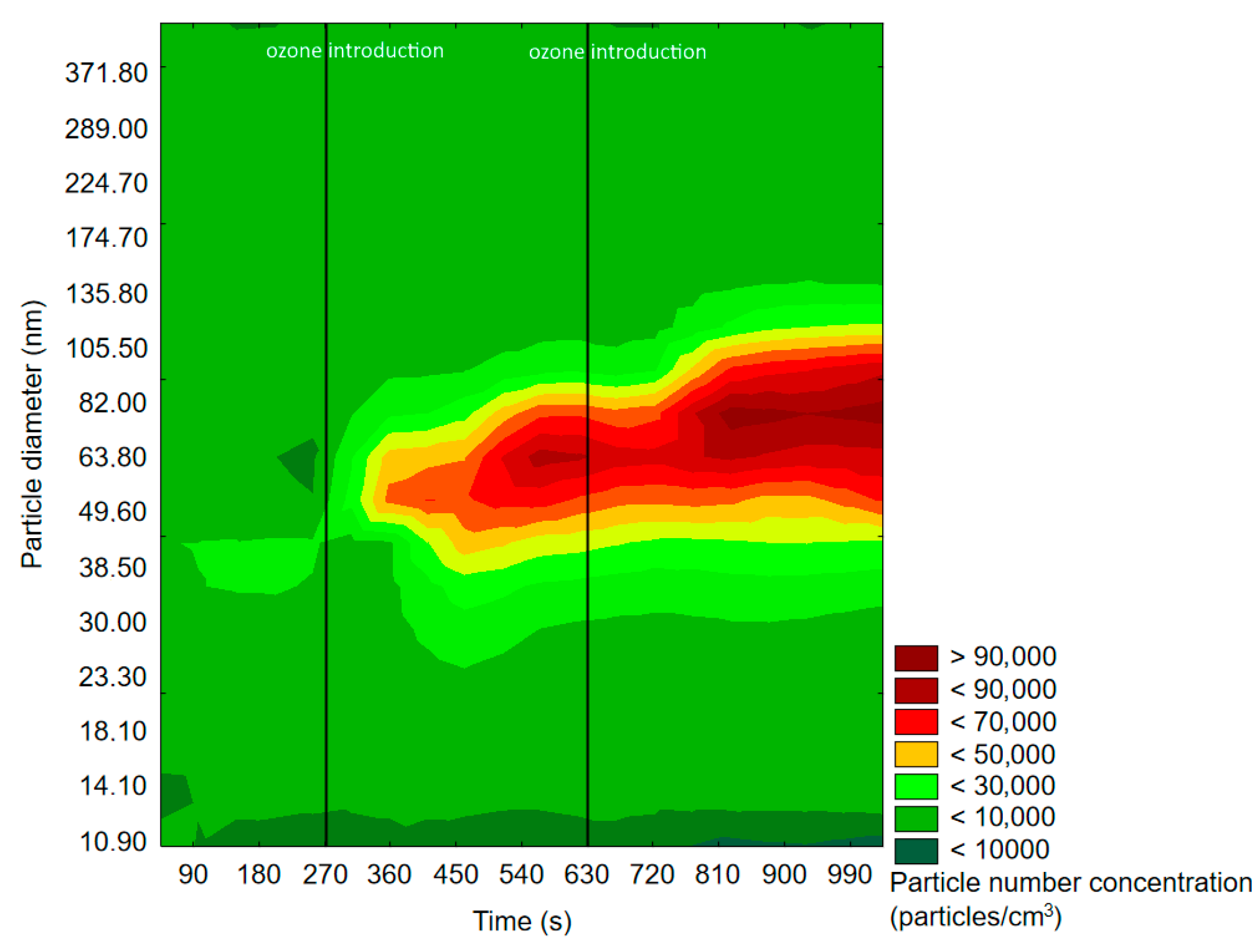
| Experiment | Cycle of 1st O3 Introduction | Cycle of 2nd O3 Introduction | RH 1 |
|---|---|---|---|
| α-pinene and O3 reaction | 4th | 11th | 59% |
| d-limonene and O3 reaction | 4th | 11th | 64% |
| α-pinene + d-limonene and O3 reaction | 3rd | 10th | 64% |
| Scots pine wooden block emitted monoterpenes and O3 reaction | 3rd | 12th | 59% |
| orange emitted monoterpenes and O3 reaction | 3rd | 7th | 66% |
| Experiment | Ion | Time Lag Between O3 Introduction and First Increase of the Oxidation Product Concentration (s) | Time Range while the Concentration of Oxidation Products Was Stable (s) | Trend at the End of the Measurement (increasing/decreasing/steady) |
|---|---|---|---|---|
| α-pinene ozonolysis | 59 | 60 | 1740–3150 | increasing |
| 151 | 30 | 1890–3840 | steady | |
| 139 | 30 | 1530–2340 | decreasing | |
| 31 | 30 | 1440–2040 | decreasing | |
| d-limonene ozonolysis | 59 | 120 | 930–2190 | decreasing/steady |
| 47 | 120 | 1290–3690 | steady | |
| 31 | 30 | 1290–2490 | steady | |
| 61 | 60 | - | increasing | |
| α-pinene + d-limonene ozonolysis | 59 | 120 | - | increasing |
| 47 | 120 | - | decreasing | |
| 31 | 120 | 990–2190 | increasing | |
| 61 | 150 | - | increasing | |
| 151 | 120 | 1590–2700 | decreasing | |
| 139 | 150 | 1290–3690 | steady |
| Experiment | Ion | Time Lag Between O3 Introduction and the First Increase of the Oxidation Product Concentration (s) | Time Range while the Concentration of Oxidation Products Was Stable | Trend at the End of the Measurement (increasing/decreasing/steady) |
|---|---|---|---|---|
| Emission from Scots pine | 59 | 30 | - | decreasing |
| 151 | 60 | 1590–2640 | decreasing/steady | |
| 139 | 90 | 1590–3090 | decreasing | |
| 31 | 30 | 990–2790 | decreasing | |
| Emission from orange peel | 59 | 30 | - | increasing |
| 47 | 60 | 1830–3690 | decreasing/steady | |
| 31 | 90 | 2430–3690 | steady | |
| 61 | 30 | - | increasing |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pytel, K.; Marcinkowska, R.; Zabiegała, B. Investigation of the Dynamism of Nanosized SOA Particle Formation in Indoor Air by a Scanning Mobility Particle Sizer and Proton-Transfer-Reaction Mass Spectrometry. Molecules 2020, 25, 2202. https://doi.org/10.3390/molecules25092202
Pytel K, Marcinkowska R, Zabiegała B. Investigation of the Dynamism of Nanosized SOA Particle Formation in Indoor Air by a Scanning Mobility Particle Sizer and Proton-Transfer-Reaction Mass Spectrometry. Molecules. 2020; 25(9):2202. https://doi.org/10.3390/molecules25092202
Chicago/Turabian StylePytel, Klaudia, Renata Marcinkowska, and Bożena Zabiegała. 2020. "Investigation of the Dynamism of Nanosized SOA Particle Formation in Indoor Air by a Scanning Mobility Particle Sizer and Proton-Transfer-Reaction Mass Spectrometry" Molecules 25, no. 9: 2202. https://doi.org/10.3390/molecules25092202
APA StylePytel, K., Marcinkowska, R., & Zabiegała, B. (2020). Investigation of the Dynamism of Nanosized SOA Particle Formation in Indoor Air by a Scanning Mobility Particle Sizer and Proton-Transfer-Reaction Mass Spectrometry. Molecules, 25(9), 2202. https://doi.org/10.3390/molecules25092202






