Trimethyl-Substituted Carbamate as a Versatile Self-Immolative Linker for Fluorescence Detection of Enzyme Reactions
Abstract
1. Introduction
2. Results
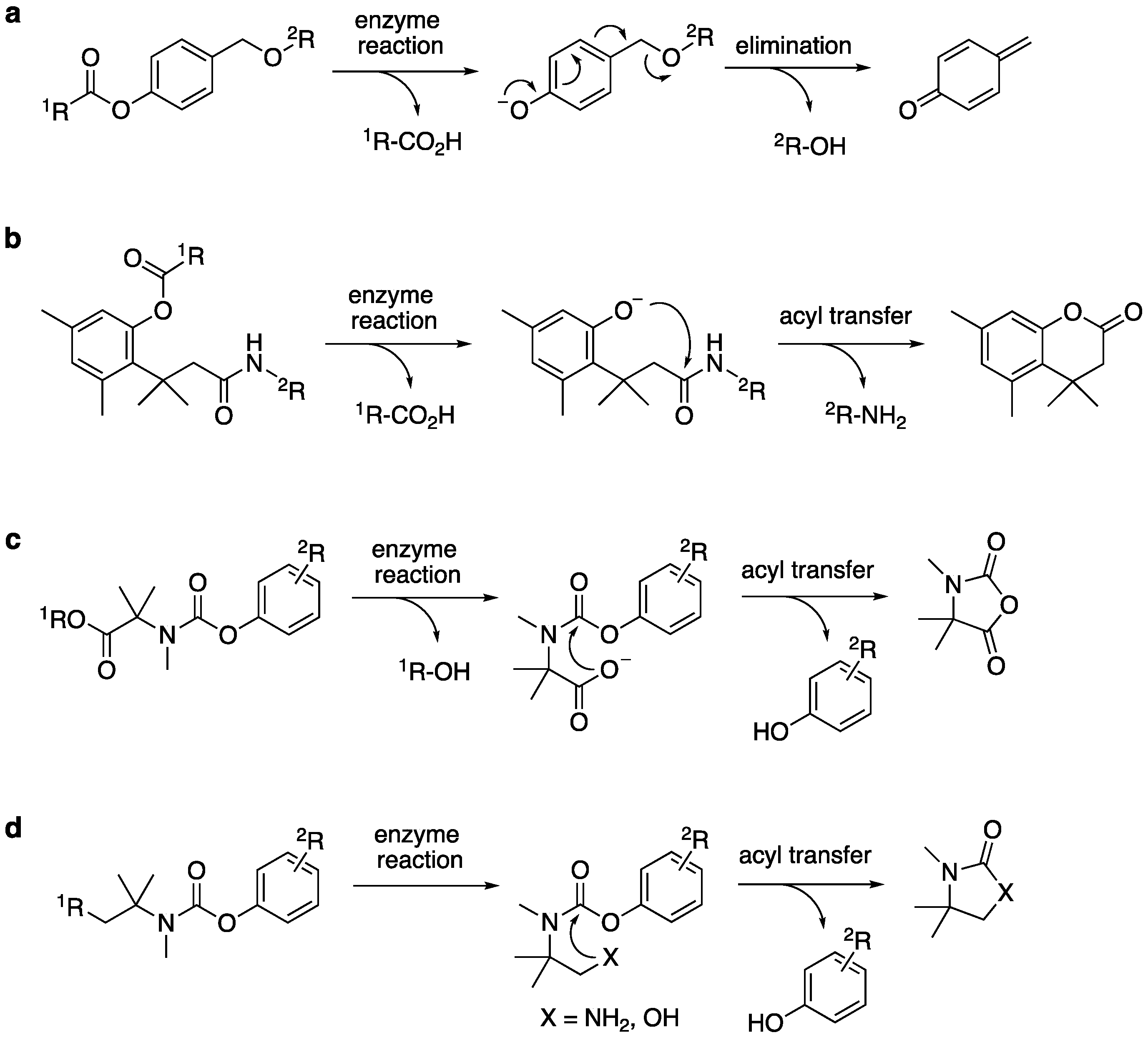
2.1. Development of N-Methyl Dimethyl Methyl Carbamate Linker
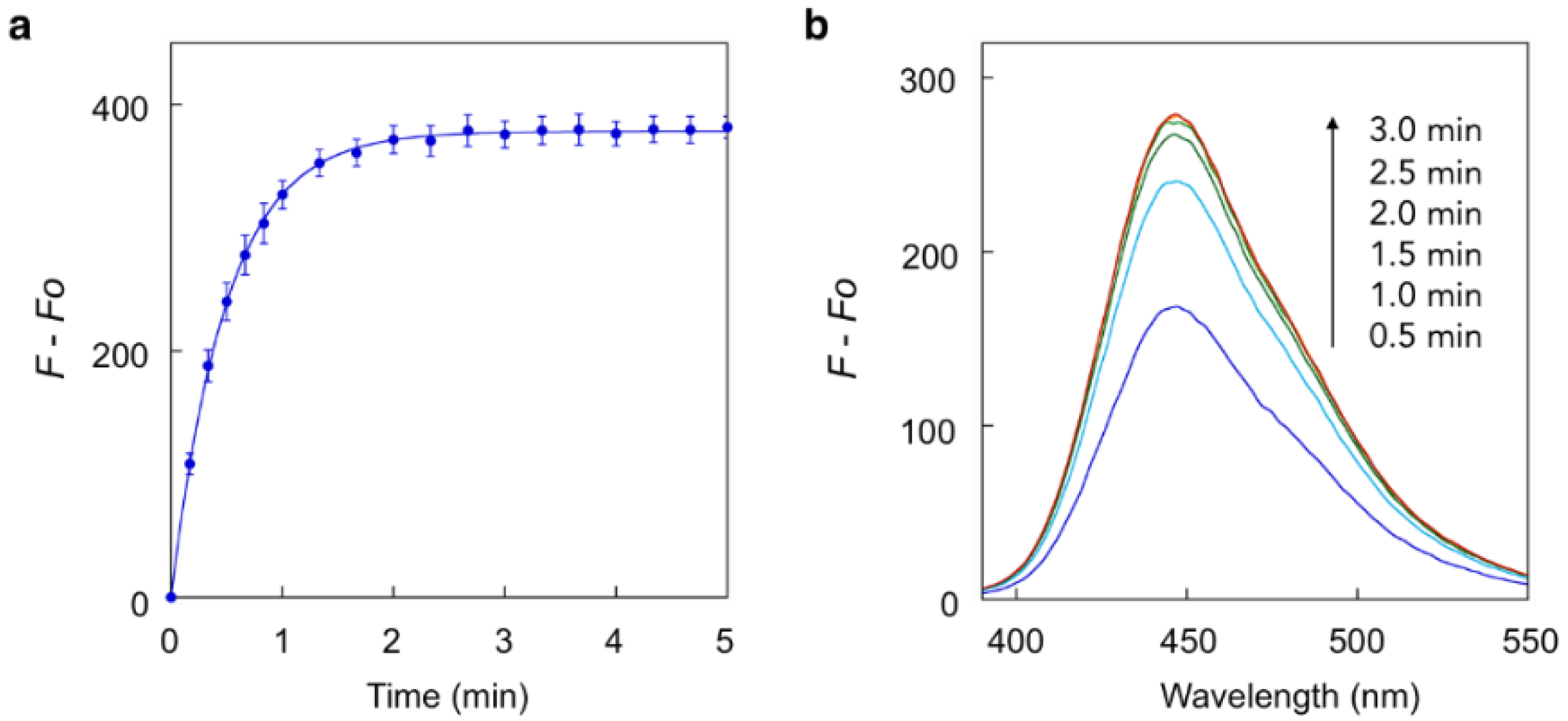
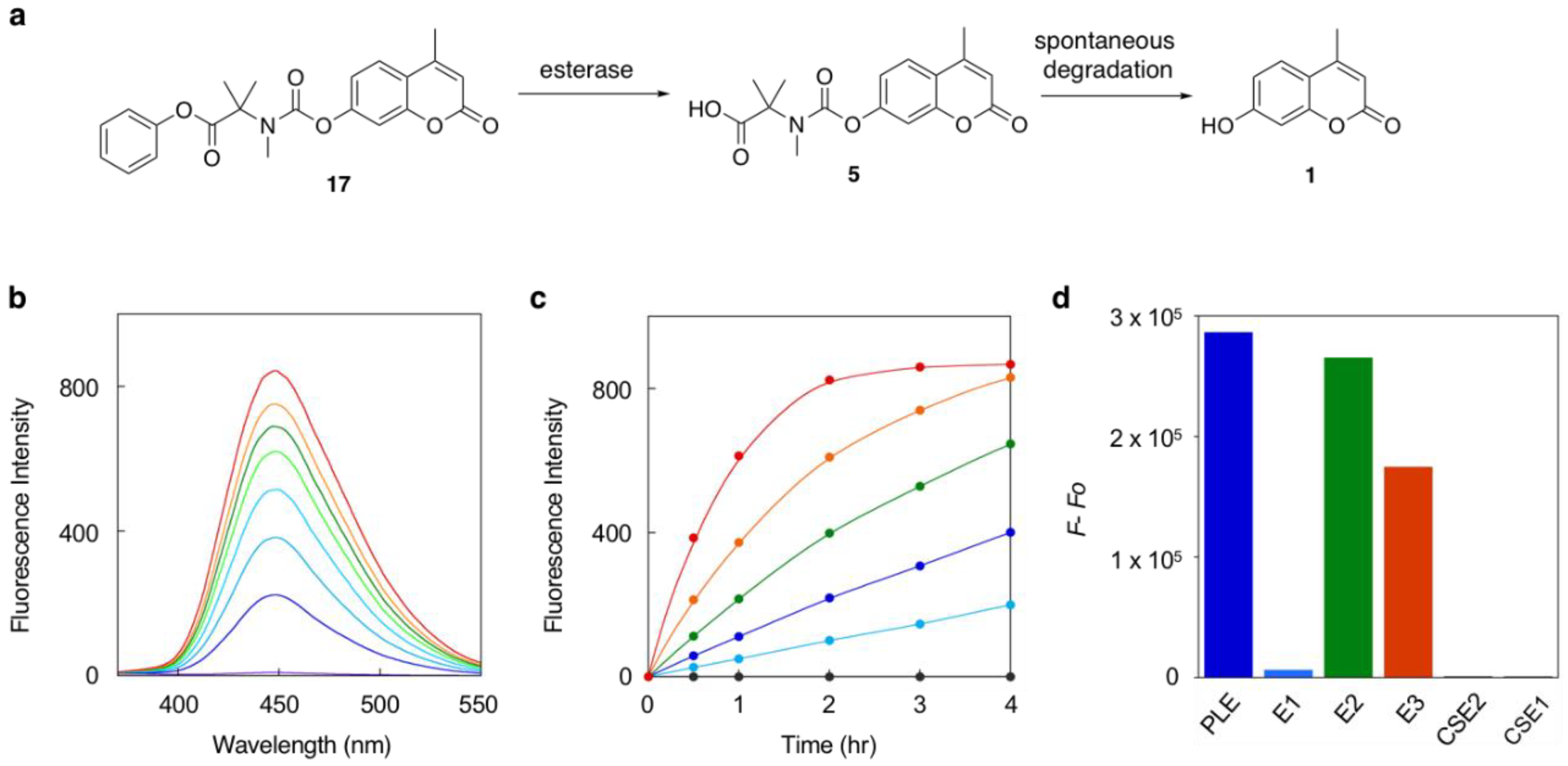
2.2. Fluorescence Detection of Enzyme Reactions with the Trimethyl Carbamate Linker
3. Materials and Methods
3.1. Fluorescence Measurement and Reaction Kinetics Analysis of the Coumarin Probes
3.2. Fluorescence Detection of Esterase Activity Using Probe 17
3.3. Fluorescence Detection of Ketoreductase Activity Using Probe 16
3.4. Fluorescence Detection of Transaminase Activity Using Probe 16
3.5. Cell Culture
3.6. Evaluation of Cell Viability after Treatment of A549 Cells with Probe 18
3.7. Evaluation of Reaction of 18 and Glutathione
3.8. Fluorescence Imaging of Intracellular Esterases in Living A549 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chyan, W.; Raines, R.T. Enzyme-Activated Fluorogenic Probes for Live-Cell and in Vivo Imaging. ACS Chem. Biol. 2018, 13, 1810–1823. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Chen, L.; Xu, C.; Li, Z.; Zhang, H.; Zhang, X.B.; Tan, W. Recent progresses in small-molecule enzymatic fluorescent probes for cancer imaging. Chem. Soc. Rev. 2018, 47, 7140–7180. [Google Scholar] [CrossRef] [PubMed]
- Garland, M.; Yim, J.J.; Bogyo, M.A. Bright Future for Precision Medicine: Advances in Fluorescent Chemical Probe Design and Their Clinical Application. Cell Chem. Biol. 2016, 23, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Ning, J.; Tian, X.; Wang, C.; Zhang, L.; Ma, X.; James, T.D. Fluorescent probes for bioactive detection and imaging of phase II metabolic enzymes. Coord. Chem. Rev. 2019, 399, 213026. [Google Scholar] [CrossRef]
- Gnaim, S.; Shabat, D. Activity-Based Optical Sensing Enabled by Self-Immolative Scaffolds: Monitoring of Release Events by Fluorescence or Chemiluminescence Output. Acc. Chem. Res. 2019, 52, 2806–2817. [Google Scholar] [CrossRef] [PubMed]
- Alouane, A.; Labruére, R.; Saux, T.L.; Schmidt, F.; Jullien, L. Self-immolative Spacers: Kinetic Aspects, Structure-Property Relationships, and Applications. Angew. Chem. Int. Ed. 2015, 54, 7492–7509. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Nicolaou, M.G.; Borchard, R.T.; Wang, B. Prodrug Strategies Based on Intramolecular Cyclization Reactions. J. Pharm. Sci. 1997, 86, 765–767. [Google Scholar] [CrossRef] [PubMed]
- Haba, K.; Popkov, M.; Shamis, M.; Lerner, R.A.; Barbas, C.F., III; Shabat, D. Single-Triggered Trimeric Prodrugs. Angew. Chem. Int. Ed. 2005, 44, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Lavine, M.N.; Hoang Trish, T.; Raines, R.T. Fluorogenic Probe for Constitutive Cellular Endocytosis. Chem. Biol. 2013, 20, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.S.; Dickson, K.A.; Raines, R.T. Latent Fluorophore Based on the Trimethyl Lock. J. Am. Chem. Soc. 2005, 127, 1652–1653. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.E.; Piizzi, G. gem-Disubstituent Effect: Theoretical Basis and Synthetic Applications. Chem. Rev. 2005, 105, 1735–1766. [Google Scholar] [CrossRef] [PubMed]
- Lightstone, F.C.; Bruice, T.C. Geminal Dialkyl Substitution, Intramolecular Reactions, and Enzyme Efficiency. J. Am. Chem. Soc. 1994, 116, 10789–10790. [Google Scholar] [CrossRef]
- Bruice, T.C.; Pandit, U.K. The Effect of Geminal Substitution Ring Size and Rotamer Distribution on the Intramolecular Nucleophilic Catalysis of the Hydrolysis of Monophenyl Esters of Dibasic Acids and the Solvolysis of the Intermediate Anhydrides. J. Am. Chem. Soc. 1960, 82, 5858–5865. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| 1R |  | 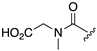 |  |  |  |
| 2 | 3 | 4 | 5 | 6 | |
| k (h−1) | n.d. b | 0.023 | 1.2 | 120 | n.d. c |
| t1/2 (h) | n.d. b | 30 | 0.58 (35 min) | 0.0058 (21 s) | > 100 h |
| 1R | 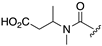 | 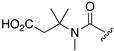 | 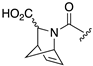 | 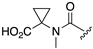 | 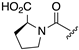 |
| 7 | 8 | 9 | 10 | 11 | |
| k (h−1) | n.d. c | 1.3 | 36 | 0.060 | n.d. c |
| t1/2 (h) | > 100 h | 0.53 (32 min) | 0.019 (1.2 min) | 11.6 | > 100 h |

| 1R | 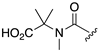 | 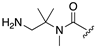 | 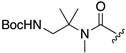 |
| 5 | 12 | 13 | |
| k (h−1) | 120 | 92 | n.d. b |
| t1/2 (h) | 0.0058 (21 s) | 0.021 (75 s) | > 100 h |
| 1R |  | 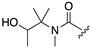 |  |
| 14 | 15 | 16 | |
| k (h−1) | 0.64 | 0.75 | n.d.b |
| t1/2 (h) | 1.1 h | 0.92 h | > 100 h |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, N.; Uchinomiya, S.; Inoue, K.; Ojida, A. Trimethyl-Substituted Carbamate as a Versatile Self-Immolative Linker for Fluorescence Detection of Enzyme Reactions. Molecules 2020, 25, 2153. https://doi.org/10.3390/molecules25092153
Nakamura N, Uchinomiya S, Inoue K, Ojida A. Trimethyl-Substituted Carbamate as a Versatile Self-Immolative Linker for Fluorescence Detection of Enzyme Reactions. Molecules. 2020; 25(9):2153. https://doi.org/10.3390/molecules25092153
Chicago/Turabian StyleNakamura, Noriaki, Shohei Uchinomiya, Kazuya Inoue, and Akio Ojida. 2020. "Trimethyl-Substituted Carbamate as a Versatile Self-Immolative Linker for Fluorescence Detection of Enzyme Reactions" Molecules 25, no. 9: 2153. https://doi.org/10.3390/molecules25092153
APA StyleNakamura, N., Uchinomiya, S., Inoue, K., & Ojida, A. (2020). Trimethyl-Substituted Carbamate as a Versatile Self-Immolative Linker for Fluorescence Detection of Enzyme Reactions. Molecules, 25(9), 2153. https://doi.org/10.3390/molecules25092153






