Effect of Four Novel Bio-Based DES (Deep Eutectic Solvents) on Hardwood Fractionation
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties
2.2. Evaluation of Olive Pomace
2.3. Instrumental Characterization
2.3.1. Gel Permeation Chromatography (GPC)
2.3.2. FT-IR Spectra
2.3.3. H NMR Spectra
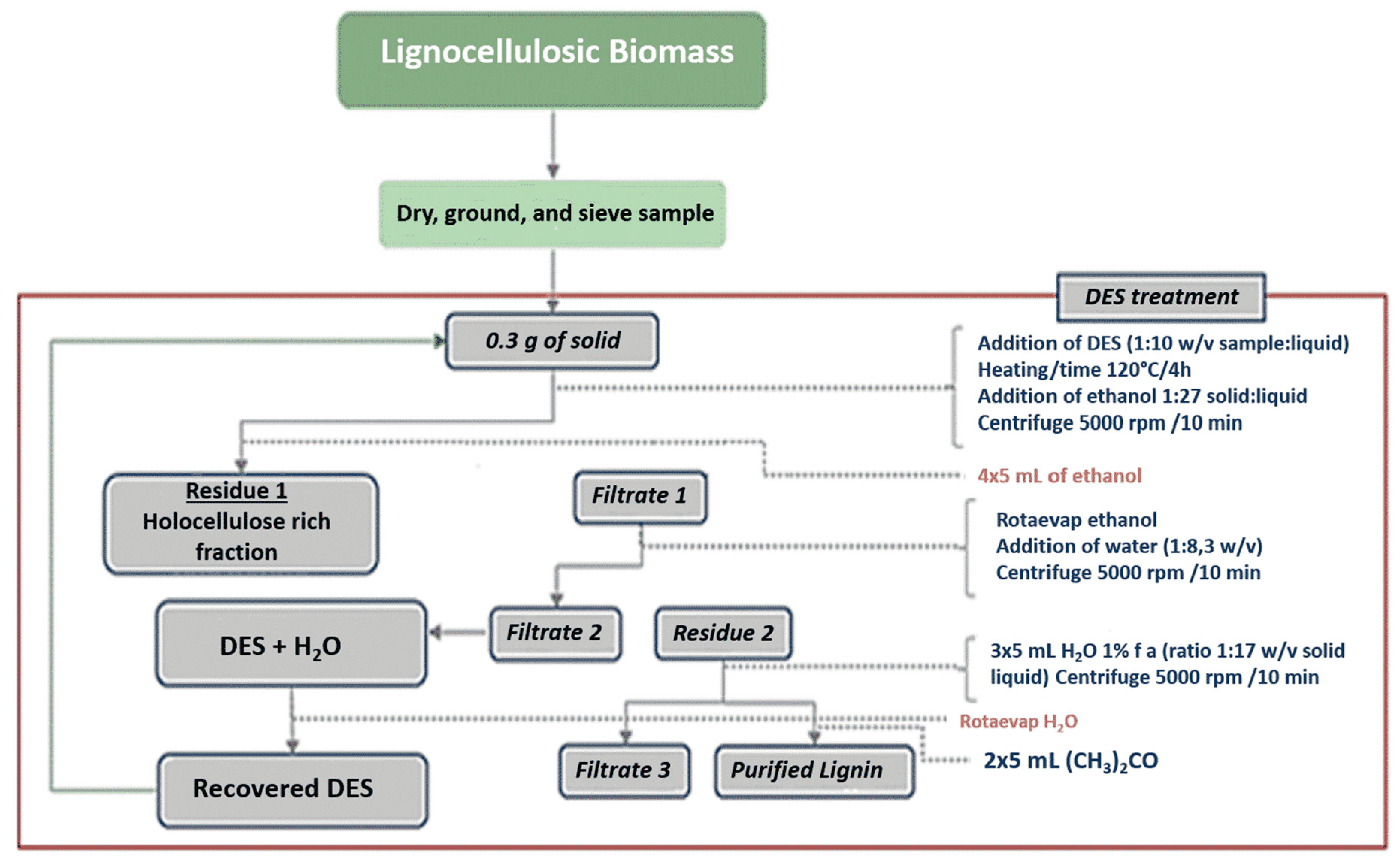
3. Materials and Methods
3.1. Materials
3.2. Solvent Evaporations
3.3. Viscosity
3.4. Melting Points
3.5. NMR Spectra
3.6. FT-IR Spectra
3.7. Mass Spectrum
3.8. Gel Permeation Chromatography (GPC)
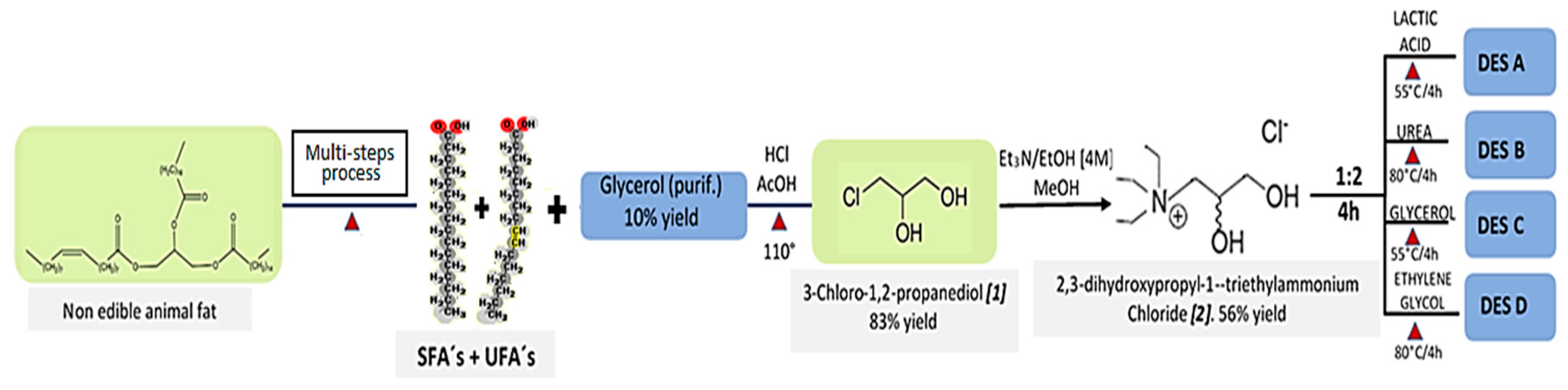
3.9. Synthesis of 3-Chloro-1,2-propanediol 1
3.10. Synthesis of [C9H22N+O2]Cl− 2
3.11. Synthesis of DESs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, S.; Wu, Y.; Chen, Q.; Yu, Z.; Wang, C.; Jin, S.; Ding, Y.; Wu, G. Dissolution of cellulose with ionic liquids and its application: A mini-review. Green Chem. 2006, 8, 325–327. [Google Scholar] [CrossRef]
- Yua, Q.; Qina, L.; Liub, Y.; Suna, Y.; Xua, H.; Wang, Z.; Yuana, Z. In situ deep eutectic solvent pretreatment to improve lignin removal from garden wastes and enhance production of bio-methane and microbial lipids. Bioresour. Technol. 2019, 271, 210–217. [Google Scholar] [CrossRef]
- Duarte, C.G.; Rios, S.M.L.; Jaramillo, D.P.M.; Ximenes, F.F.E. Biomass-Derived Inhibitors of Holocellulases. Bioenergy Res. 2012, 5, 768–777. [Google Scholar] [CrossRef]
- Domínguez de María, P. Recent trends in (ligno) cellulose dissolution using neoteric solvents: Switchable, distillable and bio-based ionic liquids. J. Chem. Technol. Biotechnol. 2014, 89, 11–18. [Google Scholar] [CrossRef]
- Smith, L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Francisco, M.; Van den Bruinhorst, A.; Kroon, M.C. New natural and renewable low transition temperature mixtures (LTTMs): Screening as solvents for lignocellulosic biomass processing. Green Chem. 2012, 14, 2153–2157. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Munro, H.L.; Rasheed, R.K.; Tambyrajah, V. Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains. Chem. Commun. 2001, 19, 2010–2011. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, C.; Crittle, T.D. Choline-based deep eutectic solvents for enzymatic preparation of biodiesel from soybean oil. J. Mol. Catal. B Enzym. 2013, 85–86, 243–247. [Google Scholar] [CrossRef]
- Satlewala, A.; Agrawala, R.; Bhagia, S.; Sangoroa, J.; Ragauskas, A.J. Natural deep eutectic solvents for lignocellulosic biomass pretreatment: Recent developments, challenges and novel opportunities. Biotechnol. Adv. 2018, 36, 2032–2050. [Google Scholar] [CrossRef]
- Mamilla, J.L.K.; Novak, U.; Grilc, M.; Likoza, B. Natural deep eutectic solvents (DES) for fractionation of waste lignocellulosic biomass and its cascade conversion to value-added bio-based chemicals. Biomass Bioenergy 2019, 120, 417–425. [Google Scholar] [CrossRef]
- Tan, Y.T.; Ngoh, G.C.; Chua, A.S.M. Effect of functional groups in acid constituent of deep eutectic solvent for extraction of reactive lignin. Bioresour. Technol. 2019, 281, 359–366. [Google Scholar] [CrossRef]
- Kumar, A.K.; Parikh, B.S.; Pravakar, M. Natural deep eutectic solvent mediated pretreatment of rice straw: Bioanalytical characterization of lignin extract and enzymatic hydrolysis of pretreated biomass residue. Environ. Sci. Pollut. Res. 2015, 23, 9265–9275. [Google Scholar] [CrossRef]
- Van Osch, D.J.G.P.; Zubeir, L.F.; Van den Bruinhorst, A.; Rocha, M.A.A.; Kroon, M.C. Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chem. 2015, 17, 4518–4521. [Google Scholar] [CrossRef]
- Shahbaz, K.; Mjalli, F.S.; Hashim, M.A.; Al-Nashef, I.M. Prediction of deep eutectic solvents densities at different temperatures. Thermochim. Acta. 2011, 515, 67–72. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Chen, Z.; Greaves, T.L.; Warr, G.; Atkin, R. Mixing cations with different alkyl chain lengths markedly depresses the melting point in deep eutectic solvents formed from alkylammonium bromide salts and urea. Chem. Commun. 2017, 53, 2375–2377. [Google Scholar] [CrossRef]
- Rengstl, D.; Fischer, V.; Kunz, W. Low-melting mixtures based on choline ionic liquids. Phys. Chem. Chem. Phys. 2014, 16, 22815–22822. [Google Scholar] [CrossRef]
- Makarov, A.; LoBrutto, R.; Karpinski, P.; Kazakevich, Y.; Christodoulatos, C.; Ganguly, A.K. Investigation of the effect of pressure and liophilic mobile phase additives on retention of small molecules and proteins using reversed-phase ultrahigh pressure liquid chromatography. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 407–427. [Google Scholar] [CrossRef]
- Cequier, E.; Aguilera, J.; Balcells, M.; Canela-Garayoa, R. Extraction and characterization of lignin from olive pomace: A comparison study among ionic liquid, sulfuric acid, and alkaline treatments. Biomass Convers. Biorefinery. 2019, 2, 241–252. [Google Scholar] [CrossRef]
- Gilli, P.; Pretto, L.; Bertolasi, V.; Gilli, G. Predicting Hydrogen-Bond Strengths from Acid−Base Molecular Properties. The pKa Slide Rule: Toward the Solution of a Long-Lasting Problem. Acc. Chem. Res. 2009, 42, 33–44. [Google Scholar] [CrossRef]
- Van Osch, D.J.G.P.; Kollau, L.J.B.M.; Van den Bruinhorst, A.; Asikainen, S.; Rocha, M.A.A.; Kroon, M.C. Ionic liquids and deep eutectic solvents for lignocellulosic biomass fractionation. Phys. Chem. Chem. Phys. 2017, 19, 2636–2665. [Google Scholar] [CrossRef]
- Varanasi, P.; Singh, P.; Auer, M.; Adams, P.D.; Simmons, B.A.; Singh, S. Survey of renewable chemicals produced from lignocellulosic biomass during ionic liquid pretreatment. Biotechnol. Biofuels 2013, 6, 14. [Google Scholar] [CrossRef]
- Qin, H.; Hu, X.; Wang, J.; Cheng, H.; Chen, L.; Qi, Z. Overview of acidic deep eutectic solvents on synthesis, properties and applications. J. GEE 2020, 5, 8–21. [Google Scholar] [CrossRef]
- Sirvio, J.A.; Visanko, M.; Liimatainen, H. Acidic deep eutectic solvents as hydrolytic media for cellulose nanocrystal production. Biomacromolecules 2016, 17, 3025–3032. [Google Scholar] [CrossRef]
- Tan, S.S.Y.; MacFarlane, D.R.; Upfal, J.; Edye, L.A.; Doherty, W.O.S.; Patti, A.F.; Pringle, J.M.; Scotta, J.L. Extraction of lignin from lignocellulose at atmospheric pressure using alkylbenzenesulfonate ionic liquid. Green Chem. 2009, 11, 339–345. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Hsu, T.C.; Guo, G.L.; Chen, W.H.; Hwang, W.S. Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresour. Technol. 2010, 101, 4907–4913. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Li, J.; Yan, L.; Li, S.; Ye, X.; Liu, D.; Ding, T.; Linhardt, R.J.; Orfila, C. Extraction and characterization of RG-I enriched pectic polysaccharides from mandarin citrus peel. Food Hydrocoll. 2018, 79, 579–586. [Google Scholar] [CrossRef]
- Diop, A.; Jradi, K.; Daneault, C.; Montplaisir, D. Kraft Lignin Depolymerization in an Ionic Liquid without a Catalyst. BioResources 2015, 10, 4933–4946. [Google Scholar] [CrossRef]
- Gallart-Sirvent, P.; Yara, E.; Villorbina, G.; Balcells, M.; Salas, N.; Canela-Garayoa, R. Recycling Rhizopus oryzae resting cells as biocatalyst to prepare near eutectic palmitic-stearic acid mixtures from non-edible fat. J. Mol. Catal. B Enzym. 2016, 134, 172–177. [Google Scholar] [CrossRef]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Pourier, M.A.; Chunbao, X. Purification of crude glycerol using acidification: Effects of acid types and product characterization. Austin Chem. Eng. 2014, 1, 1–7. [Google Scholar]
- Conant, J.B.; Quayle, O.R. Glycerol α, γ-dichlorohydrin. Org. Synth. 1941, 1, 294. [Google Scholar]
- Beckett, M.A.; Bland, C.C.; Sukumar Varma, K. A 11B NMR study of zwitterionic and cationic monoborate complexes with cationic 1,2-diol ligands. Polyhedron 2008, 27, 2226–2230. [Google Scholar] [CrossRef]
- Abbott, A.P.; Harris, R.C.; Ryder, K.S.; D’Agostino, C.; Gladden, L.F.; Mantle, M.D. Glycerol eutectics as sustainable solvent systems. Green Chem. 2011, 13, 82–90. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds [DPTAC][LA], [DPTAC][Urea], [DPTAC][GLY] and [DPTAC][EG], are available from the authors. |

| DES and IL | δ [g.cm−3] | η a [mPa.s] | Apparentp H |
|---|---|---|---|
| [DPTAC][LA] | 1.07 | 1357 | 1.20 |
| [DPTAC][Urea] | 1.09 | 383 | 7.78 |
| [DPTAC][Gly] | 1.07 | 2146 | 2.39 |
| [DPTAC][Eg] | 1.08 | 159 | 1.15 |
| [Et3NH][HSO4] b | 1.18 | 81 | 0.5 |
| ChCl:LA c | 1.16 | 58 | 0.9 |
| HBD | pKa | mp [°C] | DES | mp [°C] |
|---|---|---|---|---|
| Lactic acid (LA) | 3.86 a | 18 | [DPTAC][LA] | −32 |
| Urea | 14.4b | 133 | [DPTAC][Urea] | 65–75 |
| Glycerol (Gly) | 14.4 a | 18 | [DPTAC][Gly] | −28 |
| Ethylene glycol (Eg) | 15.1 a | −13 | [DPTAC][Eg]) | <−56 |
| Holocellulose-Rich Fraction | Lignin | ||||||
|---|---|---|---|---|---|---|---|
| DESs and IL | Weight [mg] | Recovery a [%] | Total b [%] | Weight [mg] | Recovery a [%] | Total b [%] | Klason e [%] |
| [DPTAC][LA] | 23 | 39 | 8 | 42 | 38 | 14 | 60 |
| [DPTAC][LA] (g scale) | 3.02 × 103 | 44 | 7.6 | 5.6 × 103 | 39 | 14 | 59 |
| [DPTAC][LA] g | 13 | 25 | 4.3 | 29 | 27 | 10 | 56 |
| [DPTAC][LA] h | 15 | 29 | 5 | 34 | 30 | 12 | 52 |
| [DPTAC][LA] i | 18 | 27 | 6 | 9 | 8 | 3 | |
| [DPTAC][LA] j | 19 | 32 | 6.5 | 47 | 43 | 16 | 63 |
| [DPTAC][Urea] | 27 | 45 | 9 | 30 | 27 | 10 | 57 |
| [DPTAC][Gly] | 23 | 38 | 8 | 30 | 27 | 10 | 57 |
| [DPTAC][Eg] | 20 | 34 | 7 | 37 | 34 | 12 | 58 |
| Cellulose Rich Fraction | |||||||
| [Et3NH][HSO4] c | 159 | N/A d | 53 | 67 | 60 | 22 | 98 f |
| ChCl:LA | 239 | N/A d | 76 | 26 | 23 | 9 | -- |
| [DPTAC][LA] | Holocellulose-Rich Fraction | Lignin | |||||
|---|---|---|---|---|---|---|---|
| Sample | Weight [mg] | Recovery [%] | Total [%] | Weight [mg] | Recovery [%] | Total [%] | Klason [%] |
| Apricot | 52 | 27 | 17 | 24 | 57 | 8 | 45 |
| Plum | 15 | 10 | 5 | 9 | 12 | 3 | 48 |
| Peach | 34 | 23 | 11 | 13 | 25 | 5 | 48 |
| Nectarine | 27 | 18 | 9 | 19 | 40 | 7 | 45 |
| Flat peach | 26 | 19 | 9 | 16 | 20 | 6 | 46 |
| A) IR Bands Assignments for Lignin | B) IR bands Assignments for the Holocellulose Rich Fraction | ||
|---|---|---|---|
| Wavenumber (cm−1) | Band Assignments | Wavenumber (cm−1) | Band Assignments |
| 3600–3000 | ν OH aromatic and aliphatic | 3350 | ν OH |
| 2960–2925 | ν CH3-CH2 | 2925 | ν methylene and methyl groups |
| 2921 | ν methyl and methylene | 2800 | ν CH2 stretch |
| 2860, 1460 | ν and deform CH. | 1642 | H2O |
| 1720 | C=O fatty acid band | 1605 | ν cellulose-H2O |
| 1657 | ν C=O carbonyl-carboxyl | 1430 | CH2 def. |
| 1639 | ν C=O alkyl group | 1368 | C-H def. |
| 1610 | ν aromatic | 1200–1000 | ν typical bands cellulose |
| 1516 | ν aromatic | 1161 | ν C-O-C glucosidic |
| 1597 | ν aromatic | 1107 | ν C-O-C ring |
| 1506 | ν aromatic | 1033 | ν cellulose and hemicell. (broad band) |
| 1427 | CH def. | 1058,1159, 1157 | ν C-O-C pyranose ring |
| 1425 | ν aromatic ring | 910 | β(1-4) C-O-C |
| 1375, 1330 | ν OH aromatics | 895 | β-glucosidic |
| 1364 | ν CH | ||
| 1370 | ν Syringyl groups | ||
| 1264 | ν Guaiacyl groups | ||
| 1200 | OH carbohydrates | ||
| 1120 | ν Syringyl groups | ||
| 1111 | ν glucose ring | ||
| 825 | ν Syringyl group | ||
| 916, 810 | ν guaiacyl group | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, P.; Balcells, M.; Cequier, E.; Canela-Garayoa, R. Effect of Four Novel Bio-Based DES (Deep Eutectic Solvents) on Hardwood Fractionation. Molecules 2020, 25, 2157. https://doi.org/10.3390/molecules25092157
Torres P, Balcells M, Cequier E, Canela-Garayoa R. Effect of Four Novel Bio-Based DES (Deep Eutectic Solvents) on Hardwood Fractionation. Molecules. 2020; 25(9):2157. https://doi.org/10.3390/molecules25092157
Chicago/Turabian StyleTorres, Paulo, Mercè Balcells, Enrique Cequier, and Ramon Canela-Garayoa. 2020. "Effect of Four Novel Bio-Based DES (Deep Eutectic Solvents) on Hardwood Fractionation" Molecules 25, no. 9: 2157. https://doi.org/10.3390/molecules25092157
APA StyleTorres, P., Balcells, M., Cequier, E., & Canela-Garayoa, R. (2020). Effect of Four Novel Bio-Based DES (Deep Eutectic Solvents) on Hardwood Fractionation. Molecules, 25(9), 2157. https://doi.org/10.3390/molecules25092157






