Elemental Content in Pleurotus ostreatus and Cyclocybe cylindracea Mushrooms: Correlations with Concentrations in Cultivation Substrates and Effects on the Production Process
Abstract
1. Introduction
2. Results and Discussion
2.1. Elemental Content in Cultivation Substrates
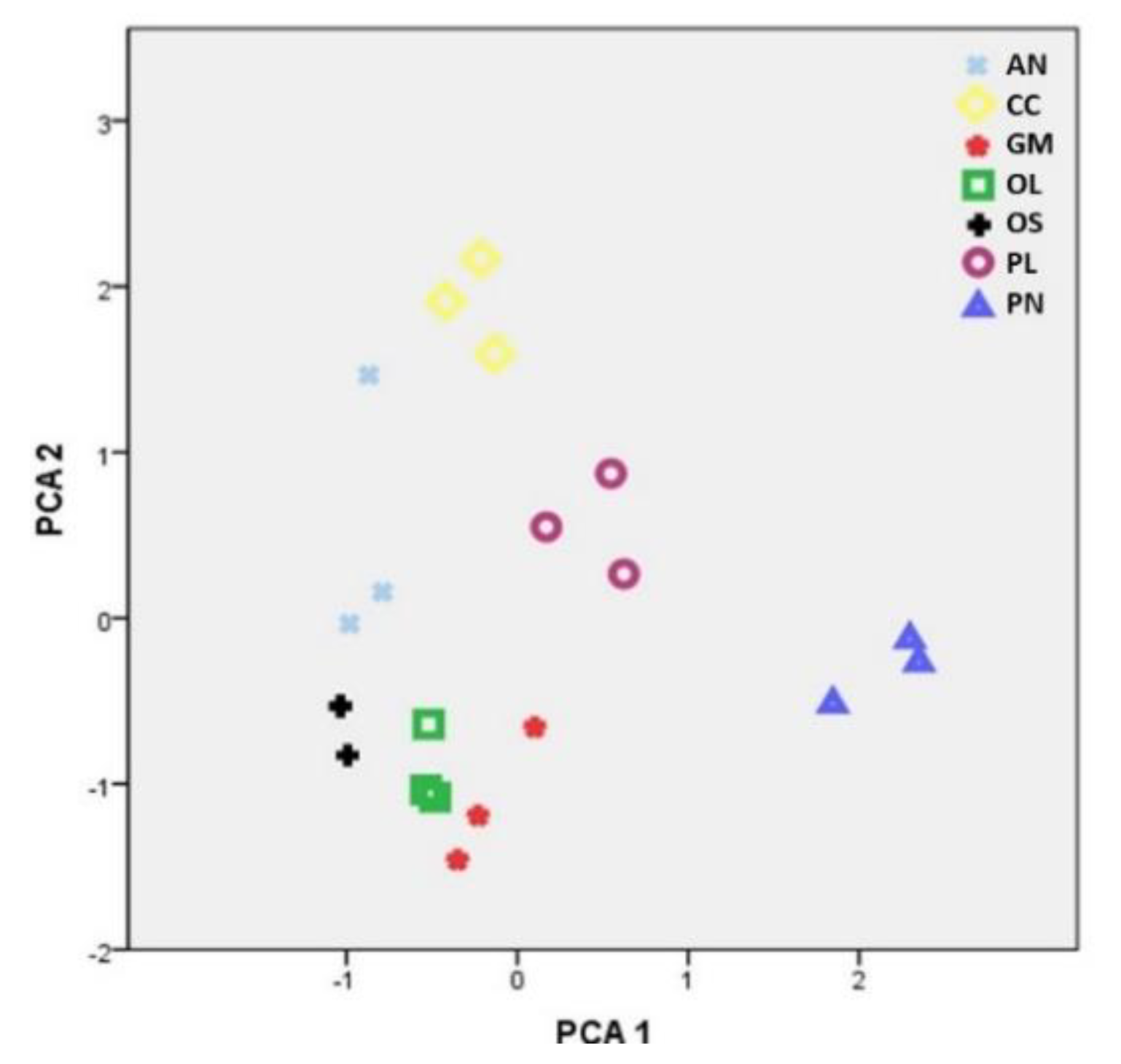
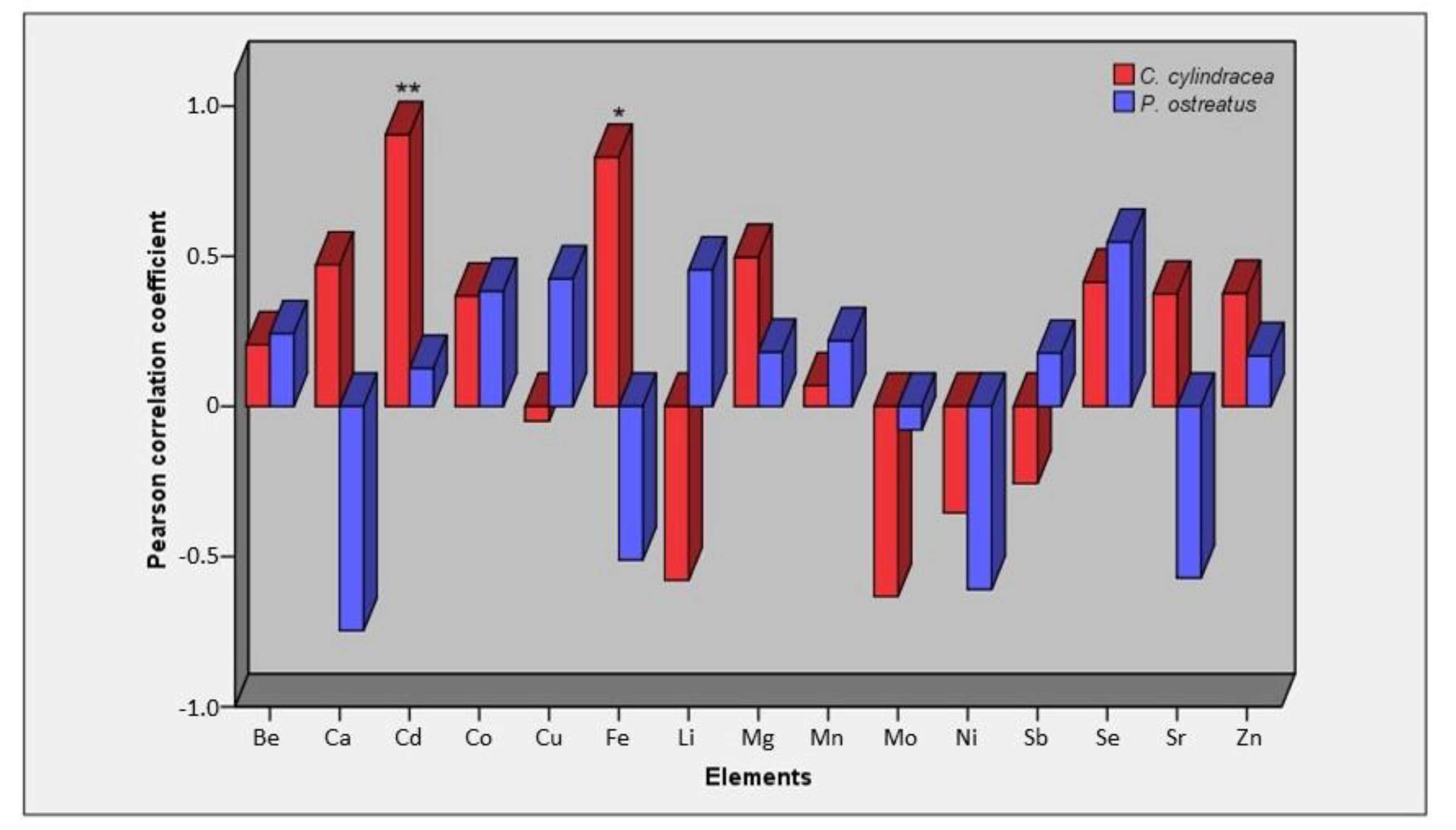
2.2. Concentration of Elements in Mushrooms and Pertinent Correlations
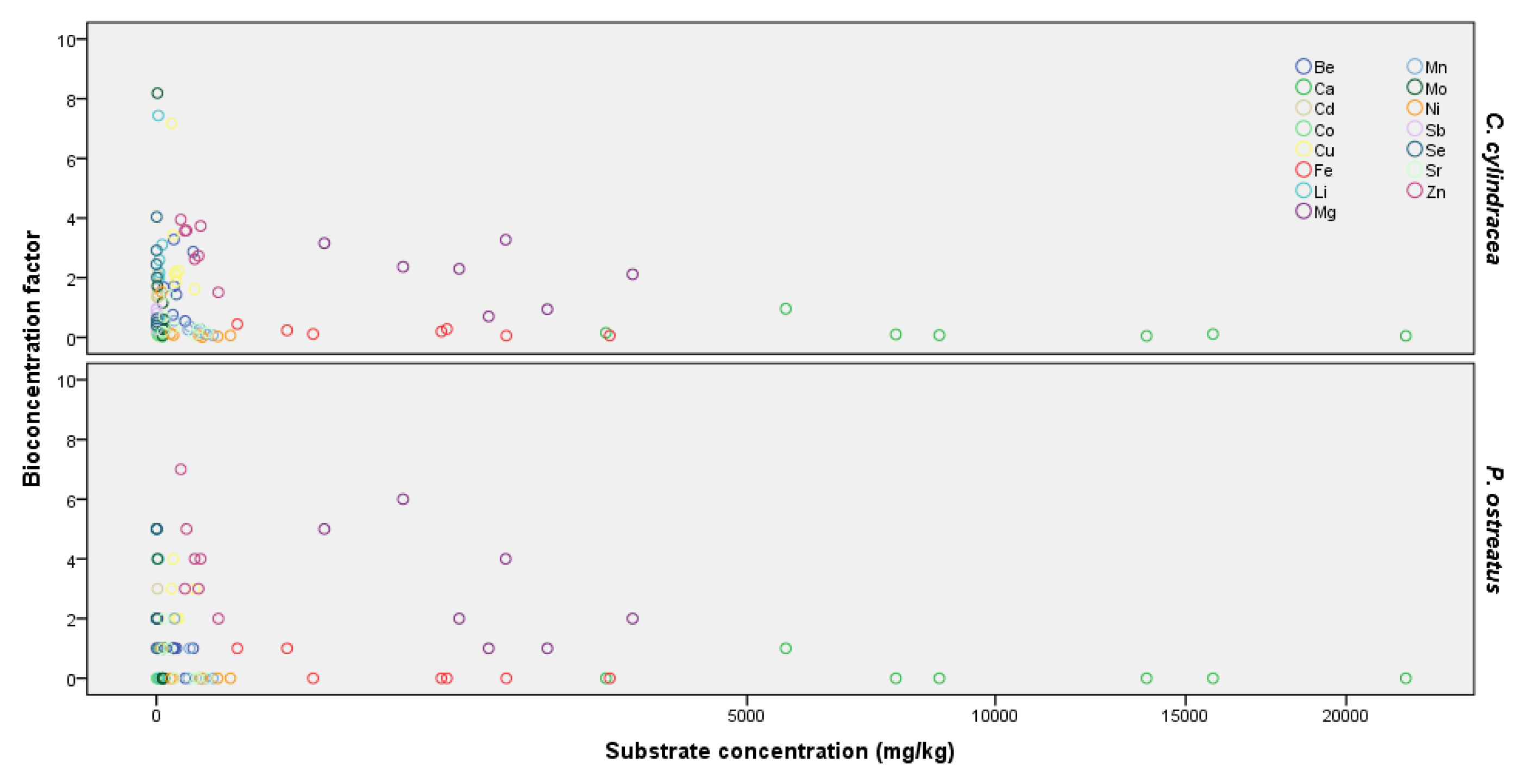
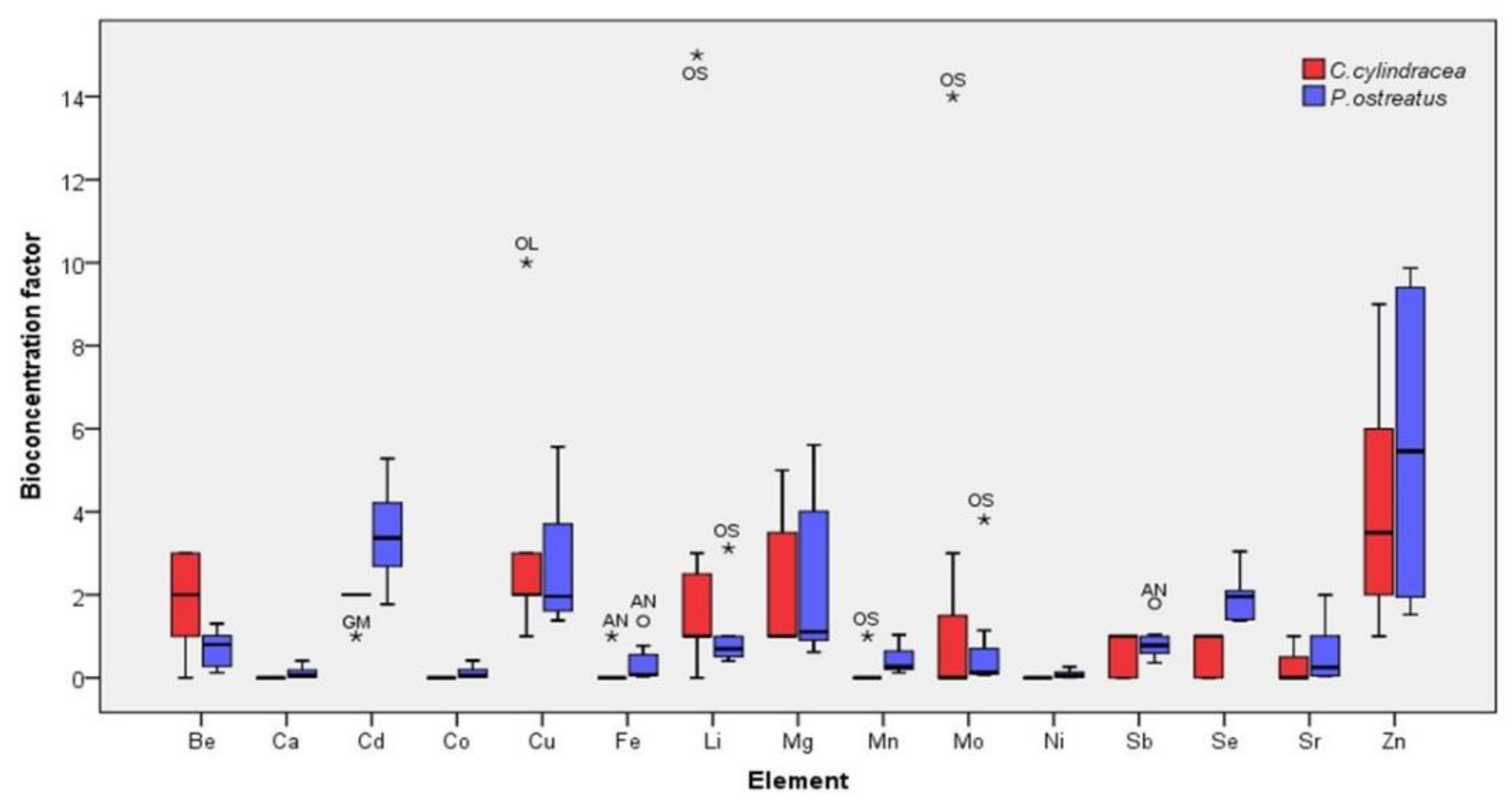
2.3. Bioconcentration Factors (BCFs)
2.4. Effect of Substrates Elements on Cultivation Parameters and Lignocellulosics Content
2.5. Consumption of Mushrooms—Dietary Intake
3. Materials and Methods
3.1. Biological Material and Mushroom Cultivation Substrates
3.2. Chemicals and Standard Solutions
3.3. Samples Preparation
3.4. ICP-MS Analysis
3.5. Health Risk Assessment of Mushroom Consumption
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zervakis, G.I.; Koutrotsios, G. Solid-State Fermentation of Plant Residues and Agro-industrial Wastes for the Production of Medicinal Mushrooms. In Medicinal and Aromatic Plants of the Middle-East; Springer Science and Business Media LLC: Singapore, 2017; Volume 4, pp. 365–396. [Google Scholar]
- Kalač, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2012, 93, 209–218. [Google Scholar] [CrossRef]
- Friedman, M. Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans. Foods 2016, 5, 80. [Google Scholar] [CrossRef]
- Gargano, M.L.; Van Griensven, L.J.L.D.; Isikhuemhen, O.S.; Lindequist, U.; Venturella, G.; Wasser, S.P.; Zervakis, G.I. Medicinal mushrooms: Valuable biological resources of high exploitation potential. Plant Biosyst. 2017, 151, 548–565. [Google Scholar] [CrossRef]
- Meneses, M.E.; Carrera, D.M.; Torres, N.; Sánchez-Tapia, M.; Aguilar-López, M.; Morales, P.; Sobal, M.; Bernabé, T.; Escudero, H.; Granados-Portillo, O.; et al. Hypocholesterolemic Properties and Prebiotic Effects of Mexican Ganoderma lucidum in C57BL/6 Mice. PLoS ONE 2016, 11, e0159631. [Google Scholar] [CrossRef] [PubMed]
- Singdevsachan, S.K.; Auroshree, P.; Mishra, J.; Baliyarsingh, B.; Tayung, K.; Thatoi, H. Mushroom polysaccharides as potential prebiotics with their antitumor and immunomodulating properties: A review. Bioact. Carbohydrates Diet. Fibre 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Smolskaitė, L.; Venskutonis, P.R.; Talou, T. Comprehensive evaluation of antioxidant and antimicrobial properties of different mushroom species. LWT 2015, 60, 462–471. [Google Scholar] [CrossRef]
- Anthes, C.; De Schutter, O. The Food and Agriculture Organization of the United Nations; Oxford University Press (OUP): Oxford, UK, 2018. [Google Scholar]
- Royse, D.J.; Baars, J.; Tan, Q. Current overview of mushroom production in the world. In Edible and Medicinal Mushrooms: Technology and Applications; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 5–13. [Google Scholar]
- Philippoussis, A.; Zervakis, G.I.; Diamantopoulou, P. Bioconversion of agricultural lignocellulosic wastes through the cultivation of the edible mushrooms Agrocybe aegerita, Volvariella volvacea and Pleurotus spp. World J. Microbiol. Biotechnol. 2001, 17, 191–200. [Google Scholar] [CrossRef]
- Uhart, M.; Piscera, J.M.; Albertó, E. Utilization of new naturally occurring strains and supplementation to improve the biological efficiency of the edible mushroom Agrocybe cylindracea. J. Ind. Microbiol. Biotechnol. 2008, 35, 595–602. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Kalogeropoulos, N.; Kaliora, A.C.; Zervakis, G.I. Toward an Increased Functionality in Oyster (Pleurotus) Mushrooms Produced on Grape Marc or Olive Mill Wastes Serving as Sources of Bioactive Compounds. J. Agric. Food Chem. 2018, 66, 5971–5983. [Google Scholar] [CrossRef]
- Zervakis, G.I.; Koutrotsios, G.; Katsaris, P. Composted versus Raw Olive Mill Waste as Substrates for the Production of Medicinal Mushrooms: An Assessment of Selected Cultivation and Quality Parameters. BioMed Res. Int. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Hoa, H.T.; Wang, C.-L.; Wang, C.-H. The Effects of Different Substrates on the Growth, Yield, and Nutritional Composition of Two Oyster Mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 423–434. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Mountzouris, K.C.; Chatzipavlidis, I.; Zervakis, G.I. Bioconversion of lignocellulosic residues by Agrocybe cylindracea and Pleurotus ostreatus mushroom fungi – Assessment of their effect on the final product and spent substrate properties. Food Chem. 2014, 161, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Koutrotsios, G.; Larou, E.; Mountzouris, K.; Zervakis, G.I. Detoxification of Olive Mill Wastewater and Bioconversion of Olive Crop Residues into High-Value-Added Biomass by the Choice Edible Mushroom Hericium erinaceus. Appl. Biochem. Biotechnol. 2016, 180, 195–209. [Google Scholar] [CrossRef]
- Baldrian, P. Interactions of heavy metals with white-rot fungi. Enzym. Microb. Technol. 2003, 32, 78–91. [Google Scholar] [CrossRef]
- Baldrian, P. Effect of Heavy Metals on Saprotrophic Soil Fungi. In Biocontrol of Lepidopteran Pests; Springer Science and Business Media LLC: Berlin & Heidelberg, Germany, 2009; Volume 19, pp. 263–279. [Google Scholar]
- Baldrian, P.; Gabriel, J. Lignocellulose degradation by Pleurotus ostreatus in the presence of cadmium. FEMS Microbiol. Lett. 2003, 220, 235–240. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2009, 156, 609–643. [Google Scholar] [CrossRef]
- Jellison, J.; Connolly, J.; Goodell, B.; Doyle, B.; Illman, B.; Fekete, F.; Ostrofsky, A. The role of cations in the biodegradation of wood by the brown rot fungi. Int. Biodeterior. Biodegradation 1997, 39, 165–179. [Google Scholar] [CrossRef]
- Bhatia, P.; Aureli, F.; D’Amato, M.; Prakash, R.; Cameotra, S.S.; Nagaraja, T.P.; Cubadda, F. Selenium bioaccessibility and speciation in biofortified Pleurotus mushrooms grown on selenium-rich agricultural residues. Food Chem. 2013, 140, 225–230. [Google Scholar] [CrossRef]
- Da Silva, M.C.; Naozuka, J.; Da Luz, J.M.R.; De Assunção, L.S.; Oliveira, P.V.; Vanetti, M.C.; Bazzolli, D.M.S.; Kasuya, M.C.M. Enrichment of Pleurotus ostreatus mushrooms with selenium in coffee husks. Food Chem. 2012, 131, 558–563. [Google Scholar] [CrossRef]
- De Assunção, L.S.; Da Luz, J.M.R.; Silva, M.D.C.S.D.; Vieira, P.A.F.; Bazzolli, D.M.S.; Vanetti, M.C.D.; Kasuya, M.C.M. Enrichment of mushrooms: An interesting strategy for the acquisition of lithium. Food Chem. 2012, 134, 1123–1127. [Google Scholar] [CrossRef]
- Gąsecka, M.; Mleczek, M.; Siwulski, M.; Niedzielski, P. Phenolic composition and antioxidant properties of Pleurotus ostreatus and Pleurotus eryngii enriched with selenium and zinc. Eur. Food Res. Technol. 2015, 242, 723–732. [Google Scholar] [CrossRef]
- Muñoz, A.H.S.; Kubachka, K.; Wrobel, K.; Corona, J.F.G.; Yathavakilla, S.K.V.; Caruso, J.A.; Wrobel, K. Se-Enriched Mycelia of Pleurotus ostreatus: Distribution of Selenium in Cell Walls and Cell Membranes/Cytosol. J. Agric. Food Chem. 2006, 54, 3440–3444. [Google Scholar] [CrossRef] [PubMed]
- Vieira, P.A.F.; Gontijo, D.C.; Vieira, B.C.; Fontes, E.A.; De Assunção, L.S.; Leite, J.; Oliveira, M.G.D.A.; Kasuya, M.C.M. Antioxidant activities, total phenolics and metal contents in Pleurotus ostreatus mushrooms enriched with iron, zinc or lithium. LWT 2013, 54, 421–425. [Google Scholar] [CrossRef]
- Zięba, P.; Kała, K.; Włodarczyk, A.; Szewczyk, A.; Kunicki, E.; Sekara, A.; Muszyńska, B. Selenium and Zinc Biofortification of Pleurotus eryngii Mycelium and Fruiting Bodies as a Tool for Controlling Their Biological Activity. Mol. 2020, 25, 889. [Google Scholar] [CrossRef]
- Sakellari, A.; Karavoltsos, S.; Tagkouli, D.; Rizou, C.; Sinanoglou, V.J.; Zoumpoulakis, P.; Koutrotsios, G.; Zervakis, G.I.; Kalogeropoulos, N. Trace Elements in Pleurotus ostreatus, P. eryngii, and P. nebrodensis Mushrooms Cultivated on Various Agricultural By-Products. Anal. Lett. 2019, 52, 2692–2709. [Google Scholar] [CrossRef]
- Siwulski, M.; Rzymski, P.; Budka, A.; Kalač, P.; Budzyńska, S.; Dawidowicz, L.; Hajduk, E.; Kozak, L.; Budzulak, J.; Sobieralski, K.; et al. The effect of different substrates on the growth of six cultivated mushroom species and composition of macro and trace elements in their fruiting bodies. Eur. Food Res. Technol. 2018, 245, 419–431. [Google Scholar] [CrossRef]
- Campos, J.; Tejera, N.A.; Sanchez, C.J. Substrate role in the accumulation of heavy metals in sporocarps of wild fungi. BioMetals 2009, 22, 835–841. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Park, J.-E.; Kim, B.-B.; Kim, S.-M.; Ro, H.-S. Determination of Mineral Components in the Cultivation Substrates of Edible Mushrooms and Their Uptake into Fruiting Bodies. Mycobiology 2009, 37, 109–113. [Google Scholar] [CrossRef]
- Aloupi, M.; Koutrotsios, G.; Koulousaris, M.; Kalogeropoulos, N. Trace metal contents in wild edible mushrooms growing on serpentine and volcanic soils on the island of Lesvos, Greece. Ecotoxicol. Environ. Saf. 2012, 78, 184–194. [Google Scholar] [CrossRef]
- Fang, Y.; Sun, X.; Yang, W.; Ma, N.; Xin, Z.; Fu, J.; Liu, X.; Liu, M.; Mariga, A.M.; Zhu, X.; et al. Concentrations and health risks of lead, cadmium, arsenic, and mercury in rice and edible mushrooms in China. Food Chem. 2014, 147, 147–151. [Google Scholar] [CrossRef]
- Širić, I.; Humar, M.; Kasap, A.; Kos, I.; Mioč, B.; Pohleven, F. Heavy metal bioaccumulation by wild edible saprophytic and ectomycorrhizal mushrooms. Environ. Sci. Pollut. Res. 2016, 23, 18239–18252. [Google Scholar] [CrossRef]
- Borovička, J.; Kotrba, P.; Gryndler, M.; Mihaljevič, M.; Řanda, Z.; Rohovec, J.; Cajthaml, T.; Stijve, T.; Dunn, C.E. Bioaccumulation of silver in ectomycorrhizal and saprobic macrofungi from pristine and polluted areas. Sci. Total Environ. 2010, 408, 2733–2744. [Google Scholar] [CrossRef]
- Campos, J.; De Toro, J.A.; Reyes, C.P.D.L.; Amorós, J.A.; Garcia-Moreno, R. Lifestyle Influence on the Content of Copper, Zinc and Rubidium in Wild Mushrooms. Appl. Environ. Soil Sci. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Christ, S.; Wubet, T.; Theuerl, S.; Herold, N.; Buscot, F. Fungal communities in bulk soil and stone compartments of different forest and soil types as revealed by a barcoding ITS rDNA and a functional laccase encoding gene marker. Soil Boil. Biochem. 2011, 43, 1292–1299. [Google Scholar] [CrossRef]
- Huang, Q.; Jia, Y.; Wan, Y.; Li, H.; Jiang, R. Market Survey and Risk Assessment for Trace Metals in Edible Fungi and the Substrate Role in Accumulation of Heavy Metals. J. Food Sci. 2015, 80, H1612–H1618. [Google Scholar] [CrossRef] [PubMed]
- Kokkoris, V.; Massas, I.; Polemis, E.; Koutrotsios, G.; Zervakis, G.I. Accumulation of heavy metals by wild edible mushrooms with respect to soil substrates in the Athens metropolitan area (Greece). Sci. Total Environ. 2019, 685, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Lavola, A.; Aphalo, P.J.; Lehto, T. Boron and other elements in sporophores of ectomycorrhizal and saprotrophic fungi. Mycorrhiza 2010, 21, 155–165. [Google Scholar] [CrossRef]
- Cindrić, I.J.; Zeiner, M.; Starčević, A.; Stingeder, G. Metals in pine needles: Characterisation of bio-indicators depending on species. Int. J. Environ. Sci. Technol. 2018, 16, 4339–4346. [Google Scholar] [CrossRef]
- Kandziora-Ciupa, M.; Ciepał, R.; Nadgórska-Socha, A.; Barczyk, G. Accumulation of heavy metals and antioxidant responses in Pinus sylvestris L. needles in polluted and non-polluted sites. Ecotoxicology 2016, 25, 970–981. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Danezis, G.; Georgiou, C.; Zervakis, G.I. Rare earth elements concentration in mushroom cultivation substrates affects the production process and fruit-bodies content of Pleurotus ostreatus and Cyclocybe cylindracea. J. Sci. Food Agric. 2018, 98, 5418–5427. [Google Scholar] [CrossRef]
- Waters, B. Moving magnesium in plant cells. New Phytol. 2011, 190, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, D.; Jiang, X.; Cao, Z. Effects of the interactions between selenium and phosphorus on the growth and selenium accumulation in rice (Oryza sativa). Environ. Geochem. Health 2004, 26, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.D.; O’Donnell, K.C.; Dow, E.R.; Du, J.; Chen, G.; Manji, H.K. Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test. Neuropharmacology 2007, 54, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Nolen, W.A.; Licht, R.W.; Young, A.H.; Malhi, G.S.; Tohen, M.; Vieta, E.; Kupka, R.W.; Zarate, C.; Nielsen, R.E.; Baldessarini, R.J.; et al. What is the optimal serum level for lithium in the maintenance treatment of bipolar disorder? A systematic review and recommendations from the ISBD/IGSLI Task Force on treatment with lithium. Bipolar Disord. 2019, 21, 394–409. [Google Scholar] [CrossRef]
- Grünfeld, J.-P.; Rossier, B.C. Lithium nephrotoxicity revisited. Nat. Rev. Nephrol. 2009, 5, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Stone, K.A. Lithium-induced nephrogenic diabetes insipidus. J. Am. Board Fam. Pr. 1999, 12, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Carrasco-Gonzalez, J.A.; Serna-Saldívar, S.; Gutierrez-Uribe, J.A. Mycochemical Changes Induced by Selenium Enrichment in P. ostreatus Fruiting Bodies. J. Agric. Food Chem. 2017, 65, 4074–4082. [Google Scholar] [CrossRef]
- Bernaś, E.; Jaworska, G.; Lisiewska, Z. Edible mushrooms as a source of valuable nutritive constituents. Acta Sci. Pol. Technol. Aliment. 2006, 5, 5–20. [Google Scholar]
- Vetter, J. Mineral elements in the important cultivated mushrooms Agaricus bisporus and Pleurotus ostreatus. Food Chem. 1994, 50, 277–279. [Google Scholar] [CrossRef]
- Kalač, P. Trace element contents in European species of wild growing edible mushrooms: A review for the period 2000–2009. Food Chem. 2010, 122, 2–15. [Google Scholar] [CrossRef]
- Keles, A.; Genccelep, H.; Demirel, K. Elemental composition of naturally growing wild edible mushroom. J. Nat. Prod. Plant Resour. 2017, 7, 37–44. [Google Scholar]
- Komık, M.; Afyon, A.; Yağız, D. Minor element and heavy metal contents of wild growing and edible mushrooms from western Black Sea region of Turkey. Fresenius Environ. Bull. 2007, 16, 1359–1362. [Google Scholar]
- Muñoz, A.H.S.; Corona, F.G.; Wrobel, K.; Soto, G.M.; Wrobel, K. Subcellular Distribution of Aluminum, Bismuth, Cadmium, Chromium, Copper, Iron, Manganese, Nickel, and Lead in Cultivated Mushrooms (Agaricus bisporus and Pleurotus ostreatus). Boil. Trace Element Res. 2005, 106, 265–278. [Google Scholar] [CrossRef]
- Muthu, N.; Shanmugasundaram, K. Proximate and mineral compositions of edible mushroom Agrocybe aegerita. J. Pharmacogn. Phytochem. 2016, 5, 116–119. [Google Scholar]
- Niedzielski, P.; Mleczek, M.; Budka, A.; Rzymski, P.; Siwulski, M.; Jasińska, A.; Gąsecka, M.; Budzyńska, S. A screening study of elemental composition in 12 marketable mushroom species accessible in Poland. Eur. Food Res. Technol. 2017, 243, 1759–1771. [Google Scholar] [CrossRef]
- Vetter, J.; Hajdu, C.; Györfi, J.; Maszlavér, P. Mineral composition of the cultivated mushrooms Agaricus bisporus, Pleurotus ostreatus and Lentinula edodes. Acta Aliment. 2005, 34, 441–451. [Google Scholar] [CrossRef]
- Zhu, F.; Qu, L.; Fan, W.; Qiao, M.; Hao, H.; Wang, X. Assessment of heavy metals in some wild edible mushrooms collected from Yunnan Province, China. Environ. Monit. Assess. 2010, 179, 191–199. [Google Scholar] [CrossRef]
- Khani, M.H.; Pahlavanzadeh, H.; Alizadeh, K. Biosorption of strontium from aqueous solution by fungus Aspergillus terreus. Environ. Sci. Pollut. Res. 2012, 19, 2408–2418. [Google Scholar] [CrossRef]
- Barker, A.V.; Pilbeam, D.J. Handbook of Plant Nutrition; CRC Press: Boca Raton, FA, USA, 2015. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FA, USA, 2001. [Google Scholar]
- Shevyakova, N.I.; Cheremisina, A.I.; Kuznetsov, V.V. Phytoremediation potential of Amaranthus hybrids: Antagonism between nickel and iron and chelating role of polyamines. Russ. J. Plant Physiol. 2011, 58, 634–642. [Google Scholar] [CrossRef]
- Abd-Elfattah, A.; Wada, K. Adsorption of Lead, Copper, Zinc, Cobalt, and Cadmium by Soils that Differ in Cation-Exchange Materials. J. Soil Sci. 1981, 32, 271–283. [Google Scholar] [CrossRef]
- Mustafa, S.; Waseem, M.; Naeem, A.; Shah, K.; Ahmad, T.; Hussain, S.Y. Selective sorption of cadmium by mixed oxides of iron and silicon. Chem. Eng. J. 2010, 157, 18–24. [Google Scholar] [CrossRef]
- Nnorom, I.C.; Eze, S.O.; O Ukaogo, P. Mineral contents of three wild-grown edible mushrooms collected from forests of south eastern Nigeria: An evaluation of bioaccumulation potentials and dietary intake risks. Sci. Afr. 2019, e00163. [Google Scholar] [CrossRef]
- Chiu, S.; Chan, Y.; Law, S.; Cheung, K.; Moore, D. Cadmium and manganese in contrast to calcium reduce yield and nutritional values of the edible mushroom Pleurotus pulmonarius. Mycol. Res. 1998, 102, 449–457. [Google Scholar] [CrossRef]
- Favero, N.; Bressa, G.; Costa, P. Response of Pleurotus ostreatus to cadmium exposure. Ecotoxicol. Environ. Saf. 1990, 20, 1–6. [Google Scholar] [CrossRef]
- Seyfferth, A.L.; McClatchy, C.; Paukett, M. Arsenic, Lead, and Cadmium in U.S. Mushrooms and Substrate in Relation to Dietary Exposure. Environ. Sci. Technol. 2016, 50, 9661–9670. [Google Scholar] [CrossRef]
- Rietra, R.; Heinen, M.; Dimkpa, C.O.; Bindraban, P.S. Effects of Nutrient Antagonism and Synergism on Yield and Fertilizer Use Efficiency. Commun. Soil Sci. Plant Anal. 2017, 48, 1895–1920. [Google Scholar] [CrossRef]
- Kurokawa, S.; Berry, M.J. Selenium. Role of the essential metalloid in health. Met. Ions Life Sci. 2013, 13, 499–534. [Google Scholar] [CrossRef]
- Baldrian, P.; Valášková, V.; Merhautová, V.; Gabriel, J. Degradation of lignocellulose by Pleurotus ostreatus in the presence of copper, manganese, lead and zinc. Res. Microbiol. 2005, 156, 670–676. [Google Scholar] [CrossRef]
- Palmieri, G.; Giardina, P.; Bianco, C.; Fontanella, B.; Sannia, G. Copper Induction of Laccase Isoenzymes in the Ligninolytic Fungus Pleurotus ostreatus. Appl. Environ. Microbiol. 2000, 66, 920–924. [Google Scholar] [CrossRef]
- Singhal, V.; Rathore, V. Effects of Zn2+ and Cu2+ on growth, lignin degradation and ligninolytic enzymes in Phanerochaete chrysosporium. World J. Microbiol. Biotechnol. 2001, 17, 235–240. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization (WHO): Geneva, Switzerland, 2011; pp. 104–108. [Google Scholar]
- Bruce, R.M.; Odin, M. Beryllium and Beryllium Compounds; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- World Health Organization; Food and Agricultural Organization of the United Nations. Vitamin and Mineral Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Martone, G. Nutritional Lithium. J. Clin. Psychiatry Neurosci. 2018, 1, 3–4. [Google Scholar]
- National Research Council Recommended Dietary Allowances; The National Academies Press: Washington, DC, USA, 1989.
- Watts, P.; Howe, P. Strontium and strontium compounds (Concise International Chemical Assessment Document 77); World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Quarcoo, A. Determination of heavy metals in Pleurotus ostreatus (Oyster mushroom) and Termitomyces clypeatus (Termite mushroom) sold on selected markets in Accra, Ghana. Mycosphere 2013, 4, 960–967. [Google Scholar] [CrossRef]
- Cocchi, L.; Vescovi, L.; Petrini, L.; Petrini, O. Heavy metals in edible mushrooms in Italy. Food Chem. 2006, 98, 277–284. [Google Scholar] [CrossRef]
- Tuzen, M.; Özdemir, M.; Demirbaş, A. Study of heavy metals in some cultivated and uncultivated mushrooms of Turkish origin. Food Chem. 1998, 63, 247–251. [Google Scholar] [CrossRef]
- E.P.A. Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures; U.S. Environmental Protection Agency: Washington, DC, USA, 2000.
- Kalač, P. A review of trace element concentrations in edible mushrooms. Food Chem. 2000, 69, 273–281. [Google Scholar] [CrossRef]
Sample Availability: Samples of the examined materials are available from the authors. |
| Element | Substrate | ||||||
|---|---|---|---|---|---|---|---|
| AN | CC | GM | OL | OS | PL | PN | |
| As | nd | nd | nd | nd | nd | 0.10 (0.03) b | 2.62 (0.28) a |
| Be | 18.63 (5.76) cd | 2.47 (0.20) e | 74.25 (4.71) a | 20.73 (2.17) c | 13.30 (0.60) d | 18.95 (1.43) cd | 40.29 (1.93) b |
| Ca* | 7.11 (0.82) e | 3.88 (0.73) f | 15.38 (1.41) c | 18.28 (1.46) b | 3.79 (0.05) f | 9.55 (1.56) d | 27.21 (1.21) a |
| Cd | 0.09 (0.00) d | 0.12 (0.00) c | 0.14 (0.01) b | 0.09 (0.00) d | 0.09 (0.01) d | 0.12 (0.01) c | 0.23 (0.01) a |
| Co | 0.08 (0.01) d | 1.52 (0.25) b | 0.63 (0.17) c | 0.30 (0.01) d | 0.12 (0.00) d | 0.80 (0.09) c | 2.15 (0.31) a |
| Cu | 7.02 (0.63) c | 8.08 (0.56) c | 12.63 (0.54) b | 5.67 (0.34) c | 7.42 (0.54) c | 10.50 (1.93) b | 27.28 (2.21) a |
| Fe* | 0.09 (0.01) c | 1.16 (0.16) b | 1.49 (0.55) b | 0.36 (0.02) c | 0.14 (0.01) c | 1.15 (0.27) b | 2.98 (0.33) a |
| Li | 0.61 (0.06) c | 0.64 (0.00) c | 1.49 (0.14) b | 0.56 (0.10) c | 0.25 (0.02) d | 0.74 (0.09) c | 1.78 (0.16) a |
| Mg * | 1.02 (0.38) d | 0.46 (0.04) e | 3.88 (0.54) a | 1.69 (0.15) c | 0.26 (0.03) e | 2.04 (0.25) bc | 2.38 (0.26) b |
| Mn | 13.87 (1.24) bc | 23.14 (0.19) bc | 37.40 (1.48) bc | 41.32 (1.84) b | 7.88 (0.54) c | 43.76 (8.66) b | 97.40 (31.63) a |
| Mo | 2.54 (1.24) a | 2.33 (0.43) a | 0.88 (0.00) bc | 0.14 (0.03) c | 0.13 (0.01) c | 1.86 (0.18) ab | 1.84 (0.32) ab |
| Ni | 2.59 (0.09) c | 93.95 (3.48) a | 12.29 (1.40) c | 8.09 (0.08) c | 3.46 (0.30) c | 36.39 (2.82) b | 50.46 (17.35) b |
| Sb | 0.19 (0.01) de | 0.34 (0.02) c | 0.26 (0.02) cd | 0.28 (0.02) cd | 0.17 (0.03) e | 0.52 (0.04) b | 0.79 (0.09) a |
| Se | 0.11 (0.03) b | 0.14 (0.06) ab | 0.15 (0.04) ab | 0.13 (0.03) ab | 0.10 (0.01) b | 0.22 (0.05) a | 0.21 (0.06) a |
| Sr | 4.85 (0.60) e | 2.65 (0.22) e | 25.42 (2.21) c | 28.31 (2.41) b | 3.75 (0.27) e | 9.03 (1.48) d | 47.20 (1.38) a |
| Zn | 11.19 (4.85) d | 29.70 (0.24) bc | 15.40 (2.80) cd | 11.99 (1.29) d | 1.76 (0.29) d | 37.64 (10.93) b | 75.03 (14.22) a |
| Element | Substrate | |||||||
|---|---|---|---|---|---|---|---|---|
| Species | AN | CC | GM | OL | OS | PL | PN | |
| As | P.os | 0.03 (0.00) c | 0.01 (0.00) d | 0.07 (0.00) b | nd | nd | nd | 0.19 (0.03) a |
| C.cl | nd | nd | nd | nd | nd | nd | nd | |
| Be | P.os | 6.45 (2.49) bc | 3.23 (1.25) c | 15.53 (2.46) abc | 16.65 (9.91) ab | 12.31 (1.28) abc | 20.85 (7.25) a | 4.97 (0.52) bc |
| C.cl | 10.37 (5.29) d | 7.74 (0.03) d | 41.39 (9.93) bc | 53.20 (0.10) ab | 31.12 (0.46) c | 62.81 (13.64) a | 15.47 (4.16) d | |
| Ca* | P.os | 0.73 (0.09) ab | 1.02 (0.31) a | 0.17 (0.13) b | 0.29 (0.13) b | 1.57 (0.52) a | 0.52 (0.18) ab | 0.36 (0.06) b |
| C.cl | 1.16 (0.02) a | 0.52 (0.08) c | 0.67 (0.31) abc | 0.63 (0.09) bc | 0.36 (0.08) c | 0.31 (0.02) c | 1.07 (0.41) ab | |
| Cd | P.os | 0.38 (0.06) b | 0.62 (0.21) a | 0.38 (0.01) b | 0.39 (0.13) b | 0.30 (0.02) b | 0.31 (0.08) b | 0.41 (0.01) b |
| C.cl | 0.20 (0.03) bc | 0.28 (0.03) b | 0.18 (0.01) c | 0.21 (0.02) bc | 0.21 (0.11) bc | 0.20 (0.01) bc | 0.54 (0.03) a | |
| Co | P.os | 0.03 (0.00) c | 0.04 (0.01) abc | 0.03 (0.01) bc | 0.02 (0.00) c | 0.05 (0.02) a | 0.02 (0.01) c | 0.05 (0.00) ab |
| C.cl | 0.02 (0.00) b | 0.02 (0.00) b | 0.03 (0.00) a | 0.02 (0.00) b | 0.02 (0.01) b | 0.01 (0.00) b | 0.03 (0.01) a | |
| Cu | P.os | 39.05 (11.39) a | 15.86 (0.11) c | 21.13 (1.94) bc | 18.97 (1.36) bc | 30.16 (6.90) ab | 16.33 (5.87) c | 37.72 (1.89) a |
| C.cl | 23.88 (2.33) b | 18.83 (1.75) b | 24.08 (2.70) b | 57.55 (35.77) a | 25.47 (0.60) b | 19.02 (2.13) b | 32.83 (2.90) b | |
| Fe * | P.os | 0.13 (0.01) a | 0.08 (0.01) bc | 0.09 (0.02) abc | 0.12 (0.02) ab | 0.11 (0.01) abc | 0.08 (0.02) c | 0.10 (0.01) abc |
| C.cl | 0.05 (0.01) b | 0.07 (0.00) b | 0.04 (0.00) b | 0.05 (0.00) b | 0.04 (0.01) b | 0.05 (0.01) b | 0.10 (0.03) a | |
| Li | P.os | 0.29 (0.04) b | 0.63 (0.06) ab | 0.61 (0.28) ab | 0.56 (0.21) ab | 0.79 (0.17) ab | 0.52 (0.04) ab | 0.97 (0.55) a |
| C.cl | 0.95 (0.07) a | 0.39 (0.16) a | 1.15 (0.12) a | 1.63 (1.21) a | 3.87 (2.35) a | 1.05 (0.15) a | 0.37 (0.08) a | |
| Mg * | P.os | 2.80 (0.04) a | 2.44 (0.65) ab | 2.40 (0.40) ab | 1.87 (0.12) bc | 1.47 (0.30) c | 1.94 (0.36) bc | 2.08 (0.11) abc |
| C.cl | 2.56 (0.53) a | 1.85 (0.24) bc | 2.26 (0.31) ab | 1.71 (0.03) bc | 1.29 (0.14) c | 2.04 (0.29) ab | 2.21 (0.37) ab | |
| Mn | P.os | 13.76 (0.85) a | 6.27 (2.00) d | 11.06 (1.33) abc | 10.33 (0.72) bc | 8.14 (1.46) cd | 7.05 (1.45) d | 11.70 (0.7) ab |
| C.cl | 5.64 (0.57) ab | 4.71 (1.28) ab | 5.13 (1.03) ab | 6.36 (1.32) a | 5.20 (0.15) ab | 4.26 (0.65) b | 5.42 (1.07) ab | |
| Mo | P.os | 0.19 (0.03) ab | 0.29 (0.02) ab | 0.09 (0.02) b | 0.16 (0.08) ab | 0.48 (0.11) a | 0.16 (0.03) ab | 0.49 (0.36) a |
| C.cl | 0.42 (0.27) a | 0.07 (0.05) a | 0.31 (0.03) a | 0.42 (0.28) a | 1.74 (0.98) a | 0.04 (0.02) a | 0.06 (0.02) a | |
| Ni | P.os | 0.69 (0.21) ab | 0.41 (0.02) ab | 0.52 (0.22) ab | 0.83 (0.43) a | 0.59 (0.15) ab | 0.28 (0.08) b | 0.49 (0.05) ab |
| C.cl | 0.34 (0.12) c | 0.41 (0.09) bc | 0.55 (0.06) abc | 0.71 (0.06) a | 0.65 (0.31) ab | 0.49 (0.07) abc | 0.58 (0.17) abc | |
| Sb | P.os | 0.35 (0.07) a | 0.32 (0.01) a | 0.21 (0.03) bc | 0.22 (0.04) bc | 0.17 (0.03) c | 0.22 (0.03) bc | 0.29 (0.10) ab |
| C.cl | 0.18 (0.01) a | 0.17 (0.03) a | 0.23 (0.04) a | 0.26 (0.16) a | 0.20 (0.04) a | 0.21 (0.04) a | 0.18 (0.03) a | |
| Se | P.os | 0.34 (0.05) ab | 0.29 (0.01) abc | 0.21 (0.11) bc | 0.27 (0.01) abc | 0.15 (0.09) c | 0.30 (0.08) abc | 0.41 (0.00) a |
| C.cl | 0.17 (0.01) ab | 0.07 (0.01) c | 0.08 (0.02) c | 0.07 (0.00) c | 0.04 (0.03) c | 0.10 (0.02) bc | 0.18 (0.07) a | |
| Sr | P.os | 2.57 (0.48) bc | 3.93 (1.04) b | 1.25 (0.65) c | 1.40 (0.34) c | 7.49 (2.75) a | 2.27 (0.68) bc | 1.97 (0.21) bc |
| C.cl | 3.79 (0.08) a | 2.19 (0.24) abc | 2.12 (0.17) abc | 2.02 (0.98) bc | 1.43 (0.22) c | 1.33 (0.10) c | 3.59 (1.37) ab | |
| Zn | P.os | 110.41 (4.07) a | 96.15 (24.74) ab | 118.26 (2.87) a | 112.70 (1.00) a | 73.87 (2.19) b | 73.38 (11.25) b | 114.15 (6.36) a |
| C.cl | 104.29 (1.69 a | 50.48 (0.46) c | 76.65 (11.19) b | 74.63 (5.16) b | 54.18 (13.61) c | 72.51 (2.05) b | 99.46 (4.89) a | |
| Element | C. cylindracea | P. ostreatus | TDI | RDI | Reference |
|---|---|---|---|---|---|
| As | nd | nd | 0.128 | [79] | |
| Be | 0.23–1.88 (0.8–6.8%) | 0.10–0.63 (0.4–2.3%) | 27.6 | [80] | |
| Ca | 9.21–34.90 (0.9–3.5%) | 5.11–47.0 (0.5–4.7%) | nr | 1000 | [81] |
| Cd | 0.01–0.02 (9.0–27.0%) | 0.01–0.02 (15.0–31.0%) | 0.060 | [79] | |
| Co | 0.00–0.00 | 0.00–0.00 | nr | ||
| Cu | 0.56–1.73 (5.7–17.3%) | 0.48–1.17 (4.8–11.7%) | 10 | 2.2 | [79] |
| Fe | 1.07–2.98 (3.6–9.9%) | 2.40–3.76 (8.0–12.5%) | 48 | 10–50 | [79] |
| Li | 0.01–0.12 (1.1–11.6%) | 0.01–0.03 (0.9–2.9%) | nr | 1 | [82] |
| Mg | 38.85–76.65 (16.2–31.9%) | 44.07–84.14 (18.4–35.1%) | nr | 240 | [81] |
| Mn | 0.13–0.19 (4.3–6.4%) | 0.19–0.41 (6.3–13.8%) | 11 | 3 | [79,83] |
| Mo | 0.00–0.05 (0.6–26.1) | 0.00–0.01 (1.4–7.4%) | nr | 0.1–0.3 | [79] |
| Ni | 0.01–0.02 (1.4–3.0%) | 0.01–0.02 (1.2–3.5%) | 0.720 | [79] | |
| Sb | 0.01–0.01 (1.4–2.2%) | 0.01–0.01 (1.4–2.9%) | 0.36 | [79] | |
| Se | 0.00–0.01 (3.4–15.4%) | 0.00–0.01 (12.9–35.1) | 320–480 | 0.026–0.035 | [79] |
| Sr | 0.04–0.11 (0.0–0.0%) | 0.04–0.22 (0.0–0.0%) | 2400 | [84] | |
| Zn | 1.51–3.13 (8.7–17.9%) | 2.20–3.55 (12.6–20.3%) | 60 | 15–20 | [79] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koutrotsios, G.; Danezis, G.; Georgiou, C.; Zervakis, G.I. Elemental Content in Pleurotus ostreatus and Cyclocybe cylindracea Mushrooms: Correlations with Concentrations in Cultivation Substrates and Effects on the Production Process. Molecules 2020, 25, 2179. https://doi.org/10.3390/molecules25092179
Koutrotsios G, Danezis G, Georgiou C, Zervakis GI. Elemental Content in Pleurotus ostreatus and Cyclocybe cylindracea Mushrooms: Correlations with Concentrations in Cultivation Substrates and Effects on the Production Process. Molecules. 2020; 25(9):2179. https://doi.org/10.3390/molecules25092179
Chicago/Turabian StyleKoutrotsios, Georgios, Georgios Danezis, Constantinos Georgiou, and Georgios I. Zervakis. 2020. "Elemental Content in Pleurotus ostreatus and Cyclocybe cylindracea Mushrooms: Correlations with Concentrations in Cultivation Substrates and Effects on the Production Process" Molecules 25, no. 9: 2179. https://doi.org/10.3390/molecules25092179
APA StyleKoutrotsios, G., Danezis, G., Georgiou, C., & Zervakis, G. I. (2020). Elemental Content in Pleurotus ostreatus and Cyclocybe cylindracea Mushrooms: Correlations with Concentrations in Cultivation Substrates and Effects on the Production Process. Molecules, 25(9), 2179. https://doi.org/10.3390/molecules25092179






