A Two-Step Ferric Chloride and Dilute Alkaline Pretreatment for Enhancing Enzymatic Hydrolysis and Fermentable Sugar Recovery from Miscanthus sinensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment Operation
2.3. Enzymatic Hydrolysis of Stalk Samples
2.4. Analysis
3. Results and Discussion
3.1. Effect of Ferric Chloride Pretreatment (FP) and Dilute Alkaline Pretreatment (ALP) on Composition
3.2. Effect of FP and ALP on Enzymatic Hydrolysis
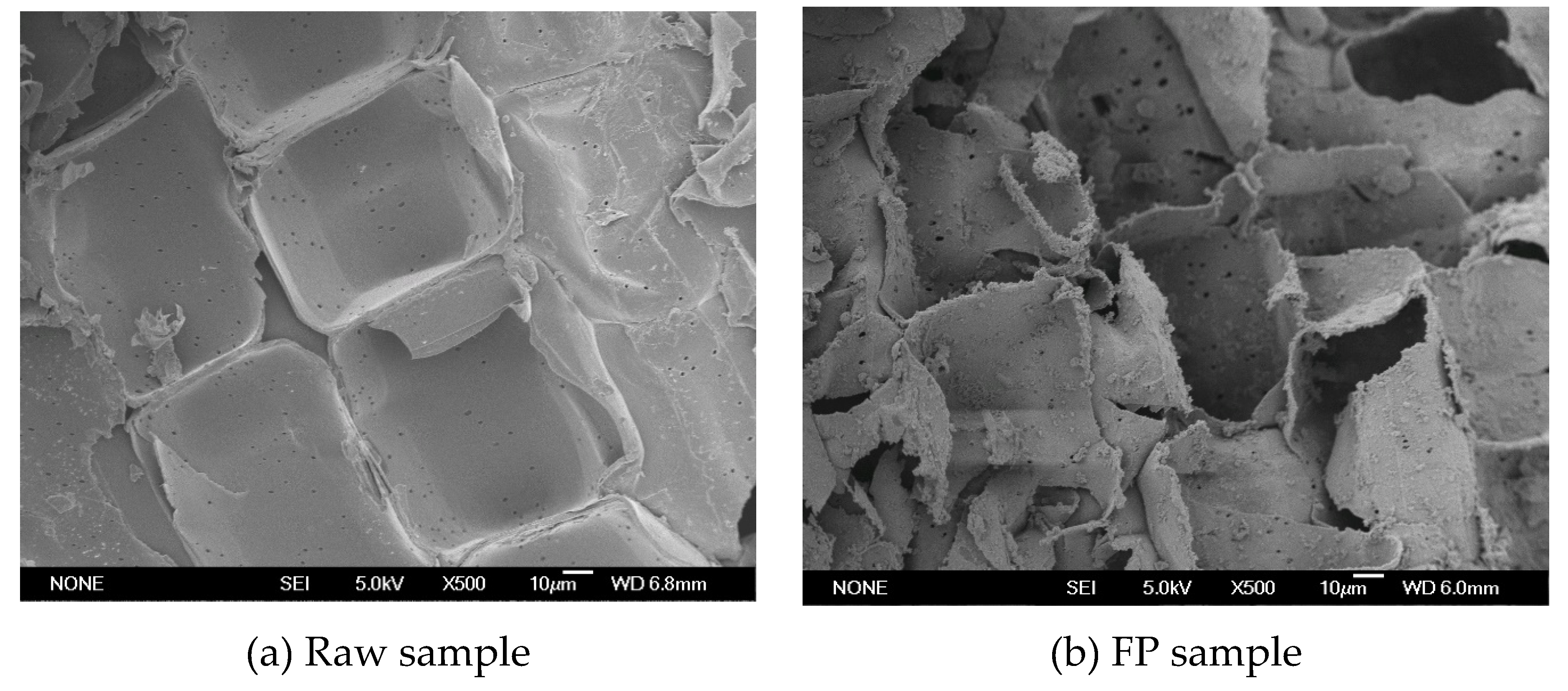
3.3. Effect of FP and ALP on Structure
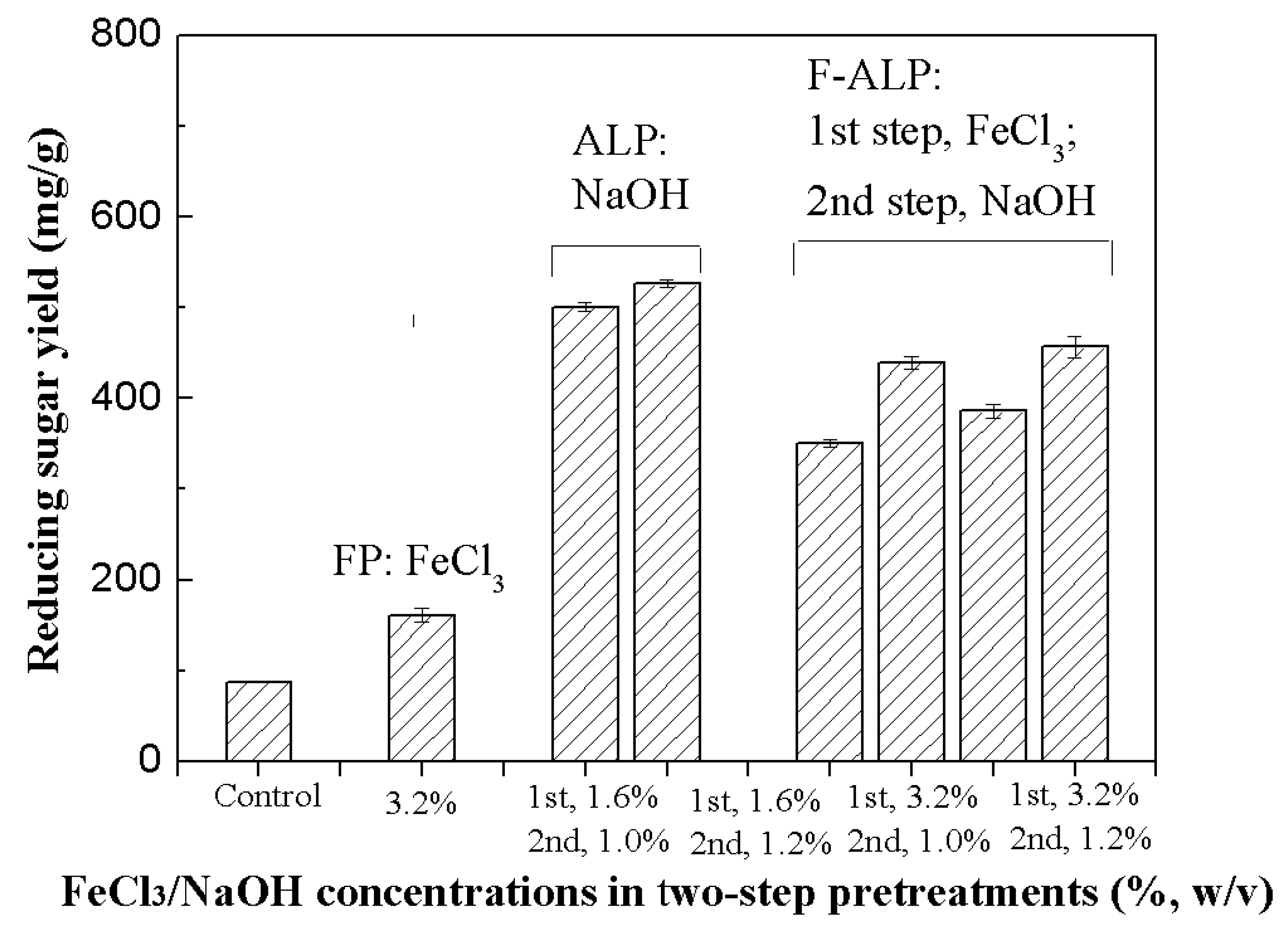
3.4. Effect of the Two-Step Process on Sugar Recovery
3.5. Mass Balance for One-Step/Two-Step Pretreatments and Enzymatic Hydrolysis Processes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duque, A.; Manzanares, P.; González, A.; Ballesteros, M. Study of the application of alkaline extrusion to the pretreatment of Eucalyptus biomass as first step in a bioethanol production process. Energies 2018, 11, 2961. [Google Scholar] [CrossRef]
- Amiri, H.; Karimi, K. Pretreatment and hydrolysis of lignocellulosic wastes for butanol production: Challenges and perspectives. Bioresour. Technol. 2018, 270, 702–721. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhao, S.S.; Rogers, K.M.; Xia, Y.A.; Zhang, B.; Suo, R.; Zhao, Y. A case of milk traceability in small-scale districts-Inner Mongolia of China by nutritional and geographical parameters. Food Chem. 2020, 316, 126332. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.F.; Chen, K.Q.; Zhu, R.; Sun, W.X.; Zou, H.; Guo, R.B. Improved anaerobic digestion performance of Miscanthus floridulus by different pretreatment methods and preliminary economic analysis. Energ. Convers. Manage. 2018, 159, 120–128. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, B.; Guo, B.; Wang, D.H.; Yang, S.M. Combination of multi-element and stable isotope analysis improved the traceability of chicken from four provinces of China. CYTA-J. Food 2016, 14, 163–168. [Google Scholar] [CrossRef]
- Li, J.B.; Zhang, H.Y.; Lu, M.S.; Han, L.J. Comparison and intrinsic correlation analysis based on composition, microstructure and enzymatichydrolysis of corn stover after different types of pretreatments. Bioresour. Technol. 2019, 293, 122016. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, B.; Chen, G.; Chen, A.L.; Yang, S.M.; Ye, Z.H. Recent developments in application of stable isotope analysis on agro-product authenticity and traceability. Food Chem. 2014, 145, 300–305. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Holowacz, I.; Lukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- He, Y.C.; Zhang, D.P.; Di, J.H.; Wu, Y.Q.; Tao, Z.C.; Liu, F.; Zhang, Z.J.; Chong, G.G.; Ding, Y.; Ma, C.L. Effective pretreatment of sugarcane bagasse with combination pretreatment and its hydrolyzates as reaction media for the biosynthesis of ethyl (S)-4-chloro-3-hydroxybutanoate by whole cells of E-coli CCZU-K14. Bioresour. Technol. 2016, 211, 720–726. [Google Scholar] [CrossRef]
- Phitsuwan, P.; Sakka, K.; Ratanakhanokchai, K. Structural changes and enzymatic response of Napier grass (Pennisetum purpureum) stem induced by alkaline pretreatment. Bioresour. Technol. 2016, 218, 247–256. [Google Scholar] [CrossRef]
- Tang, S.Y.; Xu, C.M.; Khanh Vu, L.T.; Liu, S.C.; Ye, P.; Li, L.C.; Wu, Y.X.; Chen, M.Y.; Xiao, Y.; Wu, Y.; et al. Enhanced enzymatic hydrolysis of Pennisetum alopecuroides by dilute acid, alkaline and ferric chloride pretreatments. Molecules 2019, 24, 1715. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef]
- Kang, K.E.; Park, D.H.; Jeong, G.T. Effects of inorganic salts on pretreatment of Miscanthus straw. Bioresour. Technol. 2013, 132, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Zhang, G.; Zhang, P.; Li, F.; Wang, S.; Fan, S.; Zhou, S. Microwave assisted alkaline pretreatment to enhance enzymatic saccharification of catalpa sawdust. Bioresour. Technol. 2016, 221, 26–30. [Google Scholar] [CrossRef]
- Sahoo, D.; Ummalyma, S.B.; Okram, A.K.; Pandey, A.; Sankar, M.; Sukumaran, R.K. Effect of dilute acid pretreatment of wild rice grass (Zizania latifolia) from Loktak Lake for enzymatic hydrolysis. Bioresour. Technol. 2018, 253, 252–255. [Google Scholar] [CrossRef]
- Wang, W.H.; Zhang, C.Y.; Tong, S.S.; Cui, Z.Y.; Liu, P. Enhanced enzymatic hydrolysis and structural features of corn stover by NaOH and ozone combined pretreatment. Molecules 2018, 23, 1300. [Google Scholar] [CrossRef]
- Wang, Z.N.; Hou, X.F.; Sun, J.; Li, M.; Chen, Z.Y.; Gao, Z.Z. Comparison of ultrasound-assisted ionic liquid and alkaline pretreatment of Eucalyptus for enhancing enzymatic saccharification. Bioresour. Technol. 2018, 254, 145–150. [Google Scholar] [CrossRef]
- Xia, F.; Gong, J.W.; Lu, J.; Cheng, Y.; Zhai, S.R.; An, Q.D.; Wang, H.S. Combined liquid hot water with sodium carbonate-oxygen pretreatment to improve enzymatic saccharification of reed. Bioresour. Technol. 2019, 297, 122498. [Google Scholar] [CrossRef]
- Meng, X.Z.; Wells, T.; Sun, Q.N.; Huang, F.; Ragauskas, A. Insights into the effect of dilute acid, hot water or alkaline pretreatment on the cellulose accessible surface area and the overall porosity of Populus. Green Chem. 2015, 17, 4239–4246. [Google Scholar] [CrossRef]
- Kim, D. Physico-chemical conversion of lignocellulose: Inhibitor effects and detoxification strategies: A mini review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef]
- Michelin, M.; Ximenes, E.; de Lourdes Teixeira de Moraes Polizeli, M.; Ladisch, M.R. Effect of phenolic compounds from pretreated sugarcane bagasse on cellulolytic and hemicellulolytic activities. Bioresour. Technol. 2016, 199, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Camesasca, L.; Ramı’rez, M.B.; Guigou, M.; Ferrari, M.D.; Lareo, C. Evaluation of dilute acid and alkaline pretreatments, enzymatic hydrolysis and fermentation of napiergrass for fuel ethanol production. Biomass Bioenergy 2015, 74, 193–201. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Wang, C.; Sun, S.; Sun, R. Evaluation of the two-step treatment with ionic liquids and alkali for enhancing enzymatic hydrolysis of Eucalyptus: Chemical and anatomical changes. Biotechnol. Biofuels 2016, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Li, K.N.; Wan, J.M.; Wang, X.; Wang, J.F.; Zhang, J.H. Comparison of dilute acid and alkali pretreatments in production of fermentable sugars from bamboo: Effect of Tween 80. Ind. Crops Products 2016, 83, 414–422. [Google Scholar] [CrossRef]
- Pandey, A.K.; Negi, S. Impact of surfactant assisted acid and alkali pretreatment on lignocellulosic structure of pine foliage and optimization of its saccharification parameters using response surface methodology. Bioresour. Technol. 2015, 192, 115–125. [Google Scholar] [CrossRef]
- Zhang, H.D.; Lyu, G.J.; Zhang, A.P.; Li, X.; Xie, J. Effects of ferric chloride pretreatment and surfactants on the sugar production from sugarcane bagasse. Bioresour. Technol. 2018, 265, 93–101. [Google Scholar] [CrossRef]
- Dziekonska-Kubczak, U.; Berłowska, J.; Dziugan, P.; Patelski, P.; Pielech-Przybylska, K.; Balcerek, M. Nitric Acid pretreatment of jerusalem artichoke stalks for enzymatic saccharification and bioethanol production. Energies 2018, 11, 2153. [Google Scholar] [CrossRef]
- Liu, C.Z.; Cheng, X.Y. Improved hydrogen production via thermophilic fermentation of corn stover by microwave-assisted acid pretreatment. Int. J. Hydrogen Energy 2010, 35, 8945–8952. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- SEPAC. The Methods for Water and Wastewater Monitoring and Analysis, 4th ed.; State Environmental Protection Administration of China; China Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Goering, H.K.; Van-Soest, P.J. Agricultural Handbook No. 379. Forage Fiber Analyses, Apparatus, Reagents, Procedures and Some Applications; U.S. Department of Agriculture: Washington, DC, USA, 1970. [Google Scholar]
- Pedersen, M.; Meyer, A.S. Lignocellulose pretreatment severity-relating pH to biomatrix opening. New Biotechnol. 2010, 27, 739–750. [Google Scholar] [CrossRef]
- Brienzo, M.; Fikizolo, S.; Benjamin, Y.; Tyhoda, L.; Görgens, J. Influence of pretreatment severity on structural changes, lignin content and enzymatic hydrolysis of sugarcane bagasse samples. Renew. Energy 2017, 104, 271–280. [Google Scholar] [CrossRef]
- Xin, D.; Yang, Z.; Liu, F.; Xu, X.; Zhang, J. Comparison of aqueous ammonia and dilute acid pretreatment of bamboo fractions: Structure properties and enzymatic hydrolysis. Bioresour. Technol. 2015, 175, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Du, J. Novozymes accelerates cellulosic ethanol commercialized. China WTO Tribune 2010, 10, 81. (In Chinese) [Google Scholar]
- Puligundla, P.; Smogrovicova, D.; Mok, C.; Obulam, V.S.R. A review of recent advances in high gravity ethanol fermentation. Renew. Energy 2018, 133, 1366–1379. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, Q.; Liu, H.J.; Li, S.Z.; Jiang, Z.Q. Characterization of actinidin from Chinese kiwifruit cultivars and its applications in meat tenderization and production of angiotensin I-converting enzyme (ACE) inhibitory peptides. LWT-Food Sci. Technol. 2017, 78, 1–7. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, B.; Yan, Q.J.; Jiang, Z.Q. Comparative analysis on the distribution of protease activities among fruits and vegetable resources. Food Chem. 2016, 213, 708–713. [Google Scholar] [CrossRef]
- Nosrati-Ghods, N.; Harrison, S.T.L.; Isafiade, A.J.; Tai, S.L. Ethanol from biomass hydrolysates by efficient fermentation of glucose and xylose-A Review. Chembioeng. Rev. 2018, 5, 294–311. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Yang, H.Y.; Yan, Q.J.; Yang, S.Q.; Jiang, Z.Q.; Li, S.Z. Biochemical properties and application of a novel beta-1,3-1,4-glucanase from Paenibacillus barengoltzii. Food Chem. 2017, 234, 68–75. [Google Scholar] [CrossRef]
- Si, S.L.; Chen, Y.; Fan, C.F.; Hu, H.Z.; Li, Y.; Huang, J.F.; Liao, H.F.; Hao, B.; Li, Q.; Peng, L.C.; et al. Lignin extraction distinctively enhances biomass enzymatic saccharification in hemicelluloses-rich Miscanthus species under various alkali and acid pretreatments. Bioresour. Technol. 2015, 183, 248–254. [Google Scholar] [CrossRef]
- Unrean, P.; Ketsub, N. Integrated lignocellulosic bioprocess for co-production of ethanol and xylitol from sugarcane bagasse. Ind. Crop. Prod. 2018, 123, 238–246. [Google Scholar] [CrossRef]
Sample Availability: Samples of the chemical compounds used in this study are available from the authors. |
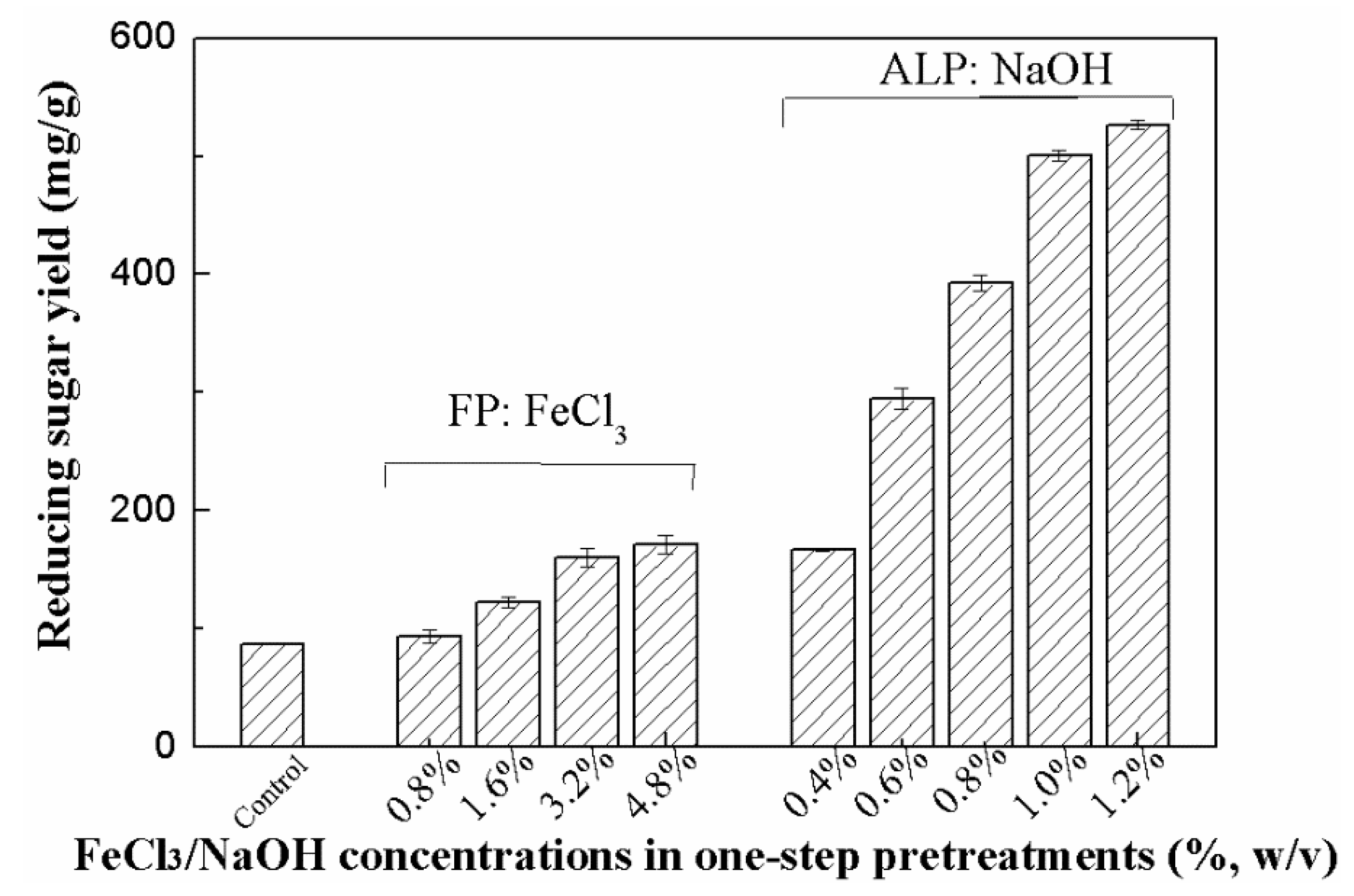

| Pretreatments | Chemical Concentrations (%) | Solid Yield (%) | SS of PTS (mg/g RS)1 | HC (%) | CC (%) | LC (%) |
|---|---|---|---|---|---|---|
| Control | -- | -- | -- | 28.3 ± 0.7 | 41.7 ± 0.4 | 20.3 ± 0.7 |
| FP | 0.8% FeCl3 | 71.5 ± 1.4 | 130.9 ± 11.5 | 13.6 ± 1.8 | 53.9 ± 0.9 | 18.7 ± 1.1 |
| 1.6% FeCl3 | 62.8 ± 3.2 | 174.8 ± 5.9 | 11.3 ± 1.5 | 55.3 ± 0.8 | 19.7 ± 1.3 | |
| 3.2% FeCl3 | 54.9 ± 1.6 | 240.2 ± 9.3 | 10.6 ± 1.4 | 58.3 ± 0.7 | 20.5 ± 1.7 | |
| 4.8% FeCl3 | 52.9 ± 2.1 | 256.9 ± 8.5 | 9.4 ± 1.7 | 58.8 ± 1.1 | 21.4 ± 1.6 | |
| ALP | 0.4% NaOH | 79.8 ± 1.0 | 52.7 ± 0.7 | 26.6 ± 0.9 | 49.6 ± 0.8 | 18.9 ± 0.6 |
| 0.6% NaOH | 75.6 ± 1.0 | 56.0 ± 1.1 | 20.5 ± 1.2 | 51.4 ± 1.4 | 16.5 ± 0.5 | |
| 0.8% NaOH | 62.0 ± 0.9 | 70.4 ± 1.0 | 15.4 ± 0.7 | 57.6 ± 0.6 | 15.6 ± 0.4 | |
| 1.0% NaOH | 59.8 ± 1.3 | 90.7 ± 1.4 | 14.6 ± 0.6 | 59.1 ± 0.3 | 13.8 ± 0.1 | |
| 1.2% NaOH | 55.0 ± 0.6 | 93.7 ± 0.5 | 14.2 ± 0.6 | 62.5 ± 0.5 | 12.0 ± 0.3 |
| Single/Two-Step Pretreatments | SS Yield of PTS | SS Yield of EH | Total SS Yield | Cellulase Amount Used2 | |||
|---|---|---|---|---|---|---|---|
| Step 1 | Step 2 | Step 1 (mg/g RS) | Step 2 (mg/g PS) | (mg/g PS) | (mg/g RS)1 | (FPU/g) | |
| Control | -- | -- | -- | -- | -- | 86.9 | 15 |
| FP | 3.2% FeCl3 | -- | 240.2 | -- | 160.7 | 328.4 | 8.2 |
| 4.8% FeCl3 | -- | 256.9 | -- | 171.0 | 347.4 | 7.9 | |
| ALP | 0.8%NaOH | -- | 70.4 | -- | 392.4 | 313.7 | 9.3 |
| 1.0%NaOH | -- | 90.7 | -- | 501.0 | 390.3 | 9.0 | |
| 1.2%NaOH | -- | 93.7 | -- | 526.5 | 383.3 | 8.3 | |
| F-ALP | 1.6% FeCl3 | 1.0%NaOH | 174.8 | 31.2 | 350.7 | 353.0 | 6.8 |
| 1.6% FeCl3 | 1.2%NaOH | 174.8 | 32.6 | 439.7 | 376.1 | 6.2 | |
| 3.2% FeCl3 | 1.0%NaOH | 240.2 | 32.0 | 386.4 | 408.8 | 5.9 | |
| 3.2% FeCl3 | 1.2%NaOH | 240.2 | 32.9 | 457.0 | 418.8 | 5.3 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Ye, P.; Chen, M.; Tang, S.; Luo, Y.; Gao, Y.; Yan, Q.; Cheng, X. A Two-Step Ferric Chloride and Dilute Alkaline Pretreatment for Enhancing Enzymatic Hydrolysis and Fermentable Sugar Recovery from Miscanthus sinensis. Molecules 2020, 25, 1843. https://doi.org/10.3390/molecules25081843
Li L, Ye P, Chen M, Tang S, Luo Y, Gao Y, Yan Q, Cheng X. A Two-Step Ferric Chloride and Dilute Alkaline Pretreatment for Enhancing Enzymatic Hydrolysis and Fermentable Sugar Recovery from Miscanthus sinensis. Molecules. 2020; 25(8):1843. https://doi.org/10.3390/molecules25081843
Chicago/Turabian StyleLi, Lingci, Peng Ye, Mengyu Chen, Shangyuan Tang, Ying Luo, Yifan Gao, Qiong Yan, and Xiyu Cheng. 2020. "A Two-Step Ferric Chloride and Dilute Alkaline Pretreatment for Enhancing Enzymatic Hydrolysis and Fermentable Sugar Recovery from Miscanthus sinensis" Molecules 25, no. 8: 1843. https://doi.org/10.3390/molecules25081843
APA StyleLi, L., Ye, P., Chen, M., Tang, S., Luo, Y., Gao, Y., Yan, Q., & Cheng, X. (2020). A Two-Step Ferric Chloride and Dilute Alkaline Pretreatment for Enhancing Enzymatic Hydrolysis and Fermentable Sugar Recovery from Miscanthus sinensis. Molecules, 25(8), 1843. https://doi.org/10.3390/molecules25081843






