Complexes of Cobalt(II) Iodide with Pyridine and Redox Active 1,2-Bis(arylimino)acenaphthene: Synthesis, Structure, Electrochemical, and Single Ion Magnet Properties
Abstract
:1. Introduction
2. Results and Discussion
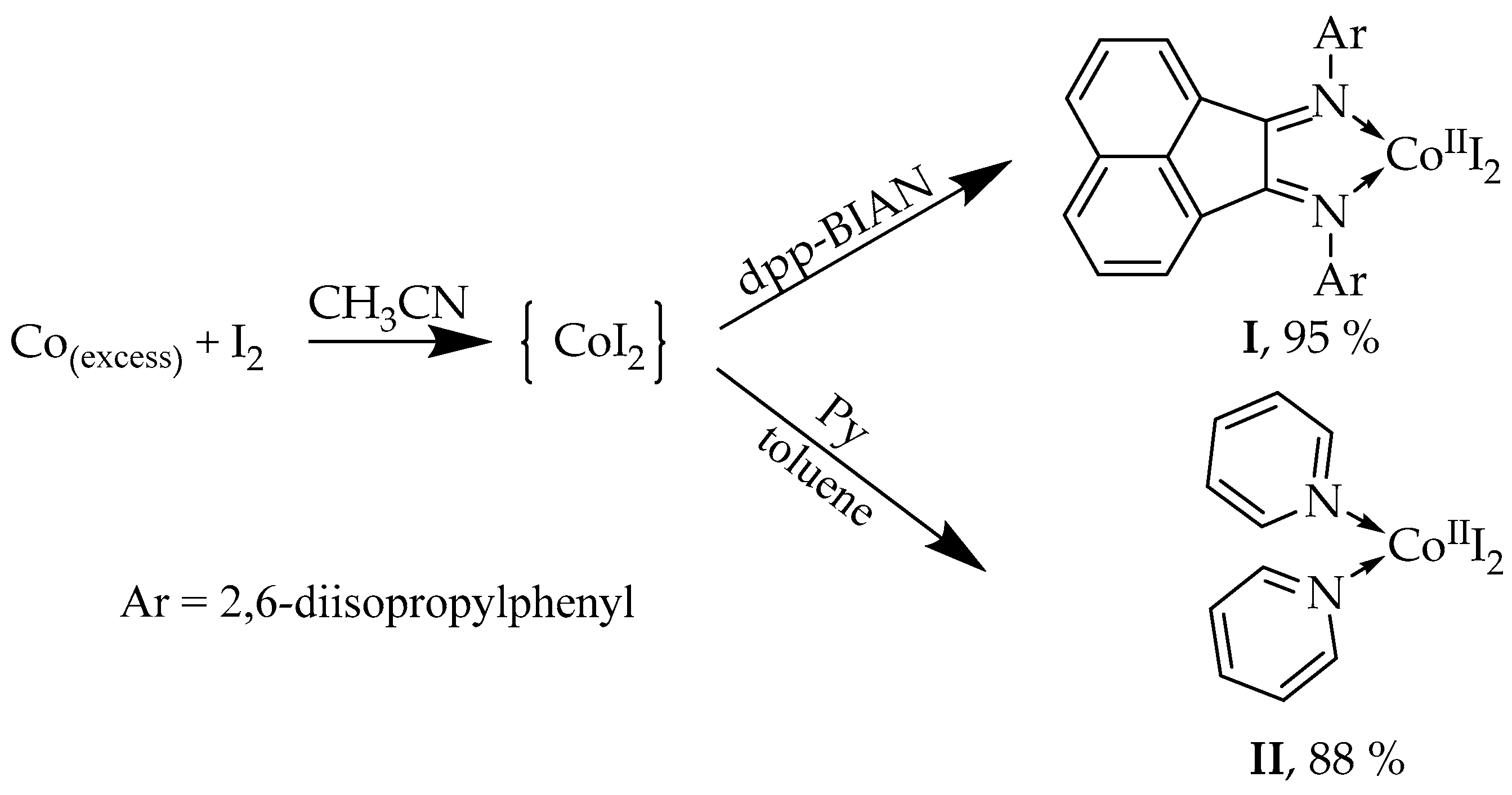
2.1. Synthesis and Characterization of [(dpp-BIAN)0CoIII2]·MeCN (I) and [(Py)2CoI2] (II)
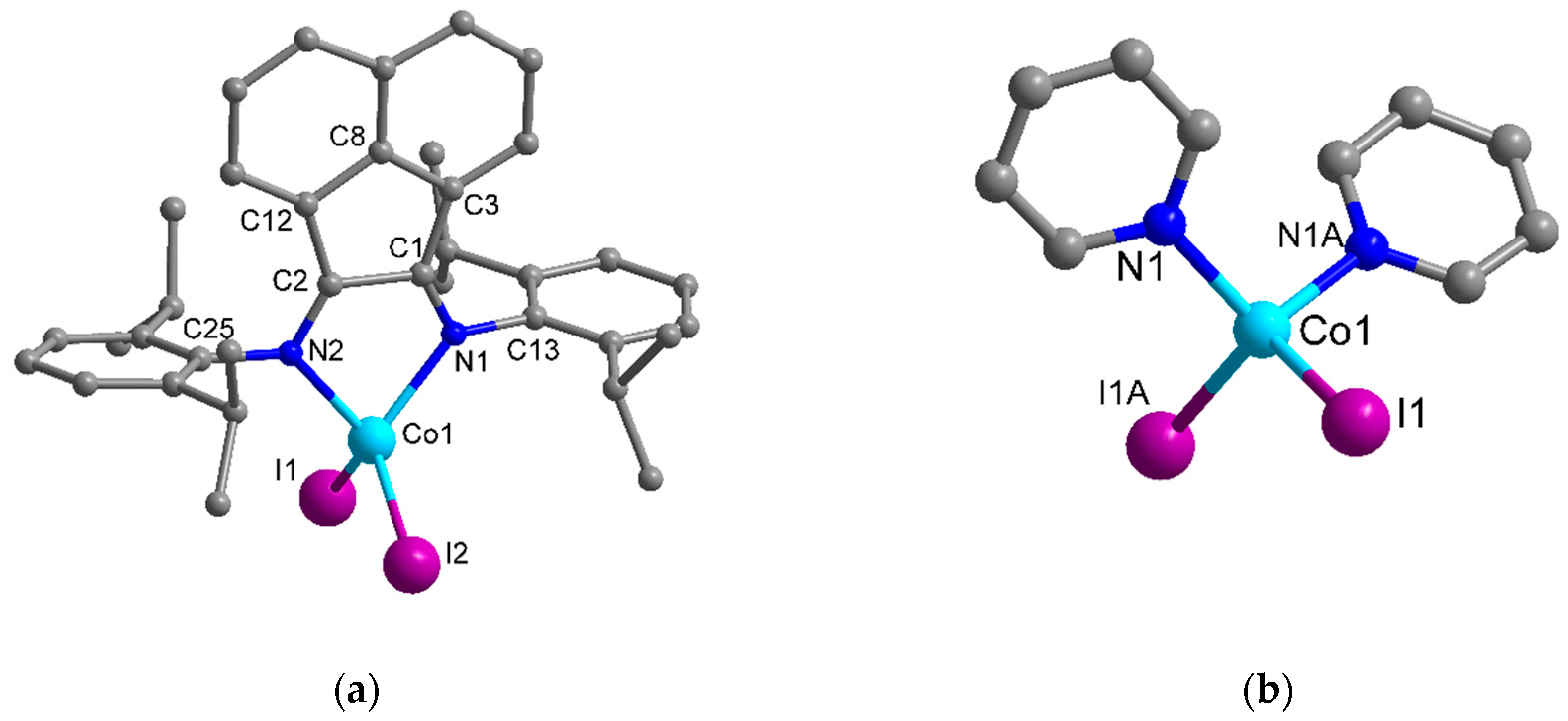
2.2. Molecular Structures
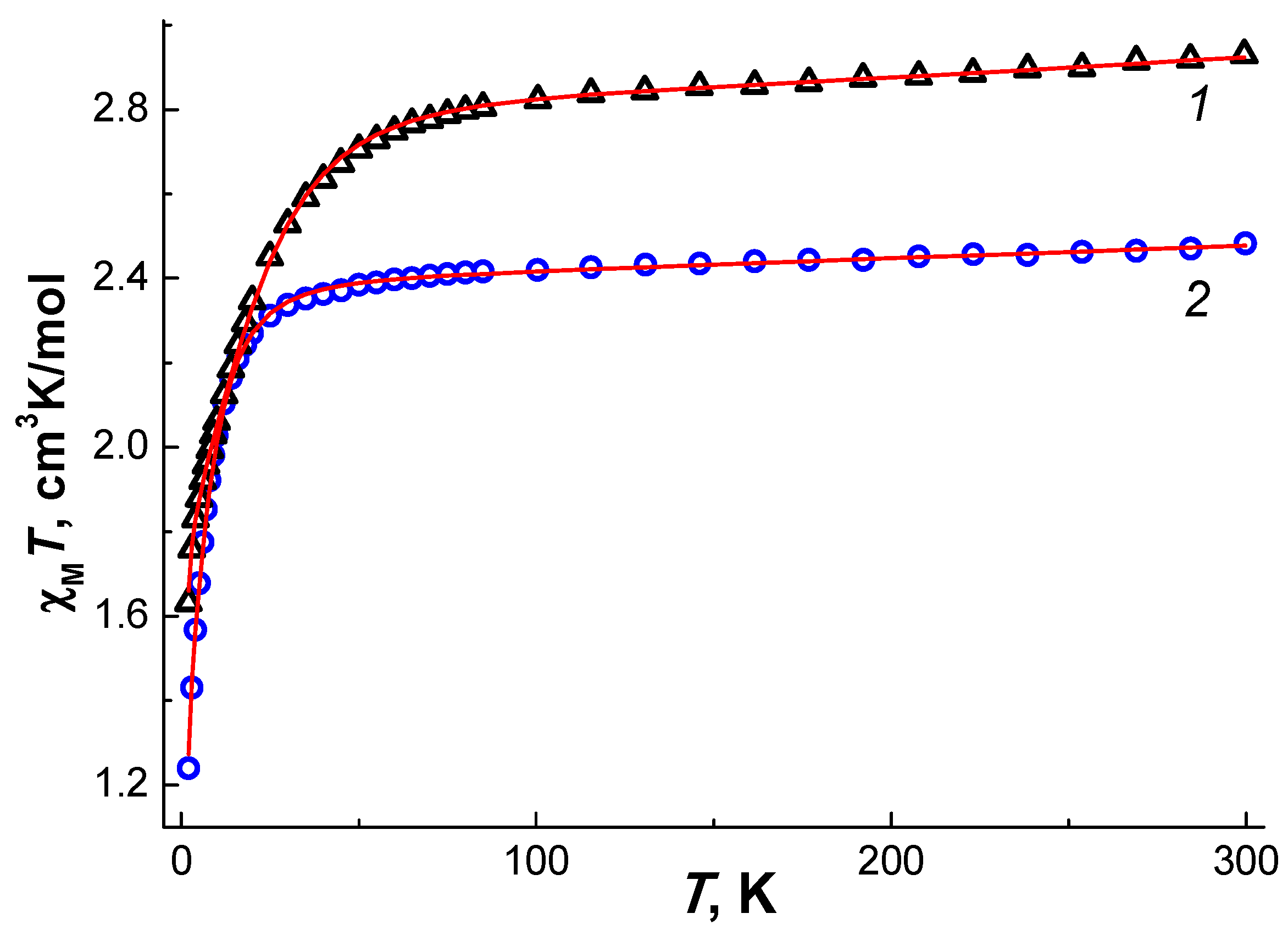
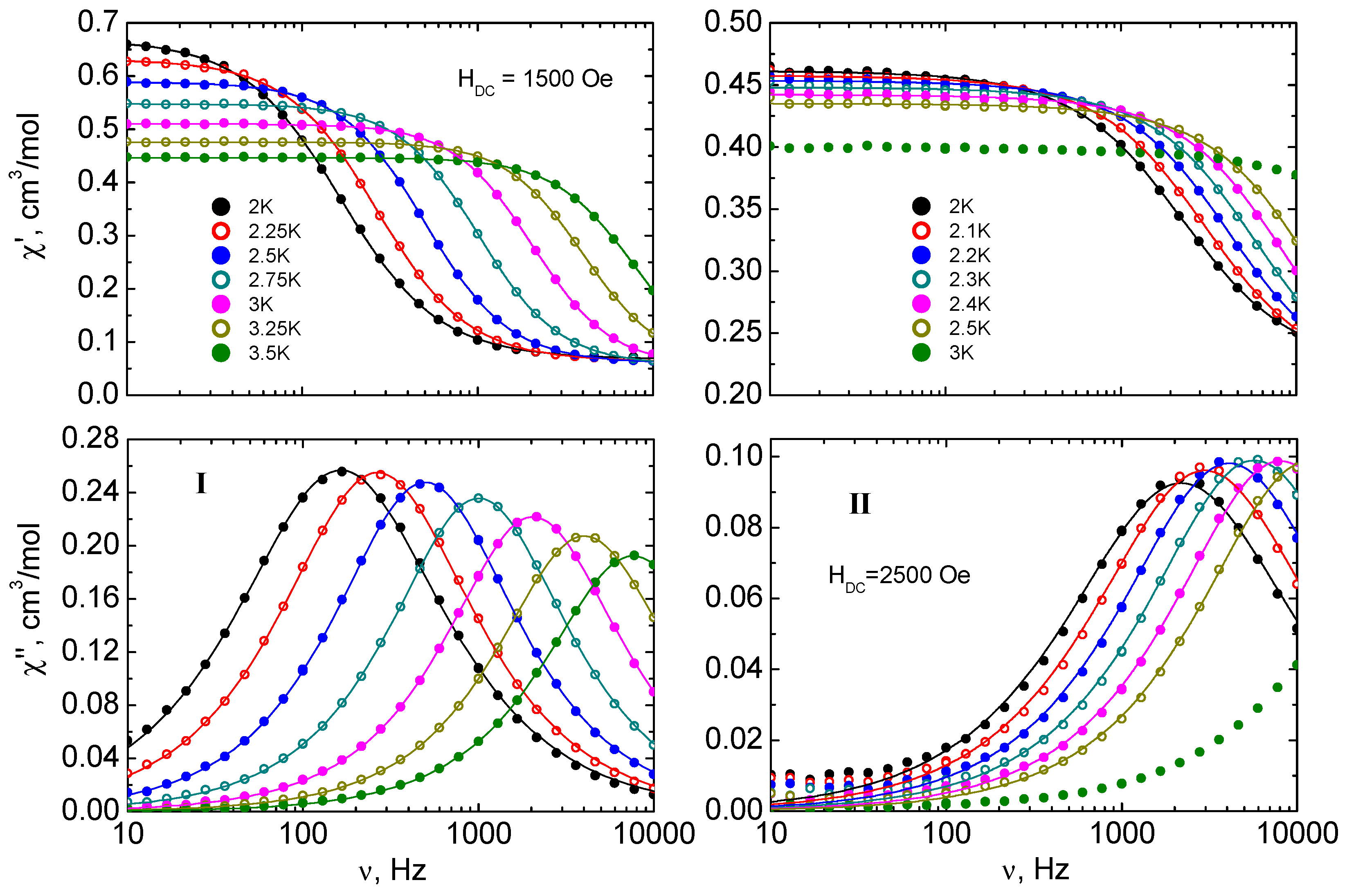
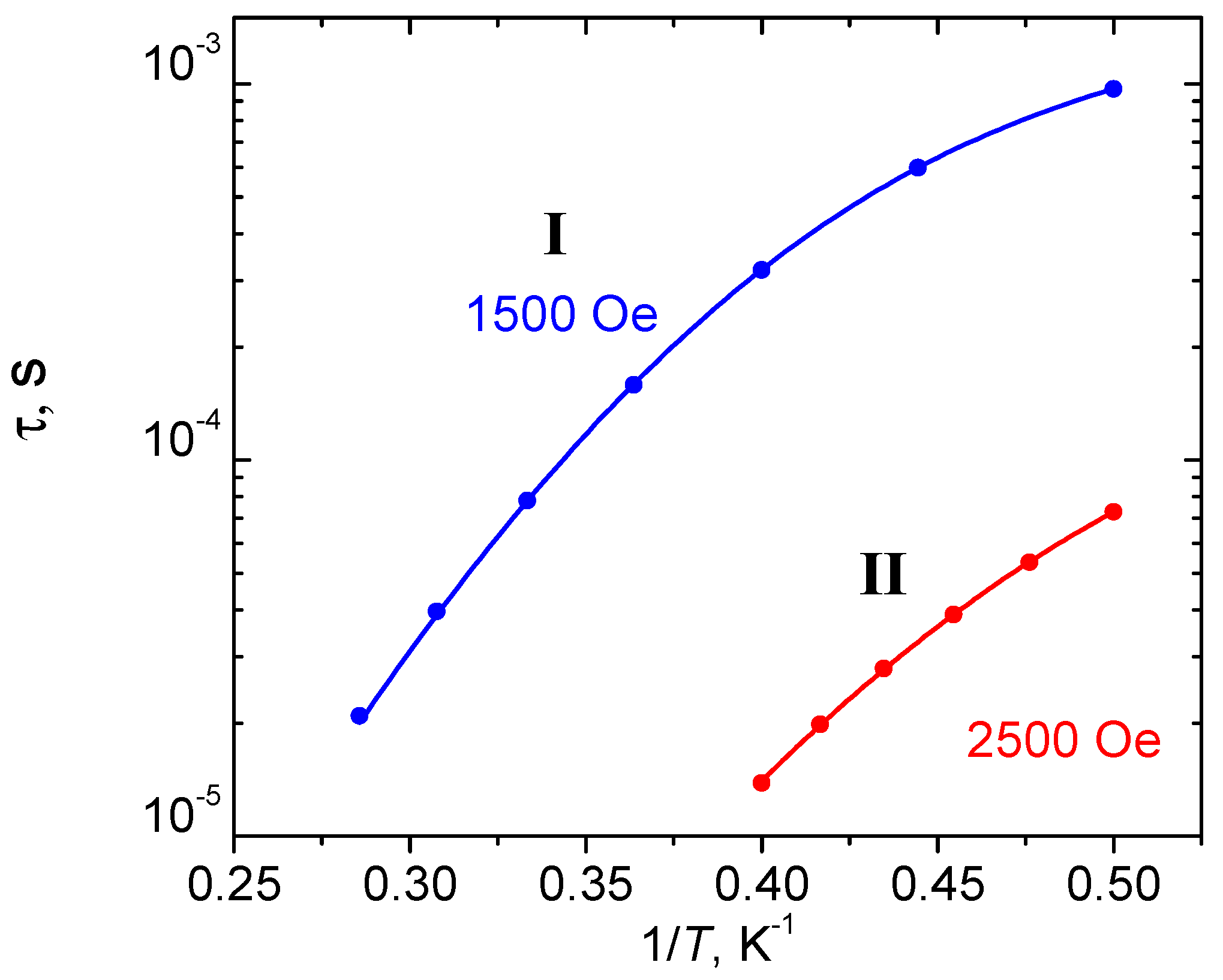
2.3. Solid State Magnetic Susceptibilities
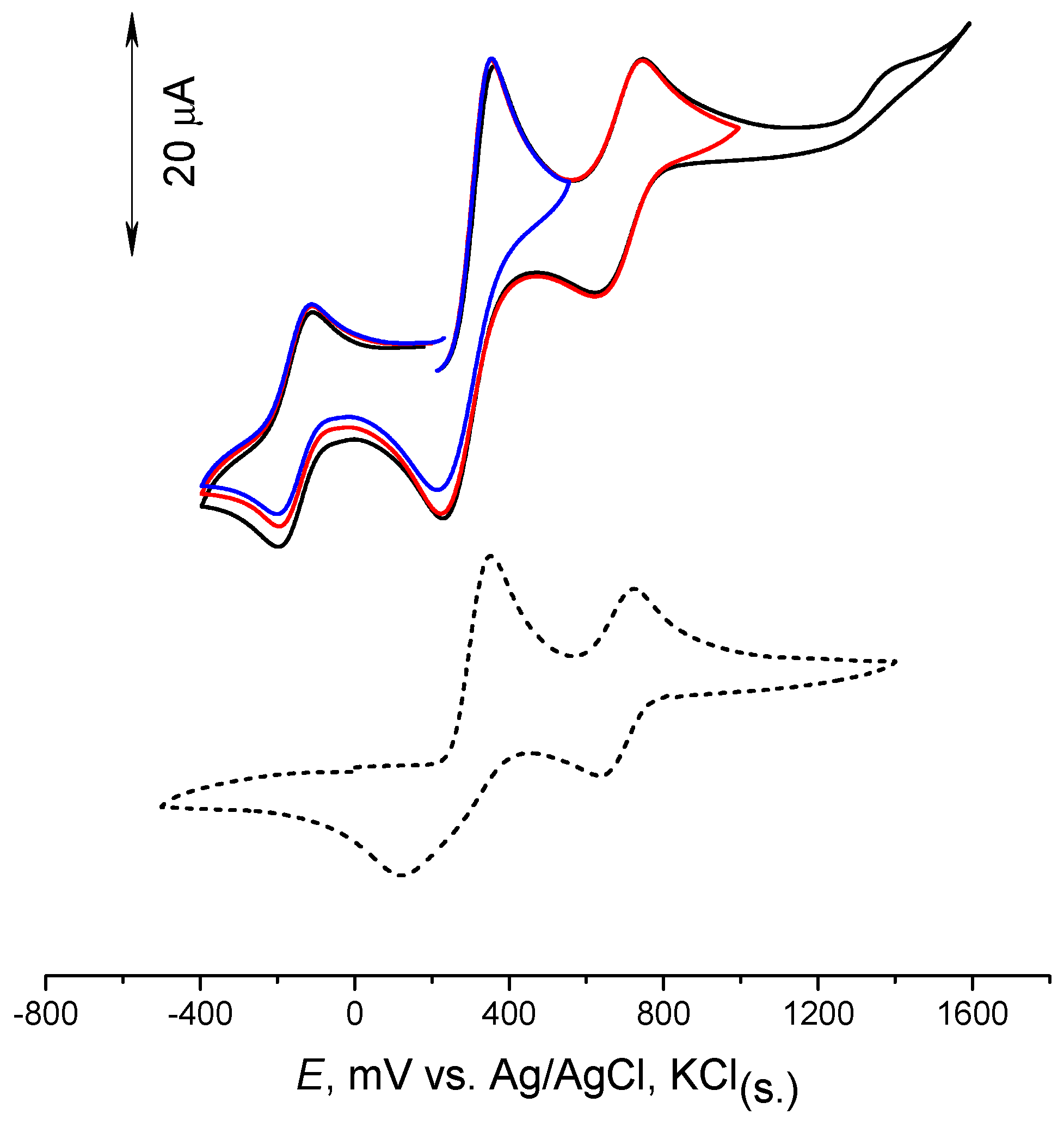
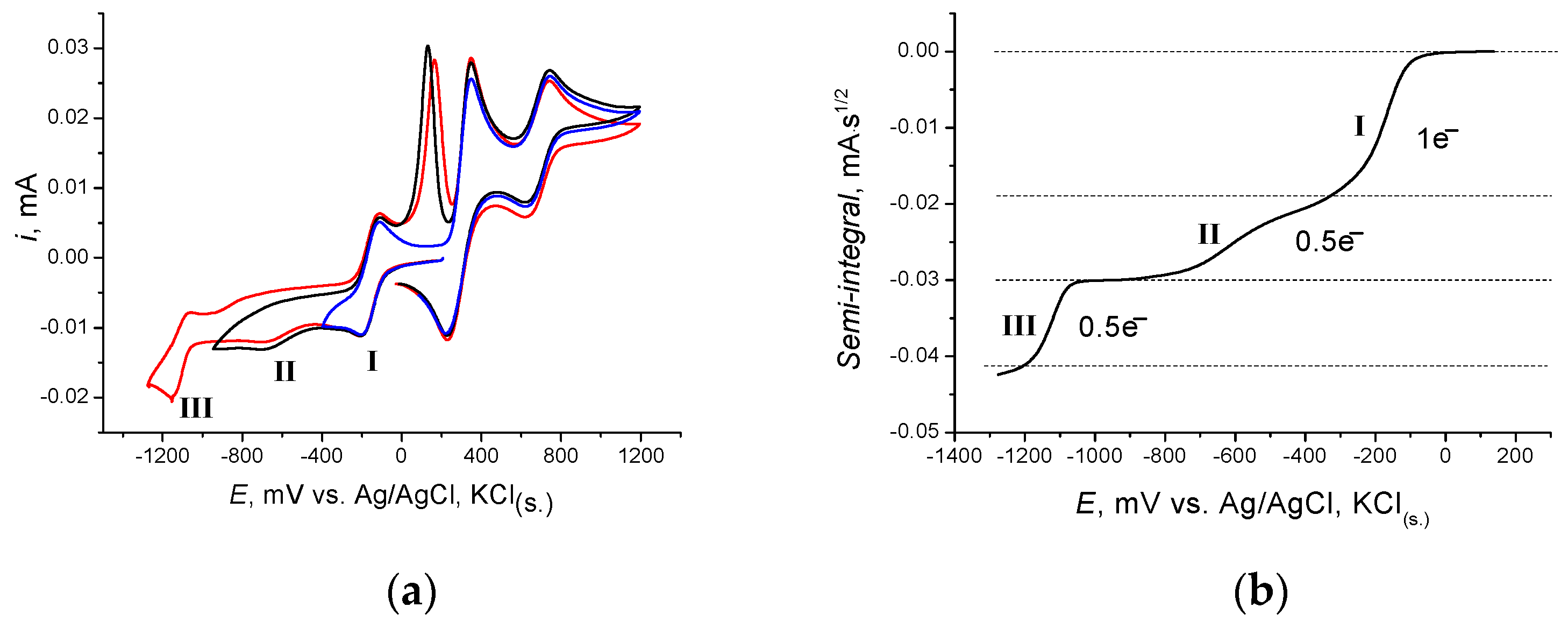
2.4. Cyclic voltammetry of [(dpp-BIAN)0CoIII2]·MeCN (I)
3. Materials and Methods
3.1. General Remarks
3.2. Synthesis of [(dpp-BIAN)0CoIII2]·MeCN (I)
3.3. Synthesis of [(Py)2CoI2] (II)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frost, J.M.; Harriman, K.; Murugesu, M. The rise of 3-d single-ion magnets in molecular magnetism: Towards materials from molecules? Chem. Sci. 2016, 7, 2470–2491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murrie, M. Cobalt(II) single-molecule magnets. Chem. Soc. Rev. 2010, 39, 1986–1995. [Google Scholar] [CrossRef]
- Tanaka, D.; Inose, T.; Tanaka, H.; Lee, S.; Ishikawa, N.; Ogawa, T. Proton-induced switching of the single molecule magnetic properties of a porphyrin based TbIII double-decker complex. Chem. Commun. 2012, 48, 7796–7798. [Google Scholar] [CrossRef]
- Horii, Y.; Horie, Y.; Katoh, K.; Breedlove, B.K.; Yamashita, M. Changing single-molecule magnet properties of a windmill-like distorted terbium(III) α-butoxy-substituted phthalocyaninato double-decker complex by protonation/deprotonation. Inorg. Chem. 2018, 57, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Damjanović, M.; Kamila, M.; Cosquer, G.; Breedlove, B.K.; Enders, M.; Yamashita, M. Proton Control of the lanthanoid single-ion magnet behavior of a double-decker complex with an indolenine-substituted annulene ligand. Inorg. Chem. 2017, 56, 6512–6521. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Cui, L.; Xie, J.-Z.; Leong, C.F.; D’Alessandro, D.M.; Zuo, J.-L. Functional coordination polymers based on redox-active tetrathiafulvalene and its derivatives. Coord. Chem. Rev. 2017, 345, 342–361. [Google Scholar] [CrossRef]
- Faust, T.B.; D’Alessandro, D.M. Radicals in metal–organic frameworks. RSC Adv. 2014, 4, 17498–17512. [Google Scholar] [CrossRef]
- Dorofeeva, V.N.; Pavlishchuk, A.V.; Kiskin, M.A.; Efimov, N.N.; Minin, V.V.; Lytvynenko, A.S.; Gavrilenko, K.S.; Kolotilov, S.V.; Novotortsev, V.M.; Eremenko, I.L. CoII Complexes with a tripyridine ligand, containing a 2,6-di-tert-butylphenolic fragment: synthesis, structure, and formation of stable radicals. ACS Omega 2019, 4, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Lv, L.; Chen, S.; You, M.; Fu, Z. Zn-MOF-based photoswitchable dyad that exhibits photocontrolled luminescence. Cryst. Growth Des. 2016, 16, 6705–6708. [Google Scholar] [CrossRef]
- Hong, J.; Zhuang, Y.; Ji, X.; Guo, X. A long-lived luminescence and EPR bimodal lanthanide-based probe for free radicals. Analyst 2011, 136, 2464–2470. [Google Scholar] [CrossRef] [Green Version]
- Ovcharenko, V.I.; Romanenko, G.V.; Maryunina, K.Y.; Bogomyakov, A.S.; Gorelik, E.V. Thermally induced magnetic anomalies in solvates of the bis(hexafluoroacetylacetonate)copper(II) complex with pyrazolyl-substituted nitronyl nitroxide. Inorg. Chem. 2008, 47, 9537–9552. [Google Scholar] [CrossRef] [PubMed]
- Fedin, M.; Veber, S.; Gromov, I.; Maryunina, K.; Fokin, S.; Romanenko, G.; Sagdeev, R.; Ovcharenko, V.; Bagryanskaya, E. Thermally induced spin transitions in nitroxide−copper(II)−nitroxide spin triads studied by EPR. Inorg. Chem. 2007, 46, 11405–11415. [Google Scholar] [CrossRef] [PubMed]
- Fedushkin, I.L.; Skatova, A.A.; Chudakova, V.A.; Fukin, G.K. Four-step reduction of dpp-bian with sodium metal: crystal structures of the sodium salts of the mono-, di-, tri- and tetraanions of dpp-bian. Angew. Chemie Int. Ed. 2003, 42, 3294–3298. [Google Scholar] [CrossRef] [PubMed]
- Sandl, S.; Maier, T.M.; van Leest, N.P.; Kröncke, S.; Chakraborty, U.; Demeshko, S.; Koszinowski, K.; de Bruin, B.; Meyer, F.; Bodensteiner, M.; et al. Cobalt-catalyzed hydrogenations via olefin cobaltate and hydride intermediates. ACS Catal. 2019, 9, 7596–7606. [Google Scholar] [CrossRef]
- Lima, G.; Nunes, E.; Dantas, R.; Simone, C.; Meneghetti, M.; Meneghetti, S. Catalytic behaviors of CoII and MnII compounds bearing α-diimine ligands for oxidative polymerization or drying oils. J. Braz. Chem. Soc. 2018, 29, 412–418. [Google Scholar] [CrossRef]
- Fomenko, I.S.; Gushchin, A.L.; Shul’pina, L.S.; Ikonnikov, N.S.; Abramov, P.A.; Romashev, N.F.; Poryvaev, A.S.; Sheveleva, A.M.; Bogomyakov, A.S.; Shmelev, N.Y.; et al. New oxidovanadium(IV) complex with a BIAN ligand: synthesis, structure, redox properties and catalytic activity. New J. Chem. 2018, 42, 16200–16210. [Google Scholar] [CrossRef]
- Singha Hazari, A.; Ray, R.; Hoque, M.A.; Lahiri, G.K. Electronic structure and multicatalytic features of redox-active bis(arylimino)acenaphthene (BIAN)-derived ruthenium complexes. Inorg. Chem. 2016, 55, 8160–8173. [Google Scholar] [CrossRef]
- Kee, J.W.; Ng, Y.Y.; Kulkarni, S.A.; Muduli, S.K.; Xu, K.; Ganguly, R.; Lu, Y.; Hirao, H.; Soo, H.S. Development of bis(arylimino)acenaphthene (BIAN) copper complexes as visible light harvesters for potential photovoltaic applications. Inorg. Chem. Front. 2016, 3, 651–662. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, C.; Wong, M.Y.; Cordes, D.B.; Slawin, A.M.Z.; Zysman-Colman, E. Cationic platinum(II) complexes bearing aryl-BIAN ligands: synthesis and structural and optoelectronic characterization. Organometallics 2015, 34, 13–22. [Google Scholar] [CrossRef] [Green Version]
- El-Ayaan, U.; Abdel-Aziz, A.A.-M. Synthesis, antimicrobial activity and molecular modeling of cobalt and nickel complexes containing the bulky ligand: bis[N-(2,6-diisopropylphenyl)imino] acenaphthene. Eur. J. Med. Chem. 2005, 40, 1214–1221. [Google Scholar] [CrossRef]
- Pelties, S.; Maier, T.; Herrmann, D.; de Bruin, B.; Rebreyend, C.; Gärtner, S.; Shenderovich, I.G.; Wolf, R. Selective P4 Activation by a highly reduced cobaltate: synthesis of dicobalt tetraphosphido complexes. Chem.–A Eur. J. 2017, 23, 6094–6102. [Google Scholar] [CrossRef]
- Fedushkin, I.L.; Makarov, V.M.; Sokolov, V.G.; Fukin, G.K. Acenaphthene-1,2-diimine chromium complexes. Dalton. Trans. 2009, 8047–8053. [Google Scholar] [CrossRef]
- Fedushkin, I.L.; Makarov, V.M.; Sokolov, V.G.; Fukin, G.K.; Maslov, M.O.; Ketkov, S.Y. Compounds of chromium, titanium, and zirconium with different reduced forms of acenaphthene-1,2-diimine. Russ. Chem. Bull. 2014, 63, 870–882. [Google Scholar] [CrossRef]
- Bendix, J.; Clark, K.M. Delocalization and valence tautomerism in vanadium tris(iminosemiquinone) complexes. Angew. Chemie Int. Ed. 2016, 55, 2748–2752. [Google Scholar] [CrossRef] [PubMed]
- Morozov, A.G.; Fedushkin, I.L.; Irran, E.; Grohmann, A. Titanium(IV) complexes supported by a dianionic acenaphthenediimine ligand: X-ray and spectroscopic studies of the metal coordination sphere. Inorg. Chem. Commun. 2018, 95, 50–55. [Google Scholar] [CrossRef]
- Abramov, P.A.; Dmitriev, A.A.; Kholin, K.V.; Gritsan, N.P.; Kadirov, M.K.; Gushchin, A.L.; Sokolov, M.N. Mechanistic study of the [(dpp-bian)Re(CO)3Br] electrochemical reduction using in situ EPR spectroscopy and computational chemistry. Electrochim. Acta 2018, 270, 526–534. [Google Scholar] [CrossRef]
- Romashev, N.F.; Gushchin, A.L.; Fomenko, I.S.; Abramov, P.A.; Mirzaeva, I.V.; Kompan’kov, N.B.; Kal’nyi, D.B.; Sokolov, M.N. A new organometallic rhodium(I) complex with dpp-bian ligand: Synthesis, structure and redox behaviour. Polyhedron 2019, 173, 114110. [Google Scholar] [CrossRef]
- Gushchin, A.L.; Romashev, N.F.; Shmakova, A.A.; Abramov, P.A.; Ryzhikov, M.R.; Fomenko, I.S.; Sokolov, M.N. Novel redox active rhodium(III) complex with bis(arylimino)acenaphthene ligand: synthesis, structure and electrochemical studies. Mendeleev Commun. 2020, 30, 81–83. [Google Scholar] [CrossRef]
- Saber, M.R.; Dunbar, K.R. Ligands effects on the magnetic anisotropy of tetrahedral cobalt complexes. Chem. Commun. 2014, 50, 12266–12269. [Google Scholar] [CrossRef]
- Kansikas, J.; Leskelä, M.; Kenessey, G.; Werner, P.-E.; Liptay, G.; Balzarini, J.; Fransson, B.; Ragnarsson, U.; Francis, G. Preparation and characterization of 2,6- and 3,5-dimethylpyridine complexes of cobalt(II) halides; the crystal structure of di(2,6-dimethylpyridinium) tetrachlorocobaltate(II) and dichlorotetrakis(3,5-dimethylpyridine)cobalt(II). Acta Chem. Scand. 1994, 48, 951–959. [Google Scholar] [CrossRef]
- Belén Lago, A.; Amoedo, A.; Carballo, R.; García-Martínez, E.; Vázquez-López, E.M. Metal coordination and in situ S–C bond cleavage of the bis(2-pyridylthio)methane ligand. Dalton. Trans. 2010, 39, 10076–10087. [Google Scholar] [CrossRef] [PubMed]
- Stauber, J.M.; Wadler, A.L.; Moore, C.E.; Rheingold, A.L.; Figueroa, J.S. Coordination properties of 2,5-dimesitylpyridine: an encumbering and versatile ligand for transition-metal chemistry. Inorg. Chem. 2011, 50, 7309–7316. [Google Scholar] [CrossRef]
- Smith, P.W.; Moore, C.E.; Rheingold, A.L.; Figueroa, J.S. Coordination and structural properties of encumbering 6-mesityl-2-picolinate complexes. Dalton. Trans. 2012, 41, 8031–8038. [Google Scholar] [CrossRef] [PubMed]
- Smolko, L.; Černák, J.; Dušek, M.; Miklovič, J.; Titiš, J.; Boča, R. Three tetracoordinate Co(II) complexes [Co(biq)X2] (X = Cl, Br, I) with easy-plane magnetic anisotropy as field-induced single-molecule magnets. Dalt. Trans. 2015, 44, 17565–17571. [Google Scholar] [CrossRef] [PubMed]
- Smolko, L.; Černák, J.; Dušek, M.; Titiš, J.; Boča, R. Tetracoordinate Co(II) complexes containing bathocuproine and single molecule magnetism. New J. Chem. 2016, 40, 6593–6598. [Google Scholar] [CrossRef]
- Smolko, L.; Černák, J.; Kuchár, J.; Rajnák, C.; Titiš, J.; Boča, R. Field-induced slow magnetic relaxation in mononuclear tetracoordinate cobalt(II) complexes containing a neocuproine ligand. Eur. J. Inorg. Chem. 2017, 2017, 3080–3086. [Google Scholar] [CrossRef]
- Perry, D.L.; Phillips, S.L. Handbook of Inorganic Compounds; Taylor & Francis: Boca Raton, FL, USA, 1995; ISBN 9780849386718. [Google Scholar]
- Paulovicova, A.; El-Ayaan, U.; Shibayama, K.; Morita, T.; Fukuda, Y. Mixed-ligand copper(II) complexes with the rigid bidentate bis(n-arylimino)acenaphthene ligand: Synthesis, spectroscopic-, and X-ray structural characterization. Eur. J. Inorg. Chem. 2001, 2001, 2641–2646. [Google Scholar] [CrossRef]
- Rosa, V.; Carabineiro, S.A.; Aviles, T.; Gomes, P.T.; Welter, R.; Campos, J.M.; Ribeiro, M.R. Synthesis, characterization and solid state structures of α-diimine cobalt(II) complexes: Ethylene polymerization tests. J. Organomet. Chem. 2008, 693, 769–775. [Google Scholar] [CrossRef]
- Fedushkin, I.L.; Skatova, A.A.; Yambulatov, D.S.; Cherkasov, A.V.; Demeshko, S.V. Europium complexes with 1,2-bis(arylimino)acenaphthenes: A search for redox isomers. Russ. Chem. Bull. 2015, 64, 38–43. [Google Scholar] [CrossRef]
- Skatova, A.A.; Yambulatov, D.S.; Fedyushkin, I.L.; Baranov, E.V. Europium and ytterbium complexes with the redox active acenaphthene-1,2-diimine ligand. Russ. J. Coord. Chem. 2018, 44, 400–409. [Google Scholar] [CrossRef]
- Rosa, V.; González, P.; Avilés, T.; Gomes, P.; Welter, R.; Rizzi, A.; Passeggi, M.; Brondino, C. Synthesis, solid-state structures, and EPR spectroscopic studies on polycrystalline and single-crystal samples of α-diimine cobalt(II) complexes. Eur. J. Inorg. Chem. 2006, 4761–4769. [Google Scholar] [CrossRef]
- Hantzsch, A. Solvatation und Komplexbildung als Ursache des Farbenwechsels der Kobaltohaloide. Zeitschrift für Anorg. und Allg. Chemie 1927, 159, 273–303. [Google Scholar] [CrossRef]
- Little, B.F.; Long, G.J. Moessbauer, electronic, and structural properties of several bis- and tetrakis(pyridine)iron(II) complexes. Inorg. Chem. 1978, 17, 3401–3413. [Google Scholar] [CrossRef]
- Bushuev, M.B.; Krivopalov, V.P.; Peresypkina, E.V.; Virovets, A.V.; Shvedenkov, Y.G.; Sheludyakova, L.A.; Semikolenova, N.V.; Zakharov, V.A.; Larionov, S.V. Complexes of copper(II) and cobalt(II) halides with 4-(3,5-dimethyl-1H-pyrazol-1-yl)-6-methyl-2-phenylpyrimidine: Synthesis, structures, and properties. Russ. J. Coord. Chem. 2009, 35, 597–608. [Google Scholar] [CrossRef]
- Bushuev, M.B.; Virovets, A.V.; Peresypkina, E.V.; Naumov, D.Y.; Potapov, A.S.; Khlebnikov, A.I.; Vasilevsky, S.F.; Lavrenova, L.G. Synthesis and structure of (bis(3,5-dimethyl-1H-pyrazol-1-yl)methane)diiodocobalt(II). J. Struct. Chem. 2005, 46, 1099–1103. [Google Scholar] [CrossRef]
- El-Ayaan, U.; Paulovicova, A.; Yamada, S.; Fukuda, Y. The crystal structure of bis[n-(2,6-diisopropylphenyl)imino] acenaphthene and studies of its copper(I) and copper(II) complexes. J. Coord. Chem. 2003, 56, 373–381. [Google Scholar] [CrossRef]
- Zhou, M.; Li, X.; Bu, D.; Lei, H. Synthesis, crystal structures and electrochemical properties of Co(II) and Mn(II) complexes with asymmetric bulky BIAN ligands. Polyhedron 2018, 148, 88–99. [Google Scholar] [CrossRef]
- Alnajrani, M.N.; Alshmimri, S.A.; Alsager, O.A. Alpha and beta diimine cobalt complexes in isoprene polymerization: a comparative study. RSC Adv. 2016, 6, 113803–113814. [Google Scholar] [CrossRef]
- Supej, M.J.; Volkov, A.; Darko, L.; West, R.A.; Darmon, J.M.; Schulz, C.E.; Wheeler, K.A.; Hoyt, H.M. Aryl-substituted BIAN complexes of iron dibromide: Synthesis, X-ray and electronic structure, and catalytic hydrosilylation activity. Polyhedron 2016, 114, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Wekesa, F.S.; Arias-Ugarte, R.; Kong, L.; Sumner, Z.; McGovern, G.P.; Findlater, M. Iron-catalyzed hydrosilylation of aldehydes and ketones under solvent-free conditions. Organometallics 2015, 34, 5051–5056. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH Publ. Inc.: New York, NY, USA, 1993; p. 393. [Google Scholar]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, A.A.; Nelyubina, Y.V.; Kats, S.V.; Penkova, L.V.; Efimov, N.N.; Dmitrienko, A.O.; Vologzhanina, A.V.; Belov, A.S.; Voloshin, Y.Z.; Novikov, V.V. Polymorphism in a cobalt-based single-ion magnet tuning its barrier to magnetization relaxation. J. Phys. Chem. Lett. 2016, 7, 4111–4116. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Cutsail III, G.E.; Aravena, D.; Amoza, M.; Rouzières, M.; Dechambenoit, P.; Losovyj, Y.; Pink, M.; Ruiz, E.; Clérac, R.; et al. A low spin manganese(IV) nitride single molecule magnet. Chem. Sci. 2016, 7, 6132–6140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polyzou, C.D.; Koumousi, E.S.; Lada, Z.G.; Raptopoulou, C.P.; Psycharis, V.; Rouzières, M.; Tsipis, A.C.; Mathonière, C.; Clérac, R.; Perlepes, S.P. “Switching on” the single-molecule magnet properties within a series of dinuclear cobalt(III)–dysprosium(III) 2-pyridyloximate complexes. Dalton. Trans. 2017, 46, 14812–14825. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Yalymov, A.I.; Korlyukov, A.A.; Long, J.; Larionova, J.; Guari, Y.; Zubavichus, Y.V.; Trigub, A.L.; Shubina, E.S.; Eremenko, I.L.; et al. Heterometallic Na6Co3 phenylsilsesquioxane exhibiting slow dynamic behavior in its magnetization. Chem.—A Eur. J. 2015, 21, 18563–18565. [Google Scholar] [CrossRef] [PubMed]
- Petrosyants, S.P.; Ilyukhin, A.B.; Efimov, N.N.; Gavrikov, A.V.; Novotortsev, V.M. Self-assembly and SMM properties of lanthanide cyanocobaltate chain complexes with terpyridine as blocking ligand. Inorganica Chim. Acta 2018, 482, 813–820. [Google Scholar] [CrossRef]
- Mamontova, E.; Long, J.; Ferreira, A.S.R.; Botas, M.P.A.; Luneau, D.; Guari, Y.; Carlos, D.L.; Larionova, J. Magneto-luminescence correlation in the textbook Dysprosium(III) nitrate single-ion magnet. Magnetochemistry 2016, 2, 41. [Google Scholar] [CrossRef]
- Gavrikov, A.V.; Koroteev, P.S.; Efimov, N.N.; Dobrokhotova, Z.V.; Ilyukhin, A.B.; Kostopoulos, A.K.; Ariciu, A.-M.; Novotortsev, V.M. Novel mononuclear and 1D-polymeric derivatives of lanthanides and (η6-benzoic acid)tricarbonylchromium: synthesis, structure and magnetism. Dalton. Trans. 2017, 46, 3369–3380. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Lin, H.; Huo, C.; Zhang, W.; Lü, J.; Yang, L.; Zhao, G.; Liu, Z.-L. Cation radical imino Diels-Alder reaction: A new approach for the synthesis of tetrahydroquinolines. Synlett 2003, 2003, 1707–1709. [Google Scholar] [CrossRef]
- Lukoyanov, A.N.; Ulivanova, E.A.; Razborov, D.A.; Khrizanforova, V.V.; Budnikova, Y.H.; Makarov, S.G.; Rumyantcev, R.V.; Ketkov, S.Y.; Fedushkin, I.L. One-electron reduction of 2-Mono(2,6-diisopropylphenylimino)acenaphthene-1-one (dpp-mian). Chem.—A Eur. J. 2019, 25, 3858–3866. [Google Scholar] [CrossRef]
- Beloglazkina, E.K.; Yudin, I.V.; Majouga, A.G.; Moiseeva, A.A.; Tursina, A.I.; Zyk, N.V. Synthesis and electrochemical study of 2-(2-pyridyl)benzothiazole complexes with transition metals (CoII, NiII, and CuII). Molecular structure of aquabis [2-(2-pyridyl)benzothiazole]copper(II) diperchlorate. Russ. Chem. Bull. 2006, 55, 1803–1809. [Google Scholar] [CrossRef]
- Khrizanforova, V.V.; Morozov, V.I.; Khrizanforov, M.N.; Lukoyanov, A.N.; Kataeva, O.N.; Fedushkin, I.L.; Budnikova, Y.H. Iron complexes of BIANs: Redox trends and electrocatalysis of hydrogen evolution. Polyhedron 2018, 154, 77–82. [Google Scholar] [CrossRef]
- SMART (control) and SAINT (integration) Software; Version 5.0.; Madison Bruker AXS Inc. Bruker: Fitchburg, WI, USA, 1997.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roos, B.O.; Taylor, P.R.; Sigbahn, P.E.M. A complete active space SCF method (CASSCF) using a density matrix formulated super-CI approach. Chem. Phys. 1980, 48, 157–173. [Google Scholar] [CrossRef]
- Siegbahn, P.; Heiberg, A.; Roos, B.; Levy, B. A comparison of the super-CI and the Newton-Raphson scheme in the complete active space SCF method. Phys. Scr. 1980, 21, 323–327. [Google Scholar] [CrossRef]
- Siegbahn, P.E.M.; Almlöf, J.; Heiberg, A.; Roos, B.O. The complete active space SCF (CASSCF) method in a Newton–Raphson formulation with application to the HNO molecule. J. Chem. Phys. 1981, 74, 2384–2396. [Google Scholar] [CrossRef]
- Angeli, C.; Cimiraglia, R.; Evangelisti, S.; Leininger, T.; Malrieu, J.-P. Introduction of n-electron valence states for multireference perturbation theory. J. Chem. Phys. 2001, 114, 10252–10264. [Google Scholar] [CrossRef]
- Angeli, C.; Cimiraglia, R.; Malrieu, J.-P. n-electron valence state perturbation theory: A spinless formulation and an efficient implementation of the strongly contracted and of the partially contracted variants. J. Chem. Phys. 2002, 117, 9138–9153. [Google Scholar] [CrossRef]
- Hess, B.A. Relativistic electronic-structure calculations employing a two-component no-pair formalism with external-field projection operators. Phys. Rev. A 1986, 33, 3742–3748. [Google Scholar] [CrossRef] [Green Version]
- Pantazis, D.A.; Chen, X.-Y.; Landis, C.R.; Neese, F. All-electron scalar relativistic basis sets for third-row transition metal atoms. J. Chem. Theory Comput. 2008, 4, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Neese, F. An improvement of the resolution of the identity approximation for the formation of the Coulomb matrix. J. Comput. Chem. 2003, 24, 1740–1747. [Google Scholar] [CrossRef]
- Ganyushin, D.; Neese, F. First-principles calculations of zero-field splitting parameters. J. Chem. Phys. 2006, 125, 24103. [Google Scholar] [CrossRef]
- Neese, F. Efficient and accurate approximations to the molecular spin-orbit coupling operator and their use in molecular g-tensor calculations. J. Chem. Phys. 2005, 122, 34107. [Google Scholar] [CrossRef]
- Maurice, R.; Bastardis, R.; de Graaf, C.; Suaud, N.; Mallah, T.; Guihéry, N. Universal Theoretical Approach to Extract Anisotropic Spin Hamiltonians. J. Chem. Theory Comput. 2009, 5, 2977–2984. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
Sample Availability: Samples of the Cobalt(II) compounds are available from the authors. |
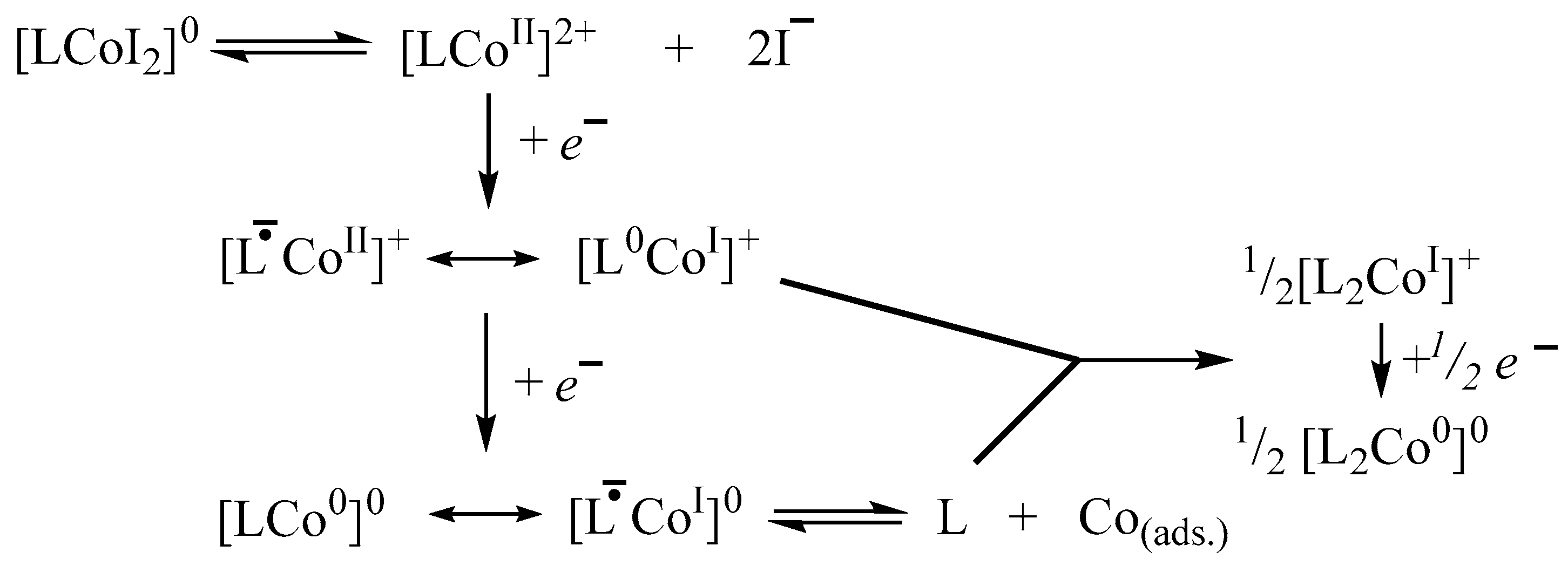
| Parameter | Value | |
|---|---|---|
| I | II | |
| Molecular formula | C38H43CoI2N3 | C10H10CoI2N2 |
| Mw | 854.48 | 470.93 |
| T, K | 296(2) | |
| Crystal system | Orthorhombic | |
| Space group | Pbca | Pbcn |
| a, Å | 19.5759(4) | 13.488(2) |
| b, Å | 19.3545(4) | 7.1497(10) |
| c, Å | 19.7703(5) | 14.588(2) |
| V, Å3 | 7490.6(3) | 1406.7(3) |
| Z | 8 | 4 |
| ρcalcd, g cm−3 | 1.515 | 2.224 |
| μ, mm−1 | 2.135 | 5.582 |
| θ range, deg | 2.06–28.28 | 2.79–30.59 |
| F(000) | 3400 | 868 |
| Index range | −26 ≤ h ≤ 25; −25 ≤ k ≤ 25; −26 ≤ l ≤ 25 | −19 ≤ h ≤ 19; −10 ≤ k ≤ 10; −20 ≤ l ≤ 20 |
| Number of reflections collected | 75256 | 15566 |
| Number of unique reflections | 9291 | 2160 |
| Rint | 0.099 | 0.162 |
| Number of reflections with I > 2σ(I) | 6692 | 1030 |
| GooF | 1.051 | 1.000 |
| R factor on F2 > 2σ(F2) | R1 = 0.062, wR2 = 0.140 | R1 = 0.074, wR2 = 0.148 |
| R factor (all data) | R1 = 0.091, wR2 = 0.155 | R1 = 0.157, wR2 = 0.182 |
| Δρmax/Δρmin, e/Å3 | −1.113/0.837 | −1.135/0.966 |
| Complex/ Parameter | Best-Fit Parameters with Equation (1) | CASSCF/NEVPT2 Calculation | ||||
|---|---|---|---|---|---|---|
| D, cm−1 | giso | zJ, cm−1 | D, cm−1 | E/D | g | |
| I | −25.7(3) | 2.481(1) | 0 | −22.6 | 0.294 | gx = 2.251 gy = 2.331 gz = 2.515 (giso = 2.366) |
| 24.6(3) | 2.481(1) | 0 | ||||
| II | −8.2(2) | 2.285(1) | −0.07(1) | −5.9 | 0.094 | gx = 2.248 gy = 2.272 gz = 2.333 (giso = 2.284) |
| 7.6(2) | 2.287(1) | −0.09(1) | ||||
| State | Ms | I (calc) | I (HDC = 0 Oe) | I (HDC = 1.5 kOe) | II (calc) | II (HDC = 0 Oe) | II (HDC = 2.5 kOe) |
|---|---|---|---|---|---|---|---|
| 1 | −3/2 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | +3/2 | 0 | 0 | 0.53 | 0 | 0 | 0.92 |
| 3 | −1/2 | 50.58 | 51.4 | 51.58 | 11.98 | 16.40 | 16.67 |
| 4 | +1/2 | 50.58 | 51.4 | 51.75 | 11.98 | 16.40 | 16.94 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yambulatov, D.S.; Nikolaevskii, S.A.; Kiskin, M.A.; Magdesieva, T.V.; Levitskiy, O.A.; Korchagin, D.V.; Efimov, N.N.; Vasil’ev, P.N.; Goloveshkin, A.S.; Sidorov, A.A.; et al. Complexes of Cobalt(II) Iodide with Pyridine and Redox Active 1,2-Bis(arylimino)acenaphthene: Synthesis, Structure, Electrochemical, and Single Ion Magnet Properties. Molecules 2020, 25, 2054. https://doi.org/10.3390/molecules25092054
Yambulatov DS, Nikolaevskii SA, Kiskin MA, Magdesieva TV, Levitskiy OA, Korchagin DV, Efimov NN, Vasil’ev PN, Goloveshkin AS, Sidorov AA, et al. Complexes of Cobalt(II) Iodide with Pyridine and Redox Active 1,2-Bis(arylimino)acenaphthene: Synthesis, Structure, Electrochemical, and Single Ion Magnet Properties. Molecules. 2020; 25(9):2054. https://doi.org/10.3390/molecules25092054
Chicago/Turabian StyleYambulatov, Dmitriy S., Stanislav A. Nikolaevskii, Mikhail A. Kiskin, Tatiana V. Magdesieva, Oleg A. Levitskiy, Denis V. Korchagin, Nikolay N. Efimov, Pavel N. Vasil’ev, Alexander S. Goloveshkin, Alexey A. Sidorov, and et al. 2020. "Complexes of Cobalt(II) Iodide with Pyridine and Redox Active 1,2-Bis(arylimino)acenaphthene: Synthesis, Structure, Electrochemical, and Single Ion Magnet Properties" Molecules 25, no. 9: 2054. https://doi.org/10.3390/molecules25092054







