Effect of (−)-Epigallocatechin Gallate to Staphylococcal Enterotoxin A on Toxin Activity
Abstract
1. Introduction
2. Results
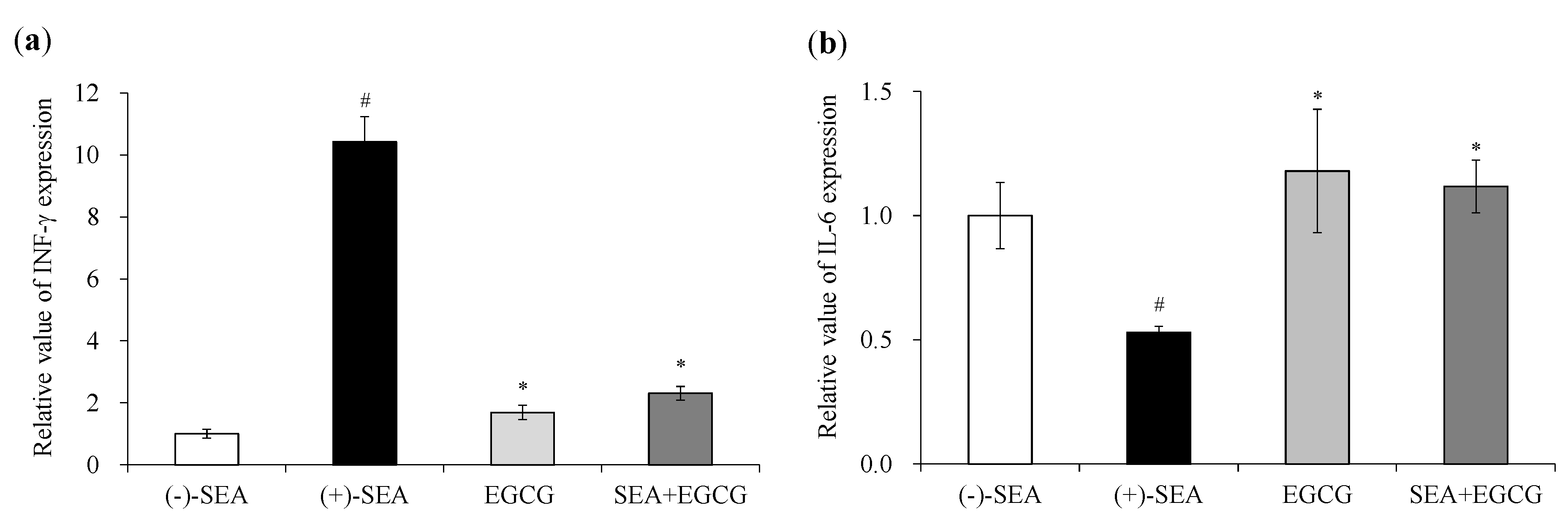
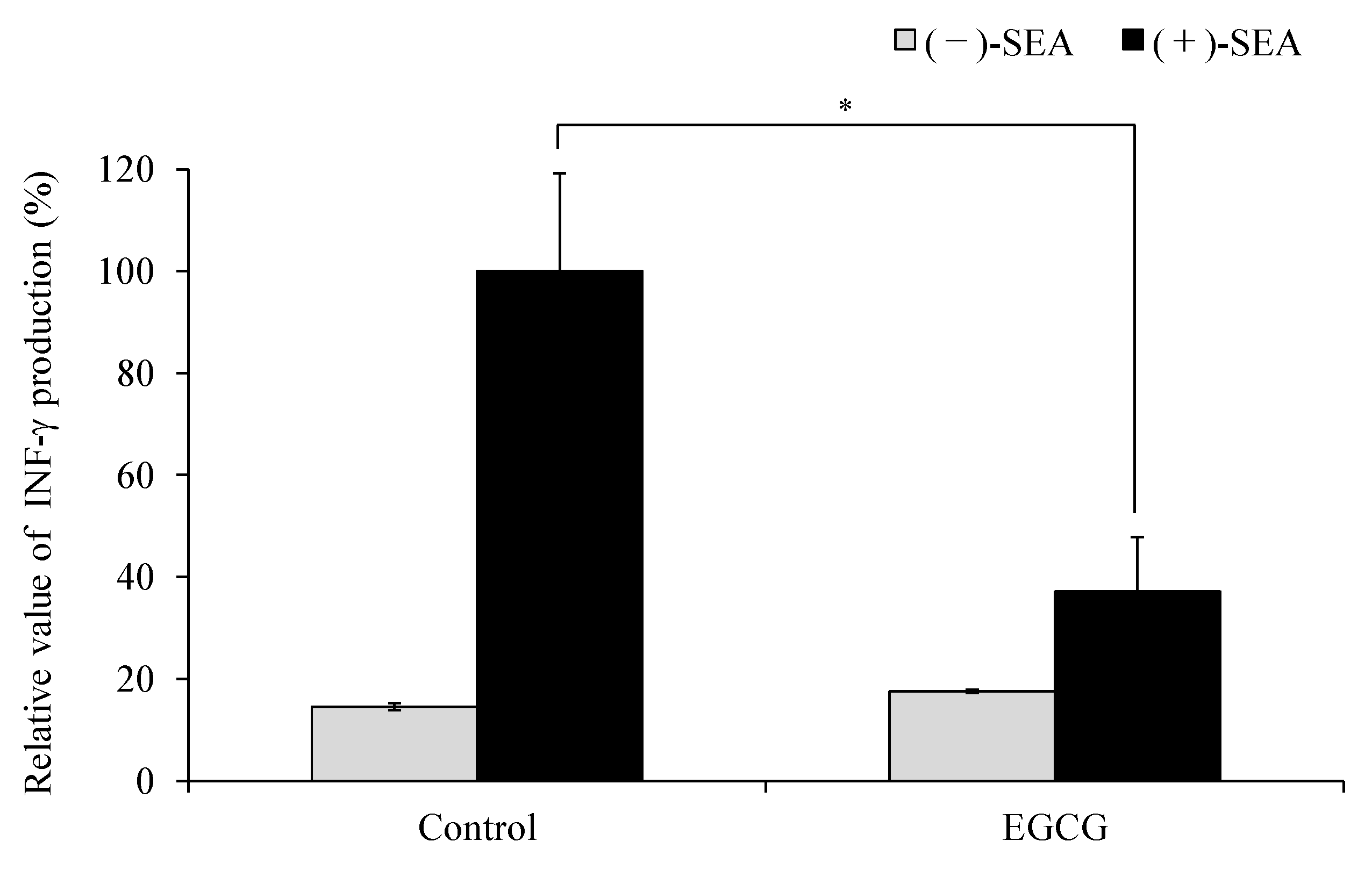
2.1. Inhibitory Effects of EGCG on SEA-Induced Cytokine Gene Expression and Production
2.2. Inhibitory Effects of EGCG on SEA-Induced the Cell Proliferation
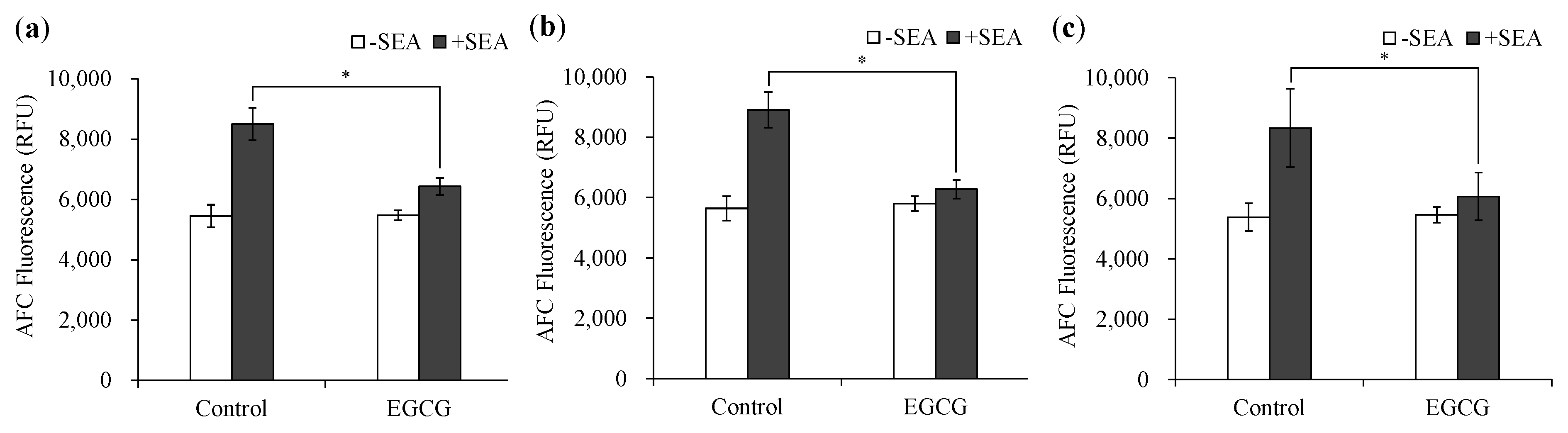
2.3. Effect of Methylated EGCG on SEA Cytotoxicity
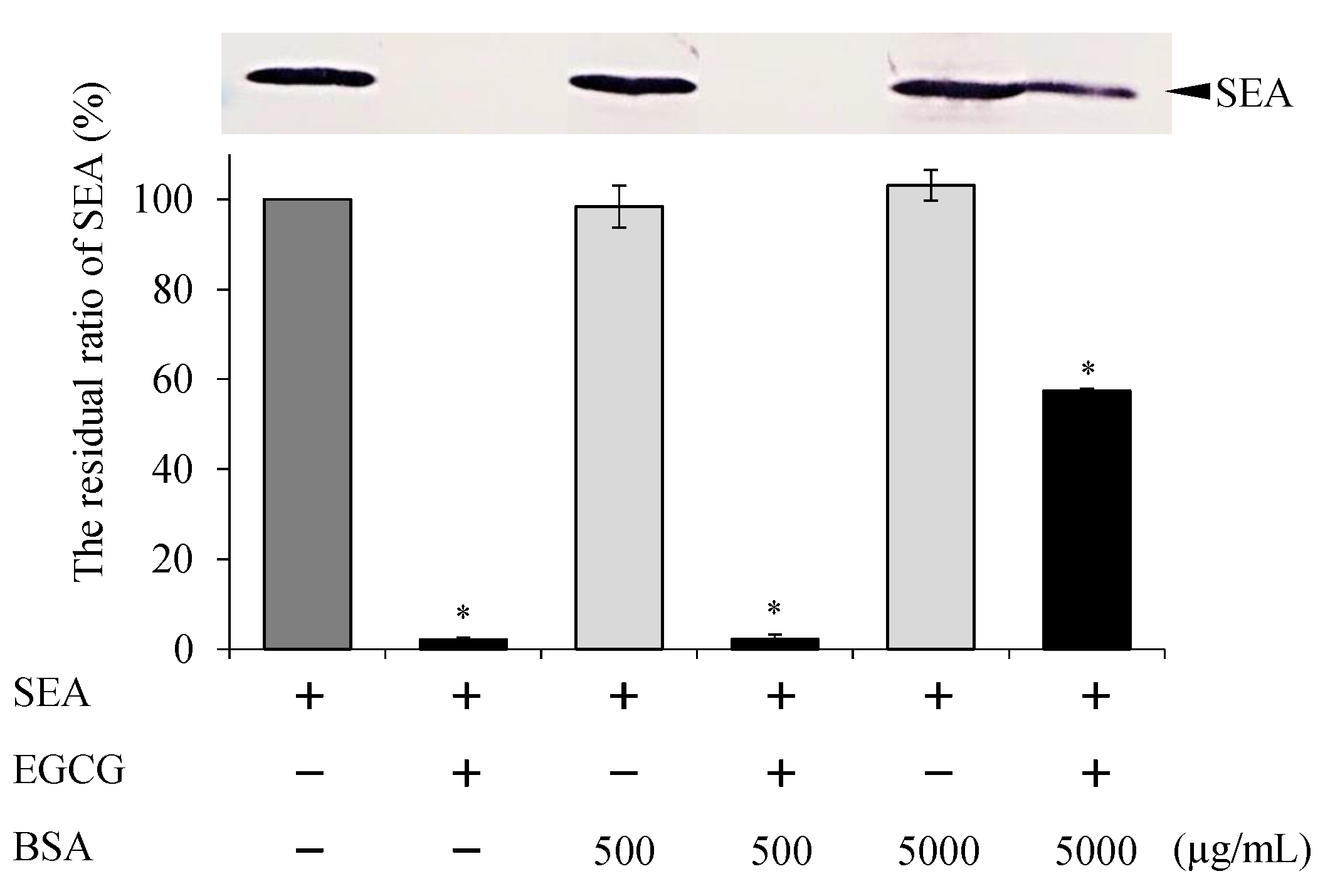
2.4. Effect of pH and Protein Treatment on Binding of EGCG with SEA
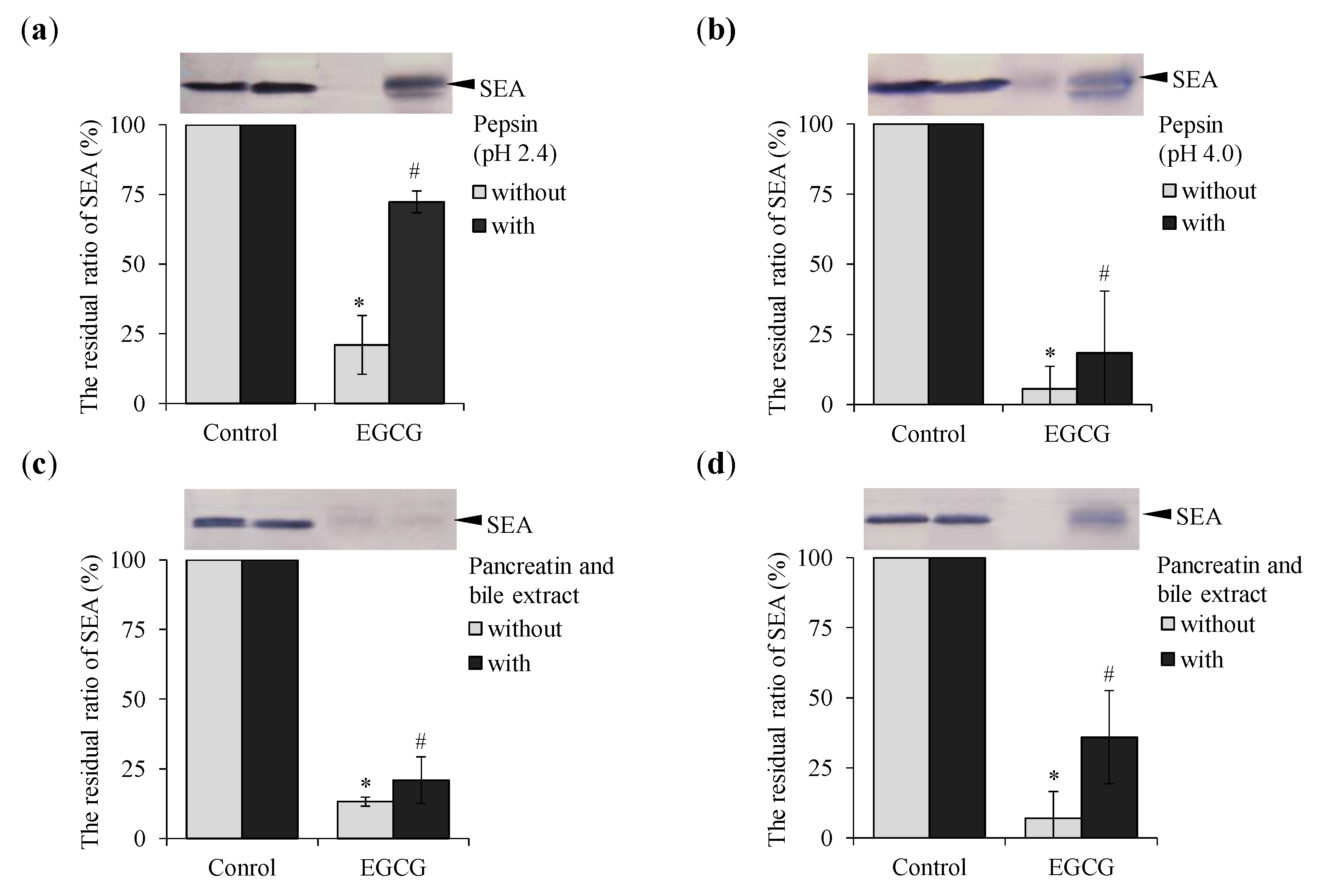
2.5. Effect of Pepsin and Pancreatin Treatment on Binding of EGCG with SEA
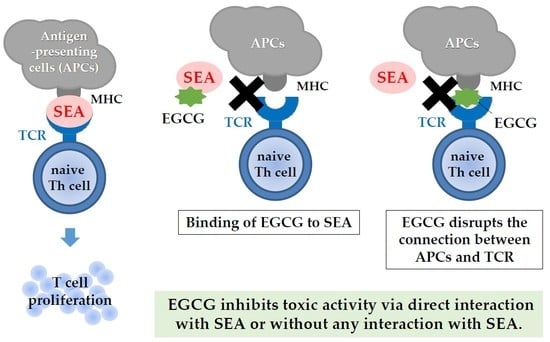
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cytokine Gene mRNA Expression
4.3. IFN-γ Detection
4.4. Isolation of Mouse Spleen Cells and SEA Cytotoxicity Assay
4.5. Effect of Methylated EGCG on SEA Cytotoxicity
4.6. Effect of pH and Protein on Binding of SEA and EGCG
4.7. Digestive Tract Model
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Taylor, T.A.; Unakal, C.G. Staphylococcus Aureus; StatPearls Publishing: Tampa, FL, USA, 2019. [Google Scholar] [PubMed]
- Balaban, N.; Rasooly, A. Staphylococcal enterotoxins. Int. J. Food Microbiol. 2000, 61, 1–10. [Google Scholar] [CrossRef]
- Muluk, N.B.; Altın, F.; Cingi, C. Role of superantigens in allergic inflammation: Their relationship to allergic rhinitis, chronic rhinosinusitis, asthma, and atopic dermatitis. Am. J. Rhinol. Allergy 2018, 32, 502–517. [Google Scholar] [CrossRef] [PubMed]
- Hisaya, K.O.; Masato, N.; Yoshio, Y.; Hu, D.L.; Akio, N.; Kunihiro, S.; Katsuhiko, O. Submucosal mast cells in the gastrointestinal tract are a target of staphylococcal enterotoxin type A. FEMS Immunol. Med. Microbiol. 2012, 64, 392–402. [Google Scholar] [CrossRef]
- Feldman, K.S. Recent progress in ellagitannin chemistry. Phytochemistry 2004, 66, 1984–2000. [Google Scholar] [CrossRef]
- Canon, F.; Paté, F.; Cheynier, V.; Sarni-Manchado, P.; Giuliani, A.; Pérez, J.; Durand, D.; Li, J.; Cabane, B. Aggregation of the salivary proline-rich protein IB5 in the presence of the tannin EgCG. Langmuir 2013, 6, 1926–1937. [Google Scholar] [CrossRef]
- Hamad, A.R.; Marrack, P.; Kappler, J.W. Transcytosis of staphylococcal superantigen toxins. J. Exp. Med. 1997, 185, 1447–1454. [Google Scholar] [CrossRef]
- Hu, D.L.; Omoe, K.; Shimoda, Y.; Nakane, A.; Shinagawa, K. Induction of emetic response to staphylococcal enterotoxins in the house musk shrew (Suncus murinus). Infect. Immun. 2003, 71, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.L.; Zhu, G.; Mori, F.; Omoe, K.; Okada, M.; Wakabayashi, K.; Kaneko, S.; Shinagawa, K.; Nakane, A. Staphylococcal enterotoxin induces emesis through increasing serotonin release in intestine and it is downregulated by cannabinoid receptor 1. Cell Microbiol. 2007, 9, 2267–2277. [Google Scholar] [CrossRef] [PubMed]
- Maina, E.K.; Hu, D.L.; Asano, K.; Nakane, A. Inhibition of emetic and superantigenic activities of staphylococcal enterotoxin A by synthetic peptides. Peptides 2012, 38, 1–7. [Google Scholar] [CrossRef]
- Dinges, M.M.; Orwin, P.M.; Schlievert, P.M. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 2000, 13, 16–34. [Google Scholar] [CrossRef]
- Fraser, J.D.; Proft, T. The bacterial superantigen and superantigen-like proteins. Immunol. Rev. 2008, 225, 226–243. [Google Scholar] [CrossRef]
- Taylor, P.W. Alternative natural sources for a new generation of antibacterial agents. Int. J. Antimicrob. Agents 2013, 42, 195–201. [Google Scholar] [CrossRef]
- Reygaert, W.C. The antimicrobial possibilities of green tea. Front. Microbiol. 2014, 5, 434. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T. Polyphenols from Asian plants, structural diversity and antitumor and antiviral activities. In Phenolic Compounds in Food and Their Effects on Health II, Antioxidants and Cancer Prevention; Huang, M.-T., Ho, C.-T., Lee, C.Y., Eds.; American Chemical Society: Washington, DC, USA, 1992; pp. 160–183. [Google Scholar] [CrossRef]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef]
- Reygaert, W.C. An update on the health benefits of green tea. Beverages 2017, 3, 6. [Google Scholar] [CrossRef]
- Shimamura, Y.; Utsumi, M.; Hirai, C.; Nakano, S.; Ito, S.; Tsuji, A.; Hosoya, T.; Kan, T.; Ohashi, N.; Masuda, S. Binding of catechins to staphylococcal enterotoxin A. Molecules 2018, 23, 1125. [Google Scholar] [CrossRef]
- Rasooly, R.; Do, P.M.; Friedman, M. Inhibition of biological activity of staphylococcal enterotoxin A (SEA) by apple juice and apple polyphenols. J. Agric. Food Chem. 2010, 58, 5421–5426. [Google Scholar] [CrossRef]
- Shimamura, Y.; Hirai, C.; Sugiyama, Y.; Utsumi, M.; Yanagida, A.; Murata, M.; Ohashi, N.; Masuda, S. Interaction between various apple procyanidin and staphylococcal enterotoxin A and their inhibitory effects on toxin activity. Toxins 2017, 9, 243. [Google Scholar] [CrossRef]
- Zimeri, J.; Tong, C. Degradation kinetics of (−)-epigallocatechin gallate as a function of pH and dissolved oxygen in a liquid model system. J. Food Sci. 1999, 64, 753–758. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986, 136, 2348. [Google Scholar]
- Li, M.; Hagerman, A.E. Role of the flavan-3-ol and galloyl moieties in the interaction of (−)-epigallocatechin gallate with serum albumin. J. Agric. Food Chem. 2014, 62, 3768–3775. [Google Scholar] [CrossRef]
- Bae, M.J.; Ishii, T.; Minoda, K.; Kawada, Y.; Ichikawa, T.; Mori, T.; Kamihira, M.; Nakayama, T. Albumin stabilizes (−)-epigallocatechin gallate in human serum: Binding capacity and antioxidant property. Mol. Nutr. Food Res. 2009, 53, 709–715. [Google Scholar] [CrossRef]
- Bourassa, P.; Bariyanga, J.; Tajmir-Riahi, H.A. Binding sites of resveratrol, genistein, and curcumin with milk α-and β-caseins. J. Phys. Chem. B 2013, 117, 1287–1295. [Google Scholar] [CrossRef]
- Takahashi, T.; Nagatoishi, S.; Kuroda, D.; Tsumoto, K. Thermodynamic and computational analyses reveal the functional roles of the galloyl group of tea catechins in molecular recognition. PLoS ONE 2018, 13, e0204856. [Google Scholar] [CrossRef]
- Ishii, T.; Ichikawa, T.; Minoda, K.; Kusaka, K.; Ito, S.; Suzuki, Y.; Akagawa, M.; Mochizuki, K.; Goda, T.; Nakayama, T. Human serum albumin as an antioxidant in the oxidation of (−)-epigallocatechin gallate: Participation of reversible covalent binding for interaction and stabilization. Biosci. Biotechnol. Biochem. 2011, 75, 100–106. [Google Scholar] [CrossRef]
- Ono, H.K.; Hirose, S.; Narita, K.; Sugiyama, M.; Asano, K.; Hu, D.L.; Nakane, A. Histamine release from intestinal mast cells induced by staphylococcal enterotoxin A (SEA) evokes vomiting reflex in common marmoset. PLoS Pathog. 2019, 15, e1007803. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Yu, P.; Zhou, W. Combined effect of pH and temperature on the stability and antioxidant capacity of epigallocatechin gallate (EGCG) in aqueous system. J. Food Eng. 2019, 250, 46–54. [Google Scholar] [CrossRef]
- Falcó, I.; Randazzo, W.; Gómez-Mascaraque, L.; Aznar, R.; López-Rubio, A.; Sánchez, G. Effect of (−)-epigallocatechin gallate at different pH conditions on enteric viruses. LWT-Food Sci. Technol 2017, 81, 250–257. [Google Scholar] [CrossRef]
- Aihara, Y.; Yoshida, A.; Furuta, T.; Wakimoto, T.; Akizawa, T.; Konishi, M.; Kan, T. Regioselective synthesis of methylated epigallocatechin gallate via nitrobenzenesulfonyl (Ns) protecting group. Bioorg. Med. Chem. Lett. 2009, 19, 4171–4174. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimamura, Y.; Utsumi, M.; Hirai, C.; Kurokawa, A.; Kan, T.; Ohashi, N.; Masuda, S. Effect of (−)-Epigallocatechin Gallate to Staphylococcal Enterotoxin A on Toxin Activity. Molecules 2020, 25, 1867. https://doi.org/10.3390/molecules25081867
Shimamura Y, Utsumi M, Hirai C, Kurokawa A, Kan T, Ohashi N, Masuda S. Effect of (−)-Epigallocatechin Gallate to Staphylococcal Enterotoxin A on Toxin Activity. Molecules. 2020; 25(8):1867. https://doi.org/10.3390/molecules25081867
Chicago/Turabian StyleShimamura, Yuko, Mio Utsumi, Chikako Hirai, Ami Kurokawa, Toshiyuki Kan, Norio Ohashi, and Shuichi Masuda. 2020. "Effect of (−)-Epigallocatechin Gallate to Staphylococcal Enterotoxin A on Toxin Activity" Molecules 25, no. 8: 1867. https://doi.org/10.3390/molecules25081867
APA StyleShimamura, Y., Utsumi, M., Hirai, C., Kurokawa, A., Kan, T., Ohashi, N., & Masuda, S. (2020). Effect of (−)-Epigallocatechin Gallate to Staphylococcal Enterotoxin A on Toxin Activity. Molecules, 25(8), 1867. https://doi.org/10.3390/molecules25081867