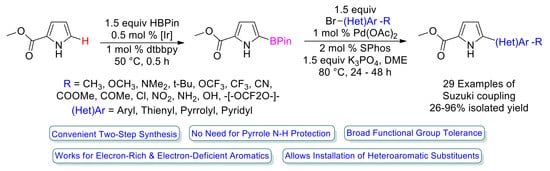
Facile Synthesis of NH-Free 5-(Hetero)Aryl-Pyrrole-2-Carboxylates by Catalytic C–H Borylation and Suzuki Coupling
Abstract
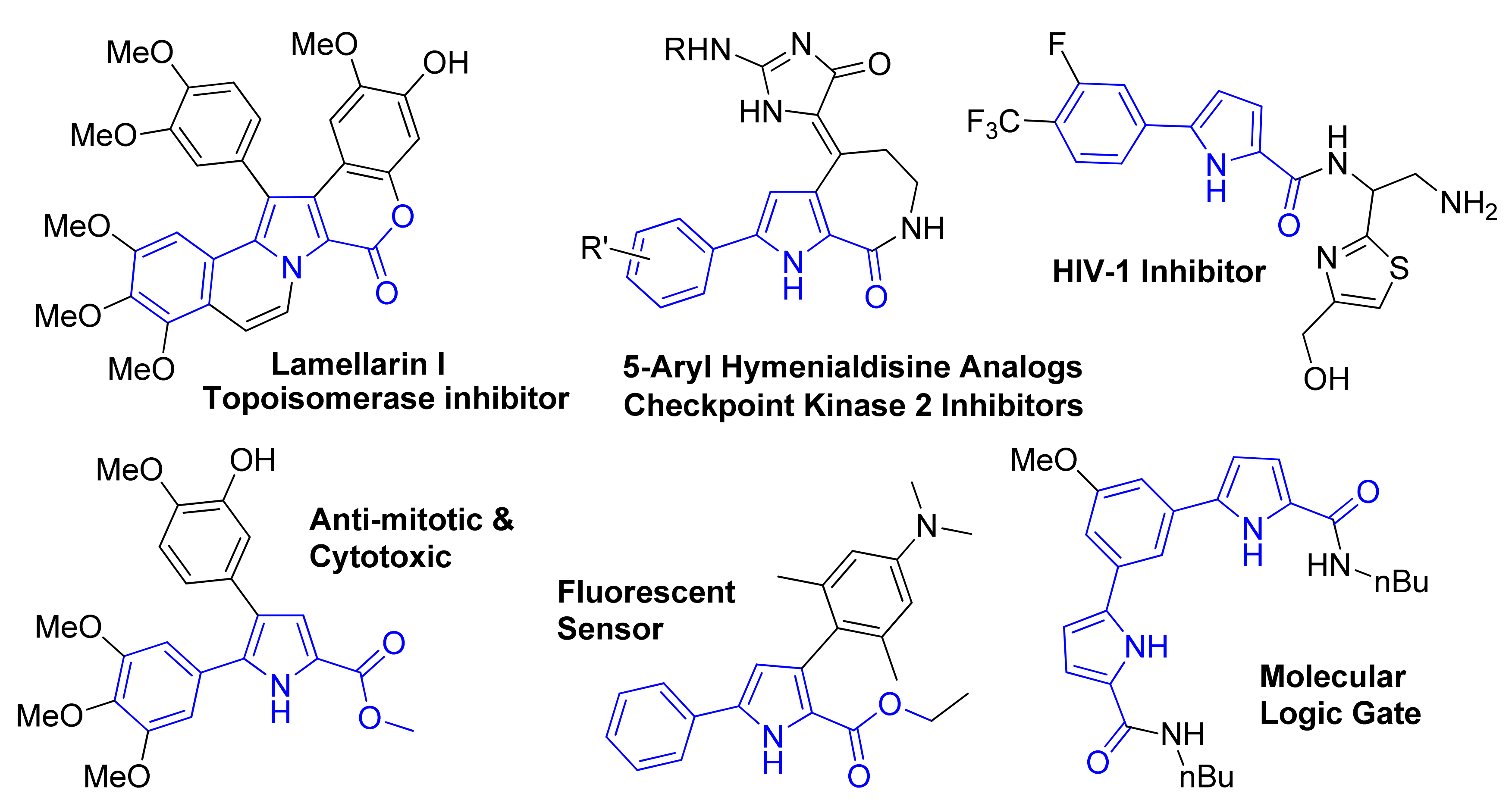
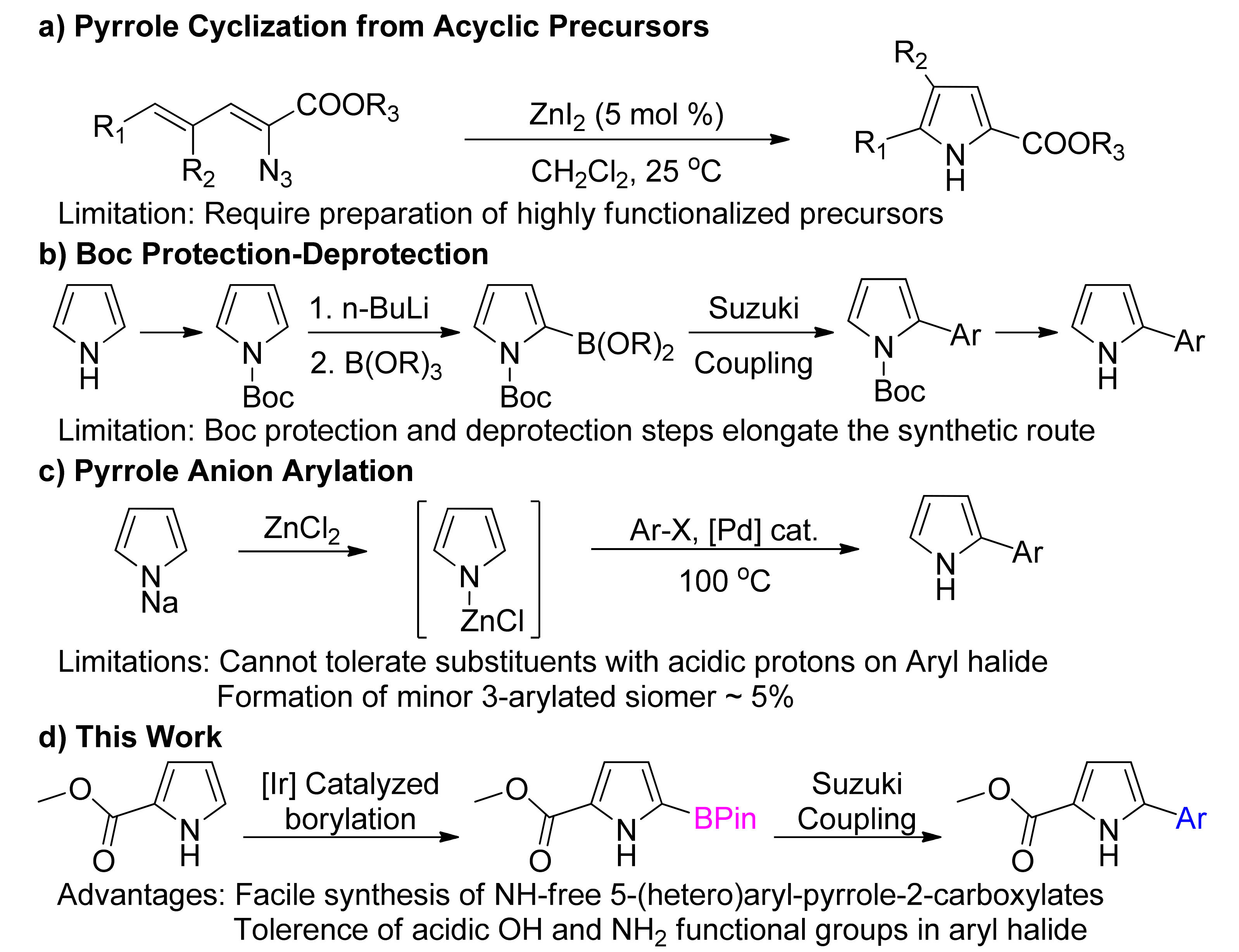
1. Introduction
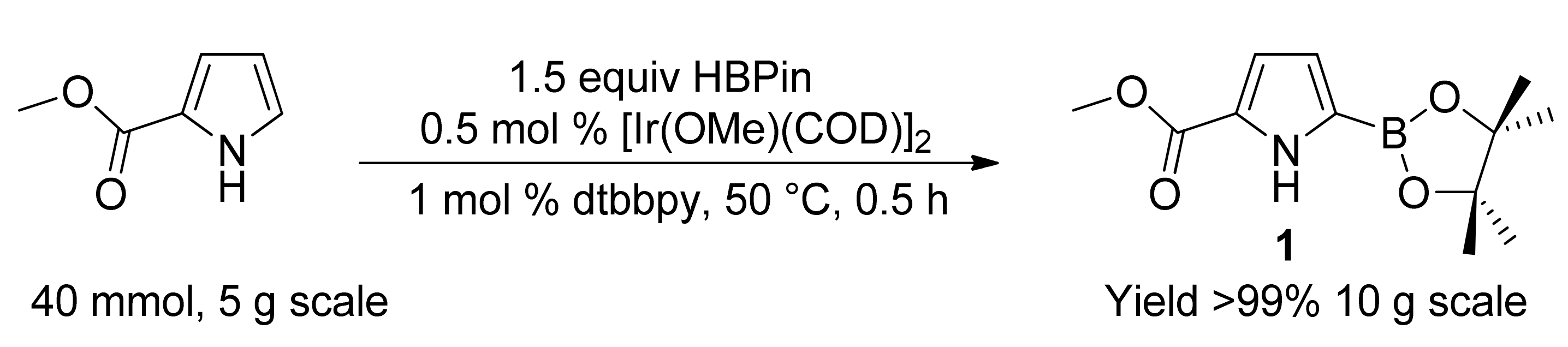
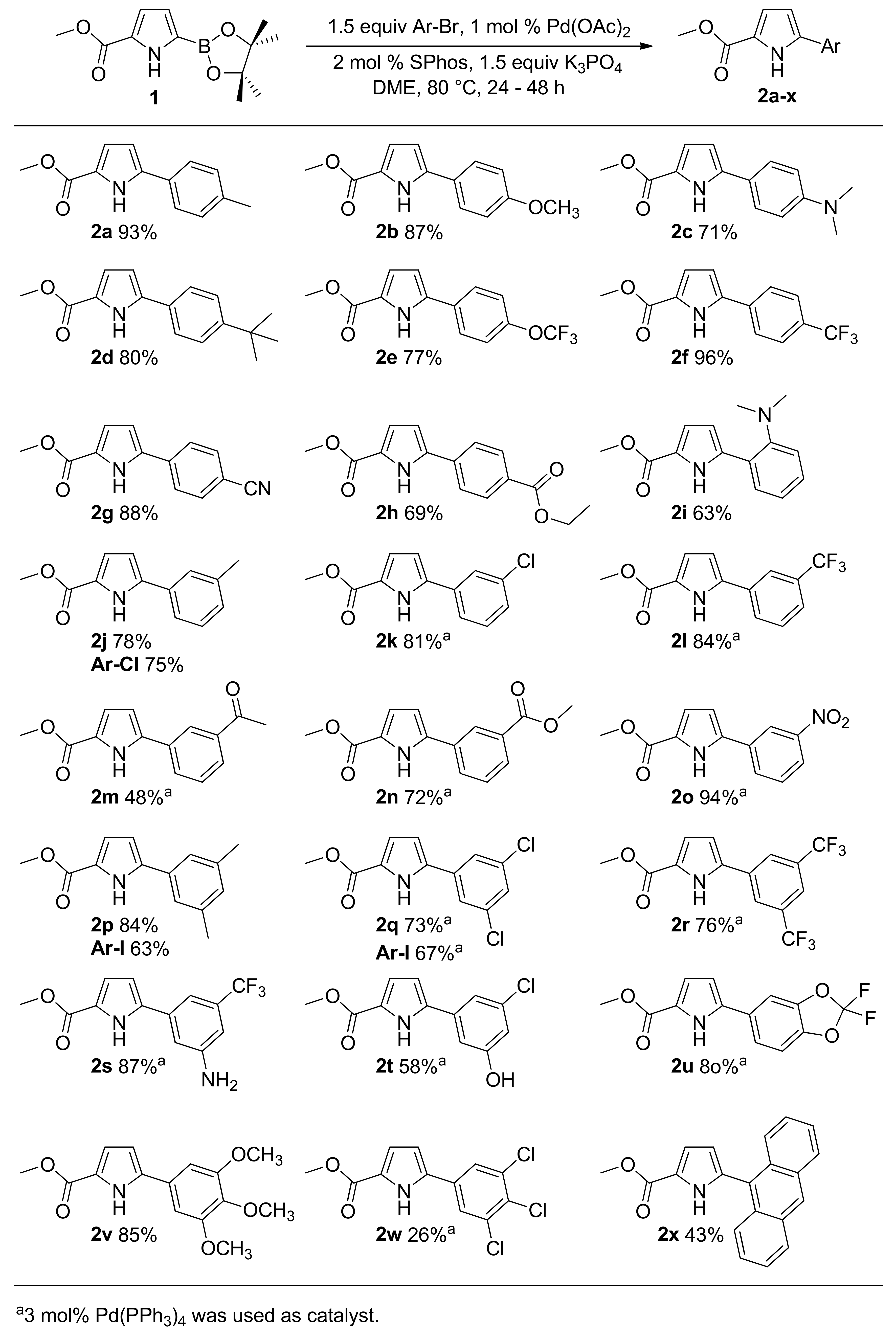
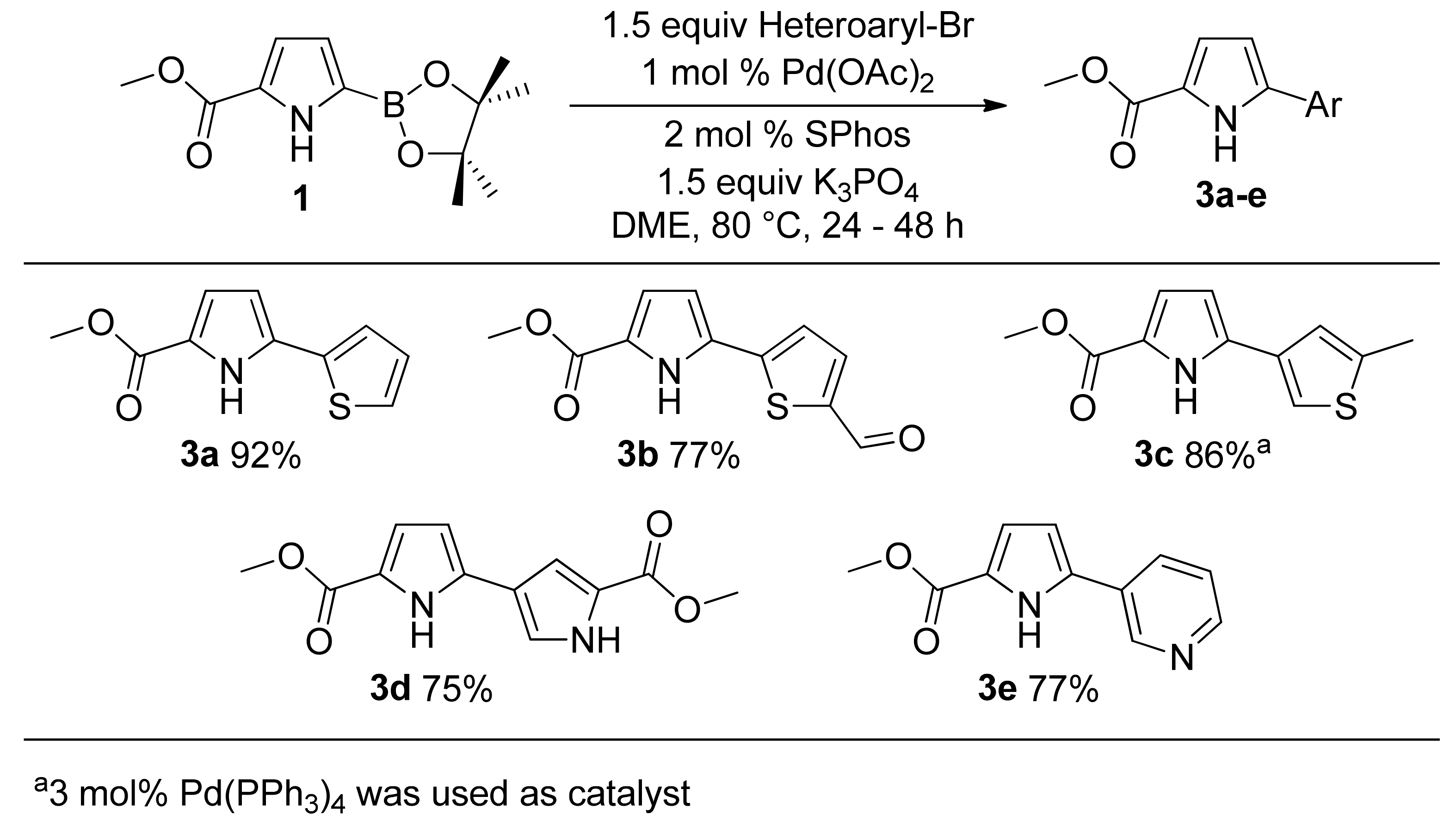
2. Results and Discussion
3. Materials and Methods
3.1. General Considerations and Starting Materials
3.2. Suzuki Coupling
3.2.1. General Suzuki Procedure A Employing Pd(OAc)2 and 2-Dicyclohexylphosphino-2′,6′-dimethoxybiphenyl (SPhos)
3.2.2. General Suzuki Procedure B Employing Palladium Tetrakistriphenylphosphine Pd(PPh3)4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Domagala, A.; Jarosz, T.; Lapkowski, M. Living on pyrrolic foundations – Advances in natural and artificial bioactive pyrrole derivatives. Eur. J. Med. Chem. 2015, 100, 176–187. [Google Scholar] [CrossRef]
- Gholap, S.S. Pyrrole: An emerging scaffold for construction of valuable therapeutic agents. Eur. J. Med. Chem. 2016, 110, 13–31. [Google Scholar] [CrossRef]
- Fukuda, T.; Umeki, T.; Tokushima, K.; Xiang, G.; Yoshida, Y.; Ishibashi, F.; Oku, Y.; Nishiya, N.; Uehara, Y.; Iwao, M. Design, synthesis, and evaluation of A-ring-modified lamellarin N analogues as noncovalent inhibitors of the EGFR T790M/L858R mutant. Bioorg. Med. Chem. 2017, 25, 6563–6580. [Google Scholar] [CrossRef]
- Lade, D.M.; Pawar, A.B.; Mainkar, P.S.; Chandrasekhar, S. Total Synthesis of Lamellarin D Trimethyl Ether, Lamellarin, D., and Lamellarin, H.J. Org. Chem. 2017, 82, 4998–5004. [Google Scholar] [CrossRef] [PubMed]
- Imbri, D.; Tauber, J.; Opatz, T. Synthetic Approaches to the Lamellarins—A Comprehensive Review. Mar. Drugs 2014, 12, 6142–6177. [Google Scholar] [CrossRef]
- Dialer, C.; Imbri, D.; Hansen, S.P.; Opatz, T. Synthesis of Lamellarin D Trimethyl Ether and Lamellarin H via 6π-Electrocyclization. J. Org. Chem. 2015, 80, 11605–11610. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Peng, J.; Hamann, M.T.; Hu, J.-F. Lamellarins and Related Pyrrole-Derived Alkaloids from Marine Organisms. Chem. Rev. 2008, 108, 264–287. [Google Scholar] [CrossRef] [PubMed]
- Saleem, R.S.Z.; Lansdell, T.A.; Tepe, J.J. Synthesis and evaluation of debromohymenialdisine-derived Chk2 inhibitors. Bioorg. Med. Chem. 2012, 20, 1475–1481. [Google Scholar] [CrossRef]
- Nguyen, T.N.T.; Saleem, R.S.Z.; Luderer, M.J.; Hovde, S.; Henry, R.W.; Tepe, J.J. Radioprotection by Hymenialdisine-Derived Checkpoint Kinase 2 Inhibitors. ACS Chem. Biol. 2012, 7, 172–184. [Google Scholar] [CrossRef]
- Curreli, F.; Belov, D.S.; Kwon, Y.D.; Ramesh, R.; Furimsky, A.M.; O’Loughlin, K.; Byrge, P.C.; Iyer, L.V.; Mirsalis, J.C.; Kurkin, A.V.; et al. Structure-based lead optimization to improve antiviral potency and ADMET properties of phenyl-1H-pyrrole-carboxamide entry inhibitors targeted to HIV-1 gp120. Eur. J. Med. Chem. 2018, 154, 367–391. [Google Scholar] [CrossRef]
- Curreli, F.; Kwon, Y.D.; Belov, D.S.; Ramesh, R.R.; Kurkin, A.V.; Altieri, A.; Kwong, P.D.; Debnath, A.K. Synthesis, Antiviral Potency, in Vitro ADMET, and X-ray Structure of Potent CD4 Mimics as Entry Inhibitors That Target the Phe43 Cavity of HIV-1 gp120. J. Med. Chem. 2017, 60, 3124–3153. [Google Scholar] [CrossRef] [PubMed]
- Curreli, F.; Belov, D.S.; Ramesh, R.R.; Patel, N.; Altieri, A.; Kurkin, A.V.; Debnath, A.K. Design, synthesis and evaluation of small molecule CD4-mimics as entry inhibitors possessing broad spectrum anti-HIV-1 activity. Bioorg. Med. Chem. 2016, 24, 5988–6003. [Google Scholar] [CrossRef]
- Curreli, F.; Kwon, Y.D.; Zhang, H.; Scacalossi, D.; Belov, D.S.; Tikhonov, A.A.; Andreev, I.A.; Altieri, A.; Kurkin, A.V.; Kwong, P.D.; et al. Structure-Based Design of a Small Molecule CD4-Antagonist with Broad Spectrum Anti-HIV-1 Activity. J. Med. Chem. 2015, 58, 6909–6927. [Google Scholar] [CrossRef] [PubMed]
- Pinna, G.; Loriga, G.; Murineddu, G.; Grella, G.; Mura, M.; Vargiu, L.; Murgioni, C.; La Colla, P. Synthesis and Anti-HIV-1 Activity of New Delavirdine Analogues Carrying Arylpyrrole Moieties. Chem. Pharm. Bull. 2001, 49, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtsev, K.V.; Bentley, M.L.; McCafferty, D.G. Probing of the cis-5-phenyl proline scaffold as a platform for the synthesis of mechanism-based inhibitors of the Staphylococcus aureus sortase SrtA isoform. Bioorg. Med. Chem. 2009, 17, 2886–2893. [Google Scholar] [CrossRef]
- Banwell, M.G.; Hamel, E.; Hockless, D.C.R.; Verdier-Pinard, P.; Willis, A.C.; Wong, D.J. 4,5-Diaryl-1H-pyrrole-2-carboxylates as combretastatin A-4/lamellarin T hybrids: Synthesis and evaluation as anti-mitotic and cytotoxic agents. Bioorg. Med. Chem. 2006, 14, 4627–4638. [Google Scholar] [CrossRef]
- Galenko, E.E.; Galenko, A.V.; Novikov, M.S.; Khlebnikov, A.F.; Kudryavtsev, I.V.; Terpilowski, M.A.; Serebriakova, M.K.; Trulioff, A.S.; Goncharov, N.V. 4-Diazo and 4-(Triaz-1-en-1-yl)-1H-pyrrole-2-carboxylates as Agents Inducing Apoptosis. ChemistrySelect 2017, 2, 7508–7513. [Google Scholar] [CrossRef]
- Galenko, E.E.; Galenko, A.V.; Khlebnikov, A.F.; Novikov, M.S.; Shakirova, J.R. Synthesis and Intramolecular Azo Coupling of 4-Diazopyrrole-2-carboxylates: Selective Approach to Benzo and Hetero [c]-Fused 6H-Pyrrolo[3,4-c]pyridazine-5-carboxylates. J. Org. Chem. 2016, 81, 8495–8507. [Google Scholar] [CrossRef]
- Killoran, J.; Gallagher, J.F.; Murphy, P.V.; O’Shea, D.F. A study of the effects of subunit pre-orientation for diarylpyrrole esters; design of new aryl-heteroaryl fluorescent sensors. New J. Chem. 2005, 29, 1258–1265. [Google Scholar] [CrossRef]
- Granda, J.M.; Staszewska-Krajewska, O.; Jurczak, J. Bispyrrolylbenzene Anion Receptor: From Supramolecular Switch to Molecular Logic Gate. Chem. Eur. J. 2014, 20, 12790–12795. [Google Scholar] [CrossRef]
- Zhang, H.; Lee, J.; Lammer, A.D.; Chi, X.; Brewster, J.T.; Lynch, V.M.; Li, H.; Zhang, Z.; Sessler, J.L. Self-Assembled Pyridine-Dipyrrolate Cages. J. Am. Chem. Soc. 2016, 138, 4573–4579. [Google Scholar] [CrossRef] [PubMed]
- Chaolu, E.; Satoshi, H.; Jun-ichiro, S. One-Handed Single Helicates of Dinickel(II) Benzenehexapyrrole-α,ω-diimine with an Amine Chiral Source. Chem. Eur. J. 2015, 21, 239–246. [Google Scholar]
- Setsune, J.-i.; Kawama, M.; Nishinaka, T. Helical binuclear CoII complexes of pyriporphyrin analogue for sensing homochiral carboxylic acids. Tetrahedron Lett. 2011, 52, 1773–1777. [Google Scholar] [CrossRef]
- Boukou-Poba, J.P.; Farnier, M.; Guilard, R. A general method for the synthesis of 2-arylpyrroles. Tetrahedron Lett. 1979, 20, 1717–1720. [Google Scholar] [CrossRef]
- Ezquerra, J.; Pedregal, C.; Rubio, A.; Valenciano, J.; Navio, J.L.G.; Alvarez-Builla, J.; Vaquero, J.J. General method for the synthesis of 5-arylpyrrole-2-carboxylic acids. Tetrahedron Lett. 1993, 34, 6317–6320. [Google Scholar] [CrossRef][Green Version]
- Queiroz, M.-J.R.P.; Begouin, A.; Pereira, G.; Ferreira, P.M.T. New synthesis of methyl 5-aryl or heteroaryl pyrrole-2-carboxylates by a tandem Sonogashira coupling/5-endo-dig-cyclization from β-iododehydroamino acid methyl esters and terminal alkynes. Tetrahedron 2008, 64, 10714–10720. [Google Scholar] [CrossRef]
- Estevez, V.; Villacampa, M.; Menendez, J.C. Recent advances in the synthesis of pyrroles by multicomponent reactions. Chem. Soc. Rev. 2014, 43, 4633–4657. [Google Scholar] [CrossRef]
- Cheng, B.-Y.; Wang, Y.-N.; Li, T.-R.; Lu, L.-Q.; Xiao, W.-J. Synthesis of Polysubstituted Pyrroles through a Formal [4 + 1] Cycloaddition/E1cb Elimination/Aromatization Sequence of Sulfur Ylides and α,β-Unsaturated Imines. J. Org. Chem. 2017, 82, 12134–12140. [Google Scholar] [CrossRef]
- Ngwerume, S.; Lewis, W.; Camp, J.E. Development of a Gold-Multifaceted Catalysis Approach to the Synthesis of Highly Substituted Pyrroles: Mechanistic Insights via Huisgen Cycloaddition Studies. J. Org. Chem. 2013, 78, 920–934. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, Y.; Luo, X.; Han, D.-M.; Deng, W.-P. Direct synthesis of pyrroles via 1,3-dipolar cycloaddition of azomethine ylides with ynones. New J. Chem. 2013, 37, 1742–1745. [Google Scholar] [CrossRef]
- Kudryavtsev, K.V.; Ivantcova, P.M.; Churakov, A.V.; Vasin, V.A. Phenyl α-bromovinyl sulfone in cycloadditions with azomethine ylides: An unexpected facile aromatization of the cycloadducts into pyrroles. Tetrahedron Lett. 2012, 53, 4300–4303. [Google Scholar] [CrossRef]
- Lade, D.M.; Pawar, A.B. Cp*Co(iii)-catalyzed vinylic C-H bond activation under mild conditions: Expedient pyrrole synthesis via (3 + 2) annulation of enamides and alkynes. Org. Chem. Front. 2016, 3, 836–840. [Google Scholar] [CrossRef]
- Imbri, D.; Netz, N.; Kucukdisli, M.; Kammer, L.M.; Jung, P.; Kretzschmann, A.; Opatz, T. One-Pot Synthesis of Pyrrole-2-carboxylates and -carboxamides via an Electrocyclization/Oxidation Sequence. J. Org. Chem. 2014, 79, 11750–11758. [Google Scholar] [CrossRef] [PubMed]
- López-Pérez, A.; Robles-Machín, R.; Adrio, J.; Carretero, J.C. Oligopyrrole Synthesis by 1,3-Dipolar Cycloaddition of Azomethine Ylides with Bissulfonyl Ethylenes. Angew. Chem. Int. Ed. 2007, 46, 9261–9264. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, C.-M.; Li, H.-L.; He, F.-S.; Luo, X.; Deng, W.-P. Regioselective Iodine-Catalyzed Construction of Polysubstituted Pyrroles from Allenes and Enamines. J. Org. Chem. 2016, 81, 8653–8658. [Google Scholar] [CrossRef]
- Galenko, E.E.; Bodunov, V.A.; Galenko, A.V.; Novikov, M.S.; Khlebnikov, A.F. Fe(II)-Catalyzed Isomerization of 4-Vinylisoxazoles into Pyrroles. J. Org. Chem. 2017, 82, 8568–8579. [Google Scholar] [CrossRef] [PubMed]
- Padwa, A.; Stengel, T. Grubbs and Wilkinson catalyzed reactions of 2-phenyl-3-vinyl substituted 2H-azirines. Arkivoc 2004, 2005, 21–32. [Google Scholar]
- Farney, E.P.; Yoon, T.P. Visible-Light Sensitization of Vinyl Azides by Transition-Metal Photocatalysis. Angew. Chem. Int. Ed. 2014, 53, 793–797. [Google Scholar] [CrossRef]
- Dong, H.; Shen, M.; Redford, J.E.; Stokes, B.J.; Pumphrey, A.L.; Driver, T.G. Transition Metal-Catalyzed Synthesis of Pyrroles from Dienyl Azides. Org. Lett. 2007, 9, 5191–5194. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ji, C.L.; Hong, X.; Szostak, M. Palladium-Catalyzed Decarbonylative Borylation of Carboxylic Acids: Tuning Reaction Selectivity by Computation. Angew. Chem. Int. Ed. 2018, 57, 16721–16726. [Google Scholar] [CrossRef]
- Akira, S.; Yasunori, Y. Cross-coupling Reactions of Organoboranes: An Easy Method for C–C Bonding. Chem. Lett. 2011, 40, 894–901. [Google Scholar]
- Lennox, A.J.J.; Lloyd-Jones, G.C. Selection of boron reagents for Suzuki–Miyaura coupling. Chem. Soc. Rev. 2014, 43, 412–443. [Google Scholar] [CrossRef] [PubMed]
- El-Maiss, J.; Mohy El Dine, T.; Lu, C.-S.; Karamé, I.; Kanj, A.; Polychronopoulou, K.; Shaya, J. Recent Advances in Metal-Catalyzed Alkyl–Boron (C(sp3)–C(sp2)) Suzuki-Miyaura Cross-Couplings. Catalysts 2020, 10, 296–320. [Google Scholar] [CrossRef]
- Shi, S.; Szostak, M. Decarbonylative Borylation of Amides by Palladium Catalysis. ACS Omega 2019, 4, 4901–4907. [Google Scholar] [CrossRef] [PubMed]
- Martina, S.; Enkelmann, V.; Wegner, G.; Schlüter, A.-D. N-Protected Pyrrole Derivatives Substituted for Metal-Catalyzed Cross-Coupling Reactions. Synthesis 1991, 1991, 613–615. [Google Scholar] [CrossRef]
- Laha, J.K.; Sharma, S.; Bhimpuria, R.A.; Dayal, N.; Dubey, G.; Bharatam, P.V. Integration of oxidative arylation with sulfonyl migration: One-pot tandem synthesis of densely functionalized (NH)-pyrroles. New J. Chem. 2017, 41, 8791–8803. [Google Scholar] [CrossRef]
- Yiğit, B.; Gürbüz, N.; Yiğit, M.; Dağdeviren, Z.; Özdemir, İ. Palladium(II) N-heterocyclic carbene complexes as catalysts for the direct arylation of pyrrole derivatives with aryl chlorides. Inorg. Chim. Acta 2017, 465, 44–49. [Google Scholar] [CrossRef]
- Laha, J.K.; Bhimpuria, R.A.; Prajapati, D.V.; Dayal, N.; Sharma, S. Palladium-catalyzed regioselective C-2 arylation of 7-azaindoles, indoles, and pyrroles with arenes. Chem. Commun. 2016, 52, 4329–4332. [Google Scholar] [CrossRef]
- Carina, S.; Karthik, D.; Andreas, O.; Gates, P.J.; Pilarski, L.T. Ru-Catalysed C-H Arylation of Indoles and Pyrroles with Boronic Acids: Scope and Mechanistic Studies. Chem. Eur. J. 2015, 21, 5380–5386. [Google Scholar]
- Wang, L.; Li, Z.; Qu, X.; Peng, W. Highly Efficient Synthesis of Arylpyrrole Derivatives via Rh(III)-Catalyzed Direct C-H Arylation with Aryl Boronic Acids. Chin. J. Chem. 2015, 33, 1015–1018. [Google Scholar] [CrossRef]
- Pla, D.; Marchal, A.; Olsen, C.A.; Albericio, F.; Álvarez, M. Modular Total Synthesis of Lamellarin, D.J. Org. Chem. 2005, 70, 8231–8234. [Google Scholar] [CrossRef] [PubMed]
- Belov, D.S.; Ivanov, V.N.; Curreli, F.; Kurkin, A.V.; Altieri, A.; Debnath, A.K. Synthesis of 5-Arylpyrrole-2-carboxylic Acids as Key Intermediates for NBD Series HIV-1 Entry Inhibitors. Synthesis 2017, 49, 3692–3699. [Google Scholar]
- Hodge, P.; Rickards, R.W. 72. The halogenation of methyl pyrrole-2-carboxylate and of some related pyrroles. J. Chem. Soc. 1965, 459–470. [Google Scholar] [CrossRef]
- Anderson, H.J.; Lee, S.-F. Pyrrole Chemistry: IV. The Preparation and some reactions of brominated pyrrole derivatives. Can. J. Chem. 1965, 43, 409–414. [Google Scholar] [CrossRef]
- Chen, W.; Cava, M.P. Convenient synthetic equivalents of 2-lithiopyrrole and 2,5-dilithiopyrrole. Tetrahedron Lett. 1987, 28, 6025–6026. [Google Scholar] [CrossRef]
- Komatsubara, M.; Umeki, T.; Fukuda, T.; Iwao, M. Modular Synthesis of Lamellarins via Regioselective Assembly of 3,4,5-Differentially Arylated Pyrrole-2-carboxylates. J. Org. Chem. 2014, 79, 529–537. [Google Scholar] [CrossRef]
- Urbano, M.; Guerrero, M.; Zhao, J.; Velaparthi, S.; Schaeffer, M.-T.; Brown, S.; Rosen, H.; Roberts, E. SAR analysis of innovative selective small molecule antagonists of sphingosine-1-phosphate 4 (S1P4) receptor. Bioorg. Med. Chem. Lett. 2011, 21, 5470–5474. [Google Scholar] [CrossRef]
- Setsune, J.-i.; Toda, M.; Watanabe, K.; Panda, P.K.; Yoshida, T. Synthesis of bis(pyrrol-2-yl)arenes by Pd-catalyzed cross coupling. Tetrahedron Lett. 2006, 47, 7541–7544. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Tse, M.K.; Holmes, D.; Maleczka, R.E., Jr.; Smith, M.R., III. Remarkably Selective Iridium Catalysts for the Elaboration of Aromatic C-H Bonds. Science 2002, 295, 305–308. [Google Scholar] [CrossRef]
- Ishiyama, T.; Takagi, J.; Ishida, K.; Miyaura, N.; Anastasi, N.R.; Hartwig, J.F. Mild Iridium-Catalyzed Borylation of Arenes. High Turnover Numbers, Room Temperature Reactions, and Isolation of a Potential Intermediate. J. Am. Chem. Soc. 2002, 124, 390–391. [Google Scholar] [CrossRef]
- Mkhalid, I.A.I.; Barnard, J.H.; Marder, T.B.; Murphy, J.M.; Hartwig, J.F. C−H Activation for the Construction of C−B Bonds. Chem. Rev. 2010, 110, 890–931. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, G.; Zhang, S.; Wang, H.; Wang, L.; Liu, L.; Jiao, J.; Li, P. Recent advances in catalytic C−H borylation reactions. Tetrahedron 2017, 73, 7123–7157. [Google Scholar] [CrossRef]
- Takagi, J.; Sato, K.; Hartwig, J.F.; Ishiyama, T.; Miyaura, N. Iridium-catalyzed C–H coupling reaction of heteroaromatic compounds with bis(pinacolato)diboron: Regioselective synthesis of heteroarylboronates. Tetrahedron Lett. 2002, 43, 5649–5651. [Google Scholar] [CrossRef]
- Ishiyama, T.; Nobuta, Y.; Hartwig, J.F.; Miyaura, N. Room temperature borylation of arenes and heteroarenes using stoichiometric amounts of pinacolborane catalyzed by iridium complexes in an inert solvent. Chem. Commun. 2003, 23, 2924–2925. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, R.; Yeung, K. The synthesis of novel pyrrololactams and their boronate ester derivatives. Tetrahedron Lett. 2016, 57, 5812–5814. [Google Scholar] [CrossRef]
- Paul, S.; Chotana, G.A.; Holmes, D.; Reichle, R.C.; Maleczka, R.E., Jr.; Smith, M.R., III. Ir-Catalyzed Functionalization of 2-Substituted Indoles at the 7-Position: Nitrogen-Directed Aromatic Borylation. J. Am. Chem. Soc. 2006, 128, 15552–15553. [Google Scholar] [CrossRef]
- Robbins, D.W.; Boebel, T.A.; Hartwig, J.F. Iridium-Catalyzed, Silyl-Directed Borylation of Nitrogen-Containing Heterocycles. J. Am. Chem. Soc. 2010, 132, 4068–4069. [Google Scholar] [CrossRef]
- Shen, F.; Tyagarajan, S.; Perera, D.; Krska, S.W.; Maligres, P.E.; Smith, M.R., III; Maleczka, R.E., Jr. Bismuth Acetate as a Catalyst for the Sequential Protodeboronation of Di- and Triborylated Indoles. Org. Lett. 2016, 18, 1554–1557. [Google Scholar] [CrossRef]
- Eastabrook, A.S.; Sperry, J. Synthetic Access to 3,5,7-Trisubstituted Indoles Enabled by Iridium Catalyzed C–H Borylation. Synthesis 2017, 49, 4731–4737. [Google Scholar]
- Chotana, G.A.; Kallepalli, V.A.; Maleczka, R.E., Jr.; Smith, M.R., III. Iridium-catalyzed borylation of thiophenes: Versatile, synthetic elaboration founded on selective C–H functionalization. Tetrahedron 2008, 64, 6103–6114. [Google Scholar] [CrossRef]
- Sadler, S.A.; Tajuddin, H.; Mkhalid, I.A.I.; Batsanov, A.S.; Albesa-Jove, D.; Cheung, M.S.; Maxwell, A.C.; Shukla, L.; Roberts, B.; Blakemore, D.C.; et al. Iridium-catalyzed C-H borylation of pyridines. Org. Biomol. Chem. 2014, 12, 7318–7327. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Emwas, A.-H.; Gao, X.; Munawar, M.A.; Chotana, G.A. Synthesis and Suzuki Cross-Coupling Reactions of 2,6-Bis(trifluoromethyl)pyridine-4-boronic Acid Pinacol Ester. Synthesis 2017, 49, 1327–1334. [Google Scholar]
- Yang, L.; Semba, K.; Nakao, Y. para-Selective C−H Borylation of (Hetero)Arenes by Cooperative Iridium/Aluminum Catalysis. Angew. Chem. Int. Ed. 2017, 56, 4853–4857. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.A.; Hartwig, J.F. Iridium-Catalyzed C–H Borylation of Heteroarenes: Scope, Regioselectivity, Application to Late-Stage Functionalization, and Mechanism. J. Am. Chem. Soc. 2014, 136, 4287–4299. [Google Scholar] [CrossRef]
- Tse, M.K.; Cho, J.-Y.; Smith, M.R., III. Regioselective Aromatic Borylation in an Inert Solvent. Org. Lett. 2001, 3, 2831–2833. [Google Scholar]
- Kallepalli, V.A.; Shi, F.; Paul, S.; Onyeozili, E.N.; Maleczka, R.E., Jr.; Smith, M.R., III. Boc Groups as Protectors and Directors for Ir-Catalyzed C−H Borylation of Heterocycles. J. Org. Chem. 2009, 74, 9199–9201. [Google Scholar] [CrossRef]
- Swartz, D.L., II; Staples, R.J.; Odom, A.L. Synthesis and hydroamination catalysis with 3-aryl substituted pyrrolyl and dipyrrolylmethane titanium(iv) complexes. Dalton Trans. 2011, 40, 7762–7768. [Google Scholar] [CrossRef]
- Robbins, D.W.; Hartwig, J.F. A C–H Borylation Approach to Suzuki–Miyaura Coupling of Typically Unstable 2–Heteroaryl and Polyfluorophenyl Boronates. Org. Lett. 2012, 14, 4266–4269. [Google Scholar] [CrossRef]
- Asghar, S.; Shahzadi, T.; Alazmi, M.; Gao, X.; Emwas, A.-H.; Saleem, R.S.Z.; Batool, F.; Chotana, G.A. Iridium-Catalyzed Regioselective Borylation of Substituted Biaryls. Synthesis 2018, 50, 2211–2220. [Google Scholar]
- Batool, F.; Parveen, S.; Emwas, A.-H.; Sioud, S.; Gao, X.; Munawar, M.A.; Chotana, G.A. Synthesis of Fluoroalkoxy Substituted Arylboronic Esters by Iridium-Catalyzed Aromatic C–H Borylation. Org. Lett. 2015, 17, 4256–4259. [Google Scholar] [CrossRef]
- Shahzadi, T.; Saleem, R.S.Z.; Chotana, G.A. Facile Synthesis of Halogen Decorated para-/meta-Hydroxy benzoates by Iridium-Catalyzed Borylation and Oxidation. Synthesis 2018, 50, 4336–4342. [Google Scholar]
- Ikram, H.M.; Rasool, N.; Ahmad, G.; Chotana, G.A.; Musharraf, S.G.; Zubarir, M.; Rana, U.A.; Zia-ul-Haq, M.; Jaafar, H.Z. Selective C-Arylation of 2,5-Dibromo-3-hexylthiophene via Suzuki Cross Coupling Reaction and Their Pharmacological Aspects. Molecules 2015, 20, 5202–5214. [Google Scholar] [CrossRef] [PubMed]
- Ikram, H.M.; Rasool, N.; Zubair, M.; Khan, K.M.; Chotana, G.A.; Akhtar, M.N.; Abu, N.; Alitheen, N.B.; Elgorban, A.M.; Rana, U.A. Efficient Double Suzuki Cross-Coupling Reactions of 2,5-Dibromo-3-hexylthiophene: Anti-Tumor, Haemolytic, Anti-Thrombolytic and Biofilm Inhibition Studies. Molecules 2016, 21, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Qazi, F.; Zakir, H.; Asghar, S.; Abbas, G.; Riaz, M. Malus domestica Mediated Synthesis of Palladium Nanoparticles and Investigation of Their Catalytic Activity Towards the Suzuki Coupling Reactions. Nanosci. Nanotechnol. Lett. 2018, 10, 373–377. [Google Scholar] [CrossRef]
- Miller, S.L.; Chotana, G.A.; Fritz, J.A.; Chattopadhyay, B.; Maleczka, R.E., Jr.; Smith, M.R., III. C-H Borylation Catalysts that Distinguish between Similarly Sized Substituents like Fluorine and Hydrogen. Org. Lett. 2019, 21, 6388–6392. [Google Scholar] [CrossRef]
- Chotana, G.A.; Montero Bastidas, J.R.; Miller, S.L.; Smith, M.R., III.; Maleczka, R.E., Jr. One-Pot Iridium-Catalyzed C–H Borylation/Sonogashira Cross-Coupling: Access to Borylated Aryl Alkynes. Molecules 2020, 25, 1754–1766. [Google Scholar] [CrossRef]
- Ishiyama, T.; Takagi, J.; Yonekawa, Y.; Hartwig, J.F.; Miyaura, N. Iridium-Catalyzed Direct Borylation of Five-Membered Heteroarenes by Bis(pinacolato)diboron: Regioselective, Stoichiometric, and Room Temperature Reactions. Adv. Synth. Catal. 2003, 345, 1103–1106. [Google Scholar] [CrossRef]
- Billingsley, K.L.; Anderson, K.W.; Buchwald, S.L. A Highly Active Catalyst for Suzuki–Miyaura Cross-Coupling Reactions of Heteroaryl Compounds. Angew. Chem. Int. Ed. 2006, 45, 3484–3488. [Google Scholar] [CrossRef]
- Rieth, R.D.; Mankad, N.P.; Calimano, E.; Sadighi, J.P. Palladium-Catalyzed Cross-Coupling of Pyrrole Anions with Aryl Chlorides, Bromides, and Iodides. Org. Lett. 2004, 6, 3981–3983. [Google Scholar] [CrossRef]
- Farnier, M.; Soth, S.; Fournari, P. Recherches en série hétérocyclique. XXVIII. Synthèse de bipyrroles. Can. J. Chem. 1976, 54, 1083–1086. [Google Scholar]
- Castro, A.J.; Giannini, D.D.; Greenlee, W.F. Synthesis of a 2,3’-bipyrrole. Denitrosation in the Knorr pyrrole synthesis. J. Org. Chem. 1970, 35, 2815–2816. [Google Scholar]
- Dohi, T.; Morimoto, K.; Maruyama, A.; Kita, Y. Direct Synthesis of Bipyrroles Using Phenyliodine Bis(trifluoroacetate) with Bromotrimethylsilane. Org. Lett. 2006, 8, 2007–2010. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanwal, S.; Ann, N.-u.-; Fatima, S.; Emwas, A.-H.; Alazmi, M.; Gao, X.; Ibrar, M.; Zaib Saleem, R.S.; Chotana, G.A. Facile Synthesis of NH-Free 5-(Hetero)Aryl-Pyrrole-2-Carboxylates by Catalytic C–H Borylation and Suzuki Coupling. Molecules 2020, 25, 2106. https://doi.org/10.3390/molecules25092106
Kanwal S, Ann N-u-, Fatima S, Emwas A-H, Alazmi M, Gao X, Ibrar M, Zaib Saleem RS, Chotana GA. Facile Synthesis of NH-Free 5-(Hetero)Aryl-Pyrrole-2-Carboxylates by Catalytic C–H Borylation and Suzuki Coupling. Molecules. 2020; 25(9):2106. https://doi.org/10.3390/molecules25092106
Chicago/Turabian StyleKanwal, Saba, Noor-ul- Ann, Saman Fatima, Abdul-Hamid Emwas, Meshari Alazmi, Xin Gao, Maha Ibrar, Rahman Shah Zaib Saleem, and Ghayoor Abbas Chotana. 2020. "Facile Synthesis of NH-Free 5-(Hetero)Aryl-Pyrrole-2-Carboxylates by Catalytic C–H Borylation and Suzuki Coupling" Molecules 25, no. 9: 2106. https://doi.org/10.3390/molecules25092106
APA StyleKanwal, S., Ann, N.-u.-, Fatima, S., Emwas, A.-H., Alazmi, M., Gao, X., Ibrar, M., Zaib Saleem, R. S., & Chotana, G. A. (2020). Facile Synthesis of NH-Free 5-(Hetero)Aryl-Pyrrole-2-Carboxylates by Catalytic C–H Borylation and Suzuki Coupling. Molecules, 25(9), 2106. https://doi.org/10.3390/molecules25092106