Anti-osteoarthritic Effects of an Herbal Composition LI73014F2 on Interleukin-1β-induced Primary Human Articular Chondrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Individual Extracts and LI73014F2
2.2. Chemicals and Reagents
2.3. Culture and Treatment of Human Articular Chondrocytes
2.4. Cell Viability Assay
2.5. Protein Extraction and Western Blot Analysis
2.6. Statistical Analysis
3. Results
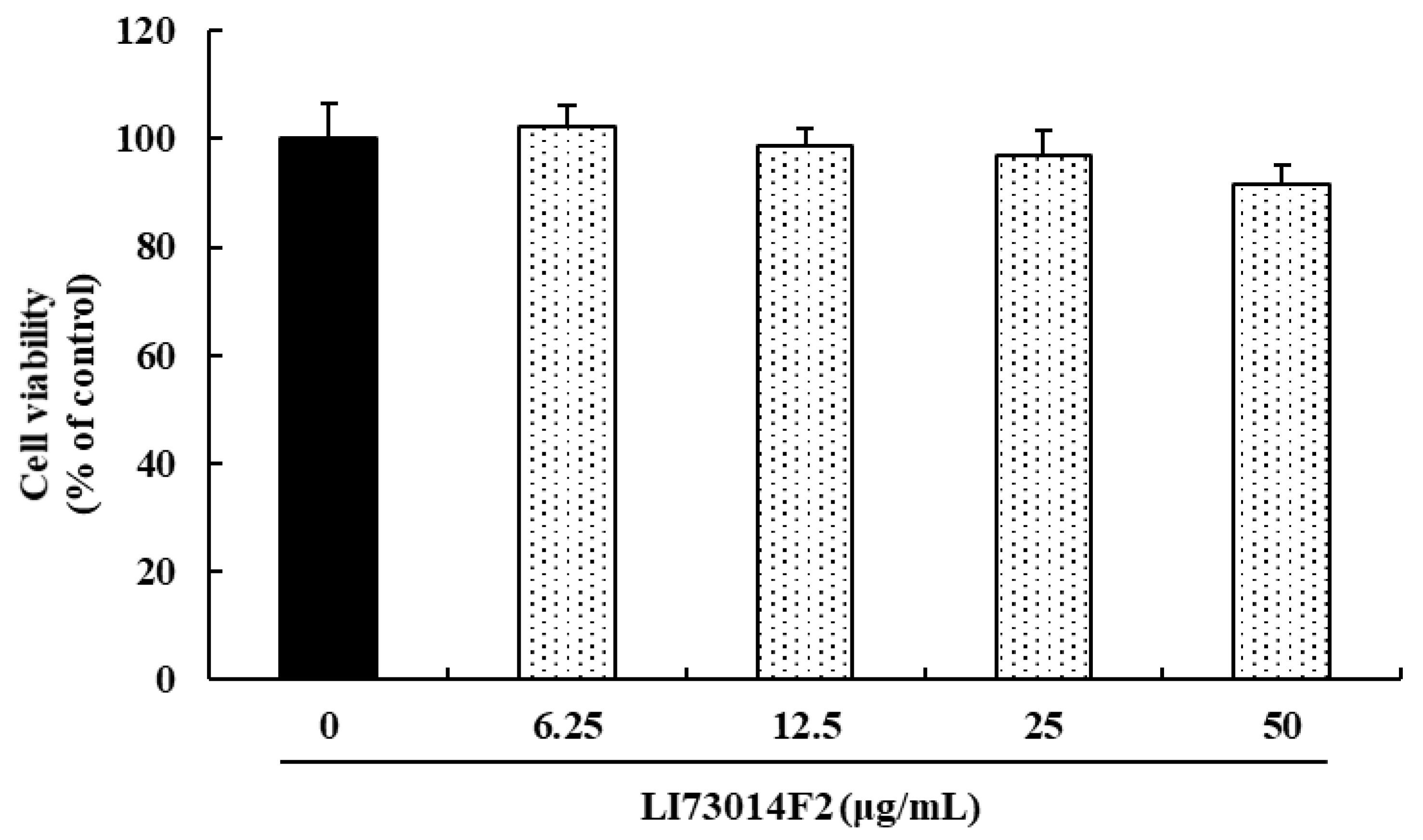
3.1. Effect of LI73014F2 on the Viability of HCH Cells
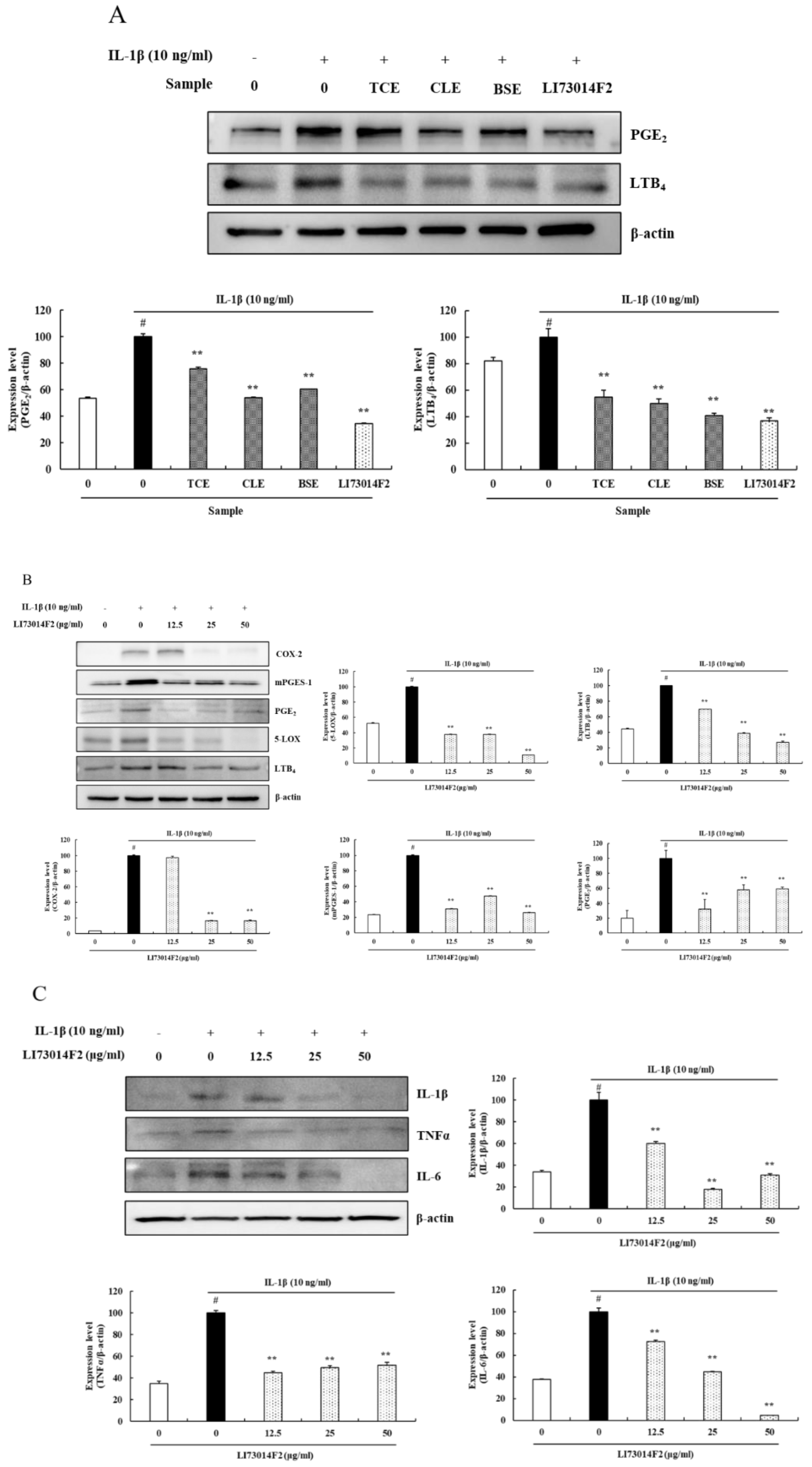
3.2. LI73014F2 Suppressed the IL-1β-induced Expression of COX-2, mPGES-1, PGE2, 5-LOX and LTB4 in HCH Cells
3.3. LI73014F2 Suppressed the IL-1β-induced Expression of Inflammatory Cytokines in HCH Cells
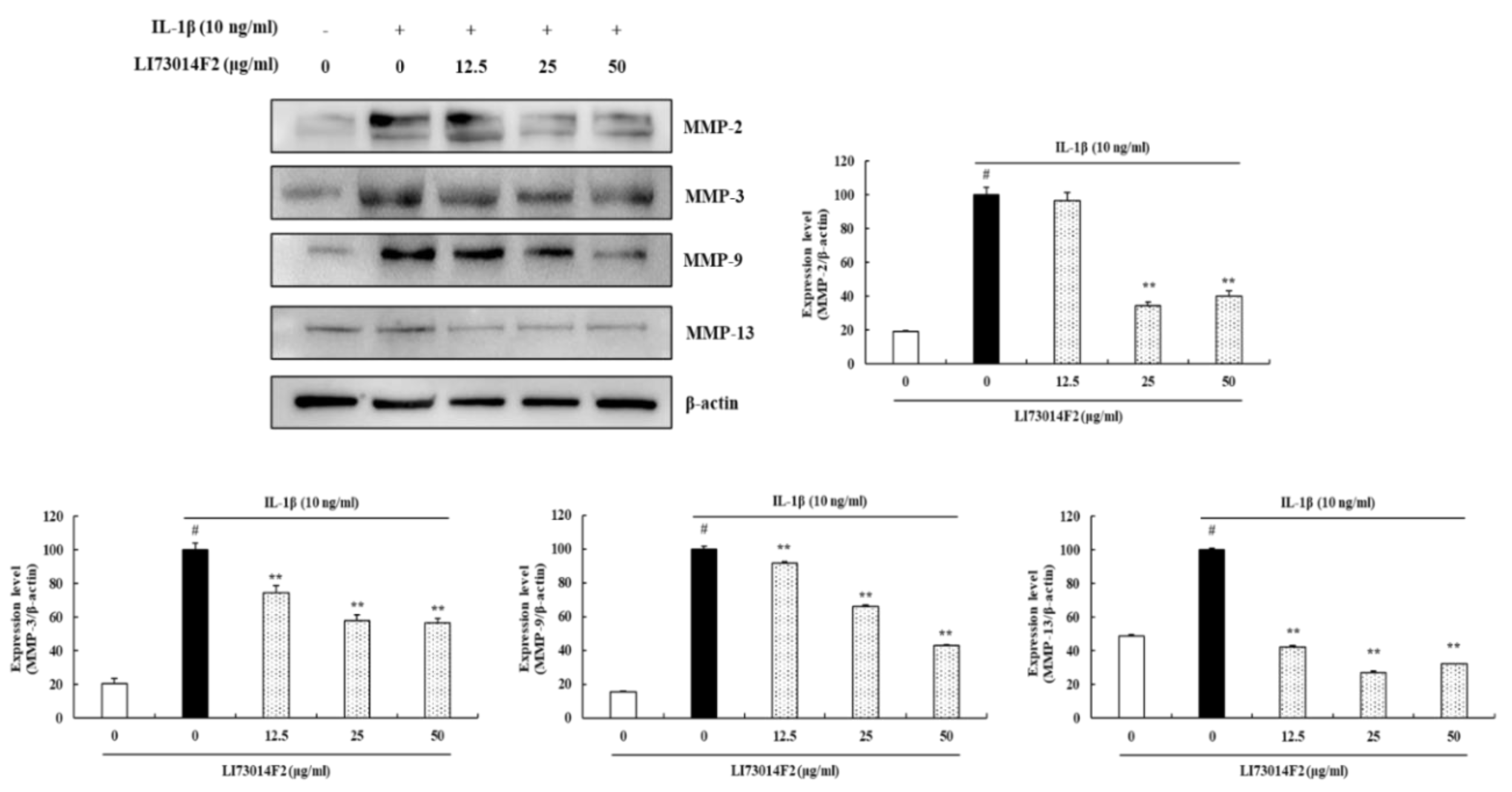
3.4. LI73014F2 Decreased the Production of MMP-2, MMP-3, MMP-9 and MMP-13 in IL-1β-stimulated HCH Cells
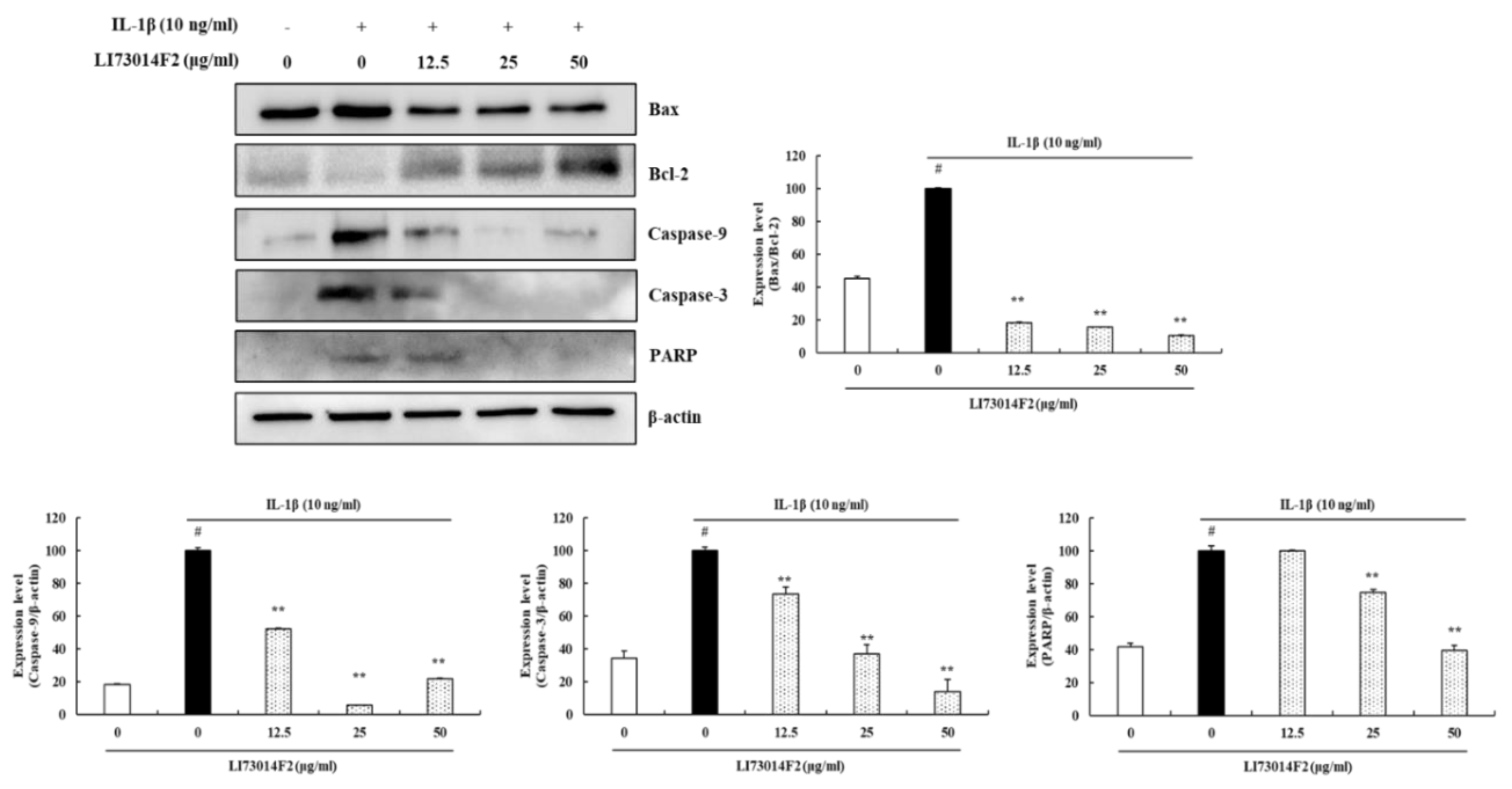
3.5. Effects of LI73014F2 on IL-1β-induced Apoptosis in HCH Cells
3.6. Effects of LI73014F2 on the NF-κB p65 and p38 MAPK Signaling Pathway in IL-1β-stimulated HCH Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernandes, J.C.; Martel-Pelletier, J.; Pelletier, J.P. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2002, 39, 237–246. [Google Scholar] [PubMed]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Cheng, Y. Inhibition of miR-20 promotes proliferation and autophagy in articular chondrocytes by PI3K/AKT/mTOR signaling pathway. Biomed. Pharmacother. 2018, 97, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Claveau, D.; Sirinyan, M.; Guay, J.; Gordon, R.; Chan, C.C.; Bureau, Y.; Riendeau, D.; Mancini, J.A. Microsomal prostaglandin E synthase-1 is a major terminal synthase that is selectively up-regulated during cyclooxygenase-2-dependent prostaglandin E2 production in the rat adjuvant-induced arthritis model. J. Immunol. 2003, 170, 4738–4744. [Google Scholar] [CrossRef]
- Shi, J.; Schmitt-Talbot, E.; DiMattia, D.A.; Dullea, R.G. The differential effects of IL-1 and TNF-α on proinflammatory cytokine and matrix metalloproteinase expression in human chondrosarcoma cells. Inflamm. Res. 2004, 53, 377–389. [Google Scholar] [CrossRef]
- Goldring, S.R.; Goldring, M.B. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin. Orthop. Relat. Res. 2004, 427, S27–S36. [Google Scholar] [CrossRef]
- Ahmed, S.; Rahman, A.; Hasnain, A.; Lalonde, M.; Goldberg, V.M.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1β-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radic. Biol. Med. 2002, 33, 1097–1105. [Google Scholar] [CrossRef]
- Boileau, C.; Pelletier, J.P.; Tardif, G.; Fahmi, H.; Laufer, S.; Lavigne, M.; Martel-Pelletier, J. The regulation of human MMP-13 by licofelone, an inhibitor of cyclo-oxygenases and 5-lipoxygenase, in human osteoarthritic chondrocytes is mediated by the inhibition of the p38 MAP kinase signalling pathway. Ann. Rheum. Dis. 2005, 64, 891–898. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, C.; Wang, X.; Huo, S. Juglanin inhibits IL-1β-induced inflammation in human chondrocytes. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3614–3620. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, Y.; Piao, T.; Liu, J. Echinocystic acid inhibits IL-1β-induced COX-2 and iNOS expression in human osteoarthritis chondrocytes. Inflammation 2016, 39, 543–549. [Google Scholar] [CrossRef]
- B Marcu, K.; Otero, M.; Olivotto, E.; Maria Borzi, R.; B Goldring, M. NF-κB signaling: Multiple angles to target OA. Curr. Drug Targets 2010, 11, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Mengshol, J.A.; Vincenti, M.P.; Coon, C.I.; Barchowsky, A.; Brinckerhoff, C.E. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor κB: Differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000, 43, 801–811. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Bhattacharyya, S.K.; Chattopadhyay, R.R. The development of Terminalia chebula Retz.(Combretaceae) in clinical research. Asian Pac. J. Trop. Biomed. 2013, 3, 244–252. [Google Scholar] [CrossRef]
- Grover, A.K.; Samson, S.E. Benefits of antioxidant supplements for knee osteoarthritis: Rationale and reality. Nutr. J. 2015, 15, 1–13. [Google Scholar] [CrossRef]
- Kimmatkar, N.; Thawani, V.; Hingorani, L.; Khiyani, R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee–a randomized double blind placebo controlled trial. Phytomedicine 2003, 10, 3–7. [Google Scholar] [CrossRef]
- Anthoni, C.; Laukoetter, M.G.; Rijcken, E.; Vowinkel, T.; Mennigen, R.; Muller, S.; Senninger, N.; Russell, J.; Jauch, J.; Bergmann, J.; et al. Mechanisms underlying the anti-inflammatory actions of boswellic acid derivatives in experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G1131–G1137. [Google Scholar] [CrossRef]
- Sengupta, K.; Alluri, K.V.; Satish, A.R.; Mishra, S.; Golakoti, T.; Sarma, K.V.; Dey, D.; Raychaudhuri, S.P. A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin® for treatment of osteoarthritis of the knee. Arthritis Res. Ther. 2008, 10, R85. [Google Scholar] [CrossRef]
- Vishal, A.A.; Mishra, A.; Raychaudhuri, S.P. A double blind, randomized, placebo controlled clinical study evaluates the early efficacy of Aflapin® in subjects with osteoarthritis of knee. Int. J. Med. Sci. 2011, 8, 615–622. [Google Scholar] [CrossRef]
- Daily, J.W.; Yang, M.; Park, S. Efficacy of turmeric extracts and curcumin for alleviating the symptoms of joint arthritis: A systematic review and meta-analysis of randomized clinical trials. J. Med. Food. 2016, 19, 717–729. [Google Scholar] [CrossRef]
- Kuptniratsaikul, V.; Dajpratham, P.; Taechaarpornkul, W.; Buntragulpoontawee, M.; Lukkanapichonchut, P.; Chootip, C.; Saengsuwan, J.; Tantayakom, K.; Laongpech, S. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: A multicenter study. Clin. Interv. Aging 2014, 9, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Singh, S.; Gupta, Y.K. Anti-arthritic and disease modifying activity of Terminalia chebula Retz. in experimental models. J. Pharm. Pharmacol. 2010, 62, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Pokuri, V.K.; Kumar, C.U.; Pingali, U. A randomized, double-blind, placebo-controlled, cross-over study to evaluate analgesic activity of Terminalia chebula in healthy human volunteers using a mechanical pain model. J. Anaesthesiol. Clin. Pharmacol. 2016, 32, 329–332. [Google Scholar] [PubMed]
- Karlapudi, V.; Prasad Mungara, A.V.V.; Sengupta, K.; Davis, B.A.; Raychaudhuri, S.P. A Placebo-Controlled Double-Blind Study Demonstrates the Clinical Efficacy of a Novel Herbal Formulation for Relieving Joint Discomfort in Human Subjects with Osteoarthritis of Knee. J. Med. Food. 2018, 21, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Chen, H. Mathematical rules for synergistic, additive, and antagonistic effects of multi-drug combinations and their application in research and development of combinatorial drugs and special medical food combinations. Food Sci. Hum. Wellness 2019, 8, 136–141. [Google Scholar] [CrossRef]
- Lee, A.S.; Ellman, M.B.; Yan, D.; Kroin, J.S.; Cole, B.J.; van Wijnen, A.J.; Im, H.J. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene. 2013, 527, 440–447. [Google Scholar] [CrossRef]
- Heraud, F.; Heraud, A.; Harmand, M.F. Apoptosis in normal and osteoarthritic human articular cartilage. Ann. Rheum. Dis. 2000, 59, 959–965. [Google Scholar] [CrossRef]
- Schuerwegh, A.J.; Dombrecht, E.J.; Stevens, W.J.; Van Offel, J.F.; Bridts, C.H.; De Clerck, L.S. Influence of pro-inflammatory (IL-1α, IL-6, TNF-α, IFN-γ) and anti-inflammatory (IL-4) cytokines on chondrocyte function. Osteoarthr. Cartil. 2003, 11, 681–687. [Google Scholar] [CrossRef]
- Loeser, R.F. Molecular mechanisms of cartilage destruction: Mechanics, inflammatory mediators, and aging collide. Arthritis Rheum. 2006, 54, 1357–1360. [Google Scholar] [CrossRef]
- Brinckerhoff, C.E.; Matrisian, L.M. Matrix metalloproteinases: A tail of a frog that became a prince. Nat. Rev. Mol. Cell Biol. 2002, 3, 207–214. [Google Scholar] [CrossRef]
- Kevorkian, L.; Young, D.A.; Darrah, C.; Donell, S.T.; Shepstone, L.; Porter, S.; Brockbank, S.M.V.; Edwards, D.R.; Parker, A.E.; Clark, I.M. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004, 50, 131–141. [Google Scholar] [CrossRef]
- Zhou, P.H.; Liu, S.Q.; Peng, H. The effect of hyaluronic acid on IL-1β-induced chondrocyte apoptosis in a rat model of osteoarthritis. J. Orthop. Res. 2008, 26, 1643–1648. [Google Scholar] [CrossRef]
- Skulachev, V.P. Cytochrome c in the apoptotic and antioxidant cascades. FEBS Lett. 1998, 423, 275–280. [Google Scholar] [CrossRef]
- Peña-Blanco, A.; García-Sáez, A.J. Bax, Bak and beyond—mitochondrial performance in apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Loreto, C.; Castorina, S.; Pichler, K.; Weinberg, A.M. Post-traumatic caspase-3 expression in the adjacent areas of growth plate injury site: A morphological study. Int. J. Mol. Sci. 2013, 14, 15767–15784. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; John, T.; Seifarth, C.; Mobasheri, A.L.I. Resveratrol inhibits IL-1β–induced stimulation of caspase-3 and cleavage of PARP in human articular chondrocytes in vitro. Ann. N. Y. Acad. Sci. 2007, 1095, 554–563. [Google Scholar] [CrossRef]
- Giunta, S.; Castorina, A.; Marzagalli, R.; Szychlinska, M.A.; Pichler, K.; Mobasheri, A.; Musumeci, G. Ameliorative effects of PACAP against cartilage degeneration. Morphological, immunohistochemical and biochemical evidence from in vivo and in vitro models of rat osteoarthritis. Int. J. Mol. Sci. 2015, 16, 5922–5944. [Google Scholar] [CrossRef]
- Loeser, R.F.; Erickson, E.A.; Long, D.L. Mitogen-activated protein kinases as therapeutic targets in osteoarthritis. Curr. Opin. Rheumatol. 2008, 20, 581–586. [Google Scholar] [CrossRef]
- Matsuo, M.; Nishida, K.; Yoshida, A.; Murakami, T.; Inoue, H. Expression of caspase-3 and-9 relevant to cartilage destruction and chondrocyte apoptosis in human osteoarthritic cartilage. Acta Med. Okayama. 2001, 55, 333–340. [Google Scholar]
- Le, D.A.; Wu, Y.; Huang, Z.; Matsushita, K.; Plesnila, N.; Augustinack, J.C.; Hyman, B.T.; Yuan, J.; Kuida, K.; Flavell, R.A.; et al. Caspase activation and neuroprotection in caspase-3-deficient mice after in vivo cerebral ischemia and in vitro oxygen glucose deprivation. Proc. Natl. Acad. Sci. USA 2002, 99, 15188–15193. [Google Scholar] [CrossRef]
- Kong, D.; Zheng, T.; Zhang, M.; Wang, D.; Du, S.; Li, X.; Fang, J.; Cao, X. Static mechanical stress induces apoptosis in rat endplate chondrocytes through MAPK and mitochondria-dependent caspase activation signaling pathways. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Abella, V.; Santoro, A.; Scotece, M.; Conde, J.; López-López, V.; Lazzaro, V.; Gómez-Reino, J.J.; Meli, R.; Gualillo, O. Non-dioxin-like polychlorinated biphenyls (PCB 101, PCB 153 and PCB 180) induce chondrocyte cell death through multiple pathways. Toxicol. Lett. 2015, 234, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.P.; Jin, G.J.; Xiong, Y.; Hu, P.F.; Bao, J.P.; Wu, L.D. Rosmarinic acid down-regulates NO and PGE 2 expression via MAPK pathway in rat chondrocytes. J. Cell. Mol. Med. 2018, 22, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Rigoglou, S.; Papavassiliou, A.G. The NF-κB signalling pathway in osteoarthritis. Int. J. Biochem. Cell Biol. 2013, 45, 2580–2584. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xi, Y.; Pan, Q.; Mao, Z.; Zhang, R.; Ma, X.; You, H. Caffeic acid protects against IL-1β-induced inflammatory responses and cartilage degradation in articular chondrocytes. Biomed. Pharmacother. 2018, 107, 433–439. [Google Scholar] [CrossRef]
- Dieppe, P.A.; Lohmander, L.S. Pathogenesis and management of pain in osteoarthritis. Lancet 2005, 365, 965–973. [Google Scholar] [CrossRef]
- Bauer, D.C.; Hunter, D.J.; Abramson, S.B.; Attur, M.; Corr, M.; Felson, D.; Heinegård, D.; Jordan, J.M.; Kepler, T.B.; Lane, N.E.; et al. Classification of osteoarthritis biomarkers: A proposed approach. Osteoarthr. Cartil. 2006, 14, 723–727. [Google Scholar] [CrossRef]
- Lee, Y.C.; Nassikas, N.J.; Clauw, D.J. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res. Ther. 2011, 13, 211. [Google Scholar] [CrossRef]
- Schaible, H.G.; Ebersberger, A.; Natura, G. Update on peripheral mechanisms of pain: Beyond prostaglandins and cytokines. Arthritis Res. Ther. 2011, 13, 210. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.L.; Lee, H.J.; Lee, D.-R.; Choi, B.-K.; Yang, S.H. Anti-osteoarthritic Effects of an Herbal Composition LI73014F2 on Interleukin-1β-induced Primary Human Articular Chondrocytes. Molecules 2020, 25, 2033. https://doi.org/10.3390/molecules25092033
Kim HL, Lee HJ, Lee D-R, Choi B-K, Yang SH. Anti-osteoarthritic Effects of an Herbal Composition LI73014F2 on Interleukin-1β-induced Primary Human Articular Chondrocytes. Molecules. 2020; 25(9):2033. https://doi.org/10.3390/molecules25092033
Chicago/Turabian StyleKim, Hae Lim, Hae Jin Lee, Dong-Ryung Lee, Bong-Keun Choi, and Seung Hwan Yang. 2020. "Anti-osteoarthritic Effects of an Herbal Composition LI73014F2 on Interleukin-1β-induced Primary Human Articular Chondrocytes" Molecules 25, no. 9: 2033. https://doi.org/10.3390/molecules25092033
APA StyleKim, H. L., Lee, H. J., Lee, D.-R., Choi, B.-K., & Yang, S. H. (2020). Anti-osteoarthritic Effects of an Herbal Composition LI73014F2 on Interleukin-1β-induced Primary Human Articular Chondrocytes. Molecules, 25(9), 2033. https://doi.org/10.3390/molecules25092033






