Organic Salts of Pharmaceutical Impurity p-Aminophenol
Abstract
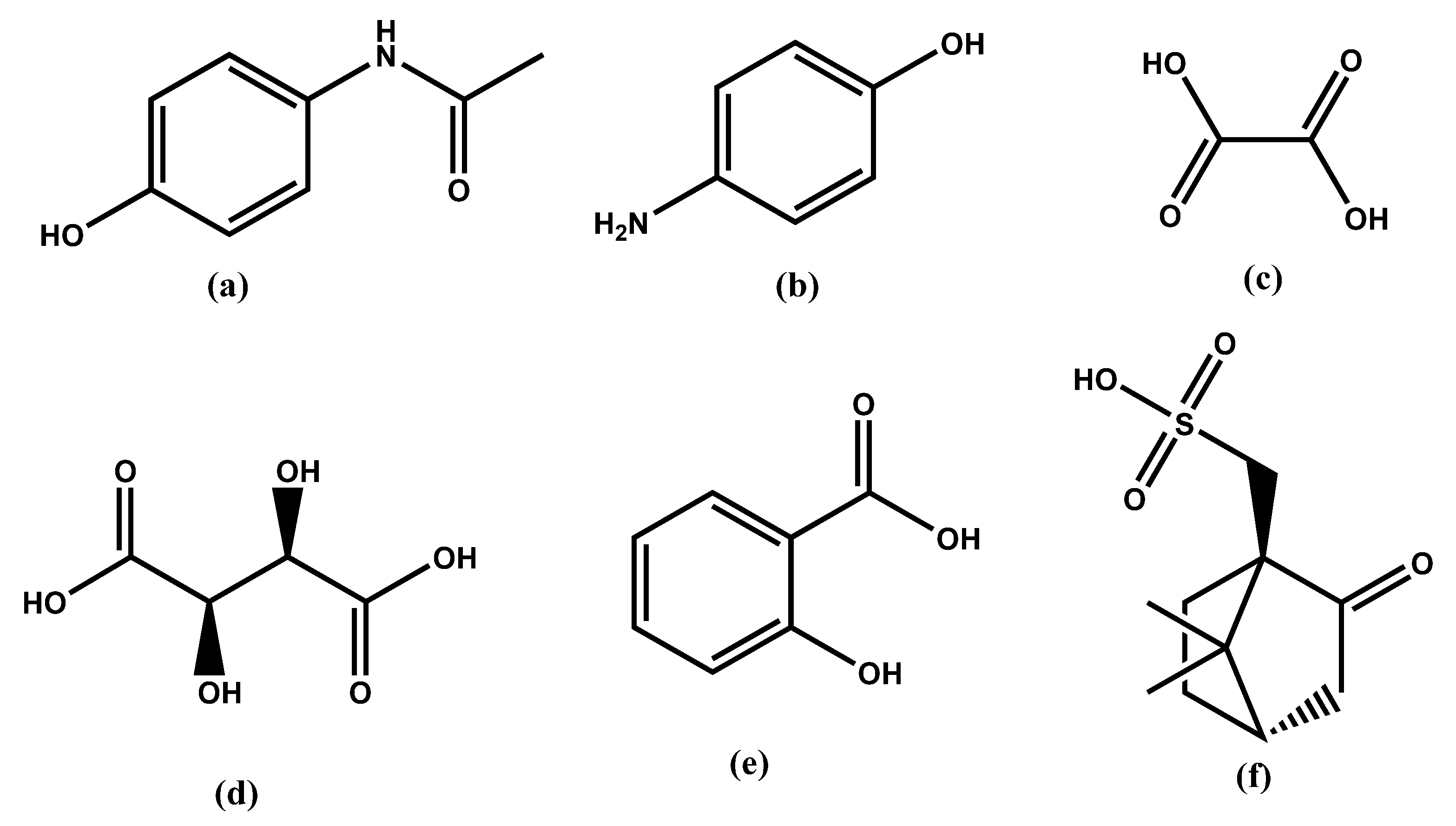
1. Introduction
2. Results and Discussion
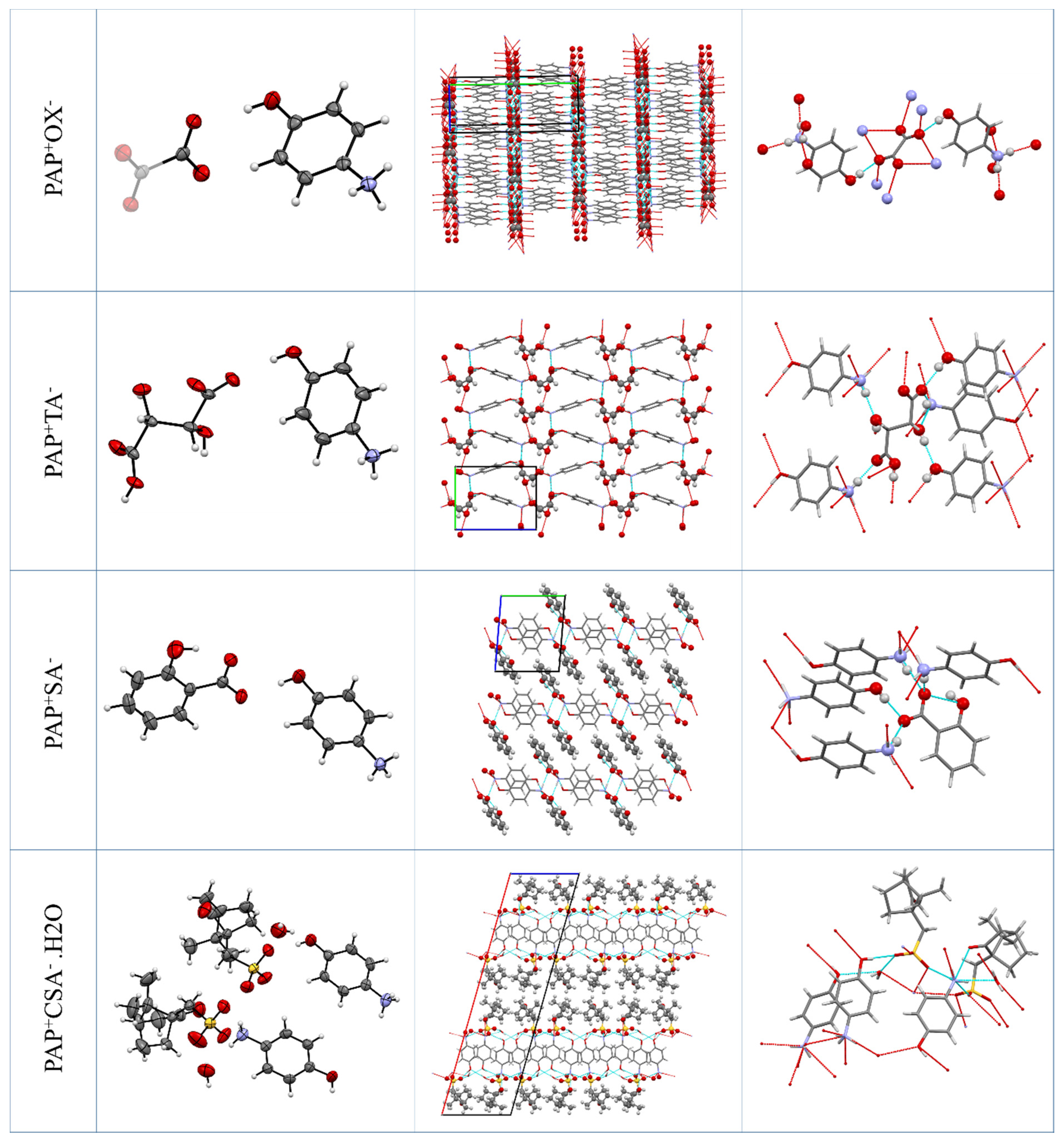
2.1. X-ray Crystallography
2.2. Crystal Structure Analysis
2.2.1. PAP+OX− Salt (2:1)
2.2.2. PAP+TA− Salt (1:1)
2.2.3. PAP+SA− Salt (1:1)
2.2.4. PAP+CSA−·H2O Salt (1:1:1)
2.3. Crystallization Experiments
2.3.1. Crystallization of Paracetamol in the Absence of PAP
2.3.2. Crystallization of Paracetamol in the Absence and Presence of PAP
2.3.3. Crystallization of PA in the Presence of PAP and a Counterion
3. Materials and Methods
3.1. Materials and General Methods
3.2. Synthesis of the PAP Salts
3.3. Impurity Spiking Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cok, I.; Emerce, E. Overview of impurities in pharmaceuticals: Toxicological aspects. Asian Chem. Lett. 2012, 16, 87–97. [Google Scholar]
- Roy, J. Pharmaceutical impurities—A mini-review. AAPS PharmSciTech 2002, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- International Conferences on Harmonization, Draft Revised Guidance on Impurities in New Drug Substances. Q3A(R). Fed. Regist. 2000, 65, 45085–45090.
- Jacobson-Kram, D.; McGovern, T. Toxicological overview of impurities in pharmaceutical products. Adv. Drug Deliv. Rev. 2007, 59, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Liu, F.; Motto, M. Identification of Pharmaceutical Impurities in Formulated Dosage forms. J. Pharm. Sci. 2011, 100, 1228–1259. [Google Scholar] [CrossRef] [PubMed]
- Kuturu, S.; Nangia, A. Lornoxicam Salts: Crystal Structures, Conformations, and Solubility. Cryst. Growth Des. 2014, 14, 2945–2953. [Google Scholar]
- Aitipamula, S.; Wong, A.B.H.; Chow, P.S.; Tan, R.B.H. Novel solid forms of oxaprozin: Cocrystals and an extended release drug–drug salt of salbutamol. RSC Adv. 2016, 6, 34110–34119. [Google Scholar] [CrossRef]
- Li, Y.; Bentzley, C.M.; Tarloffa, J.B. Comparison of para-aminophenol cyto toxicity in rat renal epithelial cells and hepatocytes. Toxicology 2004, 209, 69–76. [Google Scholar] [CrossRef]
- Fu, X.; Chen, T.S.; Ray, M.B.; Nagasawa, H.T.; Williams, W.M. p-Aminophenol-induced hepatotoxicity in hamsters: Role of glutathione. J. Biochem. Mol. Toxicol. 2004, 18, 154–161. [Google Scholar] [CrossRef]
- Bomhard, E.M.; Herbold, B.A. Genotoxic activities of aniline and its metabolites and their relationship to the carcinogenicity of aniline in the spleen of rats. Crit. Revs. Toxicol. 2005, 35, 783–835. [Google Scholar] [CrossRef]
- Song, H.; Chen, T.S. P-Aminophenol-induced liver toxicity: Tentative evidence of a role for acetaminophen. J. Biochem. Mol. Toxicol. 2001, 15, 34–40. [Google Scholar] [CrossRef]
- Newton, J.F.; Kuo, C.H.; Gemborys, M.W.; Mudge, G.H.; Hook, J.B. Nephrotoxicity of p-aminophenol, a metabolite of acetaminophen, in the Fischer 344 rat. Toxicol. Appl. Pharmacol. 1982, 65, 336–344. [Google Scholar] [CrossRef]
- Burnett, C.M.; Re, T.A.; Rodriguez, S.; Loehr, R.F.; Dressler, W.E. The toxicity of p-Aminophenol in the Sprague-Dawley rat: Effects on growth, reproduction and foetal development. Food Chem. Toxicol. 1989, 27, 691–698. [Google Scholar] [CrossRef]
- Pradeep, N.V.; Anupama, S.; Navya, K.; Shalini, H.N.; Idris, M.; Hampannavar, U.S. Biological removal of phenol from wastewaters: A mini review. Appl. Water Sci. 2015, 5, 105–112. [Google Scholar] [CrossRef]
- Sun, M.; Yao, R.; You, Y.; Deng, S.; Gao, W. Degradation of 4-aminophenol by hydrogen peroxide oxidation using enzyme from Serratia marcescens as catalyst. Front. Environ. Sci. Eng. China 2007, 1, 95–98. [Google Scholar] [CrossRef]
- Erhan, E.; Keskinler, B.; Akay, G.; Algur, O.F. Removal of phenol from water by membrane-immobilized enzymes. Part I. Dead-end filtration. J. Membr. Sci. 2002, 206, 361–373. [Google Scholar] [CrossRef]
- Snodin, D.J.; McCrossen, S.D. Guidelines and pharmacopoeial standards for pharmaceutical impurities: Overview and critical assessment. Regul. Toxicol. Pharmacol. 2012, 63, 298–312. [Google Scholar] [CrossRef]
- Khandavilli, U.B.R.; Gangavaram, S.; Rajesh Goud, N.; Cherukuvada, S.; Raghavender, S.; Nangia, A.; Manjunatha, S.G.; Nambiar, S.; Pal, S. High solubility crystalline hydrates of Na and K furosemide salts. CrystEngComm 2014, 16, 4842–4852. [Google Scholar] [CrossRef]
- Khandavilli, U.B.R.; Bhogala, B.R.; Maguire, A.R.; Lawrence, S.E. Symmetry assisted tuning of bending and brittle multi-component forms of probenecid. Chem. Commun. 2017, 53, 3881–3884. [Google Scholar] [CrossRef]
- Khandavilli, U.B.R.; Skořepová, E.; Sinha, A.S.; Bhogala, B.R.; Maguire, N.M.; Maguire, A.R.; Lawrence, S.E. Cocrystals and a Salt of the Bioactive Flavonoid: Naringenin. Cryst. Growth Des. 2018, 18, 4571–4577. [Google Scholar] [CrossRef]
- Suresh, K.; Khandavilli, U.B.R.; Gunnam, A.; Nangia, A. Polymorphism, isostructurality and physicochemical properties of glibenclamide salts. CrystEngComm 2017, 19, 918–929. [Google Scholar] [CrossRef]
- Serajuddin, A.T.M. Salt formation to improve drug solubility. Adv. Drug Deliv. Rev. 2007, 59, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Aitipamula, S.; Wong, A.B.H.; Chow, P.S.; Tan, R.B.H. Pharmaceutical salts of haloperidol with some carboxylic acids and artificial sweeteners: Hydrate formation, polymorphism, and physicochemical properties. Cryst. Growth Des. 2014, 14, 2542–2556. [Google Scholar] [CrossRef]
- Cherukuvada, S.; Nangia, A. Salts and Ionic Liquid of The Antituberculosis Drug S,S-Ethambutol. Cryst. Growth Des. 2013, 13, 152–1760. [Google Scholar] [CrossRef]
- Paluch, K.J.; McCabe, T.; Müller-Bunz, H.; Corrigan, O.I.; Healy, A.M.; Tajber, L. Formation and physicochemical properties of crystalline and amorphous salts with different stoichiometries formed between ciprofloxacin and succinic acid. Mol. Pharm. 2013, 10, 3640–3654. [Google Scholar] [CrossRef]
- His, H.K.; Chadwik, K.; Fried, A.; Kenny, M.; Myerson, A.S. Separation of impurities from solution by selective co-crystal formation. CrystEngComm 2012, 14, 2386–2388. [Google Scholar]
- Billot, P.; Hosek, P.; Perin, M.-A. Efficient Purification of an Active Pharmaceutical Ingredient via Cocrystallization: From Thermodynamics to Scale-Up Org. Process Res. Dev. 2013, 17, 505–511. [Google Scholar] [CrossRef]
- Bhogala, B.R.; Basavoju, S.; Nangia, A. Tape and layer structures in cocrystals of some di and tricarboxylic acids with 4,4-bipyridines and isonicotinamide. From binary to ternary cocrystals. CrystEngComm 2005, 7, 551–562. [Google Scholar] [CrossRef]
- Khandavilli, U.B.R.; Yousuf, M.; Schaller, B.E.; Steendam, R.R.E.; Keshavarz, L.; McArdle, P.; Frawley, P.J. Plastically bendable pregabalin multi-component systems with improved tabletability and compressibility. CrystEngComm 2020, 22, 412–415. [Google Scholar] [CrossRef]
- Wyszecka-Kaszuba, E.; Warowna-Grześkiewicz, M.; Fijałek, Z. Determination of 4-aminophenol impurities in multicomponent analgesic preparations by HPLC with amperometric detection. J. Pharm. Biomed. Anal. 2003, 32, 1081–1086. [Google Scholar] [CrossRef]
- He, Z.; Song, S.; Ying, H.; Xu, L.; Chen, J. p-Aminophenol degradation by ozonation combined with sonolysis: Operating conditions influence and mechanism. Ultrason. Sonochem. 2007, 14, 568–574. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds PA, PAP, PAP+SA−, PAP+OX−, PAP+CSA−·H2O, PAP+TA− are available from the authors. |



| Compound | PAP+SA− | PAP+OX− | PAP+CSA−·H2O | PAP+TA− |
|---|---|---|---|---|
| Formula | C13H13NO4 | C7H8NO3 | C16H25NO7S | C10H14NO7 |
| MW, g/mol | 247.25 | 154.14 | 359.43 | 259.21 |
| Crystal system | triclinic | monoclinic | monoclinic | monoclinic |
| Space group, Z | P, 2 | P 21/n, 4 | C2, 4 | P 21, 2 |
| a, Å | 6.798(7) | 6.6471(5) | 42.654(2) | 7.206(6) |
| b, Å | 8.596(1) | 17.357(7) | 7.4090(3) | 8.043(7) |
| c, Å | 10.256(1) | 6.768(5) | 11.765(5) | 10.53 (7) |
| α, ° | 93.756(4) | 90 | 90 | 90 |
| β, ° | 99.530(4) | 117.233(5) | 97.2740(1) | 98.361(8) |
| γ, ° | 94.663(4) | 90 | 90 | 90 |
| V, Å3 | 587.2(6) | 694.2(3) | 3575.7(3) | 603.9(1) |
| Dc gcm−3 | 1.398 | 1.475 | 1.335 | 1.426 |
| μ, mm−1 | 0.105 | 0.117 | 0.212 | 0.123 |
| 2θ range, ° | 3.0–26.38 | 3.56–26.36 | 2.79–26.39 | 2.86–26.85 |
| T, K | 299 | 282 | 304 | 299 |
| Total ref. | 14,425 | 7997 | 23,035 | 9330 |
| Unique ref. | 2398 | 1414 | 7278 | 2480 |
| Obs. ref., I > 2σ(I) | 2046 | 1042 | 5649 | 2347 |
| # Parameters | 178 | 117 | 464 | 191 |
| R1 [I > 2σ(I)] | 0.0393 | 0.0450 | 0.0474 | 0.0727 |
| wR2 | 0.0967 | 0.1003 | 0.1021 | 0.1601 |
| S | 0.9792 | 0.9748 | 0.9847 | 0.9779 |
| Acid | Product (Ratio in the Bracket) |
|---|---|
| Oxalic acid (OX) | PAP+OX− Salt (2:1) |
| l-tartaric acid (TA) | PAP+TA− Salt (1:1) |
| p-Salicylic acid (SA) | PAP+SA− Salt (1:1) |
| (1S)-(+)-10-Camphorsulfonic acid (CSA) | PAP+CSA−·H2O Salt hydrate(1:1:1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khandavilli, U.B.R.; Keshavarz, L.; Skořepová, E.; Steendam, R.R.E.; Frawley, P.J. Organic Salts of Pharmaceutical Impurity p-Aminophenol. Molecules 2020, 25, 1910. https://doi.org/10.3390/molecules25081910
Khandavilli UBR, Keshavarz L, Skořepová E, Steendam RRE, Frawley PJ. Organic Salts of Pharmaceutical Impurity p-Aminophenol. Molecules. 2020; 25(8):1910. https://doi.org/10.3390/molecules25081910
Chicago/Turabian StyleKhandavilli, U. B. Rao, Leila Keshavarz, Eliška Skořepová, René R. E. Steendam, and Patrick J. Frawley. 2020. "Organic Salts of Pharmaceutical Impurity p-Aminophenol" Molecules 25, no. 8: 1910. https://doi.org/10.3390/molecules25081910
APA StyleKhandavilli, U. B. R., Keshavarz, L., Skořepová, E., Steendam, R. R. E., & Frawley, P. J. (2020). Organic Salts of Pharmaceutical Impurity p-Aminophenol. Molecules, 25(8), 1910. https://doi.org/10.3390/molecules25081910






