Combination Therapy of Acarbose and Cyclosporine a Ameliorates Imiquimod-Induced Psoriasis-Like Dermatitis in Mice
Abstract
1. Introduction
2. Results
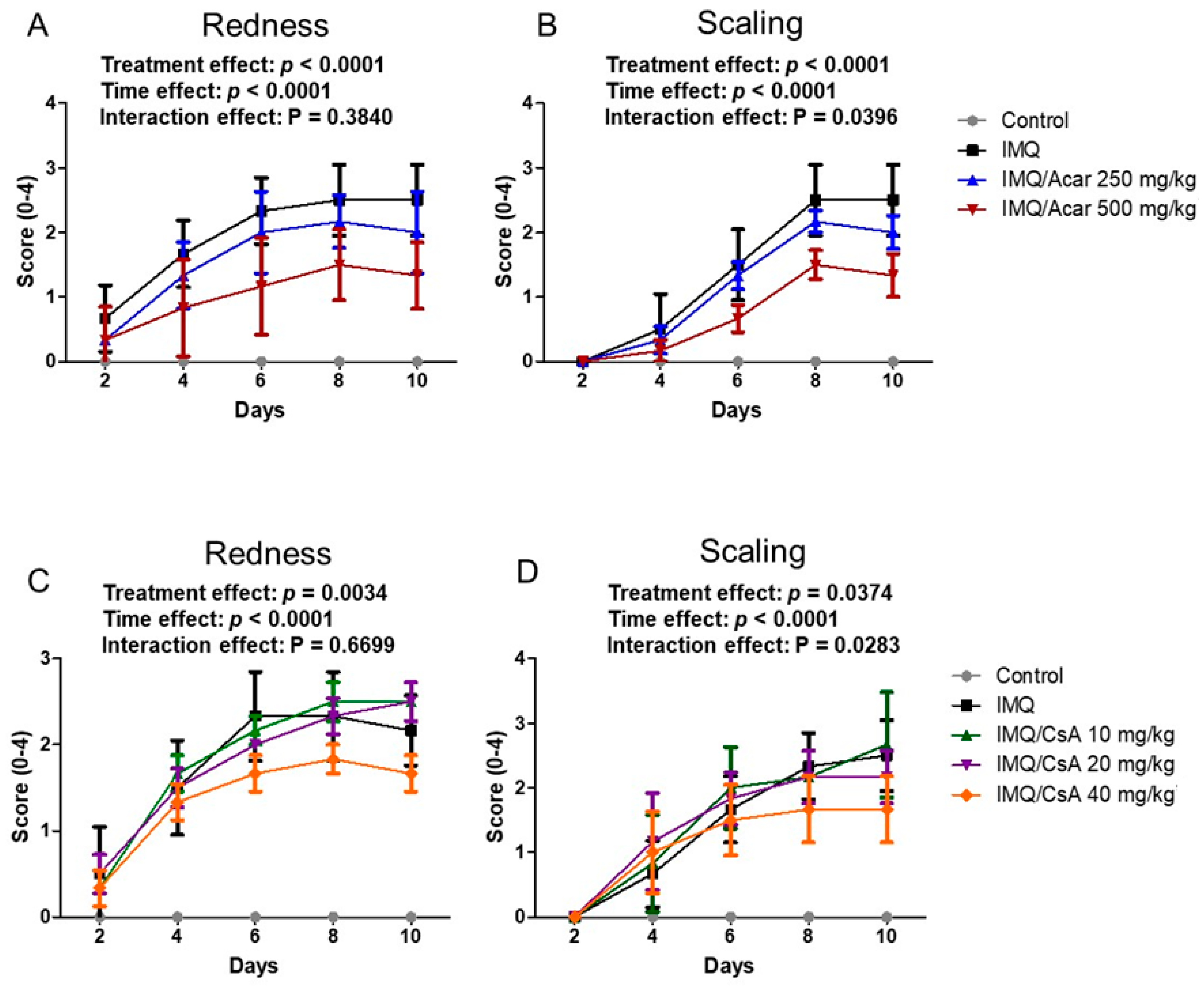
2.1. Effect of Single Doses of Acar and CsA on IMQ-Induced Psoriasis-Like Dermatitis in Mice
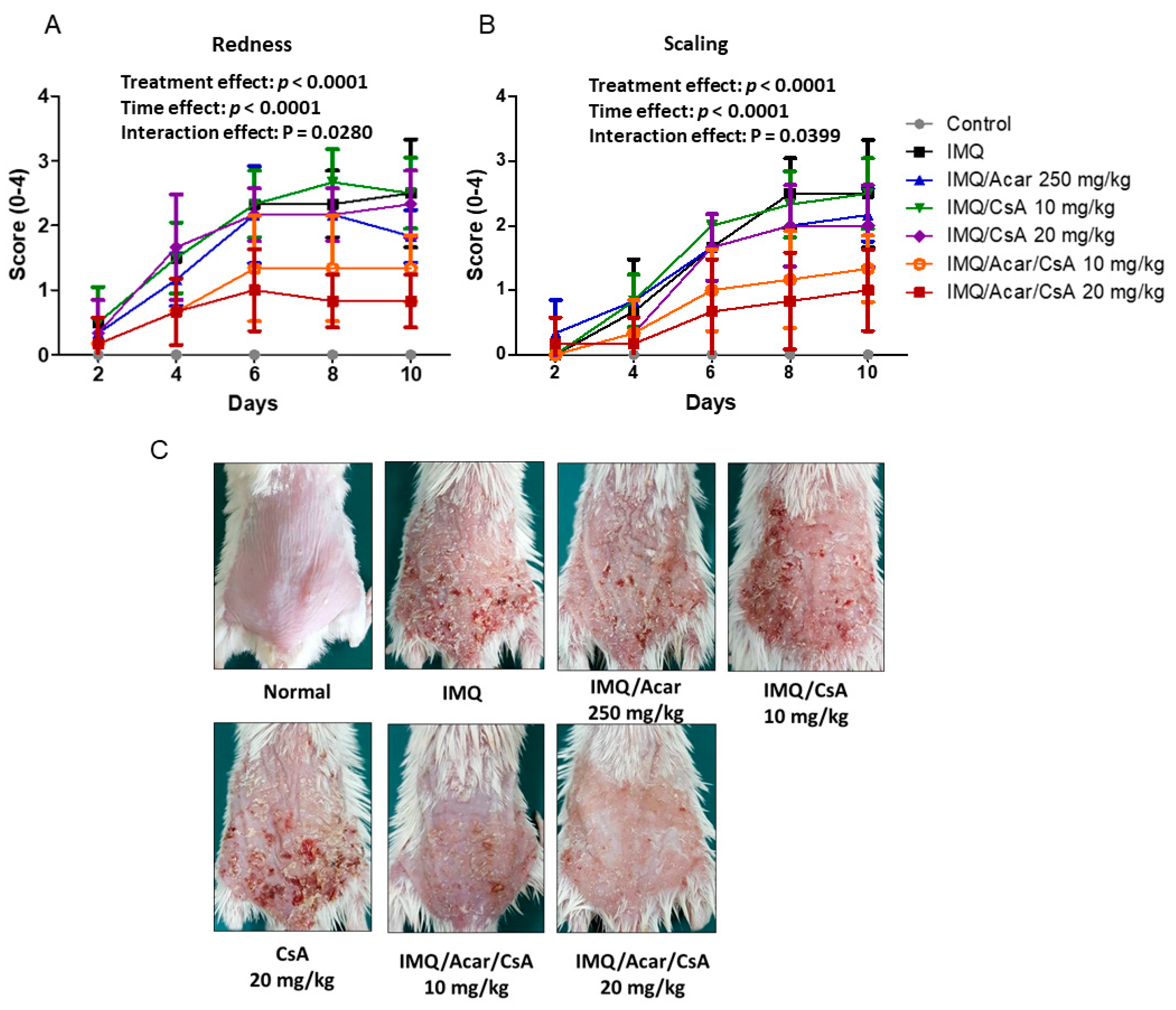
2.2. Effects of Combination of Acar and Low-Dose CsA on IMQ-Induced Psoriasis-Like Dermatitis in Mice
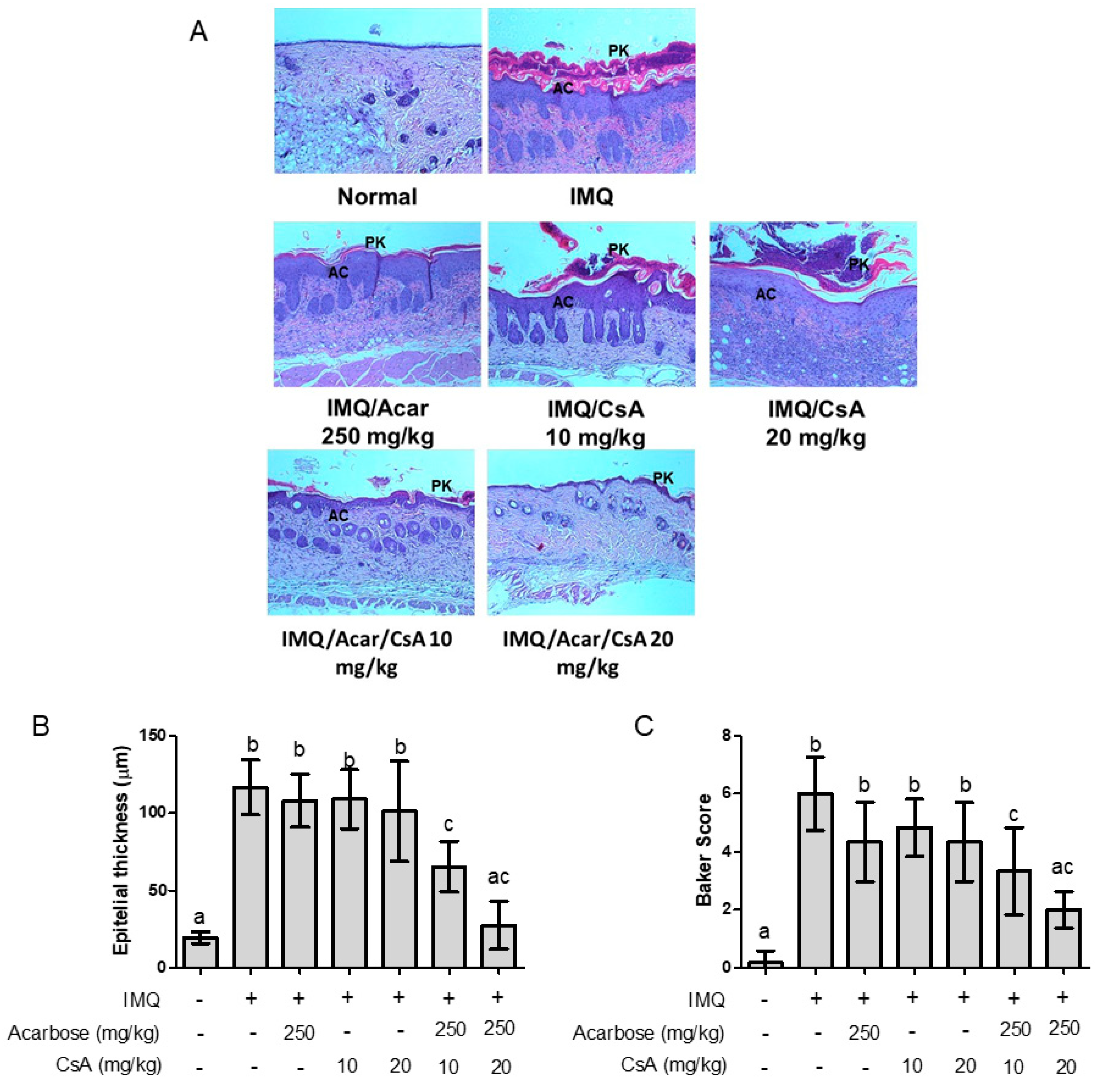
2.3. Effect of Combination of Acar and Low-Dose CsA on Histological Changes in the Skin of Mice with IMQ-Induced Psoriasis-Like Dermatitis
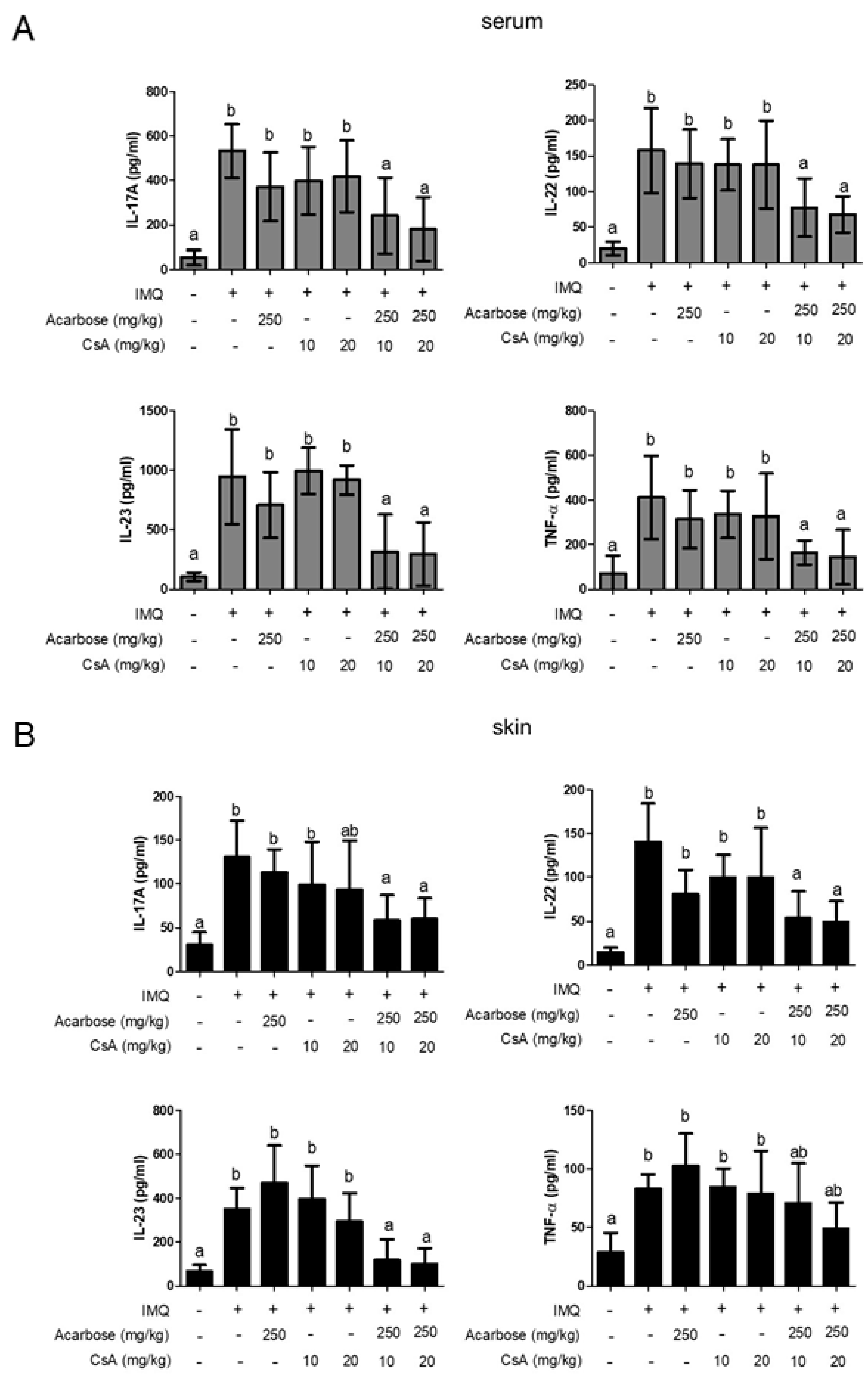
2.4. Effect of Combination of Acar and Low-Dose CsA on Levels of Inflammatory Cytokines in Serum and Skin of Mice with IMQ-Induced Psoriasis-Like Dermatitis
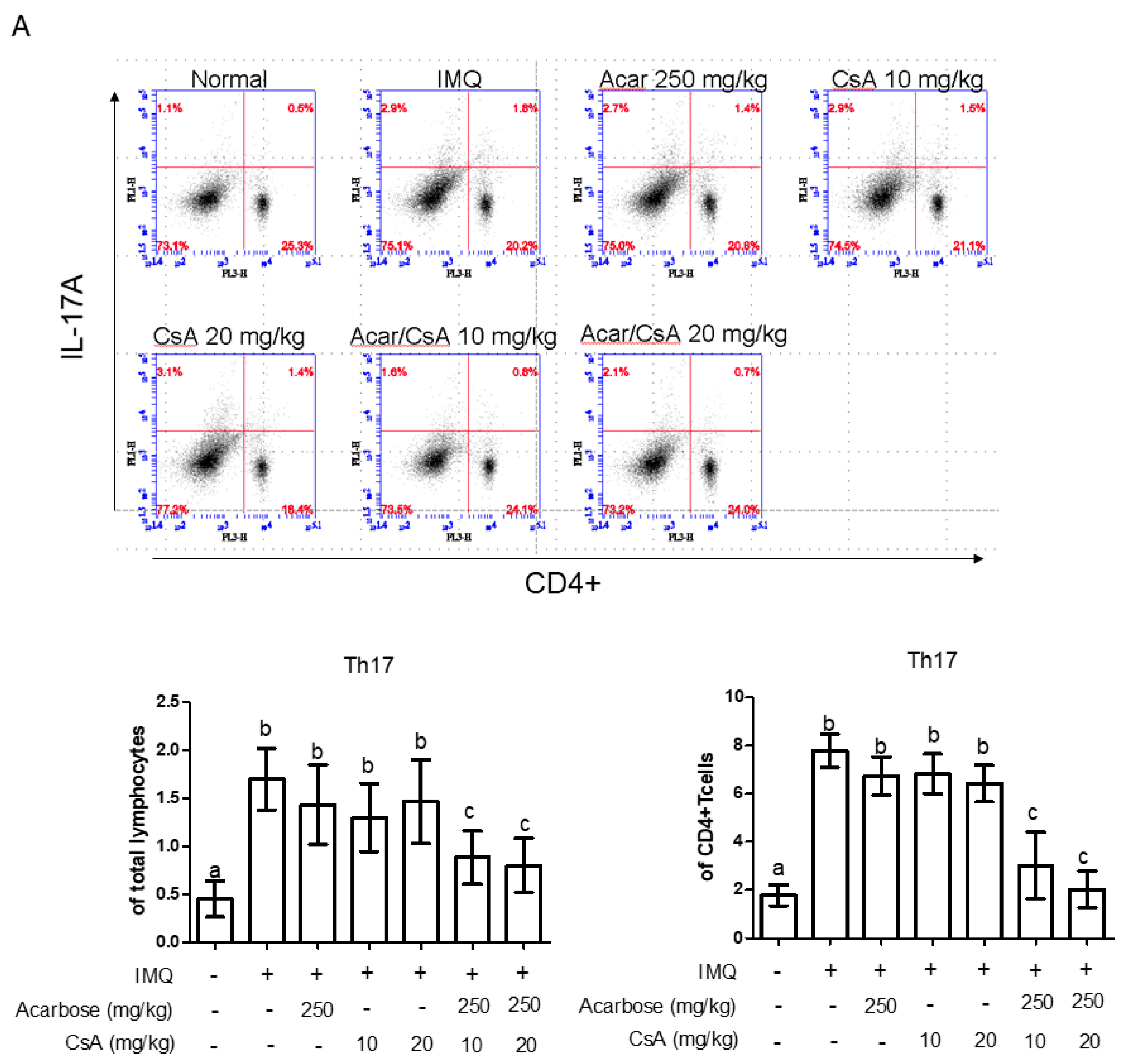
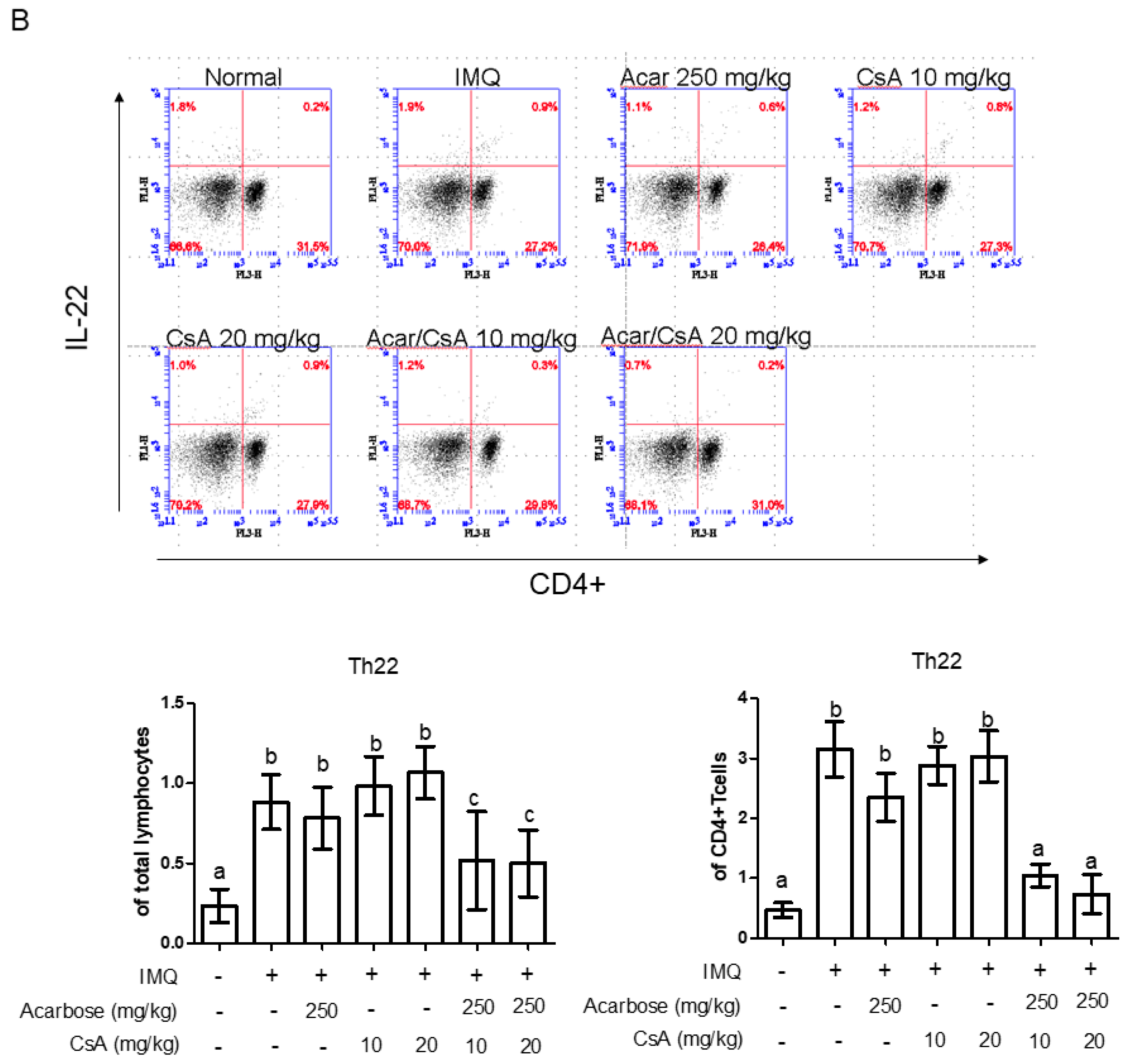
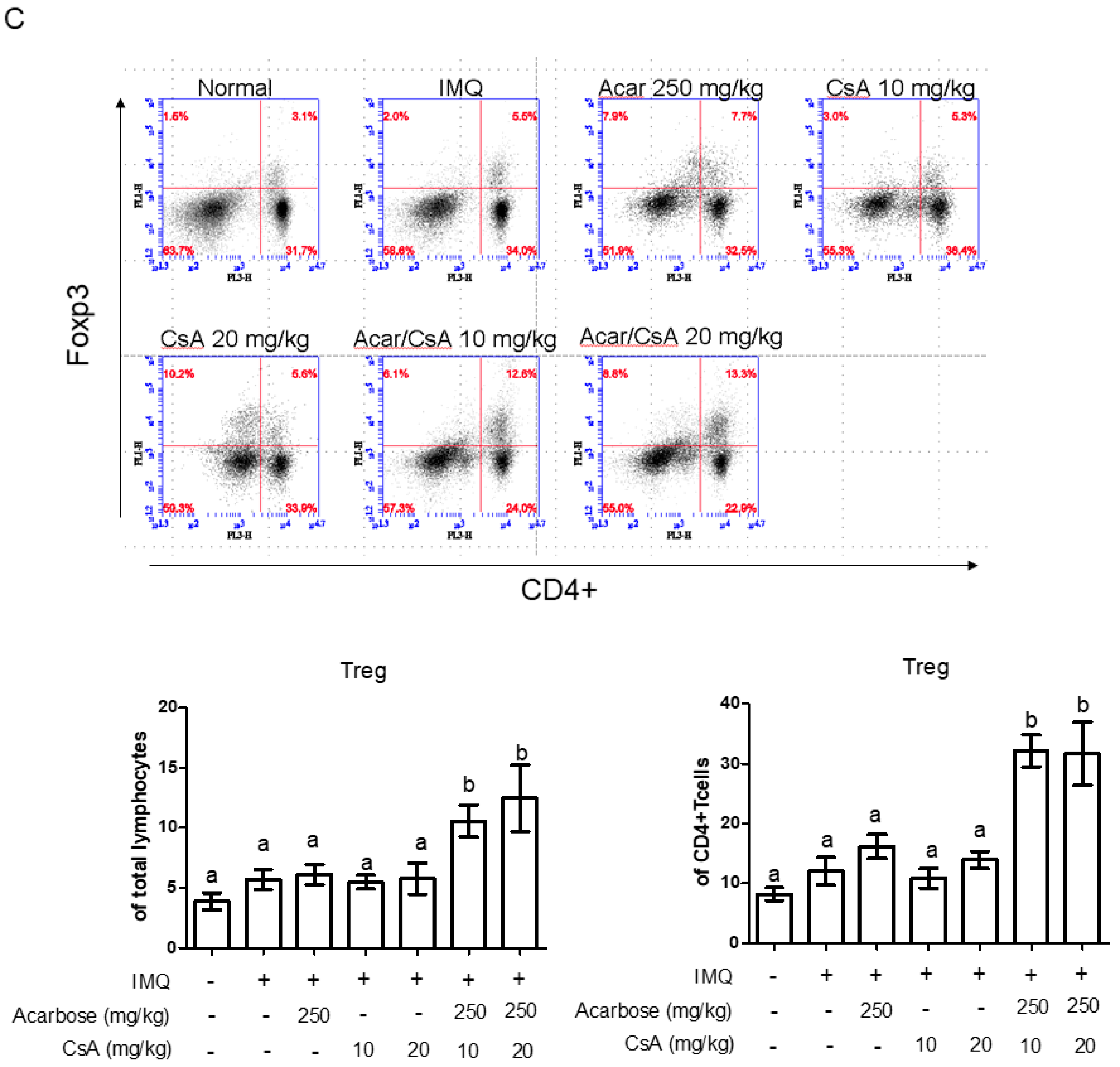
2.5. Effect of Combination of Acar and Low-Dose CsA on the Percentages of CD4+ IL-17A+ T-cells, CD4+ IL-22+ T-Cells, and Treg Cells in Mice with IMQ-Induced Psoriasis-Like Dermatitis
3. Discussion
4. Materials and Methods
4.1. Animal Treatment Procedures
4.2. Scoring of Skin Inflammation Severity
4.3. Pathological Histology
4.4. Assay of Cytokines Production
4.5. Intracellular Cytokine Staining and Flow Cytometry
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, S.; Liu, X.; Mei, L.; Wang, H.; Fang, F. Epigallocatechin-3-gallate (EGCG) inhibits imiquimod-induced psoriasis-like inflammation of BALB/c mice. BMC Complement. Altern. Med. 2016, 16, 334. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.P.; Farber, E.M. The prevalence of psoriasis in the world. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhao, Y.; Hu, J. Curcumin inhibits imiquimod-induced psoriasis-like inflammation by inhibiting IL-1beta and IL-6 production in mice. PLoS ONE 2013, 8, e67078. [Google Scholar] [CrossRef]
- Qin, S.; Wen, J.; Bai, X.C.; Chen, T.Y.; Zheng, R.C.; Zhou, G.B.; Ma, J.; Feng, J.Y.; Zhong, B.L.; Li, Y.M. Endogenous n-3 polyunsaturated fatty acids protect against imiquimod-induced psoriasis-like inflammation via the IL-17/IL-23 axis. Mol. Med. Rep. 2014, 9, 2097–2104. [Google Scholar] [CrossRef]
- Ni, C.; Chiu, M.W. Psoriasis and comorbidities: Links and risks. Clin. Cosmet. Investig. Dermatol. 2014, 7, 119–132. [Google Scholar]
- Cai, Y.; Fleming, C.; Yan, J. New insights of T cells in the pathogenesis of psoriasis. Cell Mol. Immunol. 2012, 9, 302–309. [Google Scholar] [CrossRef]
- Lowes, M.A.; Bowcock, A.M.; Krueger, J.G. Pathogenesis and therapy of psoriasis. Nature 2007, 445, 866–873. [Google Scholar] [CrossRef]
- Fantuzzi, F.; Del Giglio, M.; Gisondi, P.; Girolomoni, G. Targeting tumor necrosis factor alpha in psoriasis and psoriatic arthritis. Expert. Opin. Ther. Targets 2008, 12, 1085–1096. [Google Scholar] [CrossRef]
- Gudjonsson, J.E.; Johnston, A.; Sigmundsdottir, H.; Valdimarsson, H. Immunopathogenic mechanisms in psoriasis. Clin. Exp. Immunol. 2004, 135, 1–8. [Google Scholar] [CrossRef]
- Elloso, M.M.; Gomez-Angelats, M.; Fourie, A.M. Targeting the Th17 pathway in psoriasis. J. Leukoc. Biol. 2012, 92, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Trepicchio, W.L.; Oestreicher, J.L.; Pittman, D.; Wang, F.; Chamian, F.; Dhodapkar, M.; Krueger, J.G. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J. Exp. Med. 2004, 199, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.L.; Liang, S.; Li, J.; Napierata, L.; Brown, T.; Benoit, S.; Senices, M.; Gill, D.; Dunussi-Joannopoulos, K.; Collins, M.; et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J. Clin. Investig. 2008, 118, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Caproni, M.; Antiga, E.; Melani, L.; Volpi, W.; DelBianco, E.; Fabbri, P. Serum levels of IL-17 and IL-22 are reduced by etanercept, but not by acitretin, in patients with psoriasis: A randomized-controlled trial. J. Clin. Immunol. 2009, 29, 210–214. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, J.Y.; Lee, A.Y. Therapeutic and immunomodulatory effects of glucosamine in combination with low-dose cyclosporine a in a murine model of imiquimod-induced psoriasis. Eur. J. Pharmacol. 2015, 756, 43–51. [Google Scholar] [CrossRef]
- Jensen, P.; Skov, L.; Zachariae, C. Systemic combination treatment for psoriasis: A review. Acta Derm. Venereol. 2010, 90, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Rosak, C.; Mertes, G. Critical evaluation of the role of acarbose in the treatment of diabetes: Patient considerations. Diabetes Metab. Syndr. Obes. 2012, 5, 357–367. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; Ferrari, I.; Fogari, E.; D’Angelo, A.; Palumbo, I.; Randazzo, S.; Bianchi, L.; Cicero, A.F. Acarbose actions on insulin resistance and inflammatory parameters during an oral fat load. Eur. J. Pharmacol. 2011, 651, 240–250. [Google Scholar] [CrossRef]
- Su, B.; Liu, H.; Li, J.; Sunli, Y.; Liu, B.; Liu, D.; Zhang, P.; Meng, X. Acarbose treatment affects the serum levels of inflammatory cytokines and the gut content of bifidobacteria in Chinese patients with type 2 diabetes mellitus. J. Diabetes 2015, 7, 729–739. [Google Scholar] [CrossRef]
- Golia, E.; Limongelli, G.; Natale, F.; Fimiani, F.; Maddaloni, V.; Pariggiano, I.; Bianchi, R.; Crisci, M.; D’Acierno, L.; Giordano, R.; et al. Inflammation and cardiovascular disease: From pathogenesis to therapeutic target. Curr. Atheroscler. Rep. 2014, 16, 435. [Google Scholar] [CrossRef]
- Chen, H.H.; Chao, Y.H.; Chen, D.Y.; Yang, D.H.; Chung, T.W.; Li, Y.R.; Lin, C.C. Oral administration of acarbose ameliorates imiquimod-induced psoriasis-like dermatitis in a mouse model. Int. Immunopharmacol. 2016, 33, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Van Belle, A.B.; de Heusch, M.; Lemaire, M.M.; Hendrickx, E.; Warnier, G.; Dunussi-Joannopoulos, K.; Fouser, L.A.; Renauld, J.C.; Dumoutier, L. IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J. Immunol. 2012, 188, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Roark, C.L.; Simonian, P.L.; Fontenot, A.P.; Born, W.K.; O’Brien, R.L. gammadelta T cells: An important source of IL-17. Curr. Opin. Immunol. 2008, 20, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.; Hsu, L.; Liao, W. Phototherapy in psoriasis: A review of mechanisms of action. J. Cutan. Med. Surg. 2013, 17, 6–12. [Google Scholar] [CrossRef]
- Dubois, D.S.; Pouliot, R. Promising new treatments for psoriasis. Sci. World J. 2013, 2013, 980419. [Google Scholar]
- Villasenor-Park, J.; Wheeler, D.; Grandinetti, L. Psoriasis: Evolving treatment for a complex disease. Clevel. Clin. J. Med. 2012, 79, 413–423. [Google Scholar] [CrossRef][Green Version]
- Han, R.; Rostami-Yazdi, M.; Gerdes, S.; Mrowietz, U. Triptolide in the treatment of psoriasis and other immune-mediated inflammatory diseases. Br. J. Clin. Pharmacol. 2012, 74, 424–436. [Google Scholar] [CrossRef]
- Yuan, H.; Sun, B.; Gao, F.; Lan, M. Synergistic anticancer effects of andrographolide and paclitaxel against A549 NSCLC cells. Pharm. Biol. 2016, 54, 2629–2635. [Google Scholar] [CrossRef][Green Version]
- Boland, J.; Atkinson, K.; Britton, K.; Darveniza, P.; Johnson, S.; Biggs, J. Tissue distribution and toxicity of cyclosporin A in the mouse. Pathology 1984, 16, 117–123. [Google Scholar] [CrossRef] [PubMed]
- van der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef] [PubMed]
- Loser, K.; Beissert, S. Regulatory T cells: Banned cells for decades. J. Investig. Dermatol. 2012, 132, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Huang, L.M.; Suárez-Fariñas, M.; Sullivan-Whalen, M.; Gilleaudeau, P.; Krueger, J.G.; Lowes, M.A. Effective narrow-band UVB radiation therapy suppresses the IL-23/IL-17 axis in normalized psoriasis plaques. J. Investig. Dermatol. 2010, 130, 2654–2663. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.A.; Towne, J.E.; Kricorian, G.; Klekotka, P.; Gudjonsson, J.E.; Krueger, J.G.; Russell, C.B. The emerging role of IL-17 in the pathogenesis of psoriasis: Preclinical and clinical findings. J. Investig. Dermatol. 2013, 133, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A. T-helper 17 cells in psoriatic plaques and additional genetic links between IL-23 and psoriasis. J. Investig. Dermatol. 2008, 128, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Ito, T.; Kato, H.; Asai, S.; Tanaka, H.; Nagai, H.; Inagaki, N. Comparison of the efficacy of tacrolimus and cyclosporine A in a murine model of dinitrofluorobenzene-induced atopic dermatitis. Eur. J. Pharmacol. 2010, 645, 171–176. [Google Scholar] [CrossRef]
- Mignen, O.; Thompson, J.L.; Shuttleworth, T.J. Calcineurin directs the reciprocal regulation of calcium entry pathways in nonexcitable cells. J. Biol. Chem. 2003, 278, 40088–40096. [Google Scholar] [CrossRef]
- Park, Y.J.; Yoo, S.A.; Kim, M.; Kim, W.U. The role of calcium-calcineurin-NFAT signaling pathway in health and autoimmune diseases. Front. Immunol. 2020, 11, 195. [Google Scholar] [CrossRef]
- Karvonen, S.L.; Korkiamäki, T.; Ylä-Outinen, H.; Nissinen, M.; Teerikangas, H.; Pummi, K.; Karvonen, J.; Peltonen, J. Psoriasis and altered calcium metabolism: Downregulated capacitative calcium influx and defective calcium-mediated cell signaling in cultured psoriatic keratinocytes. J. Investig. Dermatol. 2000, 114, 693–700. [Google Scholar] [CrossRef]
- Al-Daraji, W.I.; Grant, K.R.; Ryan, K.; Saxton, A.; Reynolds, N.J. Localization of calcineurin/NFAT in human skin and psoriasis and inhibition of calcineurin/NFAT activation in human keratinocytes by cyclosporin A. J. Investig. Dermatol. 2002, 118, 779–788. [Google Scholar] [CrossRef]
- De Vuyst, E.; Salmon, M.; Evrard, C.; Lambert de Rouvroit, C.; Poumay, Y. Atopic dermatitis studies through in vitro models. Front. Med. 2017, 4, 119. [Google Scholar] [CrossRef]
- Zákostelská, Z.; Málková, J.; Klimešová, K.; Rossmann, P.; Hornová, M.; Novosádová, I.; Stehlíková, Z.; Kostovčík, M.; Hudcovic, T.; Štepánková, R.; et al. Intestinal microbiota promotes psoriasis-like skin inflammation by enhancing Th17 response. PLoS ONE 2016, 11, e0159539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiao, X.; Li, M.; Li, W.; Yu, M.; Zhang, H.; Wang, Z.; Xiang, H. Acarbose reduces blood glucose by activating miR-10a-5p and miR-664 in diabetic rats. PLoS ONE 2013, 8, e79697. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, S.; Liu, B.; Wang, Y.; Tan, W. (R)-Salbutamol improves imiquimod-induced psoriasis-like skin Ddermatitis by regulating the Th17/Tregs balance and glycerophospholipid metabolism. Cells 2020, 9, 511. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-H.; Lin, C.-C.; Tung, Y.-T.; Chao, Y.-H.; Huang, W.-C.; Lee, P.-Y. Combination Therapy of Acarbose and Cyclosporine a Ameliorates Imiquimod-Induced Psoriasis-Like Dermatitis in Mice. Molecules 2020, 25, 1822. https://doi.org/10.3390/molecules25081822
Chen H-H, Lin C-C, Tung Y-T, Chao Y-H, Huang W-C, Lee P-Y. Combination Therapy of Acarbose and Cyclosporine a Ameliorates Imiquimod-Induced Psoriasis-Like Dermatitis in Mice. Molecules. 2020; 25(8):1822. https://doi.org/10.3390/molecules25081822
Chicago/Turabian StyleChen, Hsin-Hua, Chi-Chien Lin, Yu-Tang Tung, Ya-Hsuan Chao, Wen-Ching Huang, and Po-Ying Lee. 2020. "Combination Therapy of Acarbose and Cyclosporine a Ameliorates Imiquimod-Induced Psoriasis-Like Dermatitis in Mice" Molecules 25, no. 8: 1822. https://doi.org/10.3390/molecules25081822
APA StyleChen, H.-H., Lin, C.-C., Tung, Y.-T., Chao, Y.-H., Huang, W.-C., & Lee, P.-Y. (2020). Combination Therapy of Acarbose and Cyclosporine a Ameliorates Imiquimod-Induced Psoriasis-Like Dermatitis in Mice. Molecules, 25(8), 1822. https://doi.org/10.3390/molecules25081822






