Contamination of Animal Feed with Undeclared Tetracyclines—Confirmatory Analysis by Liquid Chromatography–Mass Spectrometry after Microbiological Plate Test
Abstract
1. Introduction
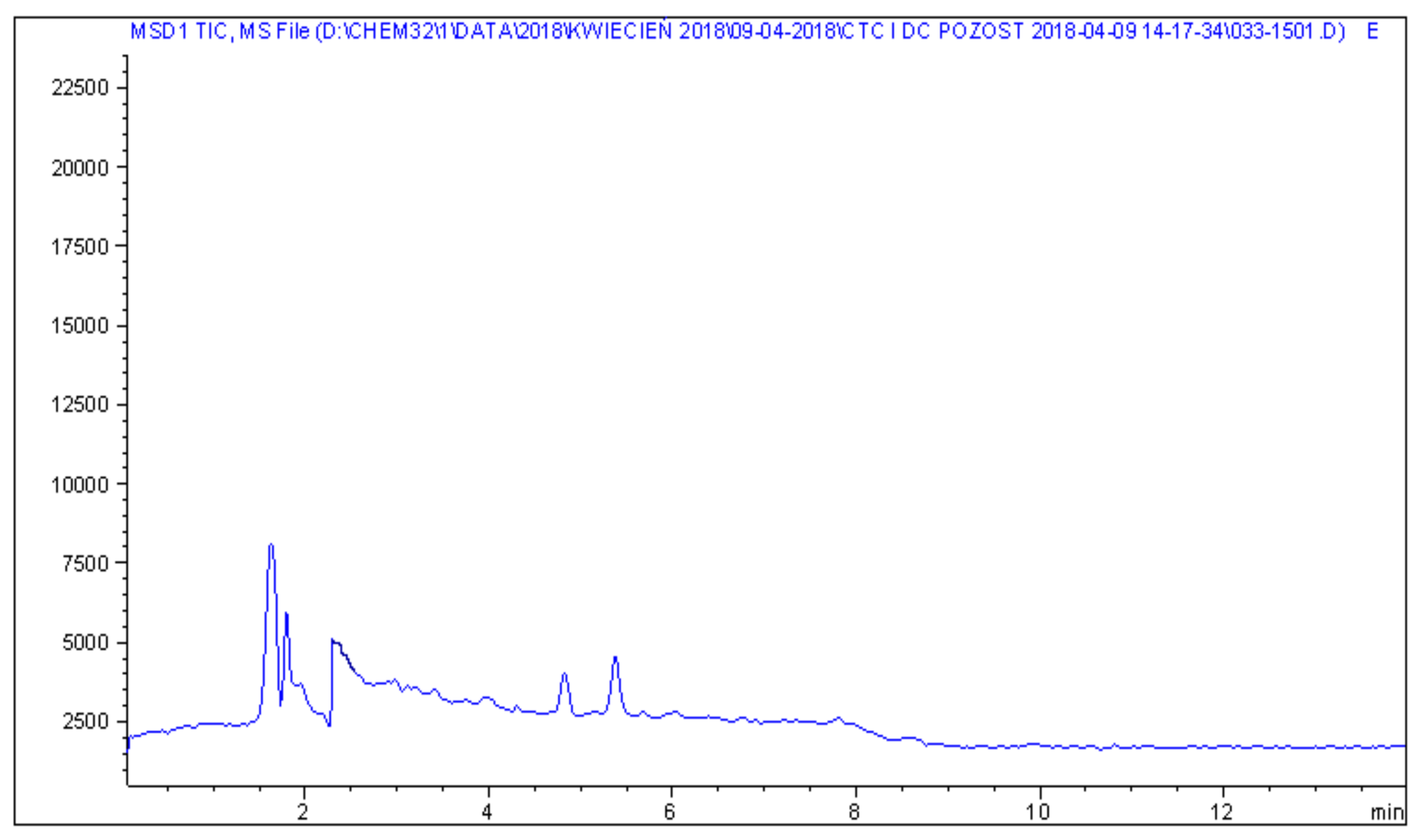
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Collection
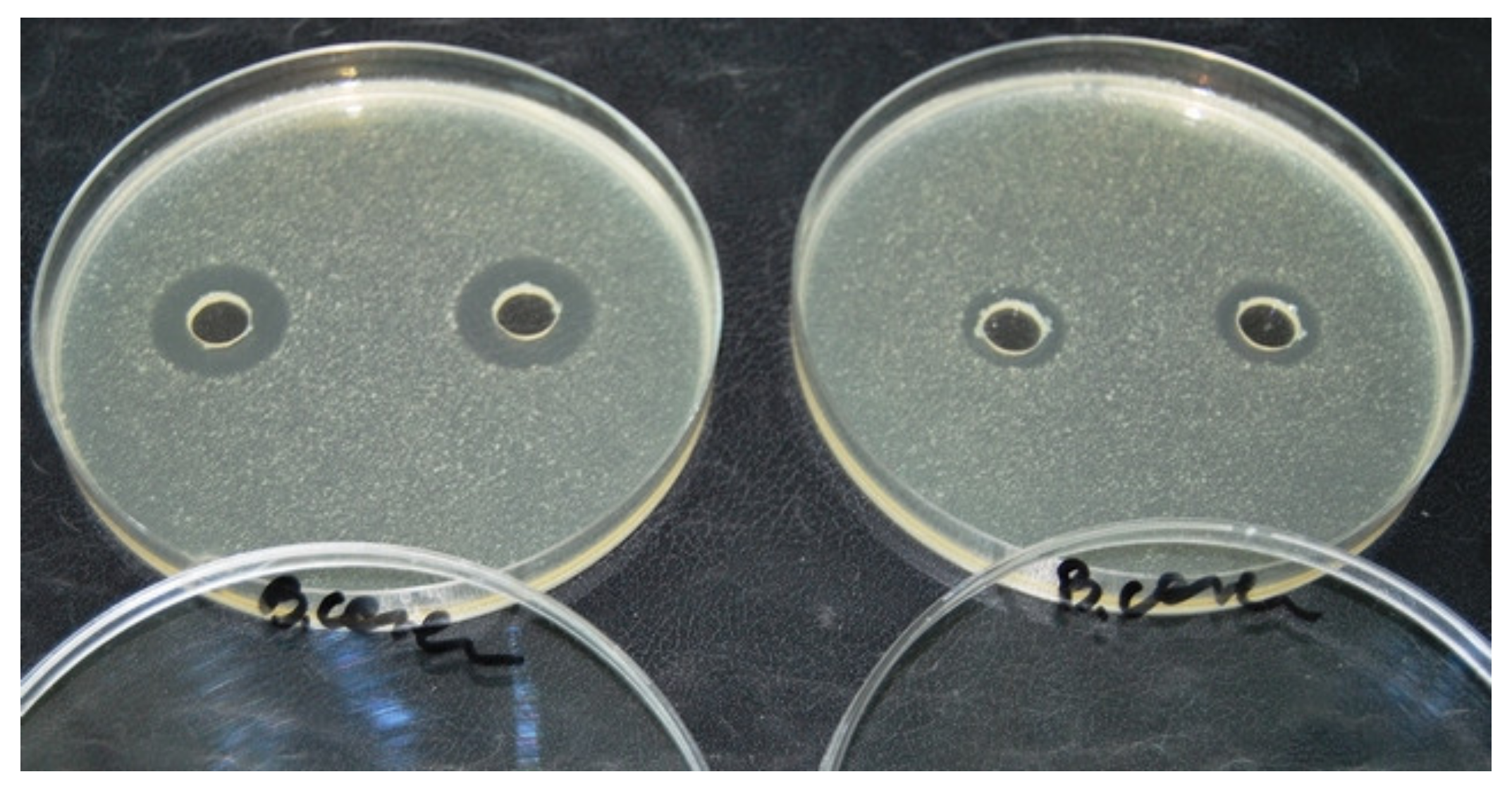
4.2. Microbiological Plate Test
4.3. Liquid Chromatography–Mass Spectrometry Analysis
4.3.1. Chemicals, Reagents and Stock Solutions
4.3.2. Sample Preparation and Clean-Up
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nielsen, P.; Gyrd-Hansen, P. Bioavailability of oxytetracycline, tetracycline and chlortetracycline after oral administration to fed and fasted pigs. J. Vet. Pharmacol. Ther. 1996, 19, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.N. Tetracyclines. In Infectious diseases, 2nd ed.; Gorbach, S.L., Bartlett, J.G., Blacklow, N.R., Eds.; The W.B. Saunders Co.: Philadelphia, P, USA, 1998; pp. 227–231. [Google Scholar]
- Chopra, I.; Roberts, M. Tetracyclines Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.F.; Nikolaidou, K.I.; Papadoyannis, I.N. Development and validation of an HPLC confirmatory method for the determination of seven tetracycline antibiotics residues in milk according to the European Union Decision 2002/657/EC. J. Sep. Sci. 2007, 30, 2430–2439. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.D. Antibiotic use in animal feed and its impact on human health. Nutr. Res. Rev. 2000, 13, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Wegener, H.C. Antibiotics in animal feed and their role in resistance development. Curr. Opin. Microbiol. 2003, 6, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.P.; Boland, J.J.; Silbergeld, E. Growth Promoting Antibiotics in Food Animal Production: An Economic Analysis. Public Health Rep. 2007, 122, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Swann, M.M. Report of the Joint Committee on the Use of Antibiotics in Animal Husbandry and Veterinary Medicine; Her Majesty’s Stationery Office: London, UK, 1969. [Google Scholar]
- Witte, W. Medical Consequences of Antibiotics Use in Agriculture. Science 1998, 279, 996–997. [Google Scholar] [CrossRef]
- Marschall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef]
- Castanon, J.I.R. History of the use of antibiotic as growth promoters in european poultry feeds. Poultry Science 2007, 86, 2466–2471. [Google Scholar] [CrossRef]
- Schnappinger, D.; Hillen, W. Tetracyclines: Antibiotic action, uptake, and resistance mechanisms. Arch. Microbiol. 1996, 165, 359–369. [Google Scholar] [CrossRef]
- Grave, K.; Torren-Edo, J.; Muller, A.; Greko, C.; Moulin, G.; Mackay, D. Variations in the sales and sales patterns of veterinary antimicrobial agents in 25 European countries. J. Antimicrob. Chemother. 2014, 69, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2017; Trends from 2010 to 2017; Ninht ESVAC Report; European Medicines Agency: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Boscher, A.; Guignard, C.; Pellet, T.; Hoffmann, L.; Bohn, T. Development of a multi-class method for the quantification of veterinary drug residues in feedingstuffs by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 6394–6404. [Google Scholar] [CrossRef] [PubMed]
- Przeniosło-Siwczyńska, M.; Patyra, E.; Chyłek-Purchała, M.; Kozak, B.; Kwiatek, K. Occurrence of tetracyclines in feedingstuffs–results of a two-year study within the official control of feed. Bull. Vet. Inst. Pulawy 2015, 59, 527–532. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition; OJ L268; European Commission: Brussels, Belgium, 2003; pp. 29–43. [Google Scholar]
- European Commission. Regulation (EU) No 2019/4 of the European Parliament and of the Council of on the manufacture, placing on the market and use of medicated feed, amending Regulation (EC) No 183/2005 of the European Parliament and of the Council and repealing Council Directive 90/167/EEC; OJ L4; European Commission: Brussels, Belgium, 2019; pp. 1–23. [Google Scholar]
- Kaklamanos, G.; Vincent, U.; von Holst, C. Analysis of antimicrobial agents in pig feed by liquid chromatography coupled to orbitrap mass spectrometry. J. Chromatogr. A 2013, 1293, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Stolker, A.A.M.; Manti, V.; Zuidema, T.; van Egmond, H.; Deckers, E.R.; Herbes, R.; Hooglugt, J.; Olde Heuvel, E.; de Jong, J. Carry-over of veterinary drugs from medicated to non-medicated feeds in commercial feed manufacturing plants. Food Addit. Contam. Part A 2013, 30, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Mevius, D.J.; Sprenger, M.J.W.; Wegener, H.C. EU conference ‘The Microbial Threat”. Int. J. Antimicrob. Ag 1999, 11, 101–105. [Google Scholar] [CrossRef]
- De Jong, J.; Tomassen, M.J.H.; van Egmond, H.J.; van Rhijn, J.A.; Zuidema, T.; Michard, J.; Genouel, C.; Brambilla, G.; Nunes da Costa, J.M.G.; Nordkvist, E.; et al. Towards a control strategy for banned antibiotics and growth promoters in feed: The SIMBAG-FEED project. In Antimicrobial Growth Promoters. Where do we go from here? 1st ed.; Barug, D., de Jong, J., Kies, A.K., Verstegen, M.W.A., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2006; pp. 211–234. [Google Scholar]
- De Wasch, K.; Okerman, L.; Croubels, S.; De Brabander, H.; Van Hoof, J.; De Backer, P. Detection of residues of tetracycline antibiotics in pork and chicken meat: Correlation between results of screening and confirmatory tests. Analyst 1998, 123, 2737–2741. [Google Scholar] [CrossRef]
- Ghidini, S.; Zanardi, E.; Varisco, G.; Chizzolini, R. Residues of β-lactams antibiotics in bovine milk: Confirmatory analysis by liquid chromatography tandem mass spectrometry after microbial assay screening. Food Addit. Contam. 2003, 20, 528–534. [Google Scholar] [CrossRef]
- Borras, S.; Companyo, R.; Granados, M.; Guiteras, J.; Perez-Vendrell, A.M.; Brufau, J.; Medina, M.; Bosch, J. Analysis of antimicrobial agents in animal feed. Trends Anal. Chem. 2011, 30, 1042–1064. [Google Scholar] [CrossRef]
- Chico, J.; van Holthoon, F.; Zuidema, T. Ion Suppression Study for Tetracyclines in Feed. Chromatogr. Res. Int. 2012, 9. [Google Scholar] [CrossRef]
- McEvoy, J.D.G. Contamination of animal feedingstuffs as a cause of residues in food: A review of regulatory aspects, incidence and control. Anal. Chim. Acta 2002, 473, 3–26. [Google Scholar] [CrossRef]
- Kantiani, L.; Farre, M.; Grases i Freixiedas, J.M.; Barcelo, D. Determination of antibacterials in animal feed by pressurized liquid extraction followed by online purification and liquid chromatography-electrospray tandem mass spectrometry. Anal. Bioanal. Chem. 2010, 398, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Kirbiš, A. Microbiological screening method for detection of aminoglycosides, β-lactams, macrolides, tetracyclines and quinolones in meat samples. Slov. Vet. Res. 2007, 44, 11–18. [Google Scholar]
- Lynas, L.; Currie, D.; McCaughey, W.J.; McEvoy, J.D.G.; Kennedy, D.G. Contamination of animal feedingstuffs with undeclared antimicrobial additives. Food Addit. Contamin. 1998, 15, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Holman, D.B.; Chenier, M.R. Impact of subtherapeutic administration of tylosin and chlortetracycline on antimicrobial resistance in farrow-to-finish swine. FEMS Microbiol. Ecol. 2013, 85, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Segato, G.; Benetti, C.; Angeletti, R.; Montesissa, C.; Biancotto, G. Doxycycline and sulfadimethoxine transfer from cross-contaminated feed to chicken tissues. Food Addit. Contamin. 2011, 28, 860–868. [Google Scholar] [CrossRef]
- World Health Organization. Critically Important Antimicrobials for Human Medicine; 6th revision; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Wasyl, D.; Hoszowski, A.; Zając, M.; Szulowski, K. Antimicrobial resistance in commensal Escherichia coli isolated from animals at slaughter. Front. Microbiol. 2013, 4, 1–12. [Google Scholar] [CrossRef]
- Przeniosło-Siwczyńska, M.; Kwiatek, K. Evaluation of multi–plate microbial assay for the screening of antibacterial substances in animal feedingstuffs. Bull. Vet. Inst. Pulawy 2007, 51, 599–602. [Google Scholar]
- Patyra, E.; Kwiatek, K. Development and validation of multi-residue analysis of tetracycline antibiotics in feed by high performance liquid chromatography coupled with mass spectrometry. Food Addit. Contam. A 2017, 34, 1553–1561. [Google Scholar] [CrossRef]
- Dewdney, J.M.; Maes, L.; Raynaud, J.P.; Blanc, F.; Scheid, J.P. Risk assessment of antibiotic residues of beta-lactams and macrolides in food products with regard to their immunoallergic potential. Food Chem. Toxicol. 1991, 29, 477–483. [Google Scholar] [CrossRef]
- Berends, B.R.; van den Bogaard, A.E.; van Knapen, F.; Snijders, J.M. Human health hazards associated with the administration of antimicrobials to slaughter animals. Part I. An assessment of the risks of residues of tetracyclines in pork. Vet. Q. 2001, 23, 2–10. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
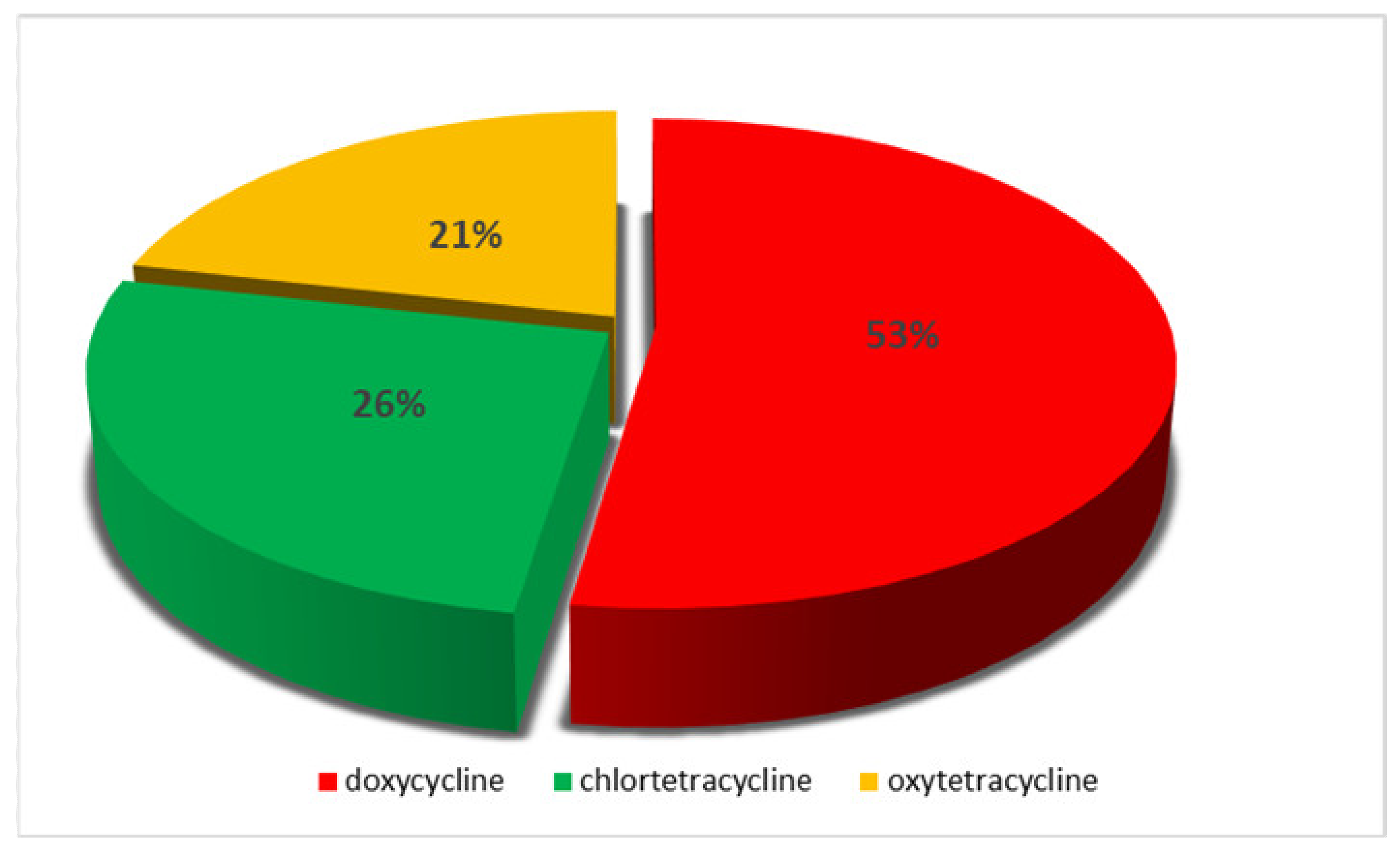

| Type of Sample | Number of Analysed Samples | Number of Samples Suspected of TCs | Number of Samples with TCs Confirmed |
|---|---|---|---|
| Cattle feed | 52 | 9 | 1 |
| Pig feed | 138 | 69 | 38 |
| Poultry feed | 114 | 79 | 0 |
| Concentrates/premixes | 21 | 19 | 0 |
| Feed Materials | 28 | 10 | 3 |
| Total | 353 | 186 (52.7%) | 42 (11.9%) |
| Sample No. | Type of Feed | Analyte | Concentration (mg kg−1) |
|---|---|---|---|
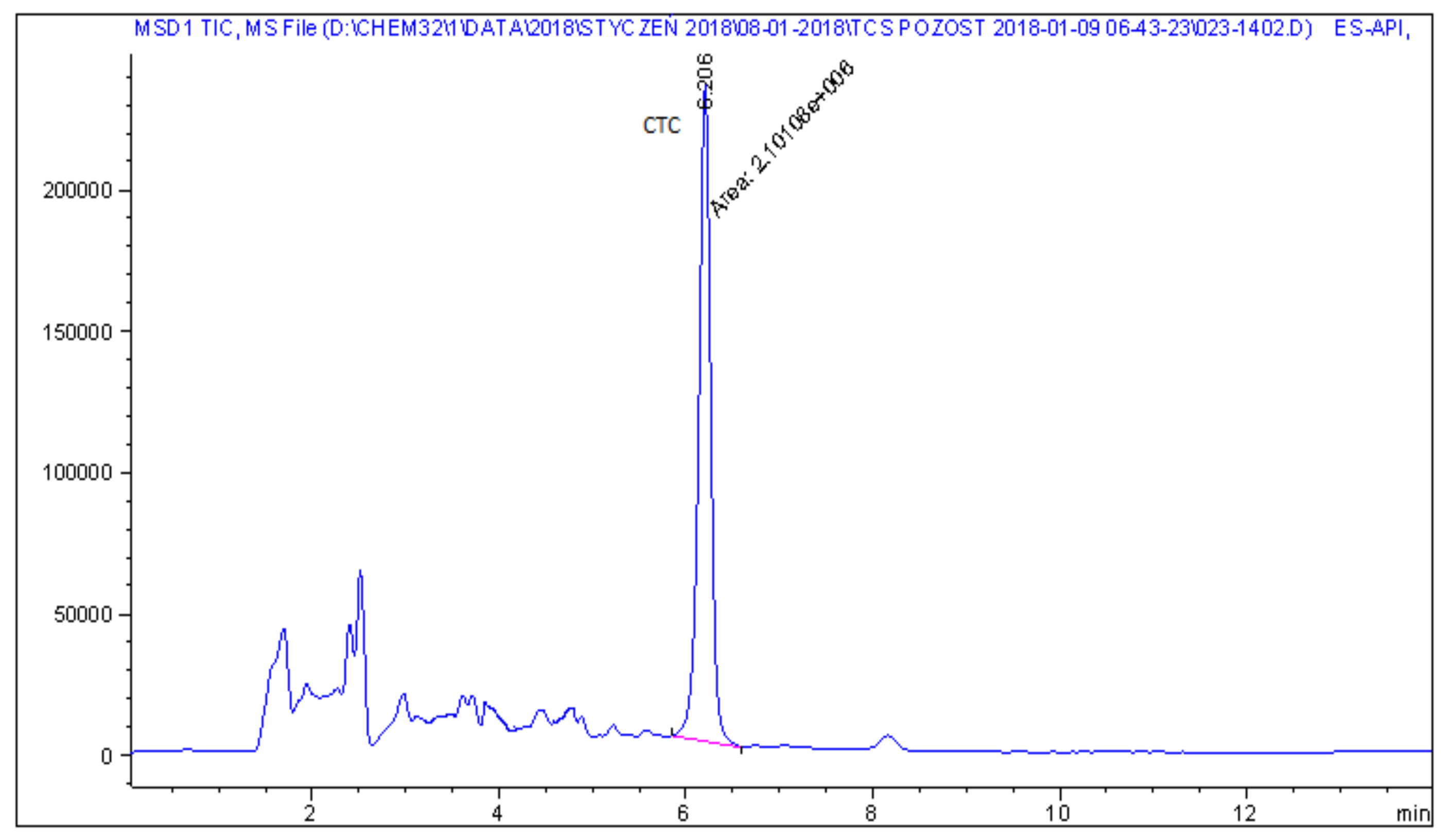
| 1 | Cattle feed | CTC | 0.3 |
| 2 | Porker feed | CTC | 0.35 |
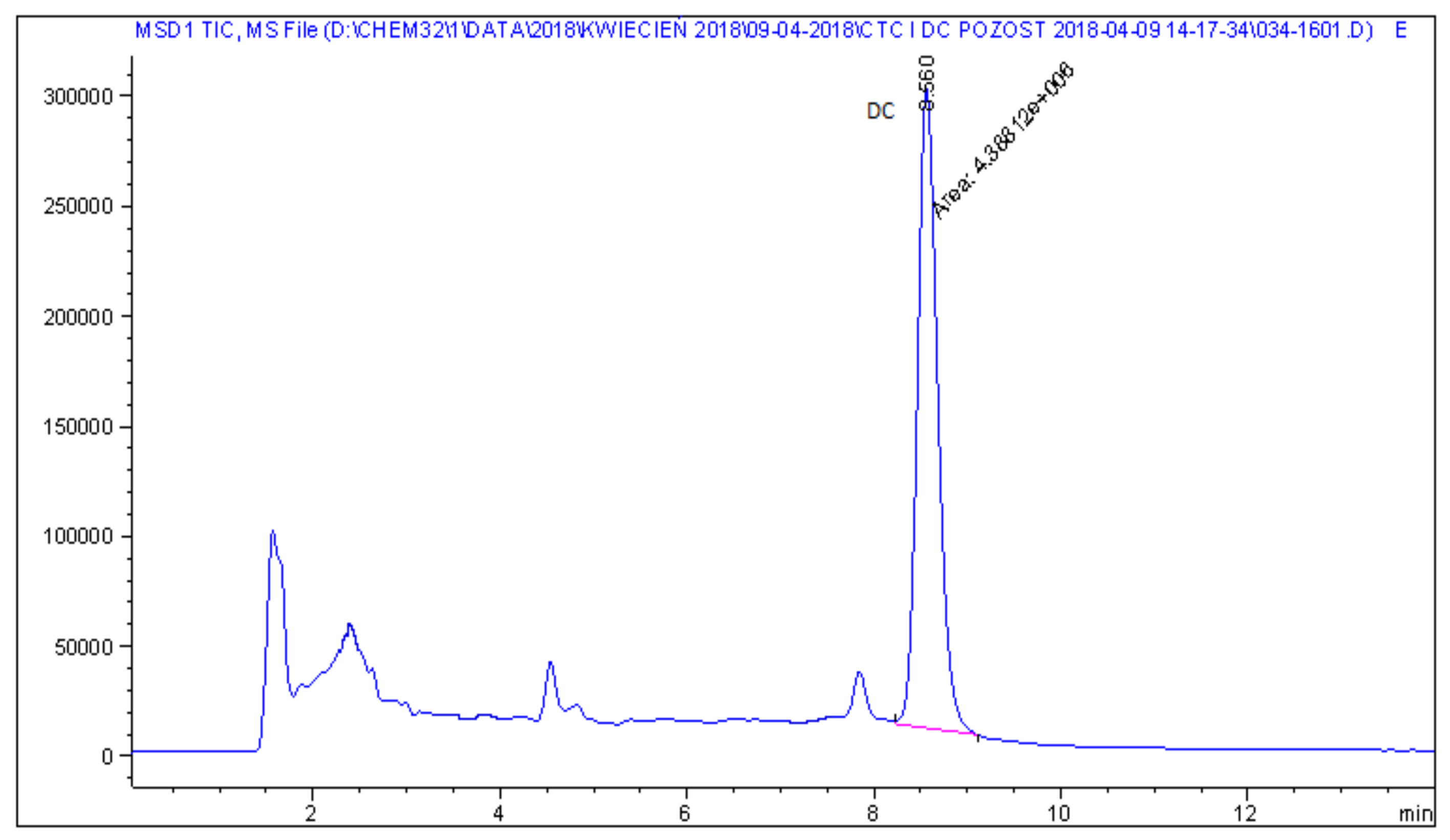
| 3 | Sow feed | DC | 0.36 |
| 4 | Porker feed | CTC | 0.85 |
| 5 | Piglet feed | DC | 4.1 |
| 6 | Pig feed | DC | 1.4 |
| 7 | Porker feed | DC | 1.4 |
| 8 | Sow feed | DC | 5.0 |
| 9 | Pig feed | OTC | 4.7 |
| 10 | Pig feed | OTC | >5.0 (49) * |
| 11 | Pig feed | OTC | 1.8 |
| 12 | Pig feed | DC | 4.6 |
| 13 | Pig feed | OTC | >5.0 (8.1) |
| 14 | Pig feed | OTC | 0.9 |
| 15 | Sow feed | DC | 1.2 |
| 16 | Porker feed | DC | <0.3 |
| 17 | Porker feed | DC | <0.3 |
| 18 | Porker feed | OTC | 2.3 |
| 19 | Porker feed | DC | 0.9 |
| 20 | Pig feed | DC | 4 |
| 21 | Pig feed | CTC | 1.2 |
| 22 | Pig feed | CTC | 1.1 |
| 23 | Pig feed | CTC | <0.3 |
| 24 | Pig feed | CTC | 2.6 |
| 25 | Pig feed | CTC | <0.3 |
| 26 | Pig feed | CTC | 2.7 |
| 27 | Pig feed | CTC | 2.6 |
| 28 | Porker feed | OTC | 2.8 |
| 29 | Piglet feed | DC | 0.57 |
| 30 | Piglet feed | DC | 1.5 |
| 31 | Pig feed | DC | 0.47 |
| 32 | Sow feed | OTC | >5.0 (5.5) |
| 33 | Pig feed | DC | 0.57 |
| 34 | Porker feed | CTC | 0.33 |
| 35 | Piglet feed | DC | 0.6 |
| 36 | Porker feed | DC | 2.4 |
| 37 | Pig feed | OTC | >5.0 (5.7) |
| 38 | Porker feed | DC (+tylosin) | >5.0 (7.9) (+10.9) |
| 39 | Piglet feed | DC (+tylosin) | 3.7 (+0.4) |
| 40 | Feed material | DC | 0.6 |
| 41 | Feed material | DC | 0.5 |
| 42 | Feed material | DC | 0.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przeniosło-Siwczyńska, M.; Patyra, E.; Grelik, A.; Chyłek-Purchała, M.; Kozak, B.; Kwiatek, K. Contamination of Animal Feed with Undeclared Tetracyclines—Confirmatory Analysis by Liquid Chromatography–Mass Spectrometry after Microbiological Plate Test. Molecules 2020, 25, 2162. https://doi.org/10.3390/molecules25092162
Przeniosło-Siwczyńska M, Patyra E, Grelik A, Chyłek-Purchała M, Kozak B, Kwiatek K. Contamination of Animal Feed with Undeclared Tetracyclines—Confirmatory Analysis by Liquid Chromatography–Mass Spectrometry after Microbiological Plate Test. Molecules. 2020; 25(9):2162. https://doi.org/10.3390/molecules25092162
Chicago/Turabian StylePrzeniosło-Siwczyńska, Monika, Ewelina Patyra, Aleksandra Grelik, Maja Chyłek-Purchała, Beata Kozak, and Krzysztof Kwiatek. 2020. "Contamination of Animal Feed with Undeclared Tetracyclines—Confirmatory Analysis by Liquid Chromatography–Mass Spectrometry after Microbiological Plate Test" Molecules 25, no. 9: 2162. https://doi.org/10.3390/molecules25092162
APA StylePrzeniosło-Siwczyńska, M., Patyra, E., Grelik, A., Chyłek-Purchała, M., Kozak, B., & Kwiatek, K. (2020). Contamination of Animal Feed with Undeclared Tetracyclines—Confirmatory Analysis by Liquid Chromatography–Mass Spectrometry after Microbiological Plate Test. Molecules, 25(9), 2162. https://doi.org/10.3390/molecules25092162





