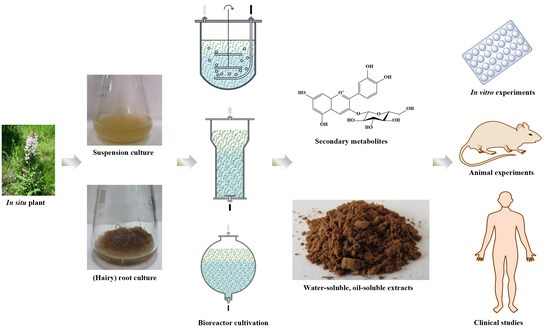
Plant In Vitro Systems as a Sustainable Source of Active Ingredients for Cosmeceutical Application
Abstract
1. Introduction
2. Plant In Vitro Systems Derived Cosmetic Ingredients
3. Small- and Large-Scale Production of Cosmetic Ingredients through Plant In Vitro Systems
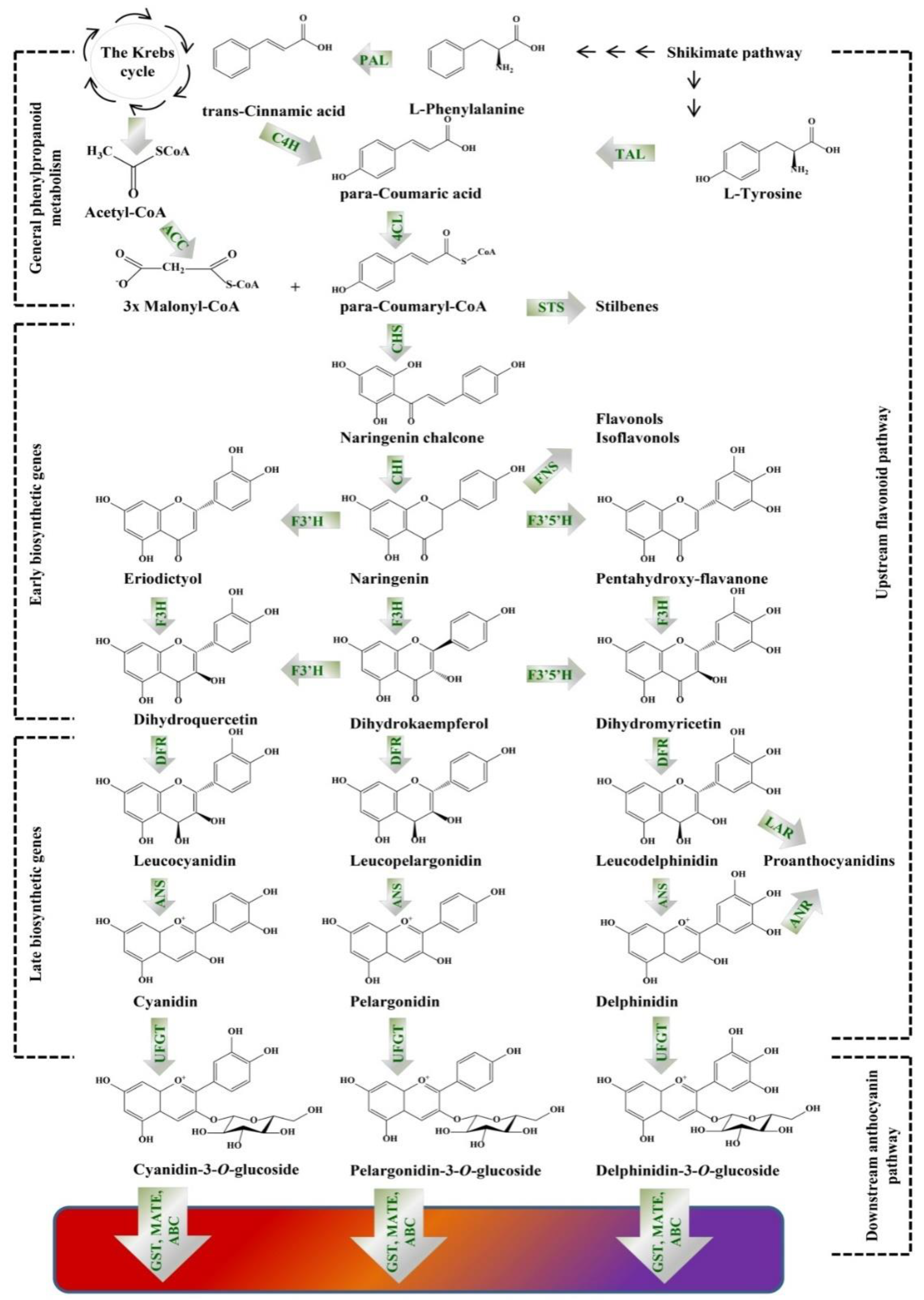
4. Metabolic Engineering of Anthocyanins Pathway in Plant In Vitro Systems
5. Extracts (Secondary Metabolites) Activities-Gene Expression and Main Activities
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmitz, C.; Fritsch, L.; Fischer, R.; Schillberg, S.; Rasche, S. Statistical experimental designs for the production of secondary metabolites in plant cell suspension cultures. Phytochem. Lett. 2016, 38, 2007–2014. [Google Scholar] [CrossRef]
- Maia, J.; Dantas, T.; da Costa Neto, B.; Borges, K.; Lima, E.; da Mata, A.; de Medeiros, M.; Pereira, C. Extract of spray-dried Malay apple (Syzygium malaccense L.) skin. J. Food Proc. Eng. 2019, 2019, e13275. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.; Garcia-Lara, S. Current methods for the discovery of new active ingredients from natural products for cosmeceutical applications. Planta Med. 2019, 85, 535–551. [Google Scholar] [CrossRef]
- Apone, F.; Tito, A.; Carola, A.; Arciello, S.; Tortora, A.; Filippini, L.; Monoli, I.; Cucchiara, M.; Gibertoni, S.; Chrispeel, M.; et al. A mixture of peptides and sugars derived from plant cell walls increases plant defense responses to stress and attenuates ageing-associated molecular changes in cultured skin cells. J. Biotechnol. 2010, 145, 367–376. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Varriale, S.; Topakas, E.; Rova, U.; Christakopoulous, P.; Faraco, V. Enzymatic synthesis of bioactive compounds with high potential for cosmeceutical application. Appl. Microbiol. Biotechnol. 2016, 100, 6519–6543. [Google Scholar] [CrossRef] [PubMed]
- Vichit, W.; Saewan, N.; Prinyarux, T. Anti-aging efficacy of Thai red rice callus cosmetic product. J. Appl. Sci. 2018, 17, 63–72. [Google Scholar] [CrossRef]
- Korkina, L.; Mayer, W.; de Luca, C. Meristem plant cells as a sustainable source of redox actives for skin rejuvenation. Biomolecules 2017, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Appelhagen, I.; Wulff-Vester, A.; Wendell, M.; Hvoslef-Eide, A.-K.; Russell, J.; Oertel, A.; Martens, S.; Mock, H.-P.; Martin, C.; Martos, A. Colour bio-factories: Towards scale-up production of anthocyanins in plant cell cultures. Metab. Eng. 2018, 48, 218–232. [Google Scholar] [CrossRef]
- Rodrigues, T.; Amore, T.; Teixeira, E.; de Medeiros Burkert, J. Carotenoid production by Rhodotorula mucilaginosa in batch and fed-batch fermentation using agroindustrial byproducts. Food Technol. Biotechnol. 2019, 57, 388–398. [Google Scholar] [CrossRef]
- Inyai, C.; Boonsnongcheep, P.; Komaikul, J.; Sritularak, B.; Tanaka, H.; Putalun, W. Alginate immobilization of Morus alba L. cell suspension cultures improved the accumulation and secretion of stilbenoids. Bioproc. Biosyst. Eng. 2019, 42, 131–141. [Google Scholar] [CrossRef]
- Crivellari, I.; Vertuani, S.; Lim, Y.; Cervellati, F.; Baldisserotto, A.; Manfredini, S.; Valacchi, G. ES2 as a novel verbascoside-derived compound in the treatment of cutaneous wound healing. Cosmetics 2018, 5, 65. [Google Scholar] [CrossRef]
- Kikowska, M.; Chmielewska, M.; Włodarczyk, A.; Studzińska-Sroka, E.; Żuchowski, J.; Stochmal, A.; Kotwicka, M.; Thiem, B. Effect of pentacyclic triterpenoids-rich callus extract of Chaenomeles japonica (Thunb.) Lindl. ex Spach on viability, morphology, and proliferation of normal human skin fibroblasts. Molecules 2018, 23, 3009. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhang, W.; Yu, X. A combination of elicitation and precursor feeding leads to increased anthocyanin synthesis in cell suspension cultures of Vitis vinifera. Plant. Cell Tissue Organ. Cult. 2011, 107, 261–269. [Google Scholar] [CrossRef]
- Saw, N.; Moser, C.; Martens, S.; Franceschi, P. Applying generalized additive models to unravel dynamic changes in anthocyanin biosynthesis in methyl jasmonate elicited grapevine (Vitis vinifera cv. Gamay) cell cultures. Hortic. Res. 2017, 4, 17038. [Google Scholar] [CrossRef]
- Jung, S.; Venkatesh, J.; Kang, M.-Y.; Kwon, J.-K.; Kang, B.-C. A non-LTR retrotransposon activates anthocyanin biosynthesis by regulating a MYB transcription factor in Capsicum annuum. Plant. Sci. 2019, 287, 110181. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyna, I.; Kochan, E.; Szymczyk, P.; Lisiecki, P.; Kuźma, Ł.; Grzegorczyk-Karolak, I. The antioxidant and antimicrobial properties of phenol-rich extracts of Dracocephalum forrestii W. W. Smith shoot cultures grown in the nutrient sprinkle bioreactor. Phytochem. Lett. 2019, 30, 254–260. [Google Scholar] [CrossRef]
- Lee, E.-K.; Jin, Y.-W.; Park, J.; Yoo, Y.; Hong, S.; Amir, R.; Yan, Z.; Elfick, A.; Tomlinson, S.; Halbritter, F.; et al. Cultured cambial meristematc cells as a source of plant natural products. Nat. Biotechnol. 2010, 28, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Trehan, S.; Michniak-Kohn, B.; Beri, K. Plant stem cells in cosmetics: Current trends and future directions. Future Sci. 2017, 3, FS0026. [Google Scholar] [CrossRef]
- Barbulova, A.; Apone, F.; Colucci, G. Plant cell cultures as source of cosmetic active ingredients. Cosmetics 2014, 1, 94–104. [Google Scholar] [CrossRef]
- Miastkowska, M.; Sikora, E. Anti-aging properties of plant stem cell extracts. Cosmetics 2018, 5, 55. [Google Scholar] [CrossRef]
- Tito, A.; Carola, A.; Bimonte, M.; Barbulova, A.; Arciello, S.; de Laurentiis, F.; Monoli, I.; Hill, J.; Gilbertoni, S.; Colucci, G.; et al. A tomato stem cell extract, containing antioxidant compounds and metal chelating factors, protects skin cells from heavy metalinduced damages. Int. J. Cosmet. Sci. 2011, 33, 543–552. [Google Scholar] [CrossRef]
- Tito, A.; Bimonte, M.; Carola, A.; De Lucia, A.; Barbulova, A.; Tortora, A.; Coluccu, G.; Apone, F. An oil-soluble extract of Rubus idaeus cells enhances hydration and water homeostasis in skin cells. Int. J. Cosmet. Sci. 2015, 37, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Schürch, C.; Blum, P.; Belser, E.; Zülli, F. Plant stem cell extract for longevity of skin and hair. Int. J. Appl. Sci. 2008, 134, 5. [Google Scholar]
- Miras-Moreno, B.; Pedreño, M.; Romero, L. Bioactivity and bioavailability of phytoene and strategies to improve its production. Phytochem. Rev. 2019, 18, 359–376. [Google Scholar] [CrossRef]
- Jeandet, P.; Clément, C.; Tisserant, L.-P.; Crouzet, J.; Courot, É. Use of grapevine cell cultures for the production of phytostilbenes of cosmetic interest. Comptes Rendus Chime. 2016, 19, 1062–1070. [Google Scholar] [CrossRef]
- Komaikul, J.; Kitisripanya, T.; Tanaka, H.; Sritularak, B.; Putalan, W. Enhanced mulberroside A production from cell suspension and root cultures of Morus alba using elicitation. Nat. Prod. Commun. 2015, 10, 1253–1256. [Google Scholar] [CrossRef]
- Korkina, L.; Mikhal’chik, E.; Suprun, M.; Pastore, S.; Daltoso, R. Molecular mechanisms underlying wound healing and anti-inflammatory properties of naturally occurring biotechnologically produced phenylpropanoid glycosides. Cell. Mol. Biol. 2007, 53, 84–91. [Google Scholar] [PubMed]
- Tofighi, Z.; Amini, M.; Shirzadi, M.; Mirhabibi, H.; Saeedi, N.; Yassa, N. Vigna radiata as a new source for biotransformation of hydroquinone arbutin. Pharm. Sci. 2016, 22, 126–131. [Google Scholar] [CrossRef][Green Version]
- Khosvari, E.; Mousavi, A.; Farhadpour, M.; Ghashghaie, J.; Ghanati, F.; Haghbeen, K. Pyrrolizidine alkaloids-free extract from the cell culture of Lithospermum officinale with high antioxidant capacity. Appl. Biochem. Biotechnol. 2019, 187, 744–752. [Google Scholar]
- Donnez, D.; Kim, K.-H.; Antoine, S.; Conreux, A.; De Luca, V.; Jeandet, P.; Clément, C. Bioproduction of resveratrol and viniferins by an elicited grapevine cell culture in a 2 L stirred bioreactor. Process. Biochem. 2011, 46, 1056–1062. [Google Scholar] [CrossRef]
- Georgiev, M.; Weber, J. Bioreactors for plant cells: Hardware configuration and internal environment optimization as tools wider commercialization. Biotechnol. Lett. 2014, 36, 1359–1367. [Google Scholar] [CrossRef]
- Chastang, T.; Pozzobon, V.; Taidi, B.; Courot, E.; Clément, C.; Pareau, D. Resveratrol production by grapevine cells in fed-batch bioreactor: Experiments and modelling. Biochem. Eng. J. 2018, 131, 9–16. [Google Scholar] [CrossRef]
- Aumont, V.; Larronde, F.; Richard, T.; Budzinski, H.; Decendit, A.; Deffieux, G.; Krisa, S.; Mérillon, J.-M. Production of highly 13C-labeled polyphenols in Vitis vinifera cell bioreactor cultures. J. Biotechnol. 2004, 109, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Decendit, A.; Ramawat, K.; Waffo, P.; Deffieux, G.; Badoc, A.; Mérillon, J.-M. Anthocyanins, catechins, condensed tannins and piceid, production in Vitis vinifera cell bioreactor culture. Biotechnol. Lett. 1996, 18, 659–662. [Google Scholar] [CrossRef]
- Honda, H.; Hiraoka, K.; Nagamori, E.; Omote, M.; Kato, Y.; Hiraoka, S.; Kobayashi, T. Enhanced anthocyanin production from grape callus in an air-lift type bioreactor using a viscous additive-supplement medium. J. Biosci. Bioengin. 2002, 94, 135–139. [Google Scholar] [CrossRef]
- Zhong, J.-J.; Yoshida, M.; Fujiyama, K.; Seki, T.; Yoshida, T. Enhancement of anthocyanin production by Perilla frutescens cells in a stirred tank bioreactor with internal light irradiation. J. Ferment. Bioeng. 1993, 75, 299–303. [Google Scholar] [CrossRef]
- Nivelle, L.; Hubert, J.; Courot, E.; Borie, N.; Renault, J.-H.; Nuzillard, J.-M.; Harakat, D.; Clément, C.; Martiny, L.; Delmas, D.; et al. Cytotoxicity of labruscol, a new resveratrol dimer produced by grapevine cell suspensions, on human skin melanoma cancer cell line HT-144. Molecules 2017, 22, 1940. [Google Scholar] [CrossRef]
- Grzegorczyk, I.; Wysokińska, H. Antioxidant compounds in Salvia officinalis L. shoot and hairy root cultures in the nutrient sprinkle bioreactor. Acta. Soc. Bot. Pol. 2010, 79, 7–10. [Google Scholar] [CrossRef]
- Sitarek, P.; Kowalczyk, T.; Picot, L.; Michalska-Hejduk, D.; Bijak, M.; Białas, A.; Wielanek, M.; Śliwiński, T.; Skała, E. Growth of Leonurus sibiricus L. roots with over-expression of AtPAP1 transcriptional factor in closed bioreactor, production of bioactive phenolic compounds and evaluation of their biological activity. Ind. Crop. Prod. 2018, 122, 732–739. [Google Scholar] [CrossRef]
- Lee, E.-J.; Park, S.-Y.; Paek, K.-Y. Enhancement strategies of bioactive compound production in adventitious root cultures of Eleutherococcus koreanum Nakai subjected to methyl jasmonate and salicylic acid elicitation through airlift bioreactors. Plant. Cell Tissue Organ. Cult. 2015, 120, 1–10. [Google Scholar] [CrossRef]
- Lee, E.-J.; Paek, K.-Y. Effect of nitrogen source on biomass and bioactive compound production in submerged cultures of Eleutherococcus koreanum Nakai adventitious roots. Biotechnol. Prog. 2012, 28, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.; Ludwig-Müller, J.; Weber, J.; Stancheva, N.; Bley, T. Bioactive metabolite production and stress-related hormones in Devil’s claw cell suspension cultures grown in bioreactors. App. Microbiol. Biotechnol. 2011, 89, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk-Karolak, I.; Rytczak, P.; Bielecki, S.; Wysokińska, H. The influence of liquid systems for shoot multiplication, secondary metabolite production and plant regeneration of Scutellaria alpina. Plant. Cell Tissue Organ. Cult. 2017, 128, 479–486. [Google Scholar] [CrossRef]
- Rani, A.; Meghana, R.; Kush, A. Squalene production in the cell suspension cultures of Indian sandalwood (Santalum album L.) in shake flasks and air lift bioreactor. Plant. Cell Tissue Organ. Cult. 2018, 135, 155–167. [Google Scholar] [CrossRef]
- Sun, D.; Li, C.; Qin, H.; Zhang, Q.; Yang, Y.; Ai, J. Somatic embryos cultures of Vitis amurensis Rupr. in air-lift bioreactors for the production of biomass and resveratrol. J. Plant. Biol. 2016, 59, 427–434. [Google Scholar] [CrossRef]
- Lee, E.-J.; Paek, K.-Y. Enhanced productivity of biomass and bioactive compounds through bioreactor cultures of Eleutherococcus koreanum Nakai adventitious roots affected by medium salt strength. Ind. Crop. Prod. 2012, 36, 460–465. [Google Scholar] [CrossRef]
- Kovatcheva-Apostolova, E.; Georgiev, M.; Ilieva, M.; Skibsted, L.; Rødtjer, A.; Andersen, M. Extracts of plant cell cultures of Lavandula vera and Rosa damascene as sources of phenolic antioxidants for use in foods. Eur. Food Res. Technol. 2008, 227, 1243–1249. [Google Scholar] [CrossRef]
- Cui, H.-Y.; Baque, A.; Lee, E.-J.; Paek, K.-Y. Scale-up of adventitious root cultures of Echinacea angustifolia in a pilot-scale bioreactor for the production of biomass and caffeic acid derivatives. Plant. Biotechnol. Rep. 2013, 7, 297–308. [Google Scholar] [CrossRef]
- Wu, C.; Tang, J.; Jin, Z.; Wang, M.; Liu, Q.; Huang, T.; Lian, M. Optimizing co-culture conditions of adventitious roots of Echinacea pallida and Echinacea purpurea in air-lift bioreactor systems. Biochem. Eng. J. 2018, 132, 206–216. [Google Scholar] [CrossRef]
- Ho, T.-T.; Lee, J.-D.; Jeong, C.-S.; Paek, K.-Y.; Park, S.-Y. Improvement of biosynthesis and accumulation of bioactive compounds by elicitation in adventitious root cultures of Polygonum multiflorum. Appl. Microbiol. Biotechnol. 2017, 102, 199–209. [Google Scholar] [CrossRef]
- Cui, X.-H.; Niranjana, H.; Murthy, N.; Paek, K.-Y. Pilot-scale culture of Hypericum perforatum L. adventitious roots in airlift bioreactors for the production of bioactive compounds. Appl. Biochem. Biotechnol. 2014, 174, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Murthy, H.; Hahn, E.-J.; Paek, K.-Y. Large-scale cultivation of adventitious roots of Echinacea purpurea in airlift bioreactors for the production of chichoric acid, chlorogenic acid and caftaric acid. Biotechnol. Lett. 2007, 29, 1179–1182. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Akita, M.; Sakamoto, K.; Liu, H.; Shigeoka, T.; Koyano, T.; Kawamura, M.; Furuya, T. Large-scale production of anthocyanin by Aralia cordata cell suspension culture. Appl. Microbiol. Biotechnol. 1993, 40, 215–218. [Google Scholar] [CrossRef]
- Ochoa-Villarreal, M.; Howat, S.; Hong, S.; Jang, M.; Jin, Y.-W.; Lee, E.-K.; Loake, G. Plant cell culture strategies for the production of natural products. Bmb Rep. 2016, 49, 149–158. [Google Scholar] [CrossRef]
- Nohynek, L.; Bailey, M.; Tähtiharju, J.; Seppänen-Laakso, T.; Rischer, H.; Oksman-Caldenty, K.-M.; Puupponen-Pimiä, R. Cloudberry (Rubus chamaemorus) cell culture with bioactive substances: Establishment and mass propagation for industrial use. Eng. Life Sci. 2014, 14, 667–675. [Google Scholar] [CrossRef]
- Schürch, C.; Blum, P.; Zülli, F. Potential of plant cells in culture for cosmetic application. Phytochem. Rev. 2008, 7, 599–605. [Google Scholar] [CrossRef]
- Lai, C.; Pan, H.; Huang, X.; Fan, L.; Duan, C.; Li, S. Validation of reference genes for gene expression analysis of response to anthocyanin induction in cell cultures of Vitis davidii (Rom. Caill.) Foëx. In Vitro Cell. Dev. Biol. Plant. 2018, 54, 642–657. [Google Scholar] [CrossRef]
- Liu, Y.; Tikunov, Y.; Schouten, R.; Marcelis, L.; Visser, R.; Bovy, A. Anthocyanin biosynthesis and degradation mechanisms in Solanaceous vegetables: A review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef]
- He, F.; Mu, L.; Yan, G.-L.; Liang, N.-N.; Pan, Q.-H.; Wang, J.; Reeves, M.; Duan, C.-Q. Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef]
- Gao, J.; Li, W.-B.; Liu, H.-F.; Chen, F.-B. De novo transcriptome sequencing of radish (Raphanus sativus L.) fleshy roots: Analysis of major genes involved in the anthocyanin synthesis pathway. Bmc Mol. Cell Biol. 2019, 20, 45. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, M.-Z.; Xie, S.-Y. Regulation of anthocyanin biosynthesis in Arabidopsis thaliana red pap1-D cells metabolically programmed by auxins. Planta 2014, 239, 765–781. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, H.; Wang, F.; Li, J.; Zhang, Y.; Wang, L.; Gao, J. Comparative transcriptome analysis reveals effects of exogenous hematin on anthocyanin biosynthesis during strawberry fruit ripening. Int. J. Genom. 2016, 676, 2731. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant. Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef]
- Piero, A. The state of the art in biosynthesis of anthocyanins and its regulation in pigmented sweet oranges [(Citrus sinensis) L. Osbeck]. J. Agric. Food Chem. 2015, 63, 4031–4041. [Google Scholar] [CrossRef]
- Soubeyrand, E.; Colombié, S.; Beauvoit, B.; Dai, Z.; Cluzet, S.; Hilbert, G.; Renaud, C.; Maneta-Peyret, L.; Dieuaide-Noubhani, M.; Mérillon, J.-M.; et al. Constraint-based modeling highlights cell energy, redox status and α-ketoglutarate availability as metabolic drivers for anthocyanin accumulation in grape cells under nitrogen limitation. Front. Plant. Sci. 2018, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Chodacka, M.; Oleszkiewicz, T.; Lowder, L.; Qi, Y.; Baranski, R. Efficient CRISPR/Cas9-based genome editing in carrot cells. Plant. Cell Rep. 2018, 37, 575–586. [Google Scholar] [CrossRef]
- Mulinacci, N.; Giaccherini, C.; Santamaria, A.; Caniato, R.; Ferrari, F.; Valletta, A.; Vincieri, F.; Pasqua, G. Anthocyanins and xanthones in the calli and regenerated shoots of Hypericum perforatum var. angustifolium (sin. Fröhlich) Borkh. Plant. Physiol. Biochem. 2008, 46, 414–420. [Google Scholar] [CrossRef]
- Nadeem, M.; Abbasi, B.; Younas, M.; Ahmad, W.; Zahir, A.; Hano, C. LED-enhanced biosynthesis of biologically active ingredients in callus cultures of Ocimum basilicum. Photochem. Photobiol. 2019, 190, 172–178. [Google Scholar] [CrossRef]
- Biswas, T.; Singh, M.; Mathur, A.; Mathur, A. A dual purpose cell line of an Indian congener of ginseng-Panax sikkimensis with distinct ginsenoside and anthocyanin production profiles. Protoplasma 2015, 252, 697–703. [Google Scholar] [CrossRef]
- Tavares, S.; Vesentini, D.; Fernandes, J.; Ferreira, R.; Laureano, O.; Ricardo-Da-Silva, J.; Amâncio, S. Vitis vinifera secondary metabolism as affected by sulfate depletion: Diagnosis through phenylpropanoid pathway genes and metabolites. Plant. Physiol. Biochem. 2013, 66, 118–126. [Google Scholar] [CrossRef]
- Yin, Y.; Borges, G.; Sakuta, M.; Crozier, A.; Ashihara, H. Effect of phosphate deficiency on the content and biosynthesis of anthocyanins and the expression of related genes in suspension-cultured grape (Vitis sp.) cells. Plant. Physiol. Biochem. 2012, 55, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Tassoni, A.; Durante, L.; Ferri, M. Combined elicitation of methyl-jasmonate and red light on stilbene and anthocyanin biosynthesis. J. Plant. Physiol. 2012, 169, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.; Ananda, A.; Krastanova, S.; Sutton, S.; Ochieng, J.; Leong, S.; Tsolova, V. Elevated gene expression in chalcone synthase enzyme suggests an increased production of flavonoids in skin and synchronized red cell cultures of North American native grape berries. DNA Cell Biol. 2012, 31, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Oglesby, L.; Ananga, A.; Obuya, J.; Ochieng, J.; Cebert, E.; Tsolova, V. Anthocyanin accumulation in muscadine berry skins is influenced by the expression of the MYB transcription factors, MybA1, and MYBCS1. Antioxidants 2016, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, W.; Huang, W.; Xu, H. Effect of salicylic acid on the gene transcript and protein accumulation of flavonoid biosynthesis-related enzymes in Vitis vinifera cell suspension cultures. Hortic. Sci. 2017, 52, 1772–1779. [Google Scholar] [CrossRef]
- Martins, V.; Garcia, A.; Costa, C.; Sottomayor, M.; Gerós, H. Calcium- and hormone-driven regulation of secondary metabolism and cell wall enzymes in grape berry cells. J. Plant. Physiol. 2018, 231, 57–67. [Google Scholar] [CrossRef]
- Sinilal, B.; Ovadia, R.; Nissim-Levi, A.; Perl, A.; Carmeli-Weissberg, M.; Oren-Shamir, M. Increased accumulation and decreased catabolism of anthocyanins in red grape cell suspension culture following magnesium treatment. Planta 2011, 234, 61–71. [Google Scholar] [CrossRef]
- Matos, M.; Romero-Díez, R.; Álvarez, A.; Bronze, M.; Rodríguez-Rojo, S.; Mato, R.; Cocero, M.; Matias, A. Polyphenol-rich extracts obtained from winemaking waste streams as natural ingredients with cosmeceutical potential. Antioxidants 2019, 8, 355. [Google Scholar] [CrossRef]
- Adhikari, D.; Panthi, V.; Pangeni, R.; Kim, H.; Park, J. Preparation, characterization, and biological activities of topical anti-aging ingredients in a Citrus junos callus extract. Molecules 2017, 22, 2198. [Google Scholar] [CrossRef]
- Di Martino, O.; Tito, A.; De Lucia, A.; Cimmino, A.; Cicotti, F.; Apone, F.; Colucci, G.; Calabrò, V. Hibiscus syriacus extract from an established cell culture stimulates skin wound healing. Biomed. Res. Int. 2017, 793, 2019. [Google Scholar] [CrossRef]
- Abbasi, B.; Siddiquah, A.; Tungmunnithum, D.; Bose, S.; Younas, M.; Garros, L.; Drouet, S.; Giglioli-Guivarćh, N.; Hano, C. Isodon rugosus (Wall. ex Benth.) Codd in vitro cultures: Establishment, phytochemical characterization and in vitro antioxidant and anti-aging activities. Int. J. Mol. Sci. 2019, 20, 452. [Google Scholar] [CrossRef]
- Portugal-Cohen, M.; Ish-Shalom, E.; Mallon, R.; Corral, P.; Michoux, F.; Ma’or, Z. Apple of Sodom (Calatropis procera) callus extract, a novel skincare active and its biological activity in skin models when combined with Dead Sea water. J. Cosmet. Derm. Sci. Appl. 2018, 8, 73–91. [Google Scholar] [CrossRef]
- Kim, H.; Park, J. Anti-aging activities of Pyrus pyrifolia var culta plant callus extract. Trop. J. Pharm. Res. 2017, 16, 1579–1588. [Google Scholar] [CrossRef][Green Version]
- Park, D.; Adhikari, D.; Pangeni, R.; Panthi, V.; Kim, H.; Park, J. Preparation and characterization of callus extract from Pyrus pyrifolia and investigation of its effects on skin regeneration. Cosmetic 2018, 5, 71. [Google Scholar] [CrossRef]
- Vertuani, S.; Beghelli, E.; Scalambra, E.; Malisardi, G.; Copetti, S.; Dal Toso, R.; Baldisserotto, A.; Manfredini, S. Activity and stability studies of verbascoside, a novel antioxidant, in dermo-cosmetic and pharmaceutical topical formulations. Moleclues 2011, 16, 7068–7080. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.; Pastore, S.; Lulli, D.; Alipieva, K.; Kostyuk, V.; Potapovich, A.; Panetta, M.; Korkina, L. Verbascum xanthophoeniceum-derived phenylethanoid glycosides are potent inhibitors of inflammatory chemokines in dormant and interferon-gamma-stimulated human keratinocytes. J. Ethnopharmacol. 2012, 144, 754–760. [Google Scholar] [CrossRef]
| Plant Species | Culture Type | Bioreactor Volume and Type | Operational Conditions | Metabolite Production, mg/L | Ref. |
|---|---|---|---|---|---|
| Anthocyanins | |||||
| Vitis vinifera (L.) cv Gamay Fréaux var. Teinturier | Suspension | Stirred tank (2-L) | 25 °C; agitation: 75 rpm; flow rate: 0.075–0.15 vvm; 5000 lux continuous fluorescent light | 387 | [33] |
| V. vinifera (L.) cv Gamay Fréaux var. Teinturier | Suspension | Stirred tank (20-L) | 25 °C; agitation: 100 rpm; flow rate: 0.2 vvm; 5000 lux continuous fluorescent light | 1200 | [34] |
| V. vinifera cv. Bailey alicant A. | Suspension | Airlift (0.5-L) | 25 °C in dark; flow rate: 80 mL/min | 33 | [35] |
| Perilla frutescens | Suspension | Stirred tank (2-L) | 25 °C; agitation: 150 rpm; flow rate: 0.1 vvm; 27 W/m2 light irradiation | 1650 | [36] |
| Resveratrol | |||||
| V. vinifera cv. Chasselas×Vitis berlandieri | Suspension | Stirred tank (2-L) | 23 °C in dark; agitation: 50 rpm; flow rate: 0.025 vvm | 209 | [30] |
| V. labrusca L | Suspension | Stirred tank (14-L) | 23 °C in dark; agitation: 50 rpm; flow rate: 0.025 vvm | 72 | [37] |
| Vitis labrusca L | Suspension | Stirred tank (5-L) | 23 °C in dark; agitation: 100 rpm; flow rate: 20.0–780.0 L/min | 66 | [32] |
| Rosmarinic acid | |||||
| Salvia officinalis L | Hairy roots Shoots | Nutrient sprinkle (5-L) | 26 °C in dark; 40 s pump operation/50 s breaks 26 °C; 16 h/8 h light/dark; 45 s pump operation/40 s breaks | 477.13 59.04 | [38] |
| Dracocephalum forrestii W. W. Smith | Shoots | Nutrient sprinkle (10-L) | 26 °C; 16 h/8 h light/dark; 25 s pump operation/2.5 s breaks | 38.26 | [16] |
| Chlorogenic acid | |||||
| Dracocephalum forrestii W. W. Smith | Shoots | Nutrient sprinkle (10-L) | 26 °C; 16 h/8 h light/dark; 25 s pump operation/2.5 s breaks | 0.07 | [16] |
| Leunorus sibiricus L. | Hairy roots | Nutrient sprinkle (5-L) | 26 °C; 40 s pump operation/1.5 min breaks | 448 | [39] |
| Eleutherococcus koreanum Nakai | Adventitious roots | Air lift (3-L) | 22 °C in dark; flow rate: 0.1 vvm | 78.22 | [40] |
| E. koreanum Nakai | Adventitious roots | Bulb type (3-L) | 22 °C in dark; 0.1 vvm flow rate | 24.68 | [41] |
| Caffeic acid | |||||
| Dracocephalum forrestii W. W. Smith | Shoots | Nutrient sprinkle (10-L) | 26 °C; 16 h/8 h light/dark; 25 s pump operation/2.5 s breaks | 0.07 | [16] |
| Leunorus sibiricus L. | Hairy roots | Nutrient sprinkle (5-L) | 26 °C; 40 s pump operation/1.5 min breaks | 302 | [39] |
| Verbascoside | |||||
| Harpagophytum procumbens | Suspension | Stirred tank (3-L) | 26 °C in dark; agitation: 100 rpm; flow rate: 1/L min | 445.44 | [42] |
| Harpagophytum procumbens | Suspension | Column bioreactor with pulsed aeration (1-L) | 26 °C in dark; 1/L min flow rate every 2 s | 496.30 | [42] |
| Scutellaria alpina | Shoots | Nutrient sprinkle (5-L) | 26 °C; 40 s pump operation/2 min breaks | 11.4 | [43] |
| Plant Species and Extract Type | Concentration of the Extract, μg/mL | Gene/Protein Expression | Main Activity | Ref. |
|---|---|---|---|---|
| Calotropis procera/aqueous extract | 8000 | IL-1β, IL-1α, TNFα PGE2 inhibition HIF1 induction | Anti-inflammatory activity Hypoxia adaptation and wound healing activity | [82] |
| Citrus junos/aqueous | 500 | Tyrosinase inhibition Procollagen type I induction | Melanin inhibition Skin regeneration | [79] |
| Hibsicus syriacus/aqueous cell extract | 20 | COL I and pro-collagen I induction AQP3 and FLG induction | Collagen synthesis and protection Skin hydration | [80] |
| Isodon rugosus (Wall. ex Benth.)/80% aqueous methanol | 50 | MMP1, hyaluronidase, elastase inhibition SIRT-1 activation | Collagen synthesis and protection DNA repair and protection | [81] |
| Lycopersicon esculentum/aqueous extract | 100 | COL I and COL III induction, MMP1, MMP3 and MPP9 inhibition GADD45α and SIRT-1 induction | Collagen synthesis and protection DNA repair and protection | [21] |
| Malus domestica/liposomal extract of whole cells | 100 | Cyclin B1, cyclin E1 induction | Retard the signs of senescence | [23] |
| Nicotiana tabacum BY2 (N. sylvestris)/aqueous cell wall extract | 3.6 | COL I and COL III induction, pro-collagen I and III induction, MMP1, MMP3 and MPP9 inhibition GADD45α, SIRT-1 and SIRT-6 induction | Collagen synthesis and protection DNA repair and protection | [4] |
| Pyrus pyrifolia var. culta/aqueous extract | 1000 | Tyrosinase inhibition Pro-collagen I induction | Melanin inhibition Collagen synthesis | [83] |
| Rubus ideaus/oil-soluble extract | 1000 | GBA, Smpd1 induction AQP3, FLG, AQP3 induction COL I and III induction | Skin lipid production Skin hydration Collagen synthesis | [22] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchev, A.S.; Georgiev, M.I. Plant In Vitro Systems as a Sustainable Source of Active Ingredients for Cosmeceutical Application. Molecules 2020, 25, 2006. https://doi.org/10.3390/molecules25092006
Marchev AS, Georgiev MI. Plant In Vitro Systems as a Sustainable Source of Active Ingredients for Cosmeceutical Application. Molecules. 2020; 25(9):2006. https://doi.org/10.3390/molecules25092006
Chicago/Turabian StyleMarchev, Andrey S., and Milen I. Georgiev. 2020. "Plant In Vitro Systems as a Sustainable Source of Active Ingredients for Cosmeceutical Application" Molecules 25, no. 9: 2006. https://doi.org/10.3390/molecules25092006
APA StyleMarchev, A. S., & Georgiev, M. I. (2020). Plant In Vitro Systems as a Sustainable Source of Active Ingredients for Cosmeceutical Application. Molecules, 25(9), 2006. https://doi.org/10.3390/molecules25092006