A Systems Biological Approach to Understanding the Mechanisms Underlying the Therapeutic Potential of Red Ginseng Supplements against Metabolic Diseases
Abstract
1. Introduction
2. Results and Discussion
2.1. Component Analysis in Red Ginseng Using Compound Combination-Oriented Natural Product Database with Unified Terminology (COCONUT) DB
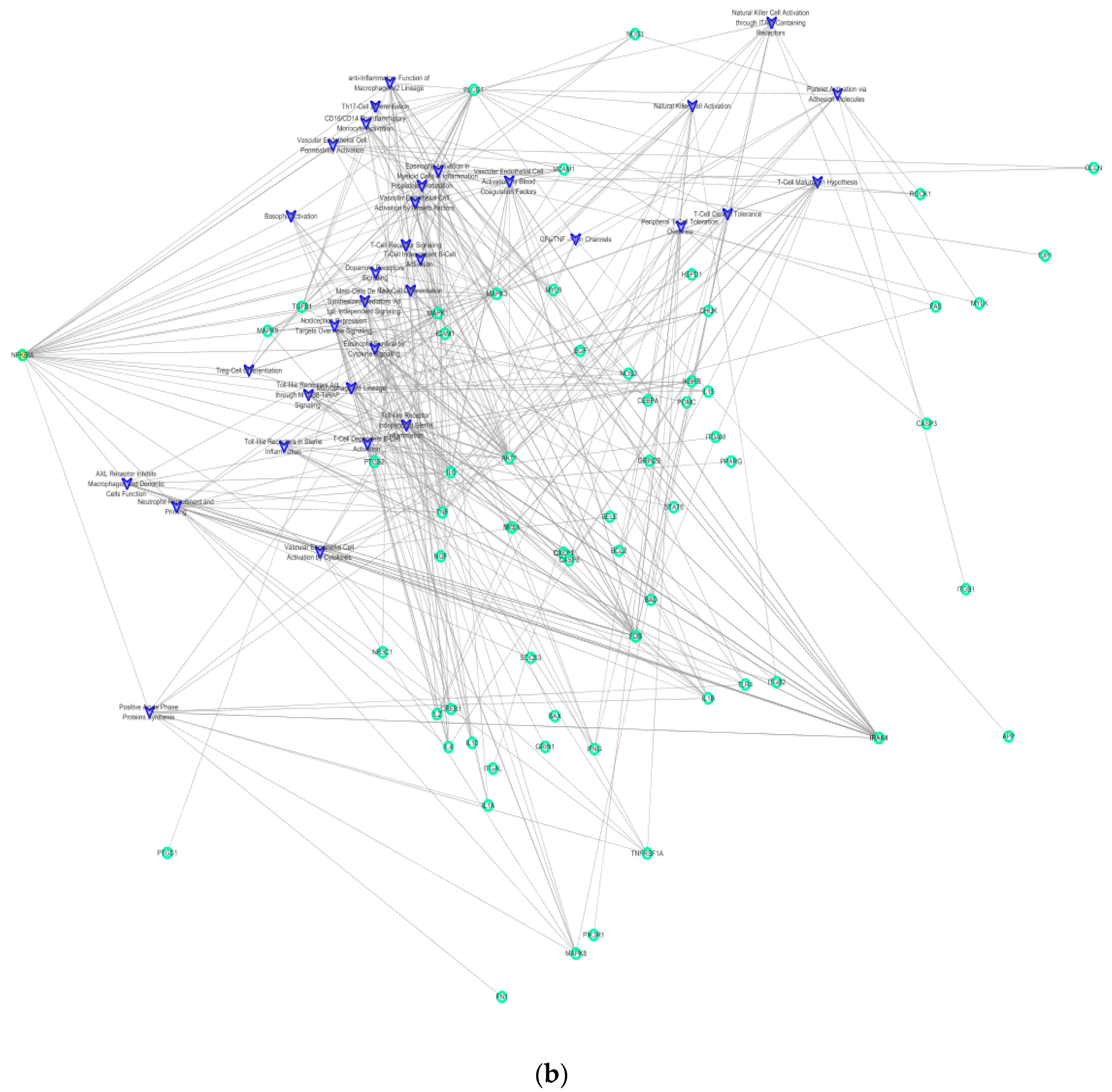
2.2. Context-Oriented Directed Associations (CODA) Network Path Exploration
2.3. Analysis of Enrichment Pathways
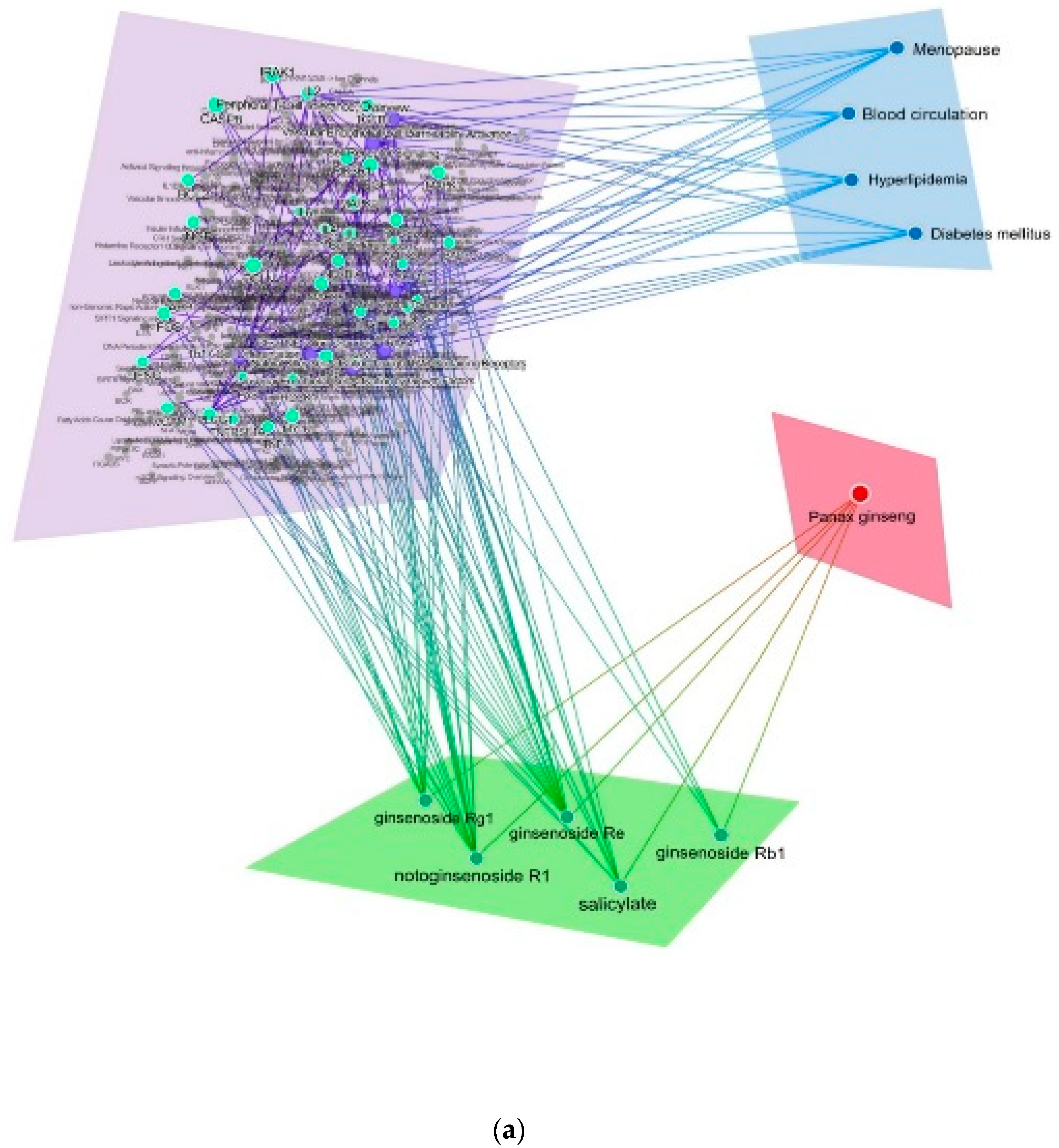
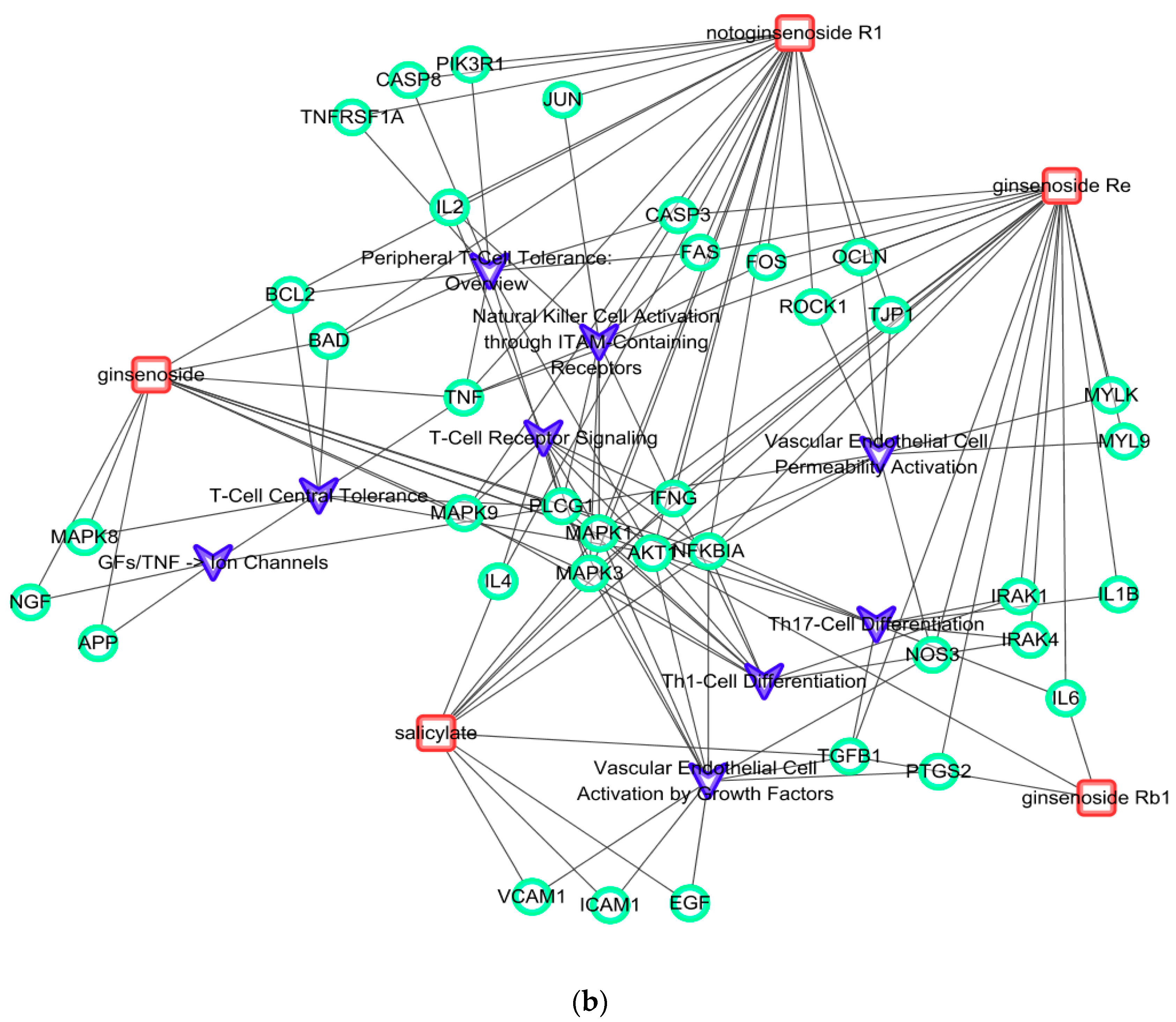
2.4. Comparison of Mechanism of Action of P. ginseng and Red Ginseng
3. Materials and Methods
3.1. Analysis of Components in Red Ginseng
3.2. Associated Phenotype Analysis
3.3. Exploration of Route With Potential Targets
3.4. Analysis of Enrichment Pathway
3.5. Network Construction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.; Dumas, M.-E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.M.; Forés, R.; Baena-Diez, J.M.; Pera, G.; Torán-Monserrat, P.; Sorribes, M.; Vicheto, M.; Reina, M.D.; Sancho, A.; Albaladejo, C.; et al. The Peripheral Arterial disease study (PERART/ARTPER): Prevalence and risk factors in the general population. BMC Public Health 2010, 10. [Google Scholar] [CrossRef]
- Bach, H.V.; Kim, J.; Myung, S.-K.; Cho, Y.A. Efficacy of Ginseng Supplements on Fatigue and Physical Performance: A Meta-analysis. J. Korean Med. Sci. 2016, 31, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Buettner, C.; Yeh, G.Y.; Phillips, R.S.; A Mittleman, M.; Kaptchuk, T.J. Systematic Review of the Effects of Ginseng on Cardiovascular Risk Factors. Ann. Pharmacother. 2006, 40, 83–95. [Google Scholar] [CrossRef]
- Hur, M.-H.; Lee, M.S.; Yang, H.-J.; Kim, C.; Bae, I.-L.; Ernst, E. Ginseng for Reducing the Blood Pressure in Patients with Hypertension: A Systematic Review and Meta-Analysis. J. Ginseng Res. 2010, 34, 342–347. [Google Scholar] [CrossRef]
- Kim, S.; Shin, B.-C.; Lee, M.S.; Lee, H.; Ernst, E. Red ginseng for type 2 diabetes mellitus: A systematic review of randomized controlled trials. Chin. J. Integr. Med. 2011, 17, 937–944. [Google Scholar] [CrossRef]
- Geng, J.; Ni, H.; Lee, M.S.; Wu, T.; Jiang, K.; Wang, G.; Zhou, A.L.; Malouf, R.; Dong, J. Ginseng for cognition. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Lee, M.S.; Yang, E.J.; Kim, J.-I.; Ernst, E. Ginseng for Cognitive Function in Alzheimer’s Disease: A Systematic Review. J. Alzheimer’s Dis. 2009, 18, 339–344. [Google Scholar] [CrossRef]
- Bahrke, M.S.; Morgan, W.P.; Stegner, A. Is Ginseng an Ergogenic Aid? Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 298–322. [Google Scholar] [CrossRef]
- Yun, T.-K. Panax ginseng—a non-organ-specific cancer preventive? Lancet Oncol. 2001, 2, 49–55. [Google Scholar] [CrossRef]
- Sun, S.; Qi, L.-W.; Du, G.-J.; Mehendale, S.R.; Wang, C.-Z.; Yuan, C.-S. Red notoginseng: Higher ginsenoside content and stronger anticancer potential than Asian and American ginseng. Food Chem. 2011, 125, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Taira, S.; Ikeda, R.; Yokota, N.; Osaka, I.; Sakamoto, M.; Kato, M.; Sahashi, Y. Mass Spectrometric Imaging of Ginsenosides Localization in Panax ginseng Root. Am. J. Chin. Med. 2010, 38, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Z.; Ni, M.; Sun, S.; Li, X.-L.; He, H.; Mehendale, S.R.; Yuan, C.-S. Detection of Adulteration of Notoginseng Root Extract with Other Panax Species by Quantitative HPLC Coupled with PCA. J. Agric. Food Chem. 2009, 57, 2363–2367. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.-Y. The Comparative Understanding between Red Ginseng and White Ginsengs, Processed Ginsengs (panax ginseng C. A. Meyer). J. Ginseng Res. 2005, 29, 1–8. [Google Scholar]
- Lee, K.J.; Lee, S.Y.; Ji, G.E. Diabetes-Ameliorating Effects of Fermented Red Ginseng and Causal Effects on Hormonal Interactions: Testing the Hypothesis by Multiple Group Path Analysis. J. Med. Food 2013, 16, 383–395. [Google Scholar] [CrossRef]
- Jovanovski, E.; Jenkins, A.; Dias, A.G.; Peeva, V.; Sievenpiper, J.; Arnason, J.T.; Rahelic, D.; Josse, R.G.; Vuksan, V. Effects of Korean Red Ginseng (Panax ginseng C.A. Mayer) and Its Isolated Ginsenosides and Polysaccharides on Arterial Stiffness in Healthy Individuals. Am. J. Hypertens. 2010, 23, 469–472. [Google Scholar] [CrossRef]
- Park, K.-S.; Park, K.-I.; Kim, J.-W.; Yun, Y.-J.; Kim, S.-H.; Lee, C.-H.; Park, J.-W.; Lee, J.-M. Efficacy and safety of Korean red ginseng for cold hypersensitivity in the hands and feet: A randomized, double-blind, placebo-controlled trial. J. Ethnopharmacol. 2014, 158, 25–32. [Google Scholar] [CrossRef]
- Kim, S.Y.; Seo, S.K.; Choi, Y.M.; Jeon, Y.E.; Lim, K.J.; Cho, S.; Lee, B.S.; Choi, Y.S. Effects of red ginseng supplementation on menopausal symptoms and cardiovascular risk factors in postmenopausal women. Menopause 2012, 19, 461–466. [Google Scholar] [CrossRef]
- Kang, J.; Lee, N.; Ahn, Y.; Lee, H. Study on improving blood flow with Korean red ginseng substances using digital infrared thermal imaging and Doppler sonography: Randomized, double blind, placebo-controlled clinical trial with parallel design. J. Tradit. Chin. Med. 2013, 33, 39–45. [Google Scholar] [CrossRef]
- Kitano, H. A robustness-based approach to systems-oriented drug design. Nat. Rev. Drug Discov. 2007, 6, 202–210. [Google Scholar] [CrossRef]
- Naylor, S.; Chen, J.Y. Unraveling human complexity and disease with systems biology and personalized medicine. Pers. Med. 2010, 7, 275–289. [Google Scholar] [CrossRef] [PubMed]
- So, S.-H.; Lee, J.W.; Kim, Y.-S.; Hyun, S.H.; Han, C.-K. Red ginseng monograph. J. Ginseng Res. 2018, 42, 549–561. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, J.; Xue, J.; Qin, Z.; Shen, F.; Liu, J.; Chen, X.; Li, X.; Wu, Z.; Xiao, W.; et al. In silico-based screen synergistic drug combinations from herb medicines: A case using Cistanche tubulosa. Sci. Rep. 2017, 7, 16364. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.-C.; Yuan, T.; Rui, B.-Y.; Zhu, Z.-Z.; Guo, S.-C.; Zhang, C. Exosomes derived from human platelet-rich plasma prevent apoptosis induced by glucocorticoid-associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the Akt/Bad/Bcl-2 signal pathway. Theranostics 2017, 7, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Toyomasu, Y.; Saravanaperumal, S.A.; Bardsley, M.R.; Smestad, J.; Lorincz, A.; Eisenman, S.T.; Cipriani, G.; Holte, M.H.N.; Al Khazal, F.J.; et al. Hyperglycemia Increases Interstitial Cells of Cajal via MAPK1 and MAPK3 Signaling to ETV1 and KIT, Leading to Rapid Gastric Emptying. Gastroenterology 2017, 153, 521–535. [Google Scholar] [CrossRef]
- Madeleine, M.M.; Johnson, L.G.; Malkki, M.; Resler, A.J.; Petersdorf, E.W.; McKnight, B.; Malone, K.E. Genetic variation in proinflammatory cytokines IL6, IL6R, TNF-region, and TNFRSF1A and risk of breast cancer. Breast Cancer Res. Treat. 2011, 129, 99–887. [Google Scholar] [CrossRef]
- Kim, I.; Moon, S.-O.; Kim, S.H.; Kim, H.J.; Koh, Y.S.; Koh, G.Y. Vascular Endothelial Growth Factor Expression of Intercellular Adhesion Molecule 1 (ICAM-1), Vascular Cell Adhesion Molecule 1 (VCAM-1), and E-selectin through Nuclear Factor-κB Activation in Endothelial Cells. J. Boil. Chem. 2000, 276, 7614–7620. [Google Scholar] [CrossRef]
- Fu, C.; Yin, D.; Nie, H.; Sun, D. Notoginsenoside R1 Protects HUVEC Against Oxidized Low Density Lipoprotein (Ox-LDL)-Induced Atherogenic Response via Down-Regulating miR-132. Cell. Physiol. Biochem. 2018, 51, 1739–1750. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, C.-Y.; Zhu, P.; Xu, S.-F.; Luo, Y.-M.; Deng, J.; Yang, D.-L. Ginsenoside Re inhibits vascular neointimal hyperplasia in balloon-injured carotid arteries through activating the eNOS/NO/cGMP pathway in rats. Biomed. Pharmacother. 2018, 106, 1091–1097. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Zhang, C.; Zhang, X.; Ye, J.; Kuang, S.; Sun, G.; Sun, X. Notoginsenoside R1 Protects Against Diabetic Cardiomyopathy Through Activating Estrogen Receptor α and Its Downstream Signaling. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Zhao, S.; Yan, L.; Li, X.; Zhang, Z.; Sun, Y.; Wang, J. Notoginsenoside R1 suppresses wear particle-induced osteolysis and RANKL mediated osteoclastogenesis in vivo and in vitro. Int. Immunopharmacol. 2017, 47, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Ji, G.E. Free-fatty-acid-regulating effects of fermented red ginseng are mediated by hormones and by the autonomic nervous system. J. Ginseng Res. 2014, 38, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.S.; Hwang, I.; Oh, K.J.; Na Lee, M.; Park, K. The Effect of Korean Red Ginseng on Sexual Function in Premenopausal Women: Placebo-Controlled, Double-Blind, Crossover Clinical Trial. Evid. Based Complement. Altern. Med. 2015, 2015, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.-S.; Lee, J.-J.; Kim, Y.-I.; Yu, J.-Y.; Park, E.-S.; Im, J.-S.; You, S.-H.; Oh, K.-W.; Lee, M.-K.; Wee, J.-J.; et al. Effect of Korean Red Ginseng Extract on Blood Circulation in Healthy Volunteers: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Ginseng Res. 2007, 31, 109–116. [Google Scholar]
- Sievenpiper, J.L.; Arnason, J.T.; Leiter, L.A.; Vuksan, V. Decreasing, null and increasing effects of eight popular types of ginseng on acute postprandial glycemic indices in healthy humans: The role of ginsenosides. J. Am. Coll. Nutr. 2004, 23, 248–258. [Google Scholar] [CrossRef]
- Cho, Y.-H.; Ahn, S.C.; Lee, S.Y.; Jeong, D.-W.; Choi, E.J.; Kim, Y.J.; Lee, J.G.; Lee, Y.-H.; Shin, B.-C. Effect of Korean red ginseng on insulin sensitivity in non-diabetic healthy overweight and obese adults. Asia Pac. J. Clin. Nutr. 2013, 22, 365–371. [Google Scholar]
- Sievenpiper, J.L.; Sung, M.-K.; Di Buono, M.; Seung-Lee, K.; Nam, K.Y.; Arnason, J.T.; Leiter, L.A.; Vuksan, V. Korean red ginseng rootlets decrease acute postprandial glycemia: Results from sequential preparation and dose-finding studies. J. Am. Coll. Nutr. 2006, 25, 100–107. [Google Scholar] [CrossRef]
- Jung, H.L.; Kwak, H.E.; Kim, S.S.; Kim, Y.C.; Lee, C.D.; Byurn, H.K.; Kang, H.Y. Effects of Panax ginseng Supplementation on Muscle Damage and Inflammation after Uphill Treadmill Running in Humans. Am. J. Chin. Med. 2011, 39, 441–450. [Google Scholar] [CrossRef]
- De Souza, L.R.; Jenkins, A.L.; Jovanovski, E.; Rahelić, D.; Vuksan, V. Ethanol extraction preparation of American ginseng (Panax quinquefolius L) and Korean red ginseng (Panax ginseng C.A. Meyer): Differential effects on postprandial insulinemia in healthy individuals. J. Ethnopharmacol. 2015, 159, 55–61. [Google Scholar] [CrossRef]
- Oh, M.-R.; Park, S.-H.; Kim, S.-Y.; Back, H.-I.; Kim, M.-G.; Jeon, J.-Y.; Ha, K.-C.; Na, W.-T.; Cha, Y.-S.; Park, B.-H.; et al. Postprandial glucose-lowering effects of fermented red ginseng in subjects with impaired fasting glucose or type 2 diabetes: A randomized, double-blind, placebo-controlled clinical trial. BMC Complement. Altern. Med. 2014, 14, 237. [Google Scholar] [CrossRef]
- De Souza, L.R.; Jenkins, A.L.; Sievenpiper, J.L.; Jovanovski, E.; Rahelić, D.; Vuksan, V. Korean red ginseng (Panax ginseng C.A. Meyer) root fractions: Differential effects on postprandial glycemia in healthy individuals. J. Ethnopharmacol. 2011, 137, 245–250. [Google Scholar] [CrossRef]
- Kim, H.-O.; Park, M.-J.; Han, J.-S. Effects of Fermented Red Ginseng Supplementation on Blood Glucose and Insulin Resistance in Type 2 Diabetic Patients. J. Korean Soc. Food Sci. Nutr. 2011, 40, 696–703. [Google Scholar] [CrossRef]
- Bang, H.; Kwak, J.H.; Ahn, H.Y.; Shin, D.Y.; Lee, J.H. Korean Red Ginseng Improves Glucose Control in Subjects with Impaired Fasting Glucose, Impaired Glucose Tolerance, or Newly Diagnosed Type 2 Diabetes Mellitus. J. Med. Food 2014, 17, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jung, J.; Yoon, S.; Kwon, M.; Bae, S.; Yim, S.; Lee, J.; Kim, S.; Kang, Y.; Lee, D.S. CODA: Integrating multi-level context-oriented directed associations for analysis of drug effects. Sci. Rep. 2017, 7, 7519. [Google Scholar] [CrossRef]
- Valdeolivas, A.; Tichit, L.; Navarro, C.; Perrin, S.; Odelin, G.; Levy, N.; Cau, P.; Remy, E.; Baudot, A. Random walk with restart on multiplex and heterogeneous biological networks. Bioinformatics 2018, 35, 497–505. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
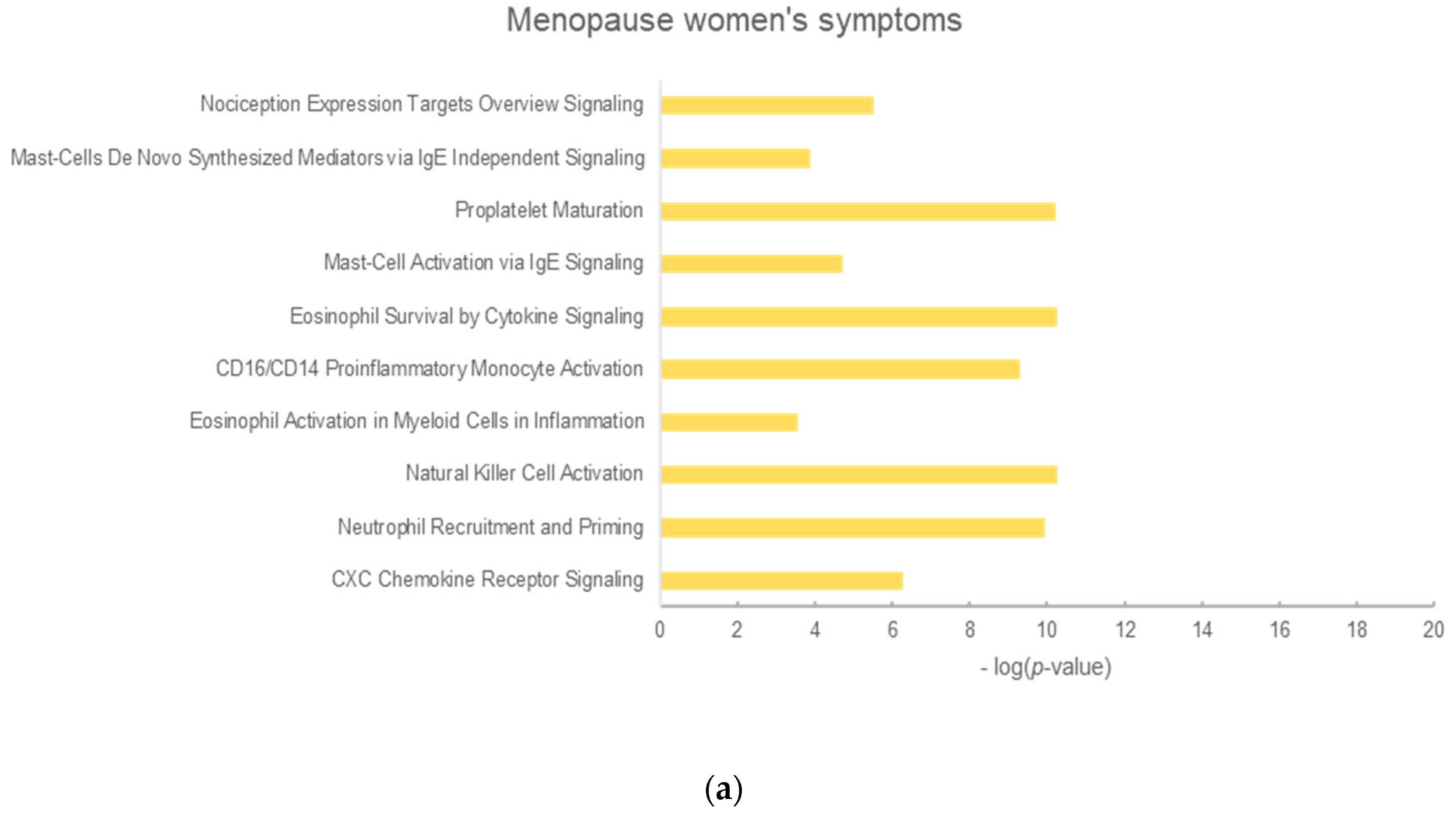

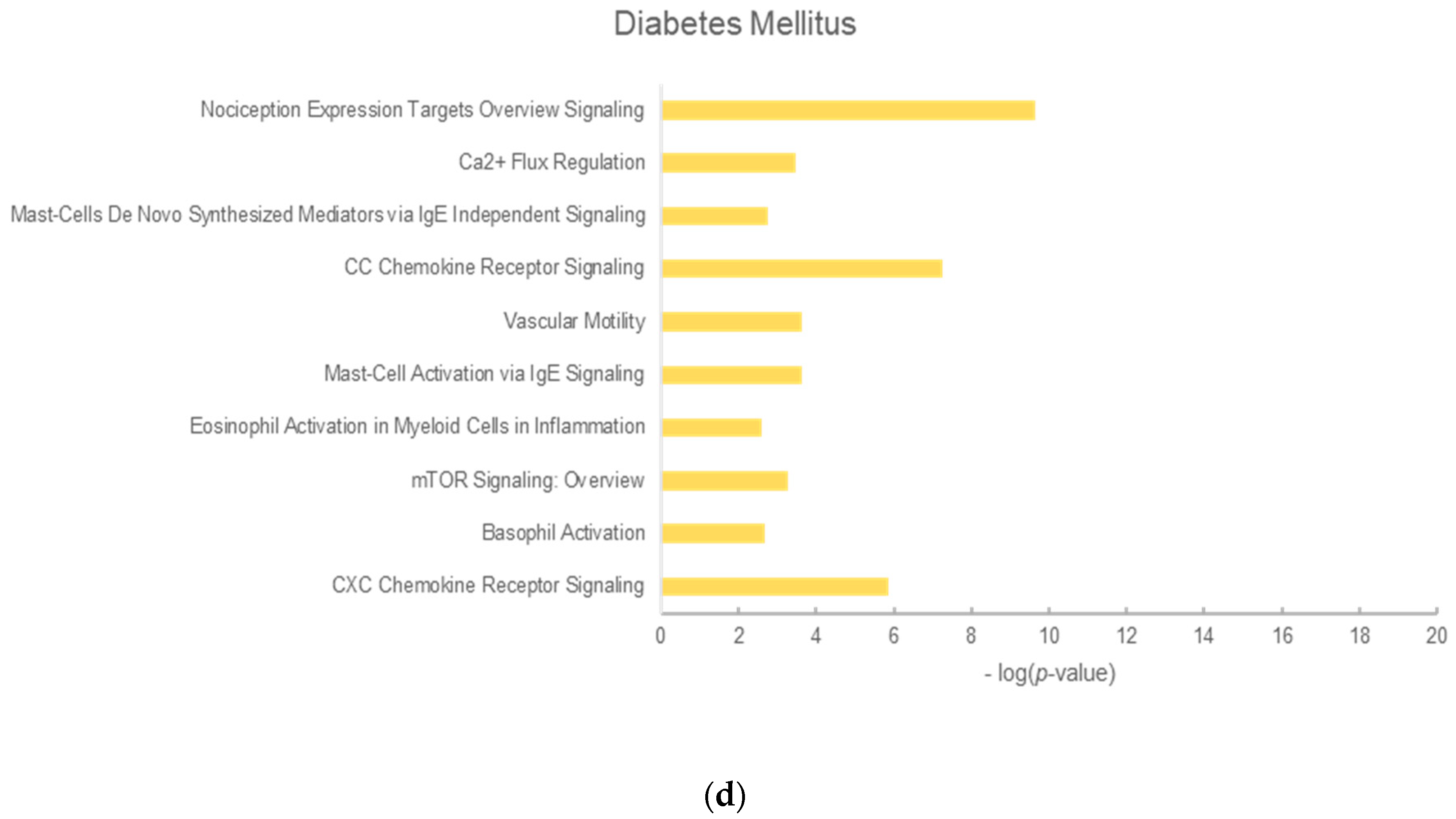


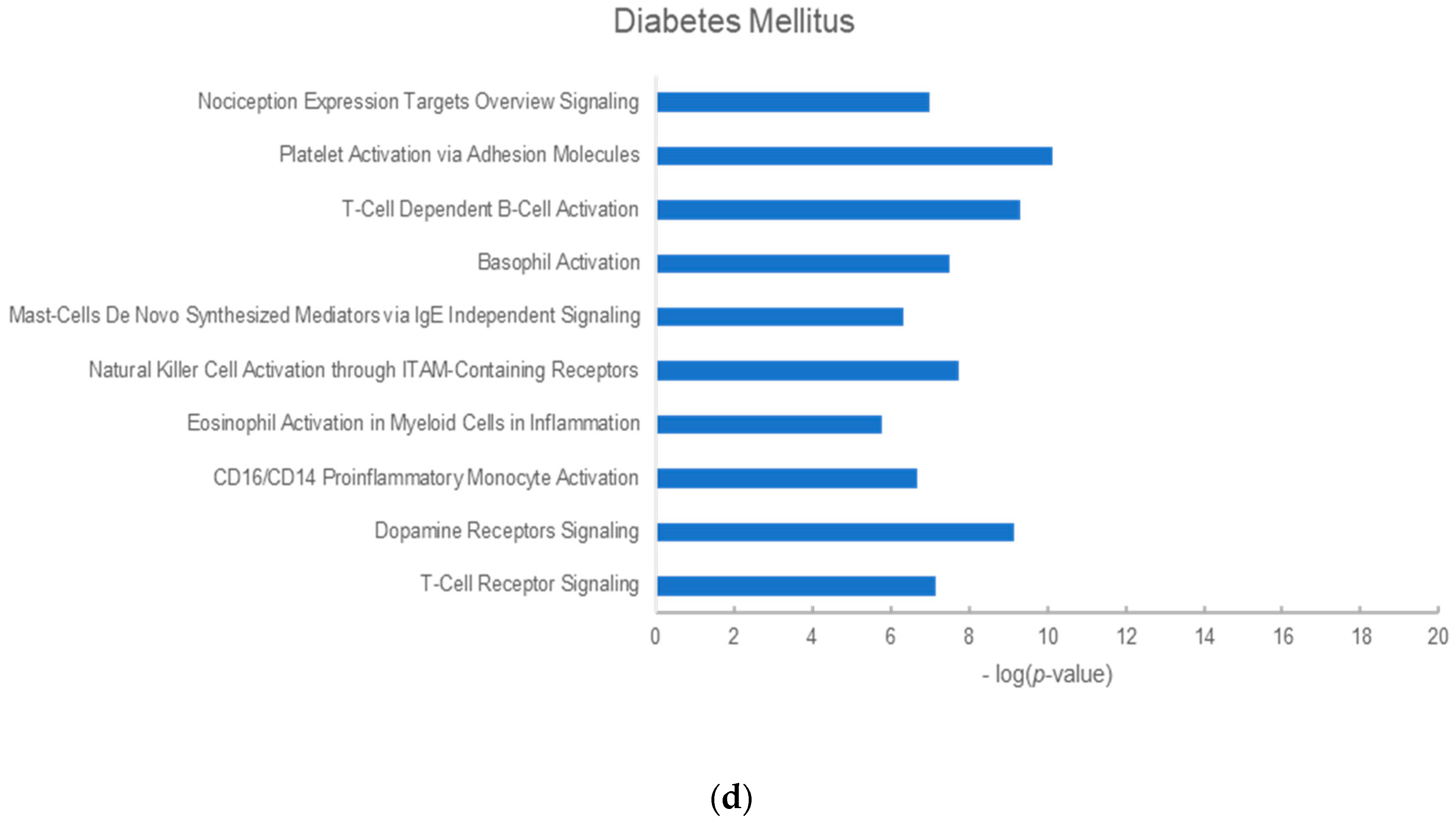
| No. | Menopausal Women’s Symptoms | Blood Circulation | Hyperlipidemia | Diabetes Mellitus |
|---|---|---|---|---|
| 1 | TNF | MAPK1 | PLCG1 | PLCG1 |
| 2 | AKT1 | TNF | IRAK4 | IRAK4 |
| 3 | FOS | MAPK3 | MAPK9 | MAPK9 |
| 4 | JUN | NFKBIA | - | - |
| 5 | MAPK1 | IL6 | - | - |
| 6 | MAPK3 | AKT1 | - | - |
| 7 | NFKBIA | FOS | - | - |
| 8 | CREB1 | TGFB1 | - | - |
| 9 | IL6 | ROCK1 | - | - |
| 10 | IRAK1 | BCL2 | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, E.; Lim, Y.; Kim, K.J.; Ki, H.-H.; Lee, D.; Suh, J.; So, S.-H.; Kwon, O.; Kim, J.Y. A Systems Biological Approach to Understanding the Mechanisms Underlying the Therapeutic Potential of Red Ginseng Supplements against Metabolic Diseases. Molecules 2020, 25, 1967. https://doi.org/10.3390/molecules25081967
Jeong E, Lim Y, Kim KJ, Ki H-H, Lee D, Suh J, So S-H, Kwon O, Kim JY. A Systems Biological Approach to Understanding the Mechanisms Underlying the Therapeutic Potential of Red Ginseng Supplements against Metabolic Diseases. Molecules. 2020; 25(8):1967. https://doi.org/10.3390/molecules25081967
Chicago/Turabian StyleJeong, Eunseon, Yeni Lim, Kyeong Jin Kim, Hyeon-Hui Ki, Doheon Lee, Jaehyun Suh, Seung-Ho So, Oran Kwon, and Ji Yeon Kim. 2020. "A Systems Biological Approach to Understanding the Mechanisms Underlying the Therapeutic Potential of Red Ginseng Supplements against Metabolic Diseases" Molecules 25, no. 8: 1967. https://doi.org/10.3390/molecules25081967
APA StyleJeong, E., Lim, Y., Kim, K. J., Ki, H.-H., Lee, D., Suh, J., So, S.-H., Kwon, O., & Kim, J. Y. (2020). A Systems Biological Approach to Understanding the Mechanisms Underlying the Therapeutic Potential of Red Ginseng Supplements against Metabolic Diseases. Molecules, 25(8), 1967. https://doi.org/10.3390/molecules25081967






