Riley Oxidation of Heterocyclic Intermediates on Paths to Hydroporphyrins—A Review
Abstract
1. Introduction
2. Results and Discussion
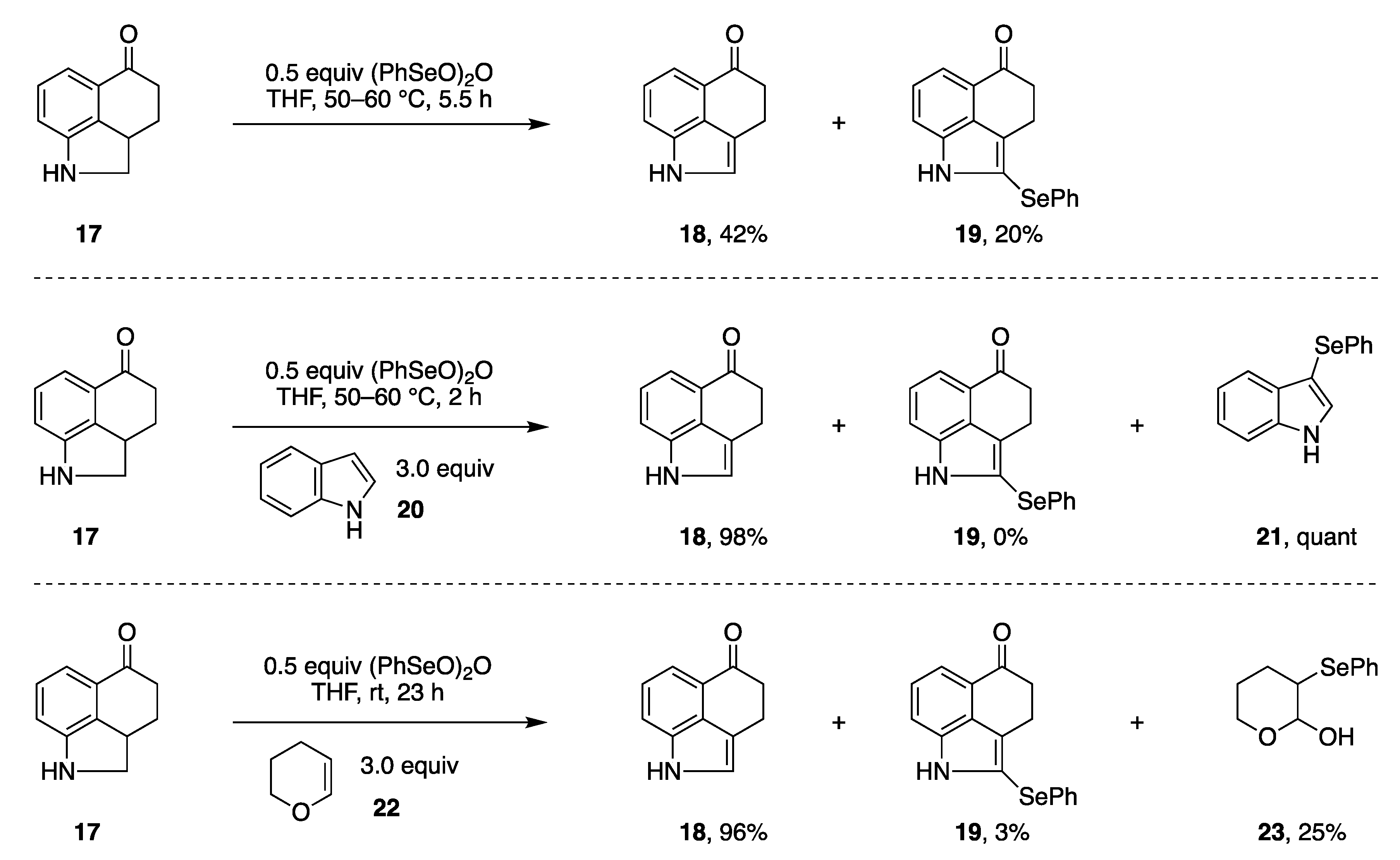
2.1. Diverse Conditions for the Riley Oxidation
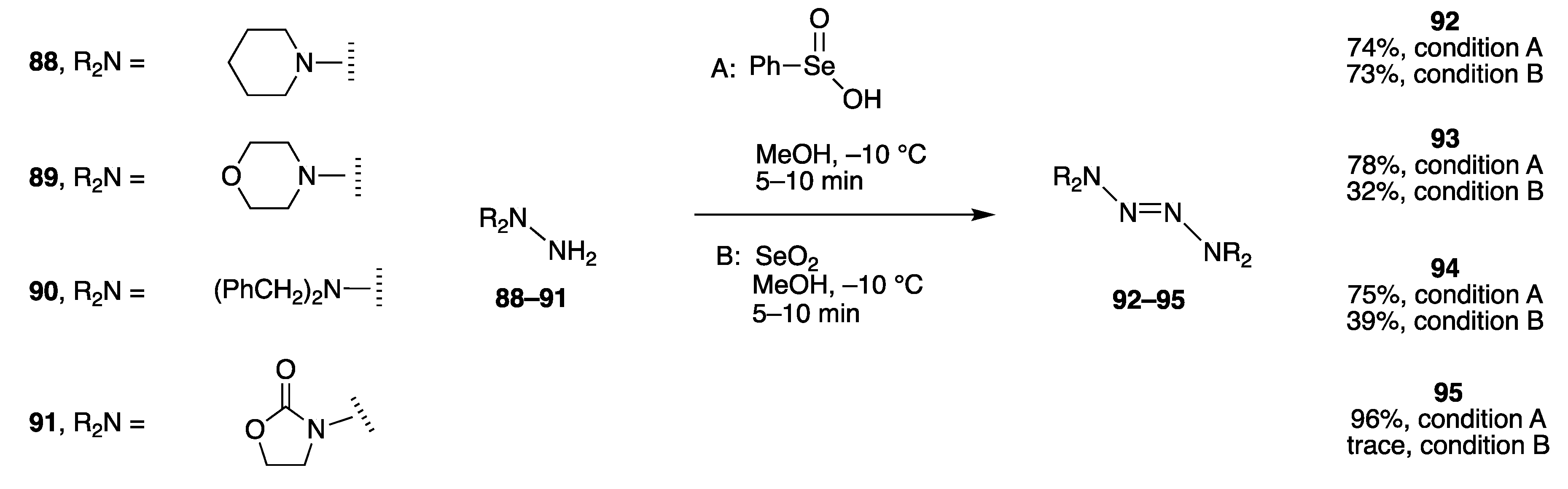
- Selenium reagent benzeneseleninic acid in methanol.
- Selenium reagent benzeneseleninic anhydride with indole or dihydropyran as a scavenger.
- SeO2 in dioxane with an added base such as pyridine.
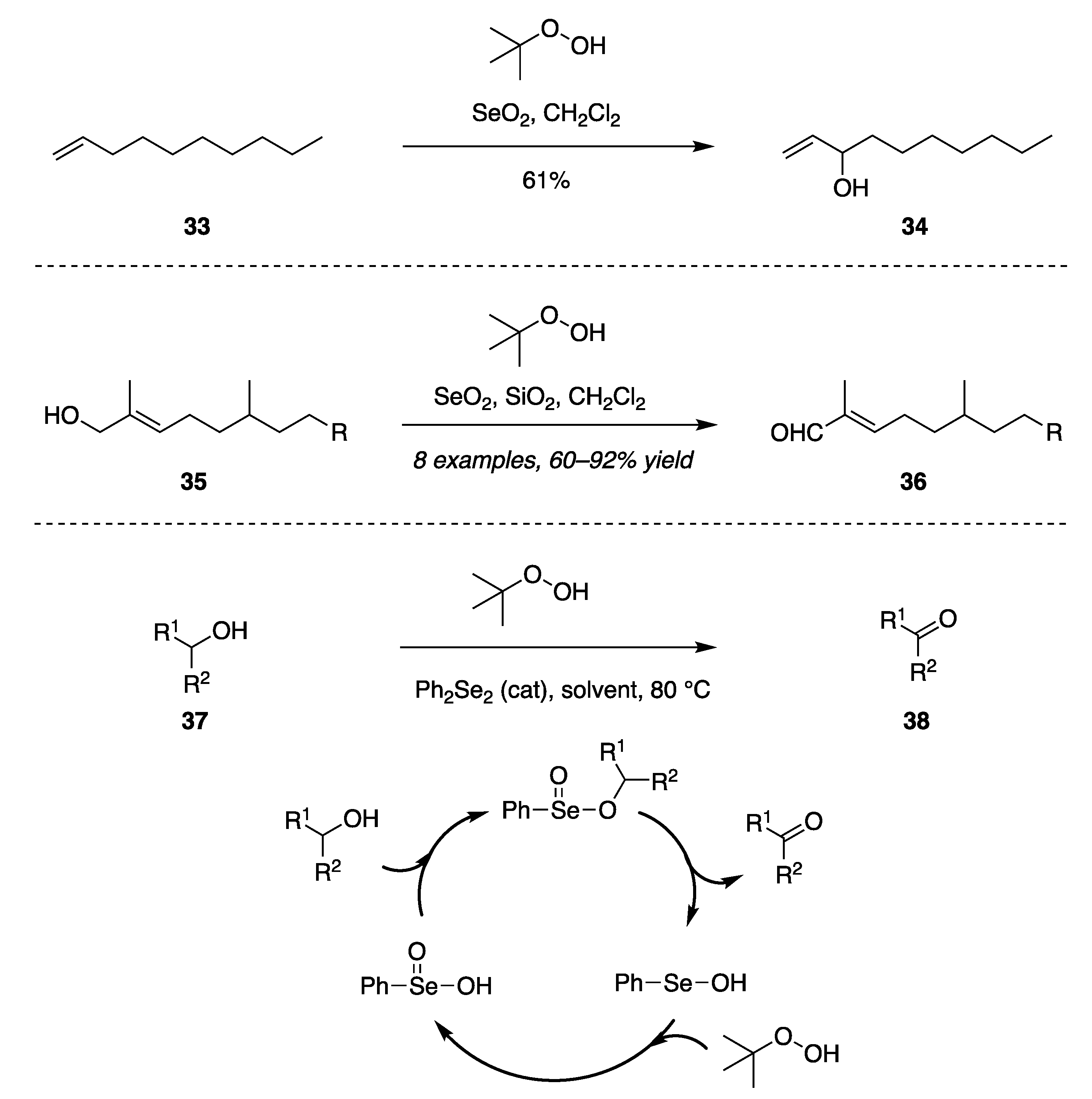
- SeO2 in CH2Cl2 with the oxygen donor TBHP.
- SeO2 in CH2Cl2 with the oxygen donor TBHP and SiO2.
- Selenium reagent Ph2Se2 in CH2Cl2 with the oxygen donor TBHP.
- SeO2 in a mixture of formic acid and dioxane.
- SeO2 in acetic anhydride under an atmosphere of O2.
- SeO2 in acetic acid or ethanol with H2SO4 as an acid catalyst.
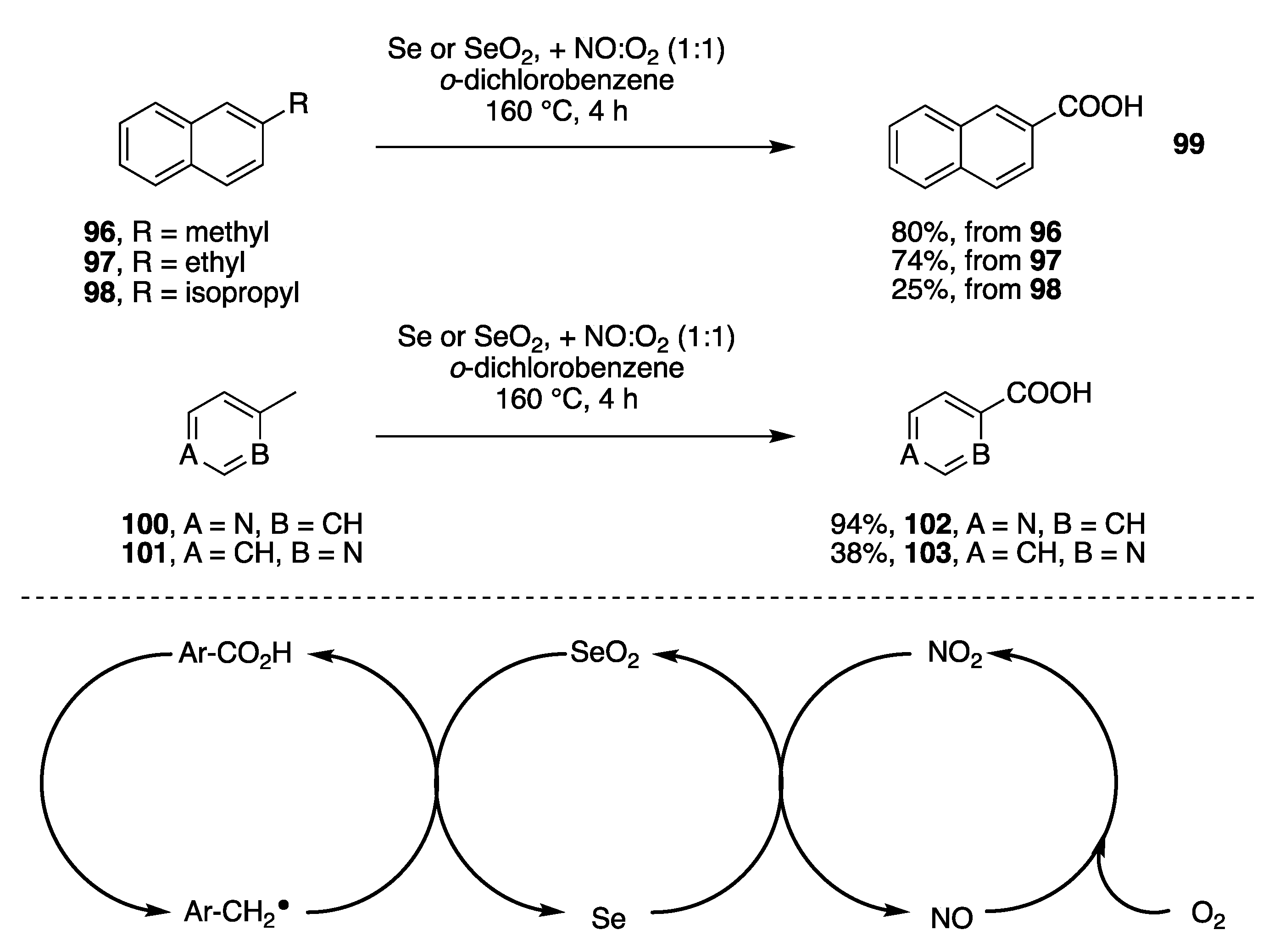
- Se or SeO2 in o-dichlorobenzene purged with a mixture of nitric oxide and O2.
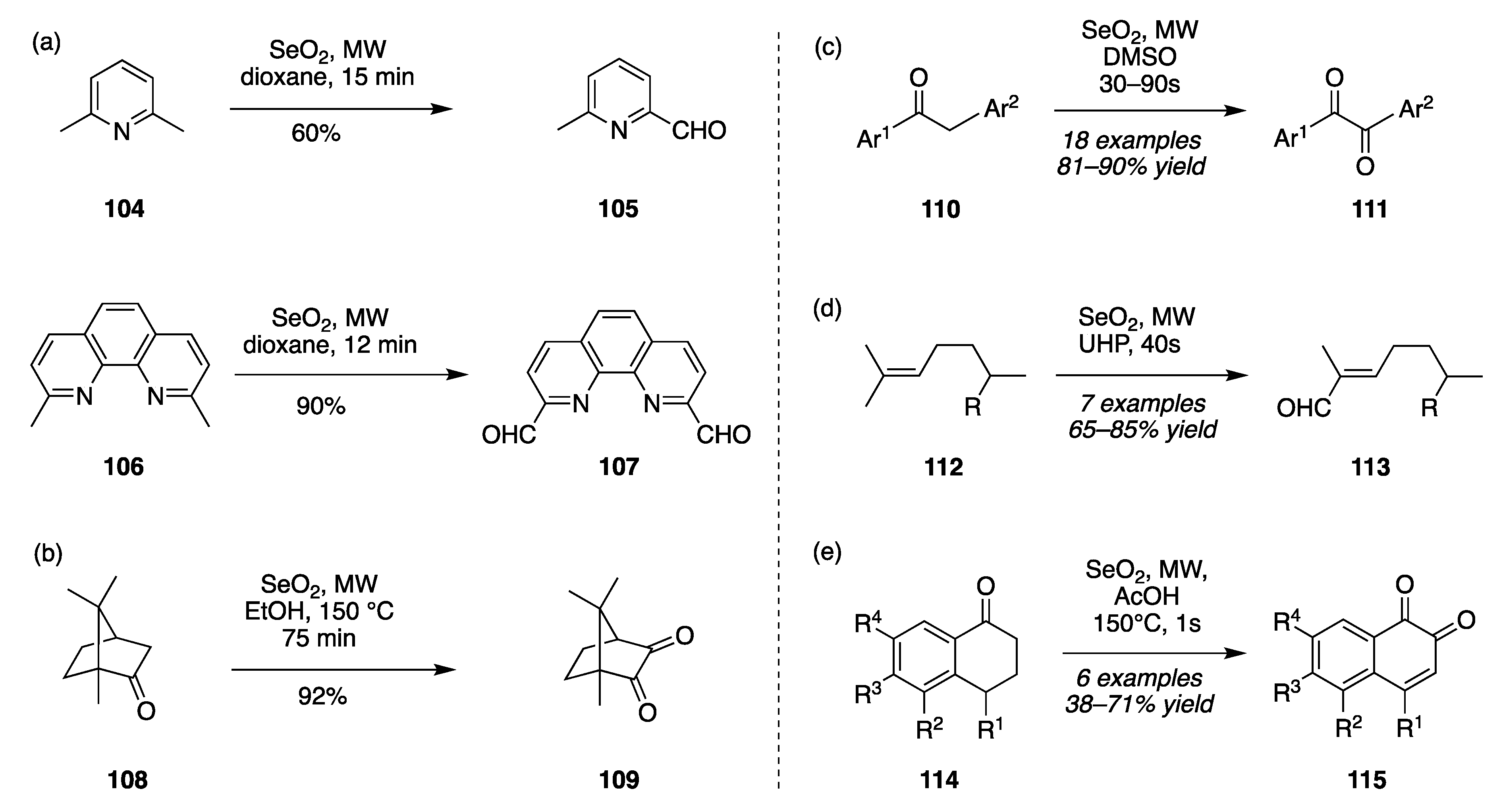
- Microwave-assisted SeO2 oxidation in dioxane.
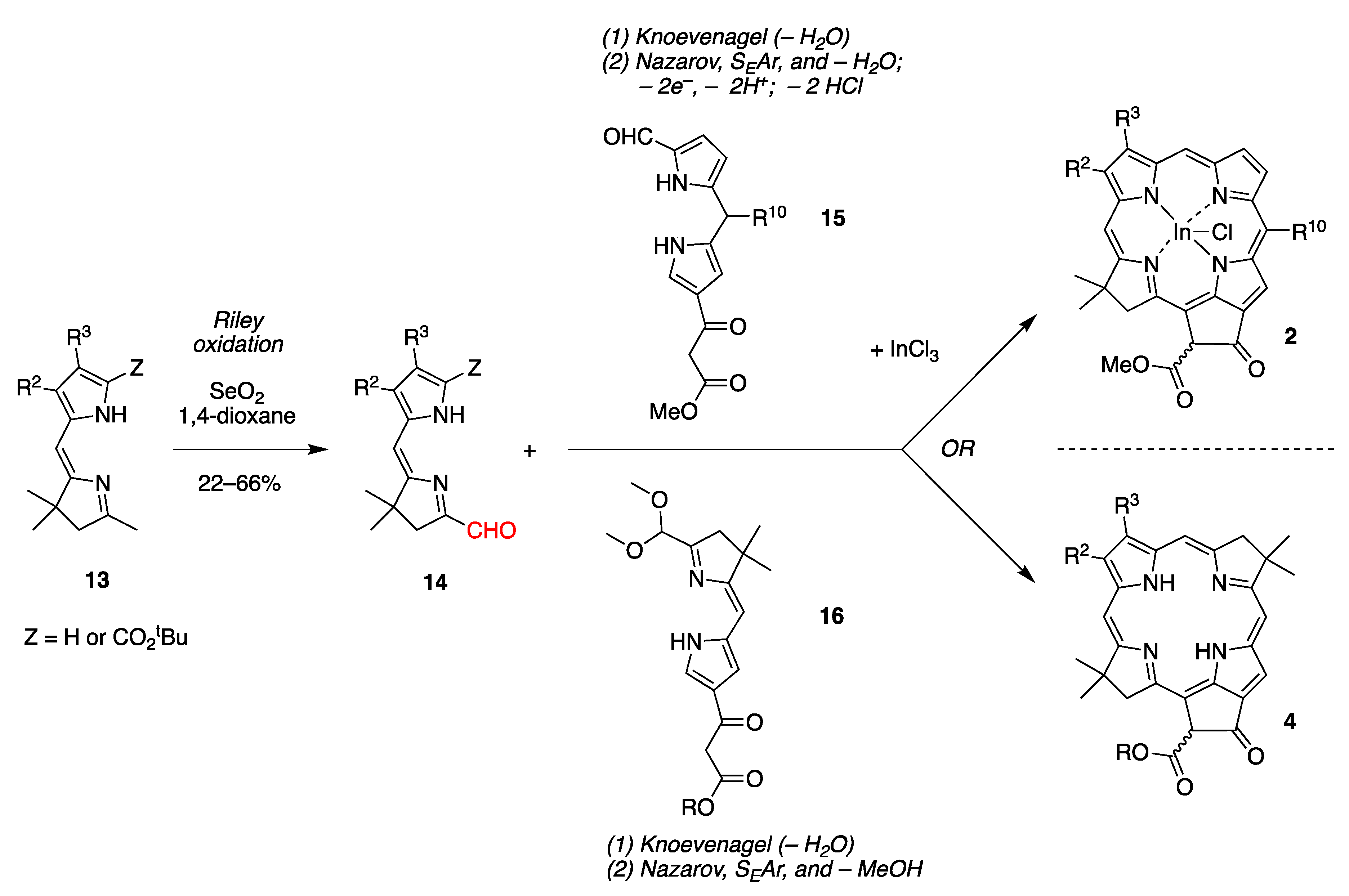
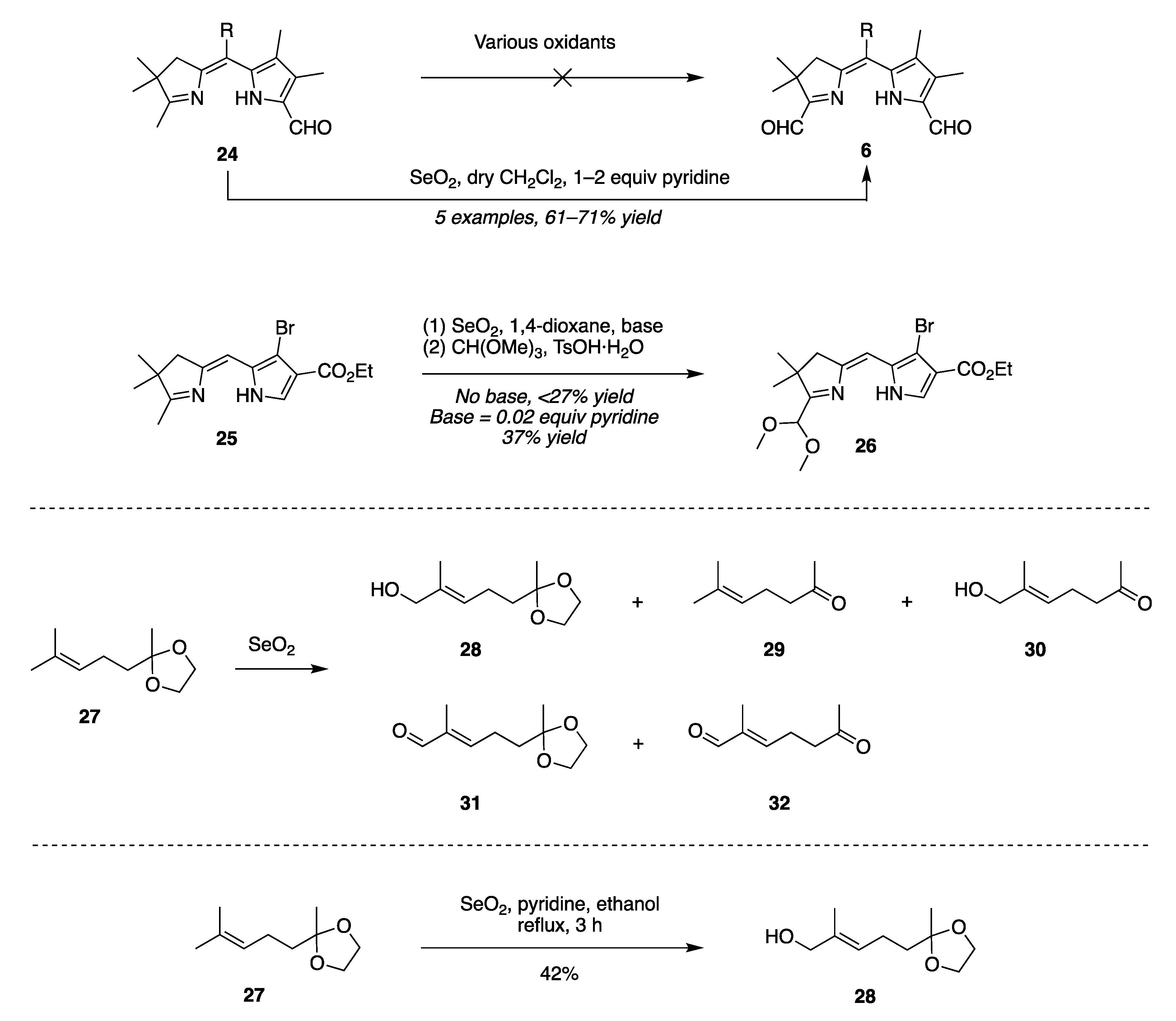
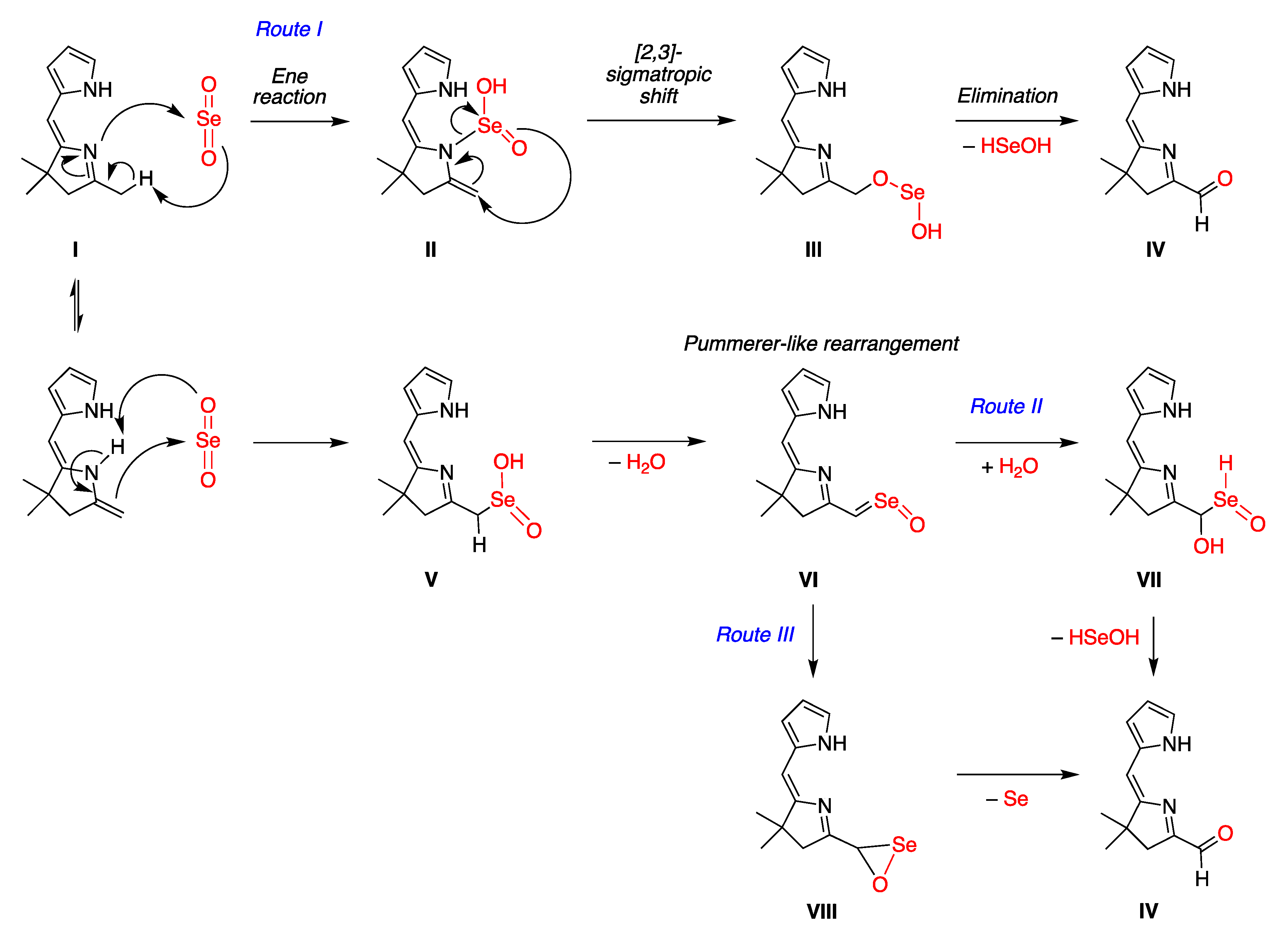
2.2. Mechanistic Considerations
- Route II begins with imine–enamine tautomerization of I. The enamine of I reacts with SeO2 to generate intermediate V, and then Pummerer-like rearrangement via intermediate VI yields VII. Subsequent elimination affords IV.
- Route III has an alternate endgame, wherein the Pummerer-like intermediate VI cyclizes to give the selaoxirane-containing VIII, which, upon loss of Se, gives IV.
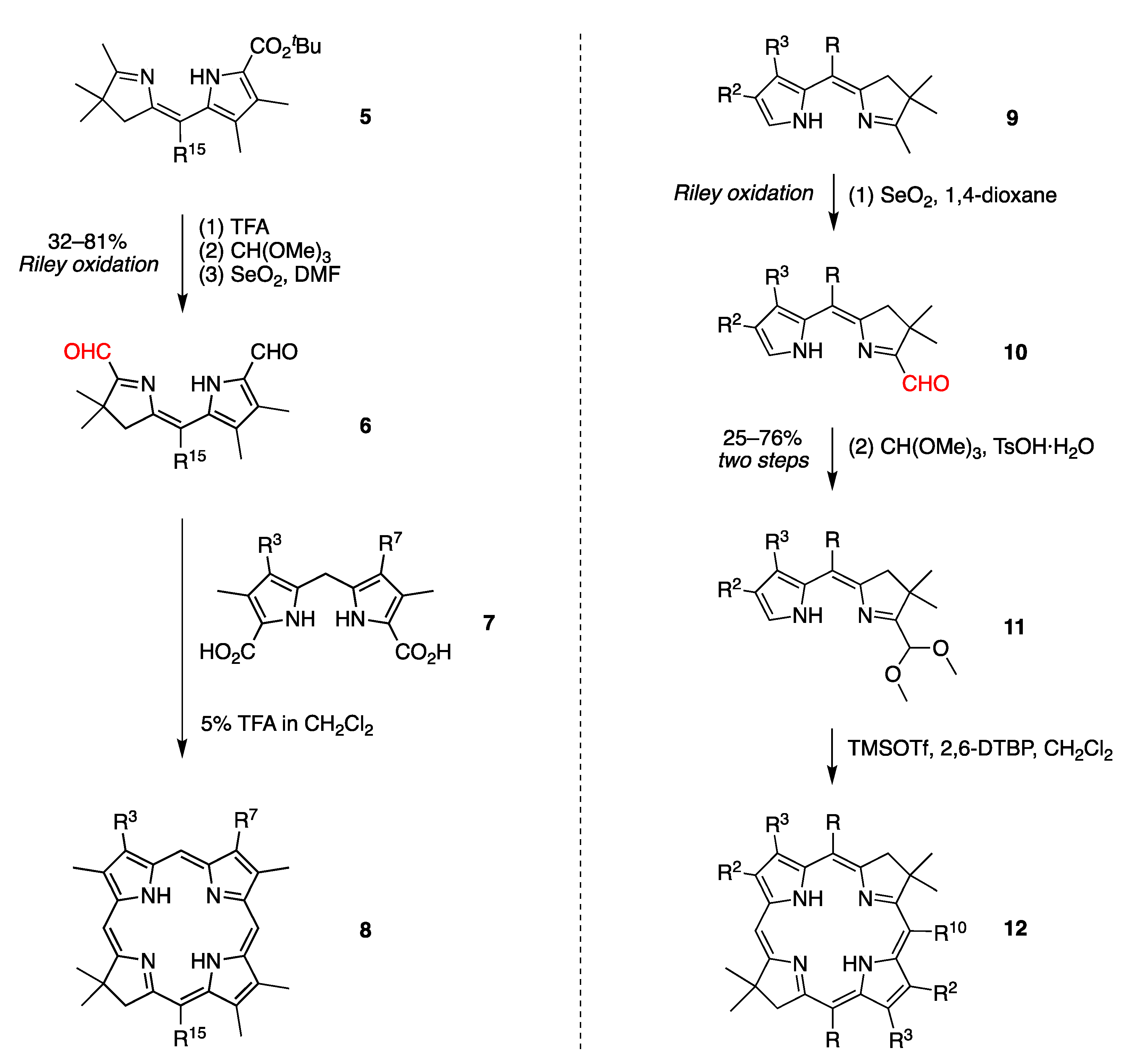
2.3. Riley Oxidation of Diverse Hydrodipyrrins
- A pyrroline N-oxide provides superior results (entry 1 versus 2, 79% versus 0%).
- Two 1-methyltetrahydrodipyrrin-N-oxides (entries 1 and 3) could be converted to the corresponding aldehyde, but neither product was subsequently converted to a hydroporphyrin. Methods for N-deoxygenation will likely be required to do so.
- β-Alkyl versus β-aryl groups afford comparable results (entry 6 versus 4, ~40%; and 26 versus 27, 31%).
- An aza-spirohexyl group in lieu of a gem-dimethyl has no adverse effect (entries 7,8 versus 4; ~40% for both; the former are dimethyl acetals).
- β,β-Dialkyl or β,β-annulated arenes afford comparable results (entries 9, 11 and 12; ~60%).
- A tert-butyl ester and ethyl ester at the 9-position afford comparable results (entries 9 and 12; ~60%).
- A pre-existing aldehyde group on the pyrrole unit survives intact and causes no adverse effect (entries 13 and 39–43; all yields >60%).
- The presence of a single aryl-substituted pyrrole gives yields of 22%–57% (entries 14–16).
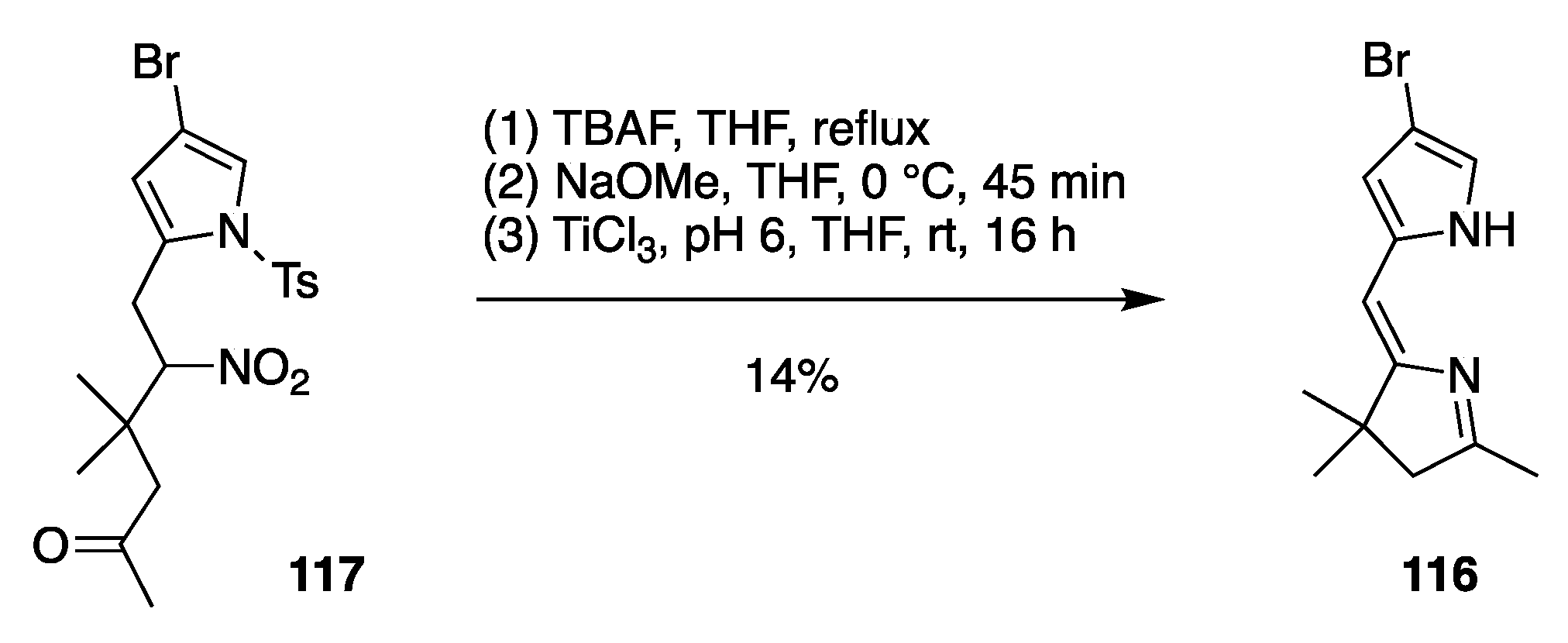
- A lone p-bromophenyl group on the pyrrole unit affords acceptable results (entry 16, 38%), as does a p-iodophenyl group (entry 15, 57%), whereas a lone bromine atom on the pyrrole unit results in failure (entry 17, 0%) unless the pyrrole is stabilized with an ester substituent (entry 25, 37%; a dimethyl acetal) or a pyrrole N-tosyl group (entry 3, 43%; also a pyrroline N-oxide). Halopyrroles lacking stabilizing (e.g., electron-withdrawing) substituents are known to be unstable [66].
- A meso-alkyl or meso-aryl group affords comparable results (entries 19 and 20; 63% and 65%; the former is a dimethyl acetal).
- A meso-alkyl group has no apparent adverse effect (entries 42 and 43 versus 39a; >60%).
- The position of the gem-dimethyl group at the 2,2- versus 3,3-site has relatively little adverse effect (entry 23 versus 4; 25% for the dimethyl acetal versus 32 or 40% for the aldehyde).
- The presence of larger 2,2-dialkyl groups is satisfactory (entries 29 and 30; yields >50%, the latter is a dimethyl acetal).
- In one case, a Z-isomer gives the Z-isomer (entry 29, 57%), whereas the E-isomer gave a ~4:1 mixture of the Z- and E-products (entry 30; 51% and 12%; both dimethyl acetals).
- In another case, the Z- and E-isomers individually each give a mixture of the Z and E products (entries 31 and 32; total yields >55%; all dimethyl acetals). In this and the preceding example, the 2-position substituents are bulky (alkyl or phenyl) groups.
- 2,2-Diphenyl substituents afford both the Z- and E-isomers in comparable quantities and nearly twice the yield of the 2,2-dimethyl unit (entry 31 versus 18; 55% total versus 30%).
- In yet another case, the Z-isomer gives a 5:1 mixture of the Z and E products (entry 40a).
- The remarkably high yields of 99% (entries 13 and 40a) are hard to reconcile with yields of ~60% for nearly identical substrates (entries 12 and 39).
- The presence of a single ester substituent on the pyrrole unit affords good yield, whereas the fully unsubstituted pyrrole does not afford product (entry 34 versus 45; 47% versus 0%; the former is a dimethyl acetal).
- The presence of an unsubstituted pyrrole affords products that are not stable or are formed in low yield (entries 44, 45; 5.8% for the bacteriochlorin product of the former, 0% for the latter).
3. Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2,6-DTBP | 2,6-di-tert-butylpyridine |
| CVMW | closed-vessel microwave |
| DMF | N,N-dimethylformamide |
| ESI-MS | electrospray ionization mass spectrometry |
| MW | microwave |
| rt | room temperature |
| SEAr | electrophilic aromatic substitution |
| TBAF | tetra-n-butylammonium fluoride |
| TBHP | tert-butyl hydroperoxide |
| TFA | trifluoroacetic acid |
| THF | tetrahydrofuran |
| TMSOTf | trimethylsilyl trifluoromethanesulfonate |
| TsOH·H2O | p-toluenesulfonic acid monohydrate |
| UHP | urea-hydrogen peroxide |
Appendix A
Preparation of 8-Bromo-1,3,3-trimethyl-2,3-dihydrodipyrrin (116).
References
- Riley, H.L.; Morley, J.F.; Friend, N.A.C. Selenium Dioxide, a New Oxidising Agent. Part I. Its Reaction with Aldehydes and Ketones. J. Chem. Soc. 1932, 1875–1883. [Google Scholar] [CrossRef]
- Riley, H.L.; Friend, N.A.C. Selenium Dioxide, a New Oxidising Agent. Part II. Its Reaction with Some Unsaturated Hydrocarbons. J. Chem. Soc. 1932, 2342–2344. [Google Scholar] [CrossRef]
- Astin, S.; Newman, A.C.C.; Riley, H.L. Selenium Dioxide, a New Oxidising Agent. Part III. Its Reaction with Some Alcohols and Esters. J. Chem. Soc. 1933, 391–394. [Google Scholar] [CrossRef]
- Emeléus, H.J.; Riley, H.L. The Luminous Reduction of Selenium Dioxide. Proc. Roy. Soc. Lond. Ser. A 1933, 140, 378–387. [Google Scholar]
- Astin, S.; Riley, H.L. Selenium Dioxide. A New Oxidising Agent. Part IV. The Preparation and Properties of Ethyl Ketohydroxysuccinate. J. Chem. Soc. 1934, 844–848. [Google Scholar] [CrossRef]
- Astin, S.; Moulds, L.D.V.; Riley, H.L. Selenium Dioxide, a New Oxidising Agent. Part V. Some Further Oxidations. J. Chem. Soc. 1935, 901–904. [Google Scholar] [CrossRef]
- Moulds, L.D.V.; Riley, H.L. The Polymerisation of Methylglyoxal. J. Chem. Soc. 1938, 621–626. [Google Scholar] [CrossRef]
- Riley, H.L. Oxidation Activity of Selenium Dioxide. Nature 1947, 159, 571–572. [Google Scholar] [CrossRef]
- Blayden, H.E. In Memoriam: HL Riley. Carbon 1987, 25, 591–592. [Google Scholar] [CrossRef]
- Rabjohn, N. Selenium Dioxide Oxidation. Org. React. 1948, 5, 331–386. [Google Scholar]
- Waitkins, G.R.; Clark, C.W. Selenium Dioxide: Preparation, Properties, and Use as Oxidizing Agent. Chem. Rev. 1945, 36, 235–289. [Google Scholar] [CrossRef]
- Nakamura, A.; Nakada, M. Allylic Oxidations in Natural Product Synthesis. Synthesis 2013, 45, 1421–1451. [Google Scholar]
- Mlochowski, J.; Wojtowicz-Mlochowska, H. Developments in Synthetic Application of Selenium(IV) Oxide and Organoselenium Compounds as Oxygen Donors and Oxygen-Transfer Agents. Molecules 2015, 20, 10205–10243. [Google Scholar] [CrossRef]
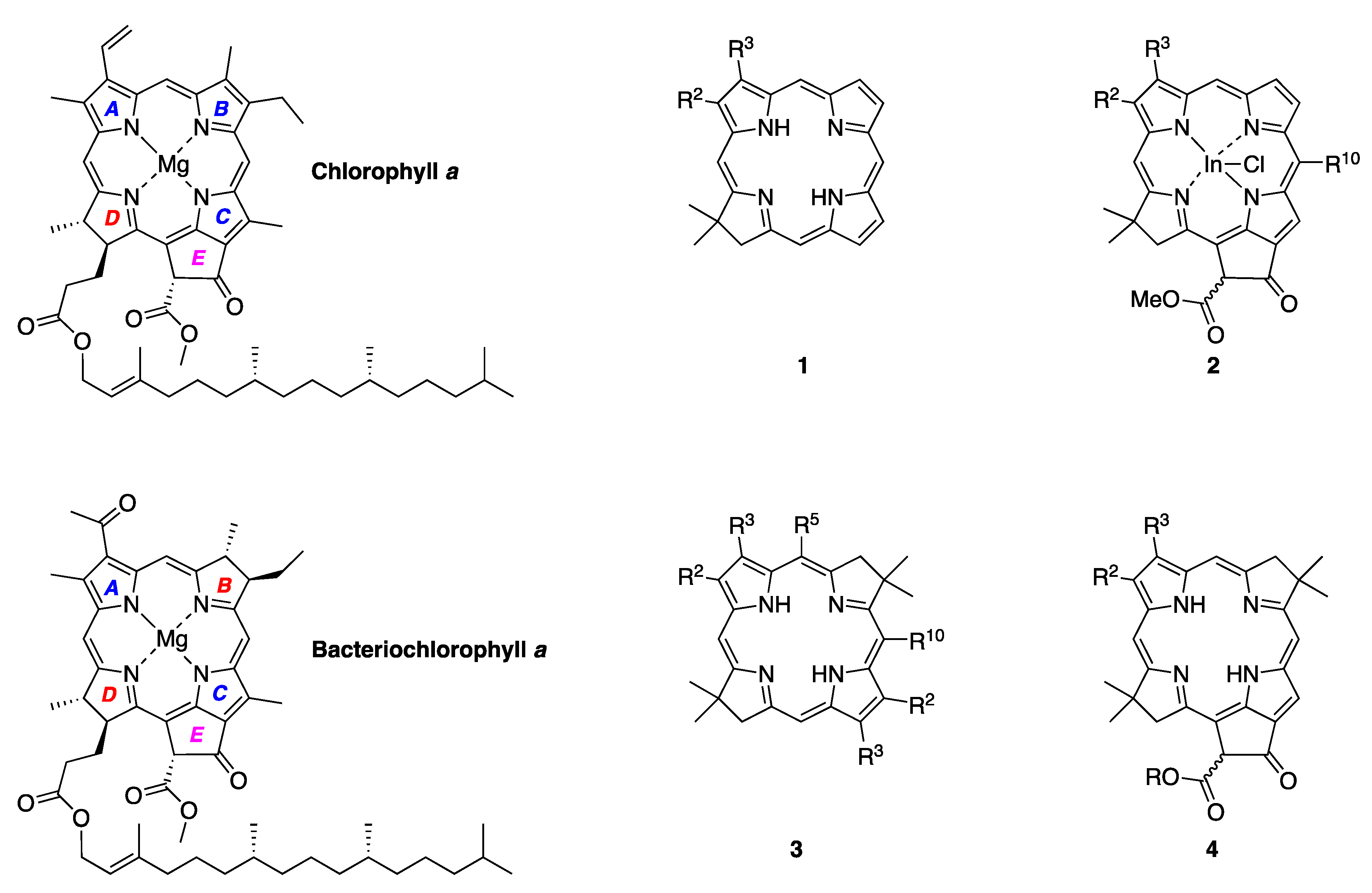
- Scheer, H. An Overview of Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications. In Chlorophylls and Bacteriochlorophylls. Biochemistry, Biophysics, Functions and Applications; Grimm, B., Porra, R.J., Rüdiger, W., Scheer, H., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 25, pp. 1–26. [Google Scholar]
- Lindsey, J.S. De Novo Synthesis of Gem-Dialkyl Chlorophyll Analogues for Probing and Emulating our Green World. Chem. Rev. 2015, 115, 6534–6620. [Google Scholar] [CrossRef]
- Jacobi, P.A.; Lanz, S.; Ghosh, I.; Leung, S.H.; Löwer, F.; Pippin, D. A New Synthesis of Chlorins. Org. Lett. 2001, 3, 831–834. [Google Scholar] [CrossRef]
- O’Neal, W.G.; Roberts, W.P.; Ghosh, I.; Wang, H.; Jacobi, P.A. Studies in Chlorin Chemistry. 3. A Practical Synthesis of C,D-Ring Symmetric Chlorins of Potential Utility in Photodynamic Therapy. J. Org. Chem. 2006, 71, 3472–3480. [Google Scholar] [CrossRef]
- O’Neal, W.G.; Jacobi, P.A. Toward a General Synthesis of Chlorins. J. Am. Chem. Soc. 2008, 130, 1102–1108. [Google Scholar]
- Zhang, S.; Kim, H.-J.; Tang, Q.; Yang, E.; Bocian, D.F.; Holten, D.; Lindsey, J.S. Synthesis and Photophysical Characteristics of 2,3,12,13-Tetraalkylbacteriochlorins. New J. Chem. 2016, 40, 5942–5956. [Google Scholar] [CrossRef]
- Liu, Y.; Lindsey, J.S. Northern–Southern Route to Synthetic Bacteriochlorins. J. Org. Chem. 2016, 81, 11882–11897. [Google Scholar] [CrossRef]
- Wang, P.; Lu, F.; Lindsey, J.S. Use of the Nascent Isocyclic Ring to Anchor Assembly of the Full Skeleton of Model Chlorophylls. J. Org. Chem. 2020, 85, 702–715. [Google Scholar] [CrossRef]
- Zhang, S.; Lindsey, J.S. Construction of the Bacteriochlorin Macrocycle with Concomitant Nazarov Cyclization to Form the Annulated Isocyclic Ring: Analogues of Bacteriochlorophyll a. J. Org. Chem. 2017, 82, 2489–2504. [Google Scholar] [CrossRef]
- Ptaszek, M.; Bhaumik, J.; Kim, H.-J.; Taniguchi, M.; Lindsey, J.S. Refined Synthesis of 2,3,4,5-Tetrahydro-1,3,3-trimethyldipyrrin, a Deceptively Simple Precursor to Hydroporphyrins. Org. Process Res. Dev. 2005, 9, 651–659. [Google Scholar] [CrossRef]
- Ninomiya, I.; Hashimoto, C.; Kiguchi, T.; Naito, T.; Barton, D.H.R.; Lusinchi, X.; Milliet, P. Dehydrogenation with Benzeneseleninic Anhydride in the Total Synthesis of Ergot Alkaloids. J. Chem. Soc. Perkin Trans 1 1990, 707–713. [Google Scholar] [CrossRef]
- Trachtenberg, E.N. Selenium Dioxide Oxidation. In Oxidation: Techniques and Applications in Organic Synthesis; Augustine, R.L., Ed.; Marcel Dekker: New York, NY, USA, 1969; pp. 19–187. [Google Scholar]
- Fujita, H.; Jing, H.; Krayer, M.; Allu, S.; Veeraraghavaiah, G.; Wu, Z.; Jiang, J.; Diers, J.R.; Magdaong, N.C.M.; Mandal, A.K.; et al. Annulated Bacteriochlorins for Near-Infrared Photophysical Studies. New J. Chem. 2019, 43, 7209–7232. [Google Scholar] [CrossRef]
- Clark, K.J.; Fray, G.I.; Jaeger, R.H.; Robinson, R. Synthesis of D- and L- Isoiridomyrmecin and Related Compounds. Tetrahedron 1959, 6, 217–224. [Google Scholar] [CrossRef]
- Bhalerao, U.T.; Rapoport, H. Stereochemistry of Allylic Oxidation with Selenium Dioxide. Stereospecific Oxidation of gem-Dimethyl Olefins. J. Am. Chem. Soc. 1971, 93, 4835–4840. [Google Scholar]
- Camps, F.; Coll, J.; Parente, A. Selenium Dioxide Oxidation of Substrates with Acid Labile Groups. Synthesis 1978, 215–216. [Google Scholar] [CrossRef]
- Umbreit, M.A.; Sharpless, K.B. Allylic Oxidation of Olefins by Catalytic and Stoichiometric Selenium Dioxide with tert-Butyl Hydroperoxide. J. Am. Chem. Soc. 1977, 99, 5526–5528. [Google Scholar] [CrossRef]
- Kalsi, P.S.; Chhabra, B.R.; Singh, J.; Vig, R. Selective Oxidation of Primary Allylic Alcohols to α,β-Unsaturated Aldehydes. Synlett 1992, 425–426. [Google Scholar] [CrossRef]
- Kuwajima, I.; Shimizu, M.; Urabe, H. Oxidation of Alcohols with tert-Butyl Hydroperoxide and Diaryl Diselenide. J. Org. Chem. 1982, 47, 837–842. [Google Scholar] [CrossRef]
- van der Toorn, J.C.; Kemperman, G.; Sheldon, R.A.; Arends, I.W.C.E. Diphenyldiselenide-Catalyzed Selective Oxidation of Activated Alcohols with tert-Butyl Hydroperoxide: New Mechanistic Insights. J. Org. Chem. 2009, 74, 3085–3089. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, H.; Shibuya, K.; Yamada, Y. Total Synthesis of Upial, A Marine Sesquiterpene Possessing Bicyclo [3.3.1]nonane Ring System. Tetrahedron 1994, 50, 661–688. [Google Scholar] [CrossRef]
- Shibuya, K. A Novel Allylic Oxidation Using a Combination of Formic Acid and Selenium Dioxide. Synth. Commun. 1994, 24, 2923–2941. [Google Scholar] [CrossRef]
- Koltun, E.S.; Kass, S.R. Synthesis of Cycloocta-3,5-dien-1-ol and Cycloocta-3,5-dien-1-one: SeO2/O2 Oxidation of Dienes. Synthesis 2000, 2000, 1366–1368. [Google Scholar] [CrossRef]
- Hanold, N.; Meier, H. Die Unsymmetrischen Cyclooctadienine: 1,3-Cyclooctadien-5-in und 1,6-Cyclooctadien-3-in. Chem. Ber. 1985, 118, 198–209. [Google Scholar] [CrossRef]
- Javaid, K.A.; Sonoda, N.; Tsutsumi, S. A New Reaction in the Selenium Dioxide Oxidation of Olefins. Tetrahedron Lett. 1969, 10, 4439–4441. [Google Scholar] [CrossRef]
- Sonoda, N.; Yamamoto, Y.; Murai, S.; Tsutsumi, S. A New Acid Catalyzed Oxidation of Some Acetylenic Compounds with Selenium Dioxide. Chem. Lett. 1972, 1, 229–232. [Google Scholar] [CrossRef]
- Bender, H.; Döpp, D. 2H-Pyrrole-l-oxides by Selenium Dioxide Dehydrogenation of Pyrroline-1-oxides. Tetrahedron Lett. 1980, 21, 1833–1836. [Google Scholar] [CrossRef]
- Sakamoto, T.; Sakasai, T.; Yamanaka, H. Studies on Pyrimidine-derivatives. XXII. Site-selective Oxidation of Dimethylpyrimidines with Selenium Dioxide to Pyrimidine-monoaldehydes. Chem. Pharm. Bull. 1981, 29, 2485–2490. [Google Scholar] [CrossRef][Green Version]
- Kim, H.-J.; Dogutan, D.K.; Ptaszek, M.; Lindsey, J.S. Synthesis of Hydrodipyrrins Tailored for Reactivity at the 1- and 9-Positions. Tetrahedron 2007, 63, 37–55. [Google Scholar] [CrossRef]
- Back, T.G.; Kerr, R.G. Oxidation of 1,l-Disubstituted Hydrazines with Benzeneseleninic Acid and Selenium Dioxide. Facile Preparation of Tetrazenes. Can. J. Chem. 1982, 60, 2711–2718. [Google Scholar] [CrossRef]
- Remias, J.E.; Sen, A. Nitrogen Oxides/Selenium Dioxide-Mediated Benzylic Oxidations. J. Mol. Catal. A Chem. 2003, 201, 63–70. [Google Scholar] [CrossRef]
- Goswami, S.; Adak, A.K. Microwave Assisted Improved Synthesis of 6-Formylpterin and Other Heterocyclic Mono- and Di-aldehydes. Synth. Commun. 2003, 33, 475–480. [Google Scholar] [CrossRef]
- Belsey, S.; Danks, T.N.; Wagner, G. Microwave-Assisted Selenium Dioxide Oxidation of Camphor Derivatives to α-Dicarbonyl Compounds and Oxoimines. Synth. Commun. 2006, 36, 1019–1024. [Google Scholar] [CrossRef]
- Shirude, S.T.; Patel, P.; Giridhar, R.; Yadav, M.R. An Efficient and Time Saving Microwave-Assisted Selenium Dioxide Oxidation of 1,2-Diarylethanones. Ind. J. Chem. 2006, 45B, 1080–1085. [Google Scholar] [CrossRef]
- Manktala, R.; Dhillon, R.S.; Chhabra, B.R. Urea-Hydrogen Peroxide and Microwave: An Eco-friendly Blend for Allylic Oxidation of Alkenes with Catalytic Selenium Dioxide. Ind. J. Chem. 2006, 45B, 1591–1594. [Google Scholar] [CrossRef]
- Gelman, D.M.; Perlmutter, P. Microwave-assisted Selenium Dioxide Mediated Selective Oxidation of 1-Tetralones to 1,2-Naphthoquinones. Tetrahedron Lett. 2009, 50, 39–40. [Google Scholar] [CrossRef]
- Riley, H.A.; Gray, A.R. Phenylglyoxal. Org. Syn. 1935, 15, 67–69. [Google Scholar]
- Riley, H.A.; Gray, A.R. Phenylglyoxal. Org. Syn. Coll. Vol. 1943, 2, 509–511. [Google Scholar]
- Trachtenberg, E.N.; Nelson, C.H.; Carver, J.R. Mechanism of Selenium Dioxide Oxidation of Olefins. J. Org. Chem. 1970, 35, 1653–1658. [Google Scholar] [CrossRef]
- Arigoni, D.; Vasella, A.; Sharpless, K.B.; Jensen, H.P. Selenium Dioxide Oxidations of Olefins. Trapping of the Allylic Seleninic Acid Intermediate as a Seleninolactone. J. Am. Chem. Soc. 1973, 95, 7917–7919. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Gordon, K.M. Selenium Dioxide Oxidation of Ketones and Aldehydes. Evidence for the Intermediacy of β-Ketoseleninic Acids. J. Am. Chem. Soc. 1976, 98, 300–301. [Google Scholar] [CrossRef]
- Stephenson, L.M.; Speth, D.R. Mechanism of Allylic Hydroxylation by Selenium Dioxide. J. Org. Chem. 1979, 44, 4683–4689. [Google Scholar] [CrossRef]
- Woggon, W.-D.; Ruther, F.; Egli, H. The Mechanism of Allylic Oxidation by Selenium Dioxide. J. Chem. Soc. Chem. Commun. 1980, 706–708. [Google Scholar] [CrossRef]
- Warpehoski, M.A.; Chabaud, B.; Sharpless, K.B. Selenium Dioxide Oxidation of Endocyclic Olefins. Evidence for a Dissociation-Recombination Pathway. J. Org. Chem. 1982, 47, 2897–2900. [Google Scholar] [CrossRef]
- Singleton, D.A.; Hang, C. Isotope Effects and the Mechanism of Allylic Hydroxylation of Alkenes with Selenium Dioxide. J. Org. Chem. 2000, 65, 7554–7560. [Google Scholar] [CrossRef]
- Ra, C.S.; Park, G. Ab Initio Studies of the Allylic Hydroxylation: DFT Calculation on the Reaction of 2-Methyl-2-butene with Selenium Dioxide. Tetrahedron Lett. 2003, 44, 1099–1102. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Butterworth Heinemann: Oxford, UK, 2001; pp. 747–788. [Google Scholar]
- Reddy, K.R.; Lubian, E.; Pavan, M.P.; Kim, H.-J.; Yang, E.; Holten, D.; Lindsey, J.S. Synthetic Bacteriochlorins with Integral Spiro-piperidine Motifs. New J. Chem. 2013, 37, 1157–1173. [Google Scholar] [CrossRef]
- Zhang, S.; Reddy, M.N.; Mass, O.; Kim, H.-J.; Hu, G.; Lindsey, J.S. Synthesis of Tailored Hydrodipyrrins and Their Examination in Directed Routes to Bacteriochlorins and Tetradehydrocorrins. New J. Chem. 2017, 41, 11170–11189. [Google Scholar] [CrossRef]
- Reddy, M.N.; Zhang, S.; Kim, H.-J.; Mass, O.; Taniguchi, M.; Lindsey, J.S. Synthesis and Spectral Properties of meso-Arylbacteriochlorins Including Insights into Essential Motifs of Hydrodipyrrin Precursors. Molecules 2017, 22, 634. [Google Scholar] [CrossRef]
- Liu, Y.; Allu, S.; Reddy, M.N.; Hood, D.; Diers, J.R.; Bocian, D.F.; Holten, D.; Lindsey, J.S. Synthesis and Photophysical Characterization of Bacteriochlorins Equipped with Integral Swallowtail Substituents. New J. Chem. 2017, 41, 4360–4376. [Google Scholar] [CrossRef]
- Noboa, M.A.; AbuSalim, D.I.; Lash, T.D. Azulichlorins and Benzocarbachlorins Derived Therefrom. J. Org. Chem. 2019, 84, 11649–11664. [Google Scholar] [CrossRef] [PubMed]
- Artico, M. Nitration, Sulfonation, and Halogenation. In Pyrroles. Part One. The Synthesis and the Physical and Chemical Aspects of the Pyrrole Ring; Jones, R.A., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1990; pp. 329–395. [Google Scholar]
- Krayer, M.; Balasubramanian, T.; Ruzié, C.; Ptaszek, M.; Cramer, D.L.; Taniguchi, M.; Lindsey, J.S. Refined Syntheses of Hydrodipyrrin Precursors to Chlorin and Bacteriochlorin Building Blocks. J. Porphyr. Phthalocyanines 2009, 13, 1098–1110. [Google Scholar] [CrossRef]
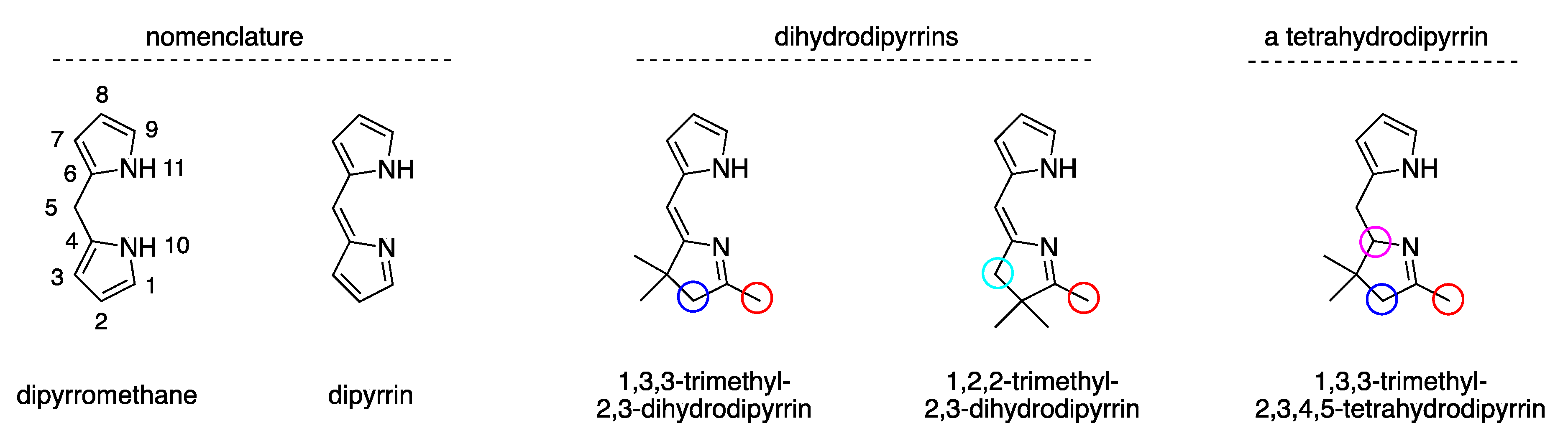
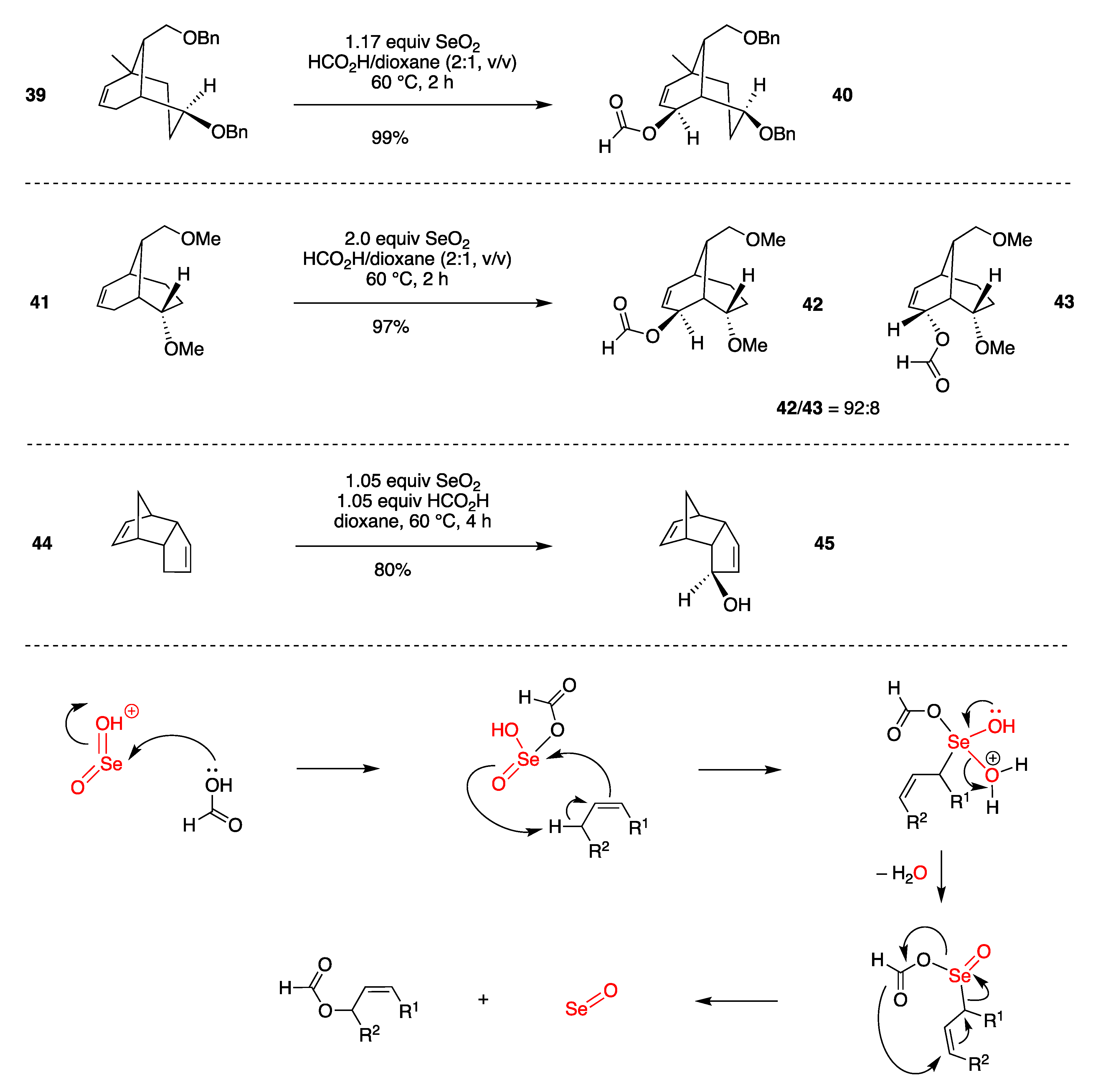
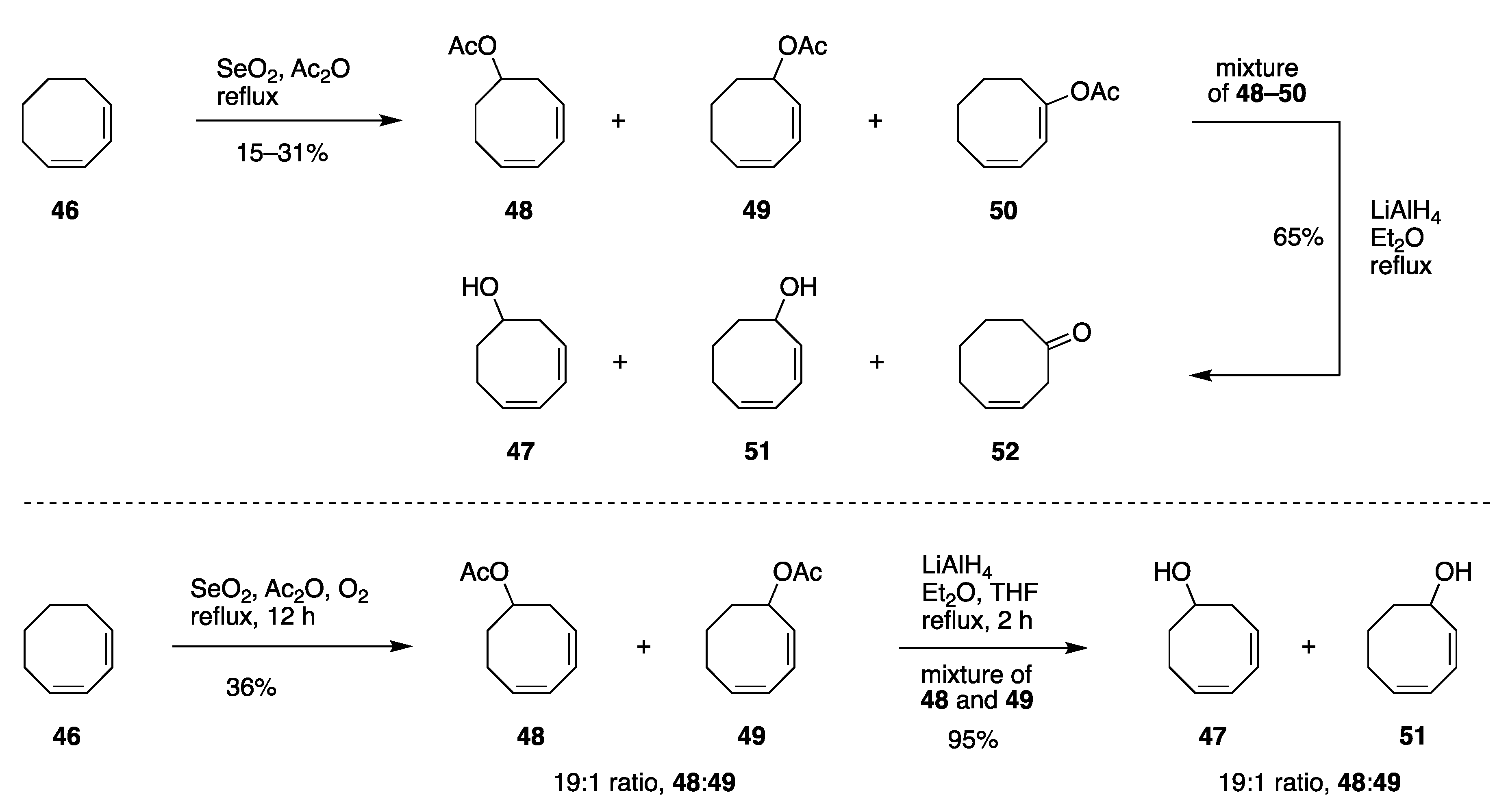
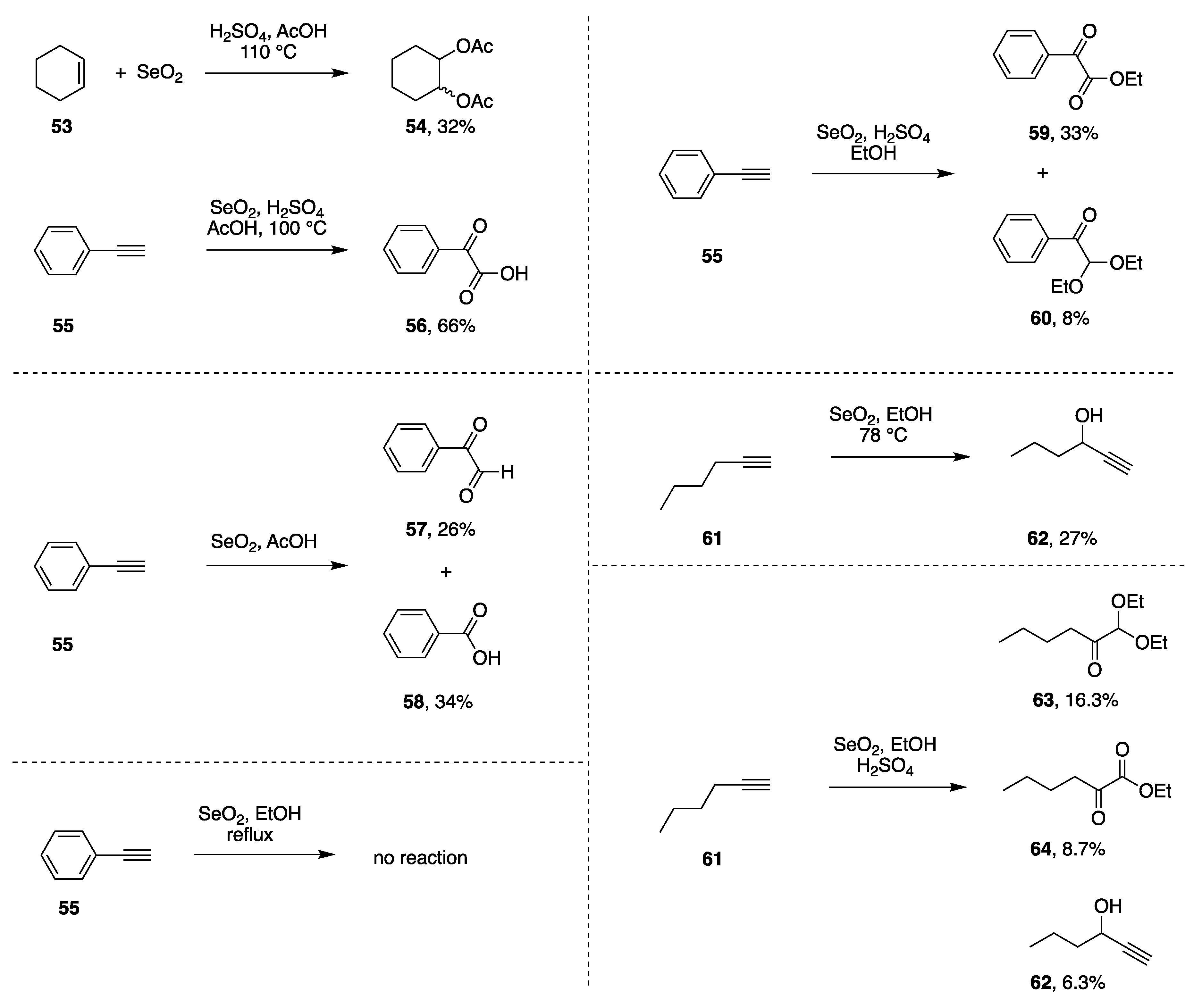
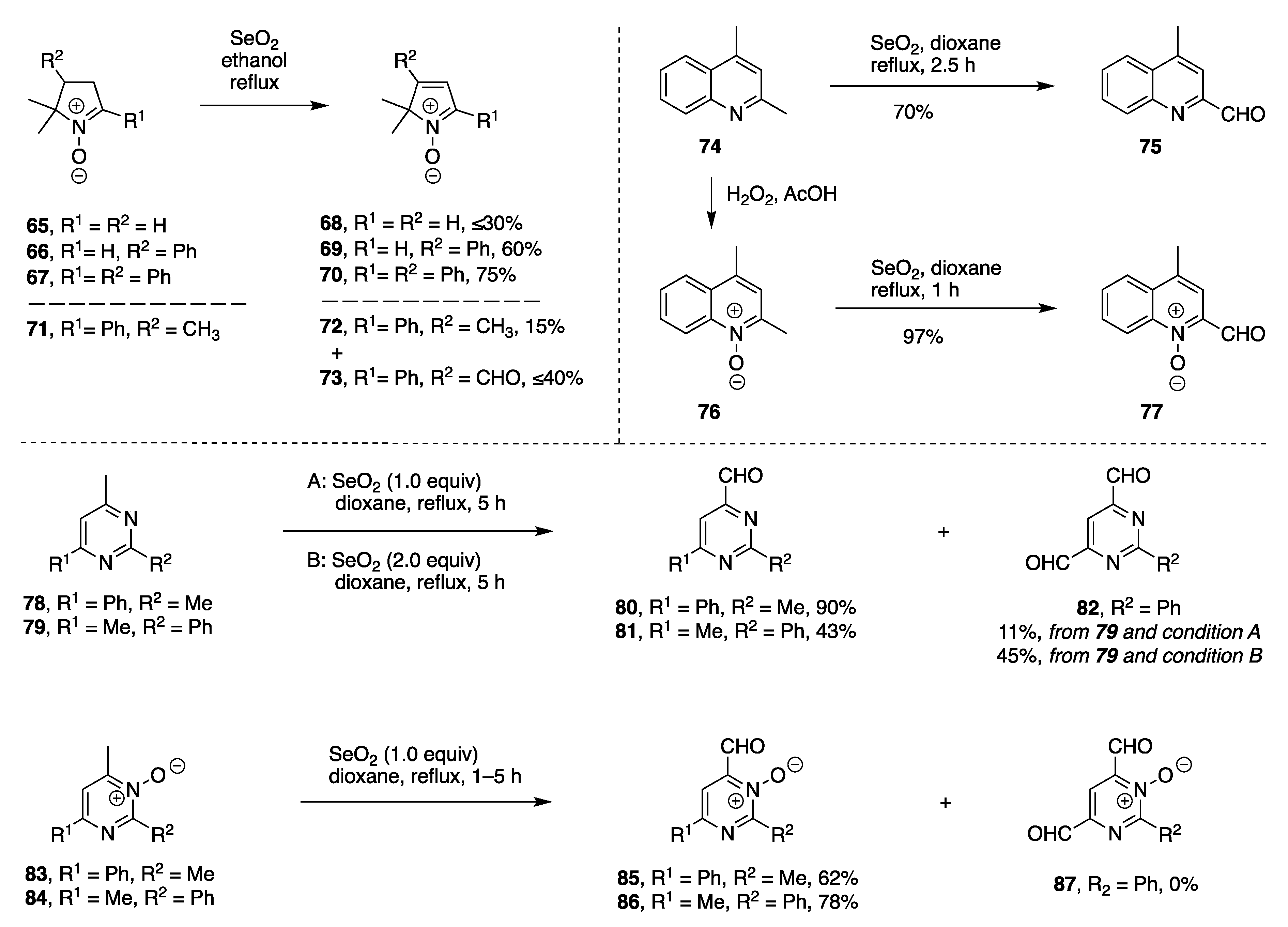

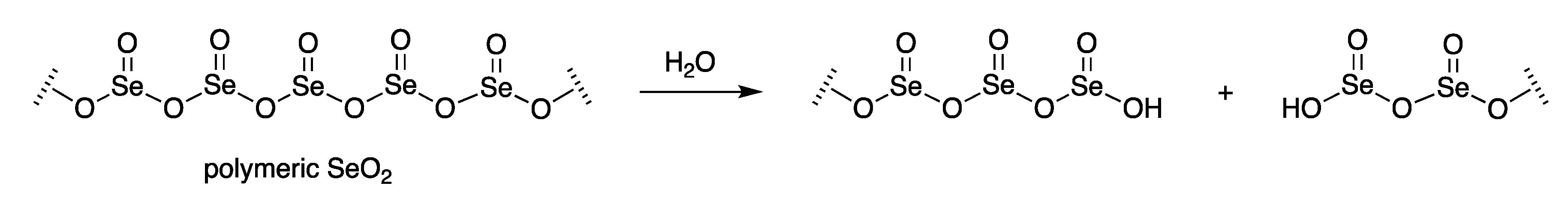
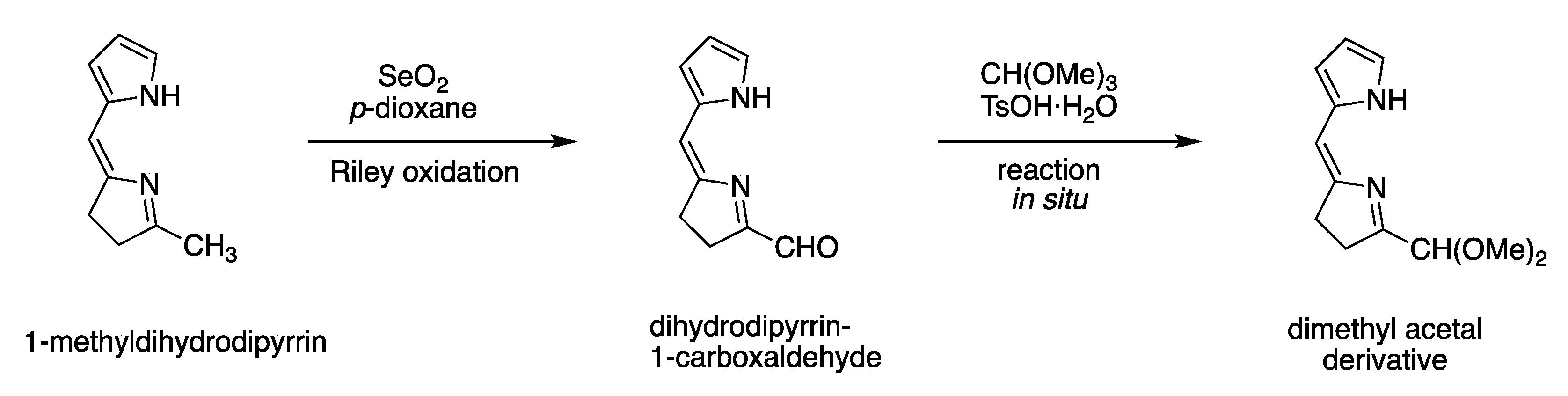
| Entry | Substrate, R = CH3 | Oxidant (Equiv) | Solvent, (Conc), and Additive (Equiv) | T (°C), Atmosphere, and Time | Product, R | Yield (%) | Ref |
|---|---|---|---|---|---|---|---|
| 1 | 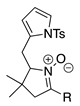 | SeO2 (1.5) | dioxane (0.08 M) | rt argon 2.5 h | –CHO | 79 | [42] |
| 2 | 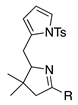 | SeO2 | – b | – b | –CHO | 0 | [42] |
| 3 | 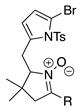 | SeO2 (1.3) | dioxane (0.10 M) | rt argon 2.5 h | –CHO | 43 | [42] |
| 4a |  | SeO2 (3.0) | dioxane (0.05 M) | rt argon 100 min | –CHO | 40 | [61] |
| 4b | Same as 4a | SeO2 (3.0) | dioxane (0.05 M) | rt argon 1.5 h | –CHO | 32 | [22] |
| 5 | 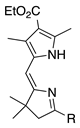 | SeO2 (3.0) | dioxane (0.04 M) | rt argon 15 min | –CHO | 66 | [62] |
| 6 | 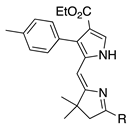 | SeO2 (3.0) | dioxane (0.05 M) | rt argon 1 h | –CHO | 39 | [62] |
| 7 | 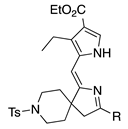 | SeO2 (3.0) | dioxane (0.05 M) | rt argon 2 h | –CH(OMe)2 | 42 | [61] |
| 8 | 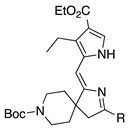 | SeO2 (3.0) | dioxane (0.05 M) | rt argon 1 h | –CH(OMe)2 | 44 | [61] |
| 9 | 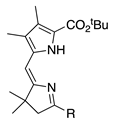 | SeO2 (3.0) | dioxane (0.05 M) | rt argon 1.5 h | –CHO | 63 | [19] |
| 10 |  | SeO2 (3.0) | dioxane (0.05 M) | rt – b 2 h | BC c | 6.6 | [19] |
| 11 |  | SeO2 (3.0) | dioxane (0.05 M) | rt argon 1.5 h | –CHO | 55 | [26] |
| 12 |  | SeO2 (3.0) | dioxane (0.04 M) | rt argon 30 min | –CHO | 59 | [62] |
| 13 | 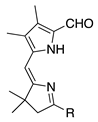 | SeO2 (1.5) | dioxane (0.12 M) | rt argon 2 h | –CHO | 99 d | [16] |
| 14 |  | SeO2 (1.5) | dioxane (0.05 M) | rt argon 1.5 h | –CHO | 47 | [19] |
| 15 | 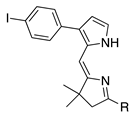 | SeO2 (1.46) | dioxane (0.05 M) | rt – b 2 h | –CHO | 57 | [19] |
| 16a | 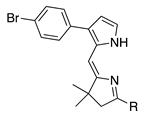 | SeO2 (3.0) | dioxane (0.05 M) | rt argon 1.5 h | –CHO | 22 | [22] |
| 16b e,f | Same as 16a | SeO2 (1.5) | dioxane (0.05 M) | rt air 0.5 h | –CHO | 36 g | this work |
| 16c e,f | Same as 16a | SeO2 (1.5) | dioxane (0.05 M) SiO2 (5.0 eq) | rt air 0.5 h | –CHO | 38 g | this work |
| 16d e,f | Same as 16a | SeO2 (1.5) | dioxane (0.05 M) pyridine (0.02 eq) | rt air 0.5 h | –CHO | 28 g | this work |
| 16e e,f | Same as 16a | SeO2 (1.5) | dioxane (0.05 M) C6F5CHO (1.5) | rt air 0.5 h | –CHO | 18 g | this work |
| 17 h | 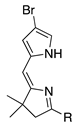 | SeO2 (1.0–3.0) | dioxane (0.05 M) | 0 °C to rt air or argon 15 min to 3 h | –CHO | 0 | this work |
| 18 | 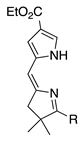 | SeO2 (3.0) | dioxane (0.06 M) | rt – b 30 min | –CH(OMe)2 | 30 | [20] |
| 19 | 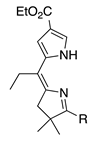 | SeO2 (3.0) | dioxane (0.08 M) | rt – b 30 min | –CH(OMe)2 | 63 | [20] |
| 20 | 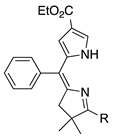 | SeO2 (3.0) | dioxane (0.05 M) | rt – b 2 h | –CHO | 65 | [63] |
| 21 |  | SeO2 (3.0) | dioxane (0.05 M) | rt – b 30 min | –CH(OMe)2 | 43 | [20] |
| 22 | 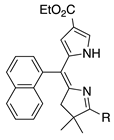 | SeO2 (3.0) | dioxane (0.07 M) | rt – b 30 min | –CH(OMe)2 | 76 | [20] |
| 23 | 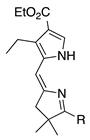 | SeO2 (3.0) | dioxane (0.06 M) | rt – b 30 min | –CH(OMe)2 | 25 | [20] |
| 24 | 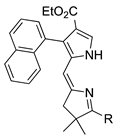 | SeO2 (3.0) | dioxane (0.06 M) | rt – b 30 min | –CH(OMe)2 | 42 | [26] |
| 25 | 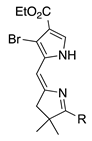 | SeO2 (3.0) | dioxane (0.05 M) pyridine (0.02 eq) | rt – b 5 h | –CH(OMe)2 | 37 | [26] |
| 26 | 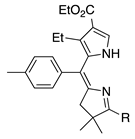 | SeO2 (3.0) | dioxane (0.02 M) | rt – b 30 min | –CH(OMe)2 | 31 | [20] |
| 27 | 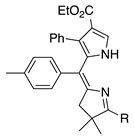 | SeO2 (3.0) | dioxane (0.02 M) | rt – b 30 min | –CH(OMe)2 | 31 | [20] |
| 28 | 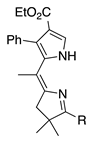 | SeO2 (2.9) | dioxane (0.01 M) | rt – b 30 min | –CH(OMe)2 | 48 | [20] |
| 29 |  | SeO2 (3.0) | dioxane (0.01 M) | rt – b 30 min | –CHO | 57 | [64] |
| 30 | 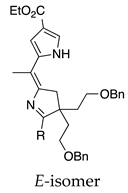 | SeO2 (3.0) | dioxane (0.01 M) | rt – b 2 h | –CH(OMe)2 | 12, E 51, Z | [64] |
| 31 | 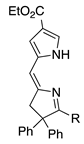 | SeO2 (3.0) | dioxane (0.02 M) | rt – b 6 h | –CH(OMe)2 | 30, Z 25, E | [64] |
| 32 | 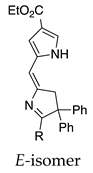 | SeO2 (3.0) | dioxane (0.01 M) | rt – b 2 h | –CH(OMe)2 | 45, E 15, Z | [64] |
| 33 | 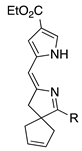 | SeO2 | – b | – b | –CHO | 0 | [64] |
| 34 | 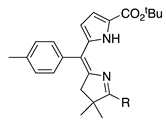 | SeO2 (3.0) | dioxane (0.04 M) | rt – b 30 min | –CH(OMe)2 | 47 | [20] |
| 35 | 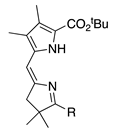 | SeO2 (1.3) | DMF (0.18 M) pyridine (1.2 eq) | rt, then 80 – b 5 h and 15 min | –CHO | 71 | [18] |
| 36a | 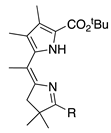 | SeO2 (1.2) | DMF (0.11 M) pyridine (1.2 eq) | rt, then 80 – b 5 h and 15 min | –CHO | 65 | [18] |
| 36b | Same as 36a | SeO2 (1.5) | dioxane (0.04 M) | reflux argon 30 min | –CHO | 32 | [16] |
| 37 |  | SeO2 | DMF (0.09 M) pyridine (1.2 eq) | rt, then 80 – b 5 h and 15 min | –CHO | 81 | [18] |
| 38 | 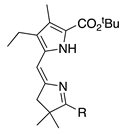 | SeO2 (1.2) | DMF (0.11 M) | rt, then 80 – b 5 h and 15 min | –CHO | 46 | [65] |
| 39a |  | SeO2 (1.6) | dioxane (0.08 M) | rt argon 2 h | –CHO | 68 d | [16] |
| 39b | 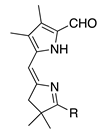 | SeO2 (1.3) | CH2Cl2 (0.05 M) pyridine (1.3 equiv), then DMF (0.10) M | rt, then 80 – b 2 h and 15 min | –CHO | 61 | [17] |
| 40a | 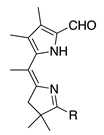 | SeO2 (1.3) | dioxane (0.09 M) | rt argon 2 h | –CHO | 99 E/Z 1:5 i | [16] |
| 40b | Same as 40a | SeO2 (1.2) | CH2Cl2 (0.05 M) pyridine (1.19 equiv), then DMF (0.10) M | rt, then 80 – b 2 h and 15 min | –CHO | 71 | [17] |
| 41a | 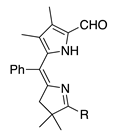 | SeO2 (1.6) | dioxane (0.12 M) | rt argon 2 h | –CHO | 62 d | [16] |
| 41b | Same as 41a | SeO2 (1.0) | CH2Cl2 (0.05 M) pyridine (1.0 eq), then DMF (0.07 M) | rt, then 80 – b 5 h and 15 min | –CHO | 70 | [17] |
| 42 | 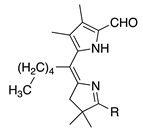 | SeO2 (1.2) | CH2Cl2 (0.05 M) pyridine (1.2 eq), then DMF | rt, then 80 – b 5 h and 15 min | –CHO | 63 | [17] |
| 43 | 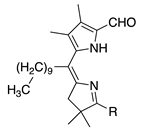 | SeO2 (1.2) | CH2Cl2 (0.05 M) pyridine (1.2 eq), then DMF | rt, then 80 – b 5 h and 15 min | –CHO | 65 | [17] |
| 44 | 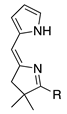 | SeO2 (2.1) | CH2Cl2 (0.02 M) | rt argon – b | BC c | 5.8 | [20] |
| 45 | 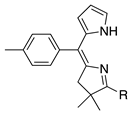 | SeO2 | – b | – b | –CHO | 0 | [20] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Lindsey, J.S. Riley Oxidation of Heterocyclic Intermediates on Paths to Hydroporphyrins—A Review. Molecules 2020, 25, 1858. https://doi.org/10.3390/molecules25081858
Wang P, Lindsey JS. Riley Oxidation of Heterocyclic Intermediates on Paths to Hydroporphyrins—A Review. Molecules. 2020; 25(8):1858. https://doi.org/10.3390/molecules25081858
Chicago/Turabian StyleWang, Pengzhi, and Jonathan S. Lindsey. 2020. "Riley Oxidation of Heterocyclic Intermediates on Paths to Hydroporphyrins—A Review" Molecules 25, no. 8: 1858. https://doi.org/10.3390/molecules25081858
APA StyleWang, P., & Lindsey, J. S. (2020). Riley Oxidation of Heterocyclic Intermediates on Paths to Hydroporphyrins—A Review. Molecules, 25(8), 1858. https://doi.org/10.3390/molecules25081858







