Natural Deep Eutectic Solvents for the Extraction of Phenyletanes and Phenylpropanoids of Rhodiola rosea L.
Abstract
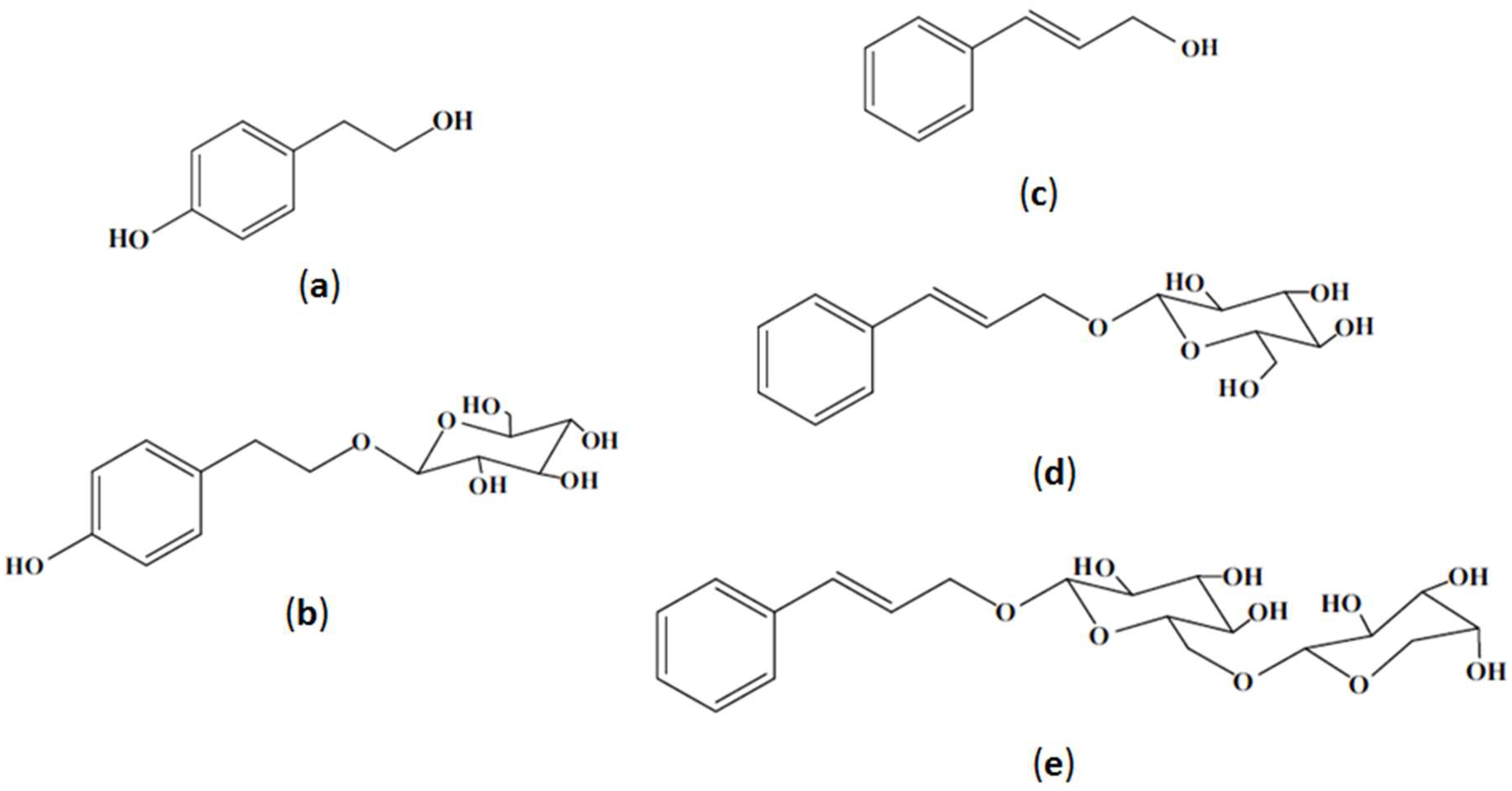
1. Introduction
2. Results and Discussion
2.1. Extractability of Phenyletanes and Phenylpropanoids from R. rosea by NADES and 40% Ethanol
2.2. Optimization of Extraction Conditions
2.3. Verification of the Models
3. Materials and Methods
3.1. Materials and Reagents
3.2. HPLC Analysis
3.3. NADES Preparation
3.4. UAE with Different Solvents
3.5. Experimental Design and Statistical Analysis
3.6. Data Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mell, C. Dyes, tannins, perfumes, and medicines from Rhodiola rosea. Text. Colorist 1938, 60, 483–484. [Google Scholar]
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 2010, 17, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Galambosi, B.; Galambosi, Z.; Hethelyi, E.; Szöke, E.; Volodin, V.; Poletaeva, I.; Iljina, I. Importance and quality of roseroot (Rhodiola rosea L.) growing in the European North. Z. Arznei Gewürzpflanz. 2010, 15, 160–169. [Google Scholar]
- Chiang, H.M.; Chen, H.C.; Wu, C.S.; Wu, P.Y.; Wen, K.C. Rhodiola plants: Chemistry and biological activity. J. Food Drug Anal. 2015, 23, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.N.; Tsitsilin, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Heinrich, M. Traditional and current food use of wild plants listed in the Russian pharmacopoeia. Front. Pharmacol. 2017, 8, 841. [Google Scholar] [CrossRef]
- Brown, R.P.; Gerbarg, P.L.; Ramazanov, Z. Rhodiola rosea. A Phytomedicinal Overview. HerbalGram 2002, 56, 40–52. [Google Scholar]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Wagner, H.; Verpoorte, R.; Heinrich, M. Medicinal plants of the Russian Pharmacopoeia; their history and applications. J. Ethnopharmacol. 2014, 154, 481–536. [Google Scholar] [CrossRef]
- Marchev, A.S.; Dinkova-Kostova, A.T.; György, Z.; Mirmazloum, I.; Aneva, I.Y.; Georgiev, M.I. Rhodiola rosea L.: From golden root to green cell factories. Phytochem. Rev. 2016, 15, 515–536. [Google Scholar] [CrossRef]
- Khanum, F.; Bawa, A.S.; Singh, B. Rhodiola rosea: A versatile adaptogen. Compr. Rev. Food Sci. Food Saf. 2005, 4, 55–62. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G. Evidence-based efficacy of adaptogens in fatigue, and molecular mechanisms related to their stress-protective activity. Curr. Clin. Pharmacol. 2009, 4, 198–219. [Google Scholar] [CrossRef]
- Panossian, A. Understanding adaptogenic activity: Specificity of the pharmacological action of adaptogens and other phytochemicals. Ann. N. Y. Acad. Sci. 2017, 1401, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.M.; Gupta, B.M.; Bansal, J.; Bansal, M. Rhodiola rosea: A quantitative and qualitative assessment of global publications output during 2003–18. Int. J. Pharm. Investig. 2019, 9, 93–100. [Google Scholar] [CrossRef]
- Rohloff, J. Volatiles from rhizomes of Rhodiola rosea L. Phytochemistry 2002, 59, 655–661. [Google Scholar] [CrossRef]
- Ioset, K.; Nyberg, N.; Van Diermen, D.; Malnoe, P.; Hostettmann, K.; Shikov, A.N.; Jaroszewski, J.W. Metabolic profiling of Rhodiola rosea rhizomes by 1H NMR spectroscopy. Phytochem. Anal. 2011, 22, 158–165. [Google Scholar] [CrossRef]
- Tao, H.; Wu, X.; Cao, J.; Peng, Y.; Wang, A.; Pei, J.; Xiao, J.; Wang, S.; Wang, Y. Rhodiola species: A comprehensive review of traditional use, phytochemistry, pharmacology, toxicity, and clinical study. Med. Res. Rev. 2019, 39, 1779–1850. [Google Scholar] [CrossRef]
- Zapesochnaya, G.G.; Kurkin, V.A. Glycosides of cinnamyl alcohol from the rhizomes of Rhodiola rosea. Chem. Nat. Compd. 1982, 18, 685–688. [Google Scholar] [CrossRef]
- Zapesochnaya, G.G.; Kurkin, V.A.; Shchavlinskii, A.N. Flavonoids of the epigeal part of Rhodiola rosea II. Structures of new glycosides of herbacetin and of gossypetin. Chem. Nat. Compd. 1985, 21, 464–473. [Google Scholar] [CrossRef]
- Kurkin, V.A.; Zapesochnaya, G.G.; Shchavlinskii, A.N. Terpenoids of the Rhizomes of Rhodiola rosea. Chem. Nat. Compd. 1985, 21, 593–597. [Google Scholar] [CrossRef]
- Dubichev, A.G.; Kurkin, V.A.; Zapesochnaya, G.G.; Vorontsov, E.D. Chemical composition of the rhizomes of the Rhodiola rosea by the HPLC method. Chem. Nat. Compd. 1991, 27, 161–164. [Google Scholar] [CrossRef]
- Marchev, A.S.; Aneva, I.Y.; Koycheva, I.K.; Georgiev, M.I. Phytochemical variations of Rhodiola rosea L. wild-grown in Bulgaria. Phytochem. Lett. 2017, 20, 386–390. [Google Scholar] [CrossRef]
- Ganzera, M.; Yayla, Y.; Khan, I.A. Analysis of the marker compounds of Rhodiola rosea L. (golden root) by reversed phase high performance liquid chromatography. Chem. Pharm. Bull. (Tokyo) 2001, 49, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Iheozor-Ejidor, P.; Dey, E.S. Extraction of rosavin from Rhodiola rosea root using supercritical carbon dioxide with water. J. Supercrit. Fluids 2009, 50, 29–32. [Google Scholar] [CrossRef]
- Ma, C.; Wang, H.; Gu, X.; Hu, L. Large-scale preparative isolation of rosavin from Rhodiola rosea via Ion liquids MAE and subsequent flash adsorption chromatography. Sep. Sci. Technol. 2012, 47, 1821–1827. [Google Scholar] [CrossRef]
- Zhu, S.; Ma, C.; Fu, Q.; Hu, L.; Lou, Z.; Wang, H.; Tao, G. Application of ionic liquids in an online ultrasonic assisted extraction and solid-phase trapping of rhodiosin and rhodionin from Rhodiola rosea for UPLC. Chromatographia 2013, 76, 195–200. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Z.; Zou, L.; Chi, R. Imidazolium-based ionic liquids with inorganic anions in the extraction of salidroside and tyrosol from Rhodiola: The role of cations and anions on the extraction mechanism. J. Mol. Liq. 2019, 275, 136–145. [Google Scholar] [CrossRef]
- Tsvetov, N.S.; Mryasova, K.P.; Korotkova, G.V.; Asming, S.V.; Nikolaev, V.G. Extraction of cinnamic alcohol from Rhodiola rosea using deep eutectic solvents. IOP Conf. Ser. Earth Environ. Sci. 2019, 315, 042002. [Google Scholar] [CrossRef]
- Tsvetov, N.S.; Mryasova, K.P.; Shavarda, A.L.; Nikolaev, V.G. Estimation of extraction efficiency of salidroside from Rhodiola rosea using deep eutectic solvents. IOP Conf. Ser. Earth Environ. Sci. 2019, 315, 042006. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for phenolic compounds extraction: Overview, challenges, and opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Skarpalezos, D.; Detsi, A. Deep Eutectic Solvents as extraction media for valuable flavonoids from natural sources. Appl. Sci. 2019, 9, 4169. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Vidović, S.; Redovniković, I.R.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Choi, Y.H.; Van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.; Witkamp, G.J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Ćurko, N.; Srček, V.G.; Bubalo, M.C.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef]
- Bosiljkov, T.; Dujmić, F.; Bubalo, M.C.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Redovnicović, I.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Wu, Y.C.; Wu, P.; Li, Y.B.; Liu, T.C.; Zhang, L.; Zhou, Y.H. Natural deep eutectic solvents as new green solvents to extract anthraquinones from Rheum palmatum L. RSC Adv. 2018, 8, 15069–15077. [Google Scholar] [CrossRef]
- Li, J.; Han, Z.; Yu, B. Efficient extraction of major catechins in Camellia sinensis leaves using green choline chloride-baseddeep eutectic solvents. RSC Adv. 2015, 5, 93937–93944. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef]
- Dai, Y.; Row, K.H. Application of Natural Deep Eutectic Solvents in the extraction of quercetin from vegetables. Molecules 2019, 24, 2300. [Google Scholar] [CrossRef]
- Oomen, W.W.; Begines, P.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvent extraction of flavonoids of Scutellaria baicalensis as a replacement for conventional organic solvents. Molecules 2020, 25, 617. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Daurtseva, A.V.; Pozharitskaya, O.N.; Flisyuk, E.V.; Shikov, A.N. Natural Deep Eutectic Solvents as alternatives for extracting phlorotannins from brown algae. Pharm. Chem. J. 2019, 53, 243–247. [Google Scholar] [CrossRef]
- Nicolae, C.V.; Trică, B.; Doncea, S.M.; Marinaș, I.C.; Oancea, F.; Constantinescu-Aruxandei, D. Evaluation and optimization of polysaccharides and ferulic acid solubility in NADES using surface response methodology. Proceedings 2019, 29, 96. [Google Scholar] [CrossRef]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. TrAC Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops—A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Najafabadi, H.; Leardi, R.; Jalali-Heravi, M. Experimental design in analytical chemistry. Part I: Theory. J. AOAC Int. 2014, 97, 3–11. [Google Scholar] [CrossRef]
- Espino, M.; Solari, M.; De los Ángeles Fernández, M.; Boiteux, J.; Gómez, M.R.; Silva, M.F. NADES-mediated folk plant extracts as novel antifungal agents against Candida albicans. J. Pharm. Biomed. Anal. 2019, 167, 15–20. [Google Scholar] [CrossRef]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Minina, S.A.; Shigarova, L.V.; Vainshtein, V.A. Optimized process of ginseng root extraction. Pharm. Chem. J. 1998, 32, 385–388. [Google Scholar] [CrossRef]
- He, X.; Yang, J.; Huang, Y.; Zhang, Y.; Wan, H.; Li, C. Green and efficient ultrasonic-assisted extraction of bioactive components from Salvia miltiorrhiza by Natural Deep Eutectic Solvents. Molecules 2020, 25, 140. [Google Scholar] [CrossRef]
- Box, G.E.P.; Hunter, W.G.; Hunter, J.S. Statistics for Experimenters; John Wiley & Sons: New York, NY, USA, 1985. [Google Scholar]
- Banik, R.M.; Pandey, D.K. Optimizing conditions for oleanolic acid extraction from Lantana camara roots using response surface methodology. Ind. Crop. Prod. 2008, 27, 241–248. [Google Scholar] [CrossRef]
- Wang, L.; Li, T.; Liu, F.; Liu, D.; Xu, Y.; Yang, Y.; Zhao, Y.; Wei, H. Ultrasonic-assisted enzymatic extraction and characterization of polysaccharides from dandelion (Taraxacum officinale) leaves. Int. J. Biol. Macromol. 2019, 126, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.G.; Zenkevich, I.G.; Shikov, A.N.; Pimenov, A.I.; Pozharitskaya, O.N.; Ivanova, S.A.; Galambosi, B. Comparative analysis of Rhodiola rosea of Scandinavian and Russian origin. In Proceedings of the Congress: Phytopharm 2003. Actual problems of creation of new medicinal preparations of natural origin, St. Petersburg-Pushkin, Russia, 3–5 July 2003; pp. 570–574. [Google Scholar]
- Kučinskaitė, A.; Pobłocka-Olech, L.; Krauze-Baranowska, M.; Sznitowska, M.; Savickas, A.; Briedis, V. Evaluation of biologically active compounds in roots and rhizomes of Rhodiola rosea L. cultivated in Lithuania. Medicina 2007, 43, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, A.; Buchwald, W.; Gryszczyńska, A. Biometric features and content of phenolic compounds of roseroot (Rhodiola rosea L.). Acta Soc. Bot. Pol. 2016, 85, 3500. [Google Scholar] [CrossRef]
- Kolodziej, B.; Sugier, D. Influence of plants age on the chemical composition of roseroot (Rhodiola rosea L.). Acta Sci. Pol. Hortorum Cultus 2013, 12, 147–160. [Google Scholar]
- Peschel, W.; Prieto, J.M.; Karkour, C.; Williamson, E.M. Effect of provenance, plant part and processing on extract profiles from cultivated European Rhodiola rosea L. for medicinal use. Phytochemistry 2013, 86, 92–102. [Google Scholar] [CrossRef]
- Elameen, A.; Klemsdal, S.S.; Dragland, S.; Fjellheim, S.; Rognli, O.A. Genetic diversity in a germplasm collection of roseroot (Rhodiola rosea) in Norway studied by AFLP. Biochem. Syst. Ecol. 2008, 36, 706–715. [Google Scholar] [CrossRef]
- Elameen, A.; Dragland, S.; Klemsdal, S.S. Bioactive compounds produced by clones of Rhodiola rosea maintained in the Norwegian germplasm collection. Pharmazie 2010, 5, 618–623. [Google Scholar] [CrossRef]
- Gomez, F.J.V.; Espino, M.; Fernández, M.A.; Silva, M.F. A greener approach to prepare natural deep eutectic solvents. ChemistrySelect 2018, 3, 6122–6125. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Makarova, M.N.; Pozharitskaya, O.N.; Shikov, A.N. Effects of ultrasound treatment on the chemical composition and anticoagulant properties of dry fucus extract. Pharm. Chem. J. 2015, 49, 183–186. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Solvent | Extraction Yield (Mean ± SD), mg/g | ||||
|---|---|---|---|---|---|
| Cinnamyl Alcohol | Rosavin | Rosin | Salidroside | Tyrosol | |
| NADES LG | 0.11 ± 0.01 | 6.28 ± 0.02 | 0.32 ± 0.01 | 4.07 ± 0.02 | 0.33 ± 0.06 |
| NADES LF1 | 0.14 ± 0.02 | 8.42 ± 0.48 | 0.42 ± 0.01 | 5.66 ± 0.01 | 0.61 ± 0.02 |
| NADES LF2 | 0.26 ± 0.03 | 10.38 ± 0.03 | 1.11 ± 0.02 | 7.01 ± 0.06 | 0.33 ± 0.01 |
| EtOH 40% | 0.50 ± 0.01 | 12.11 ± 0.05 | 1.08 ± 0.02 | 10.70 ± 0.23 | 1.25 ± 0.01 |
| Levels | Factors | ||||
|---|---|---|---|---|---|
| X1 (Particle Size, mm) | X2 (Extraction Time, min) | X3 (Temperature, °C) | X4 (Extraction Modulus) | X5 (Molar Water Content) | |
| Main level (0) | 0.5 … 2.0 | 60 | 36 | 1:30 | 5:1:5 |
| High level (+1) | 2.0 … 3.0 | 90 | 50 | 1:40 | 5:1:7 |
| Low level (−1) | 0 … 0.5 | 30 | 22 | 1:20 | 5:1:3 |
| Run | Factors | Extraction Yield (Yi, mg/g) * | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | X5 | Salidroside (Y1) | Tyrosol (Y2) | Rosavin (Y3) | Rosin (Y4) | Cinnamyl Alcohol (Y5) | Sum (Y6) | |
| 1 | +1 | +1 | +1 | +1 | +1 | 7.40 ± 0.06 | 0.25 ± 0,03 | 8.22 ± 0.07 | 0.72 ± 0.01 | 0.029 ± 0.002 | 16.62 ± 0.13 |
| 2 | −1 | −1 | +1 | +1 | +1 | 7.80 ± 0.14 | 0.25 ± 0.01 | 8.65 ± 0.30 | 0.81 ± 0.01 | 0.040 ± 0.000 | 17.55 ± 0.45 |
| 3 | −1 | +1 | +1 | −1 | −1 | 3.64 ± 0.32 | 0.17 ± 0.02 | 5.81 ± 0.12 | 0.44 ± 0.01 | 0.026 ± 0.001 | 10.07 ± 0.20 |
| 4 | +1 | −1 | +1 | −1 | −1 | 2.04 ± 0.06 | 0.10 ± 0.01 | 2.37 ± 0.12 | 0.19 ± 0.01 | 0.019 ± 0.001 | 4.71 ± 0.07 |
| 5 | +1 | +1 | −1 | −1 | +1 | 4.23 ± 0.01 | 0.13 ± 0.00 | 4.63 ± 0.01 | 0.43 ± 0.01 | 0.061 ± 0.001 | 9.47 ± 0.03 |
| 6 | −1 | −1 | −1 | −1 | +1 | 6.31 ± 0.00 | 0.11 ± 0.00 | 7.26 ± 0.01 | 0.70 ± 0.00 | 0.095 ± 0.007 | 14.47 ± 0.03 |
| 7 | −1 | +1 | −1 | +1 | −1 | 7.17 ± 0.08 | 0.29 ± 0.01 | 8.46 ± 0.01 | 0.64 ± 0.02 | 0.028 ± 0.004 | 16.57 ± 0.11 |
| 8 | +1 | −1 | −1 | +1 | −1 | 3.75 ± 0.07 | 0.15 ± 0.02 | 3.11 ± 0.14 | 0.26 ± 0.01 | 0.00 ± 0.00 | 7.26 ± 0.21 |
| Model | F-Ratio | p-Value | R2 |
|---|---|---|---|
| Y1, salidroside | 38.01 | 0.0000 | 95.00 |
| Y2, tyrosol | 45.09 | 0.0000 | 95.75 |
| Y3, rosavin | 37.29 | 0.0000 | 94.91 |
| Y4, rosin | 47.92 | 0.0000 | 95.99 |
| Y5, cinnamyl alcohol | 42.47 | 0.0000 | 95.50 |
| Y6, sum of markers | 38.11 | 0.0000 | 95.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shikov, A.N.; Kosman, V.M.; Flissyuk, E.V.; Smekhova, I.E.; Elameen, A.; Pozharitskaya, O.N. Natural Deep Eutectic Solvents for the Extraction of Phenyletanes and Phenylpropanoids of Rhodiola rosea L. Molecules 2020, 25, 1826. https://doi.org/10.3390/molecules25081826
Shikov AN, Kosman VM, Flissyuk EV, Smekhova IE, Elameen A, Pozharitskaya ON. Natural Deep Eutectic Solvents for the Extraction of Phenyletanes and Phenylpropanoids of Rhodiola rosea L. Molecules. 2020; 25(8):1826. https://doi.org/10.3390/molecules25081826
Chicago/Turabian StyleShikov, Alexander N., Vera M. Kosman, Elena V. Flissyuk, Irina E. Smekhova, Abdelhameed Elameen, and Olga N. Pozharitskaya. 2020. "Natural Deep Eutectic Solvents for the Extraction of Phenyletanes and Phenylpropanoids of Rhodiola rosea L." Molecules 25, no. 8: 1826. https://doi.org/10.3390/molecules25081826
APA StyleShikov, A. N., Kosman, V. M., Flissyuk, E. V., Smekhova, I. E., Elameen, A., & Pozharitskaya, O. N. (2020). Natural Deep Eutectic Solvents for the Extraction of Phenyletanes and Phenylpropanoids of Rhodiola rosea L. Molecules, 25(8), 1826. https://doi.org/10.3390/molecules25081826







