A Functionalized Silicate Adsorbent and Exploration of Its Adsorption Mechanism
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation of ASHMA
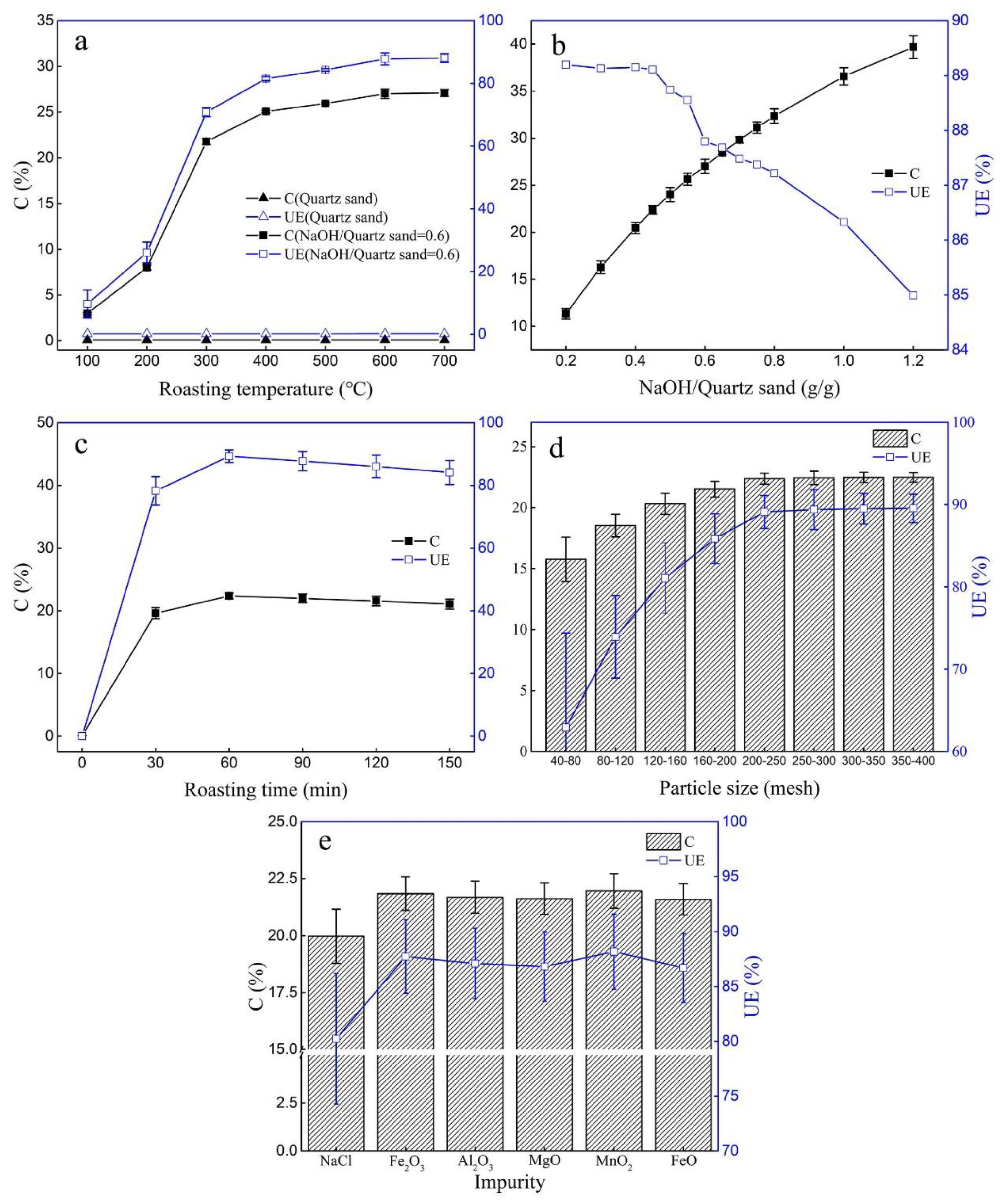
2.1.1. Production of ASHMA
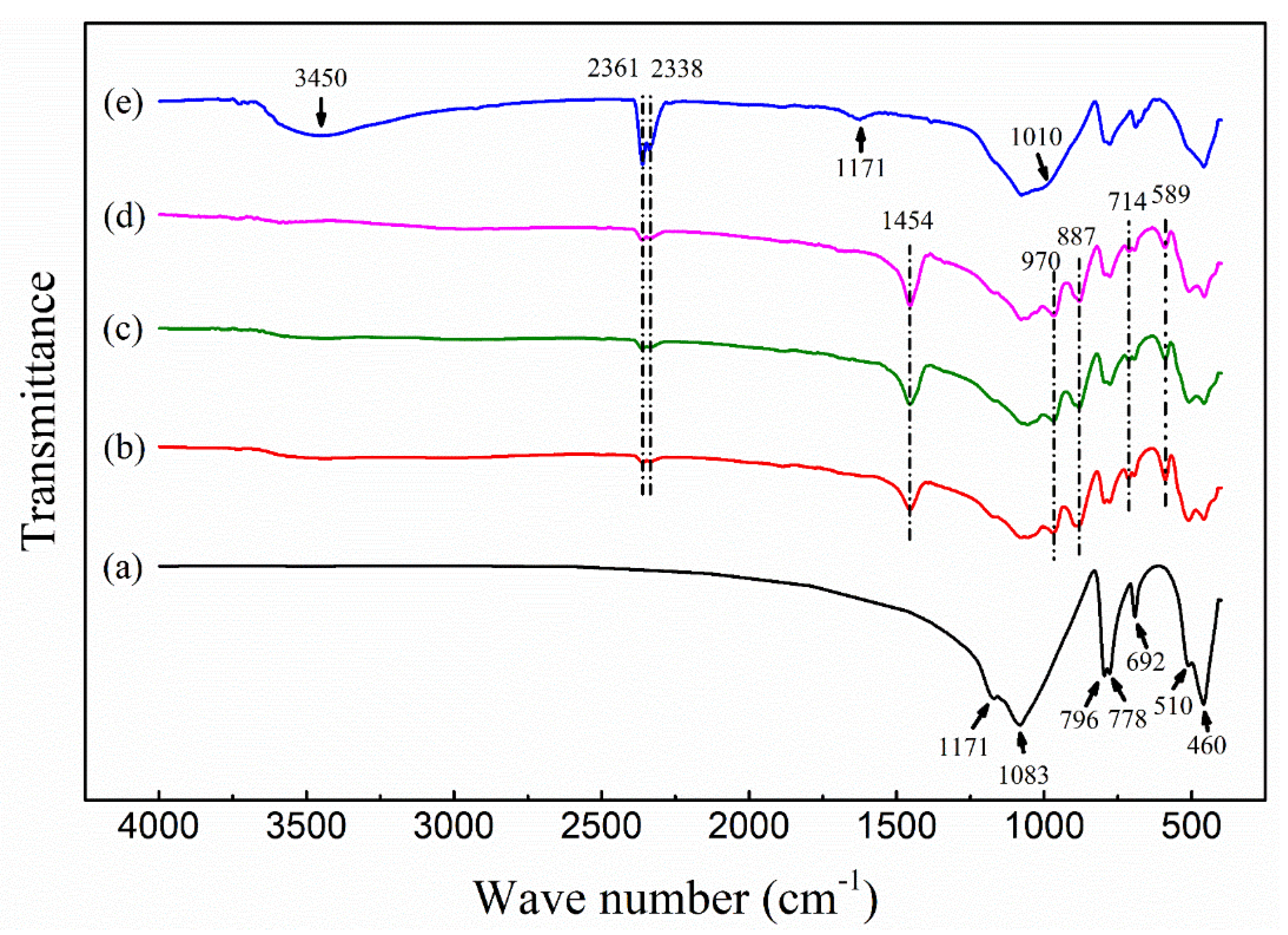
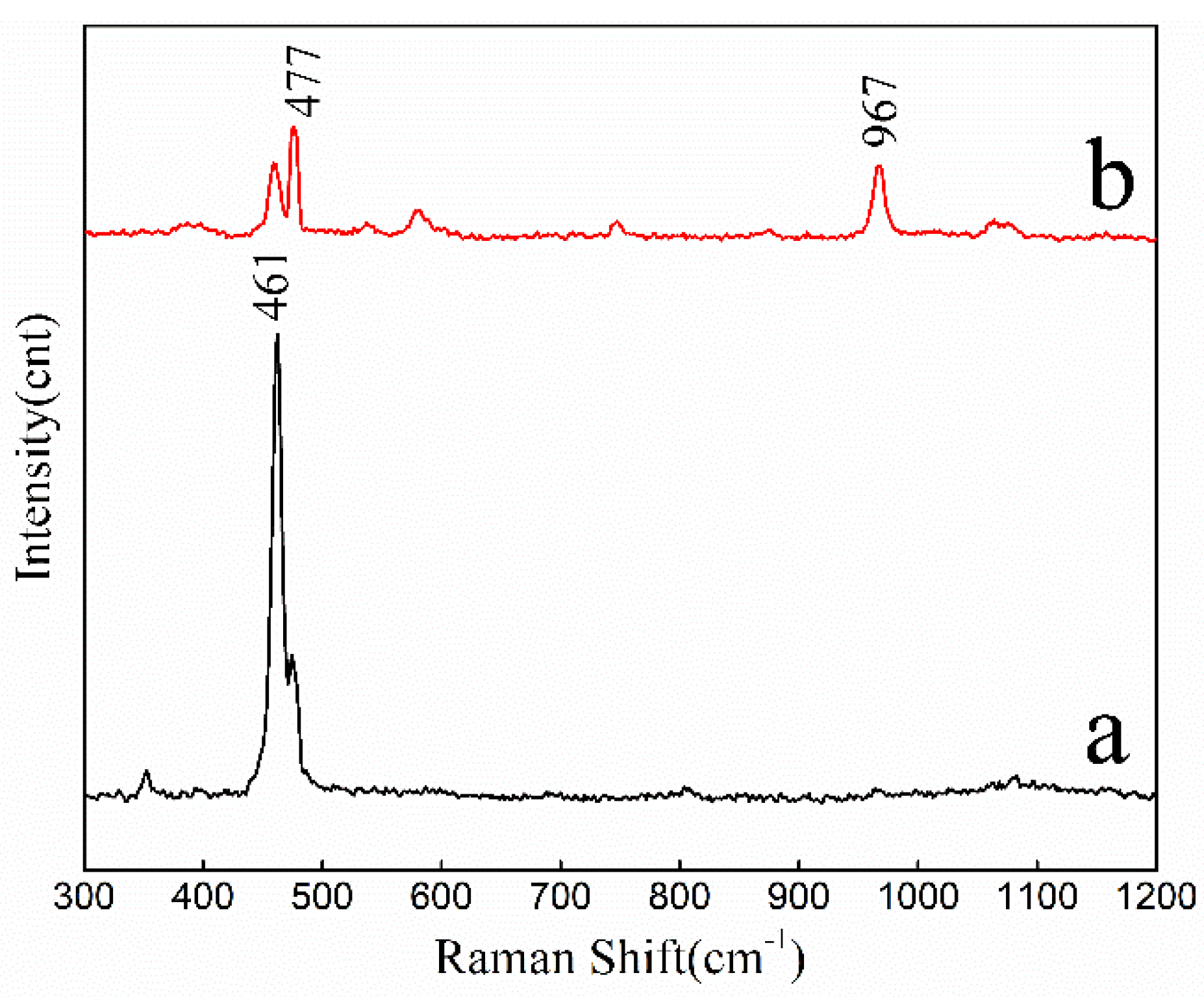
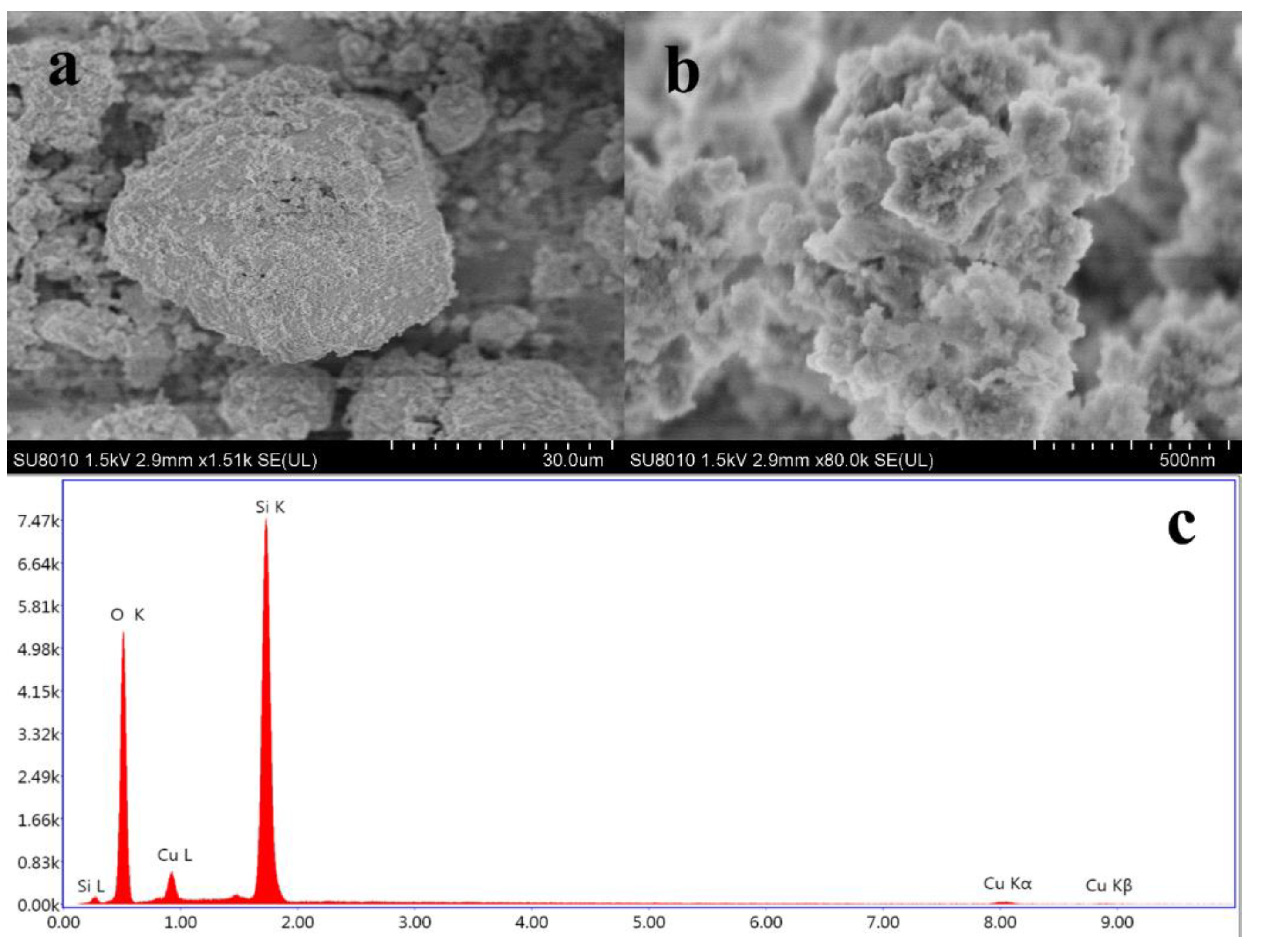
2.1.2. Characterization of ASHMA
2.2. Adsorption
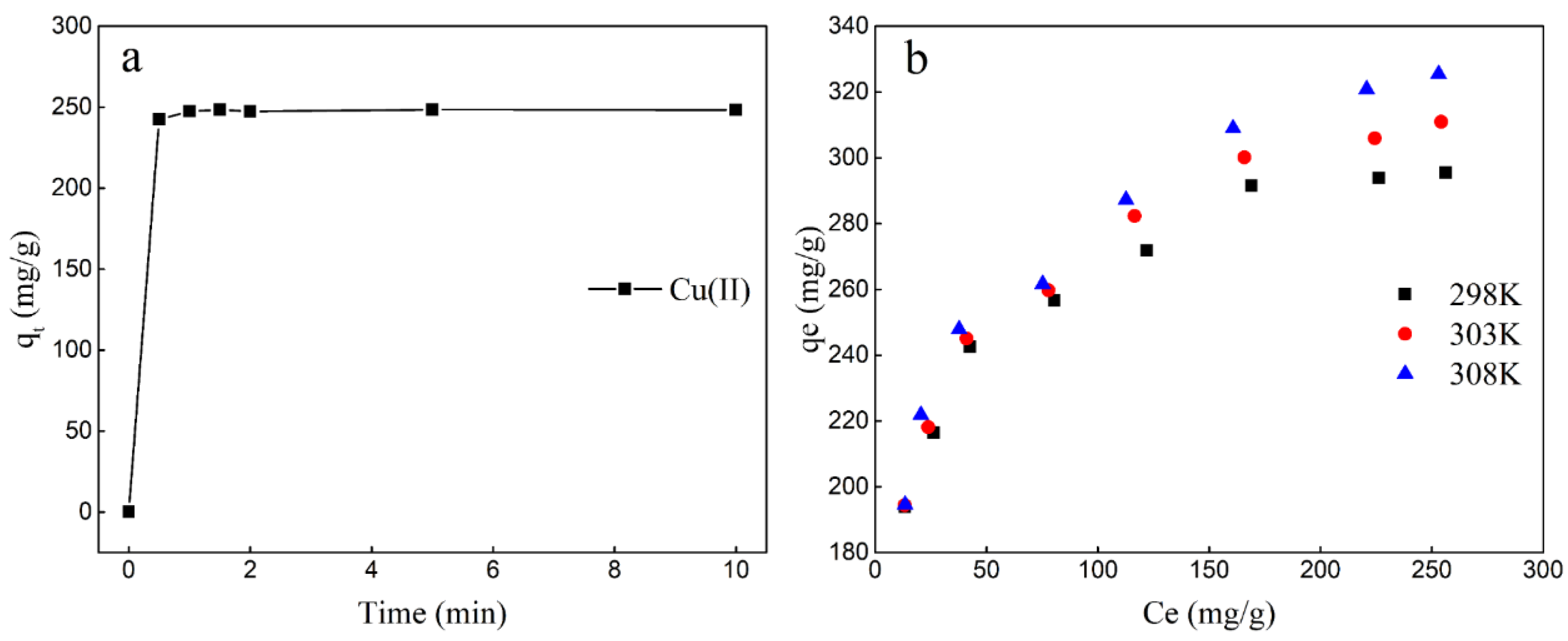
2.2.1. Adsorption Properties
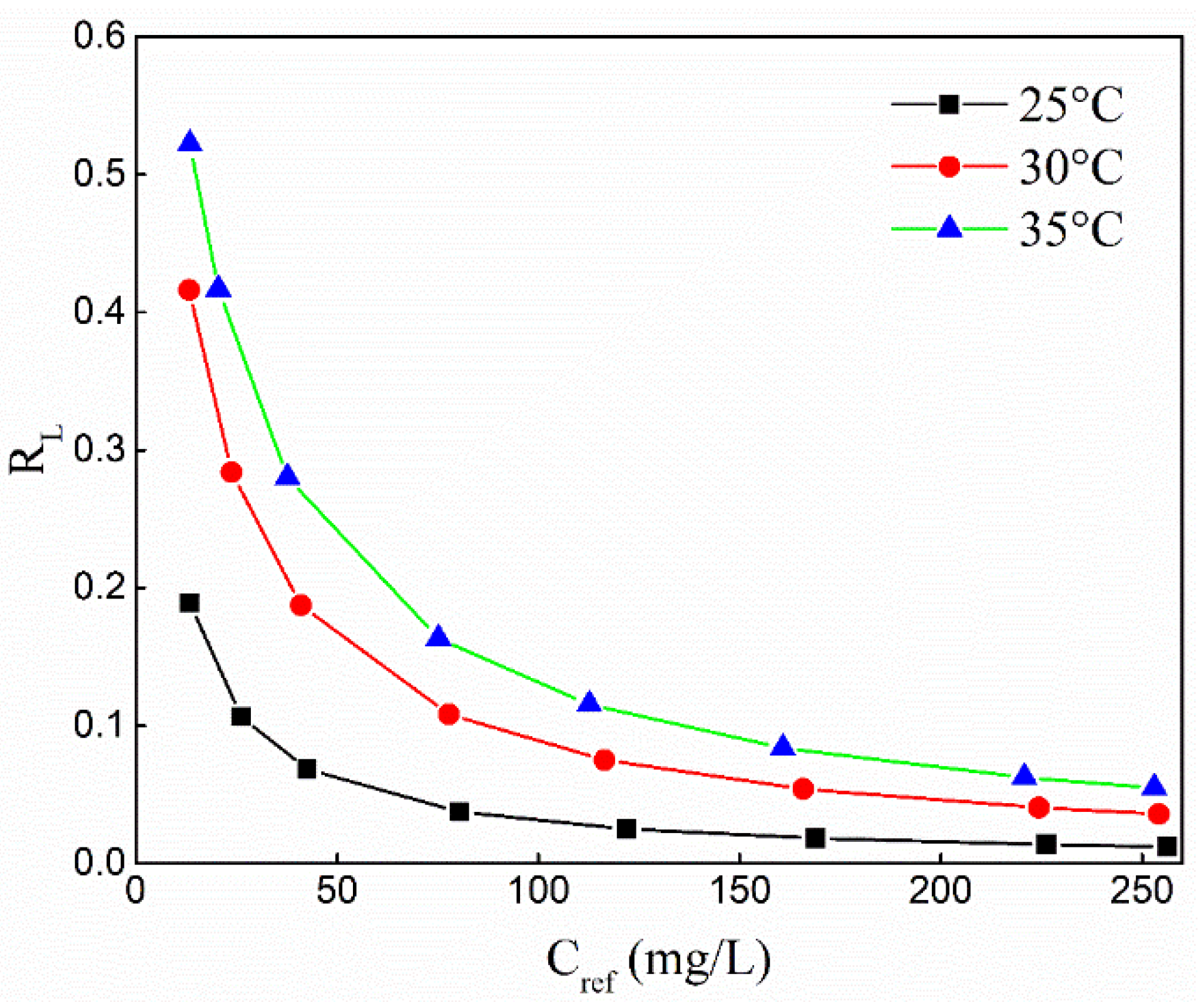
2.2.2. Adsorption Isotherm
2.2.3. Comparison with Other Adsorbents
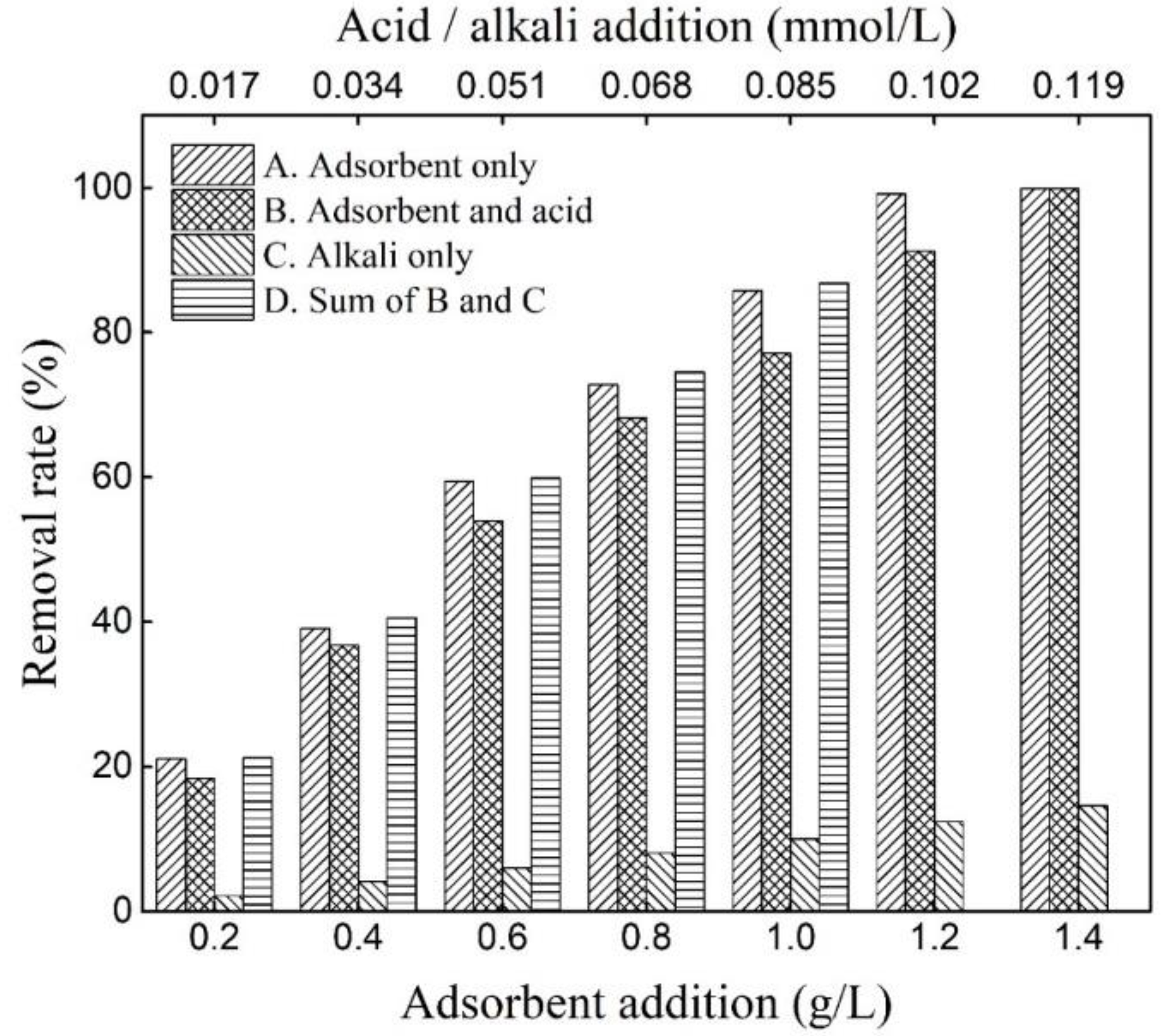
2.2.4. Adsorption Mechanism
3. Materials and Methods
3.1. Materials
3.2. Preparation Process
3.3. Adsorption Equilibrium
3.4. Analytical Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, N.; Kang, Y.; Pan, W.; Zeng, L.; Zhang, Q.; Luo, J. Concentration and transportation of heavy metals in vegetables and risk assessment of human exposure to bioaccessible heavy metals in soil near a waste-incinerator site, South China. Sci. Total Environ. 2015, 521, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yin, M.; Zhang, W.; Tsang, D.C.W.; Wei, X.; Zhou, Y.; Xiao, T.; Wang, J.; Dong, X.; Sun, Y.; et al. Response of microbial communities and interactions to thallium in contaminated sediments near a pyrite mining area. Environ. Pollut. 2019, 248, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, H.; Bai, Z.; Zhou, W.; Liu, K.; Wang, M.; He, Y. Heavy metal concentrations of soils near the large opencast coal mine pits in China. Chemosphere 2020, 244, 125360. [Google Scholar] [CrossRef] [PubMed]
- Bello, O.; Naidu, R.; Rahman, M.M.; Liu, Y.; Dong, Z. Lead concentration in the blood of the general population living near a lead-zinc mine site, Nigeria: Exposure pathways. Sci. Total Environ. 2016, 542, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Thind, P.S.; John, S. Health risk assessment of the workers exposed to the heavy metals in e-waste recycling sites of Chandigarh and Ludhiana, Punjab, India. Chemosphere 2018, 203, 426–433. [Google Scholar] [CrossRef]
- Sakamoto, M.; Itai, T.; Marumoto, K.; Marumoto, M.; Kodamatani, H.; Tomiyasu, T.; Nagasaka, H.; Mori, K.; Poulain, A.J.; Domingo, J.L.; et al. Mercury speciation in preserved historical sludge: Potential risk from sludge contained within reclaimed land of Minamata Bay, Japan. Environ. Res. 2020, 180, 108668. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Satyanarayana, K.G.; Pramada, P.N.; Raghavan, P.; Gupta, T.N. Review Processing, properties and applications of reactive silica from rice husk—an overview. J. Mater. Sci. 2003, 38, 3159–3168. [Google Scholar] [CrossRef]
- Quenneville, J.; Taylor, R.S.; van Duin, A.C.T. Reactive Molecular Dynamics Studies of DMMP Adsorption and Reactivity on Amorphous Silica Surfaces. J. Phys. Chem. C 2010, 114, 18894–18902. [Google Scholar] [CrossRef]
- Ma, J.; Qin, G.; Zhang, Y.; Sun, J.; Wang, S.; Jiang, L. Heavy metal removal from aqueous solutions by calcium silicate powder from waste coal fly-ash. J. Clean. Prod. 2018, 182, 776–782. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Y.; Cai, A.; Cheng, T.; Wu, Z.; Liu, H.; Bao, X.; Yuan, P. The mesopore-elimination treatment and silanol-groups recovery for macroporous silica microspheres and its application as an efficient support for polystyrene hydrogenation. Catal. Commun. 2018, 111, 75–79. [Google Scholar] [CrossRef]
- Dalstein, L.; Potapova, E.; Tyrode, E. The elusive silica/water interface: Isolated silanols under water as revealed by vibrational sum frequency spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 10343–10349. [Google Scholar] [CrossRef] [PubMed]
- Schindler, P.W.; Fürst, B.; Dick, R.; Wolf, P.U. Ligand properties of surface silanol groups. I. surface complex formation with Fe3+, Cu2+, Cd2+, and Pb2+. J. Colloid Interface Sci. 1976, 55, 469–475. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, G.; Duan, X.; Liang, X.; Meng, J.; Liang, J. Environmental Applications of Diatomite Minerals in Removing Heavy Metals from Water. Ind. Eng. Chem. Res. 2019, 58, 11638–11652. [Google Scholar] [CrossRef]
- Lei, C.; Yan, B.; Chen, T.; Xiao, X.M. Preparation and adsorption characteristics for heavy metals of active silicon adsorbent from leaching residue of lead-zinc tailings. Environ. Sci. Pollut. Res. Int. 2018, 25, 21233–21242. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Meunier, J.D.; Miche, H.; Keller, C. Effect of silicon on reducing cadmium toxicity in durum wheat (Triticum turgidum L. cv. Claudio W.) grown in a soil with aged contamination. J. Hazard. Mater. 2012, 209-210, 326–334. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Y.; Li, H.; Cheng, F. Evaluating heavy metal accumulation and potential risks in soil-plant systems applied with magnesium slag-based fertilizer. Chemosphere 2018, 197, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef]
- Tubana, B.S.; Babu, T.; Datnoff, L.E. A Review of Silicon in Soils and Plants and Its Role in US Agriculture. Soil Sci. 2016, 181, 393–411. [Google Scholar] [CrossRef]
- Bakhat, H.F.; Bibi, N.; Zia, Z.; Abbas, S.; Hammad, H.M.; Fahad, S.; Ashraf, M.R.; Shah, G.M.; Rabbani, F.; Saeed, S. Silicon mitigates biotic stresses in crop plants: A review. Crop Prot. 2018, 104, 21–34. [Google Scholar] [CrossRef]
- Meharg, C.; Meharg, A.A. Silicon, the silver bullet for mitigating biotic and abiotic stress, and improving grain quality, in rice? Environ. Exp. Bot. 2015, 120, 8–17. [Google Scholar] [CrossRef]
- He, H.; Tam, N.F.Y.; Yao, A.; Qiu, R.; Li, W.C.; Ye, Z. Growth and Cd uptake by rice (Oryza sativa) in acidic and Cd-contaminated paddy soils amended with steel slag. Chemosphere 2017, 189, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R′′Si(OR′)3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Tang, C.; Zhu, J.; Li, Z.; Zhu, R.; Zhou, Q.; Wei, J.; He, H.; Tao, Q. Surface chemistry and reactivity of SiO2 polymorphs: A comparative study on α-quartz and α-cristobalite. Appl. Surf. Sci. 2015, 355, 1161–1167. [Google Scholar] [CrossRef]
- Criado, M.; Fernández-Jiménez, A.; Palomo, A. Alkali activation of fly ash: Effect of the SiO2/Na2O ratio. Microporous Mesoporous Mater. 2007, 106, 180–191. [Google Scholar] [CrossRef]
- Bhaduri, S.B. THE PHYSICS AND CHEMISTRY OF SOL-GEL PROCESSING edited by C.J. Brinker and G.W. Scherer Academic Press, Inc., San Diego, CA 908 pages, hard cover, 1990. Mater. Manuf. Process. 1993, 8, 391–392. [Google Scholar] [CrossRef]
- Vidal, L.; Joussein, E.; Colas, M.; Cornette, J.; Sanz, J.; Sobrados, I.; Gelet, J.L.; Absi, J.; Rossignol, S. Controlling the reactivity of silicate solutions: A FTIR, Raman and NMR study. Colloids Surf. A Physicochem. Eng. Asp. 2016, 503, 101–109. [Google Scholar] [CrossRef]
- Kalampounias, A.G. IR and Raman spectroscopic studies of sol–gel derived alkaline-earth silicate glasses. Bull. Mater. Sci. 2011, 34, 299–303. [Google Scholar] [CrossRef]
- Goerne, T.; García, M.; Grada, G.R.; Pérez, O.; López, E.G.; Lemus, M. Obtaining of sol-gel ketorolac-silica nanoparticles: Characterization and drug release kinetics. J. Nanomater. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Hammad, A.H.; Marzouk, M.A.; ElBatal, H.A. The Effects of Bi2O3 on Optical, FTIR and Thermal Properties of SrO-B2O3 Glasses. Silicon 2015, 8, 123–131. [Google Scholar] [CrossRef]
- Yang, J.; Li, D.; Fang, Y. Synthesis of Nanoscale CaO-Al2O3-SiO2-H2O and Na2O-Al2O3-SiO2-H2O Using the Hydrothermal Method and Their Characterization. Materials 2017, 10, 695. [Google Scholar] [CrossRef]
- Babushkina, M.S.; Ugolkov, V.L.; Marin, Y.B.; Nikitina, L.P.; Goncharov, A.G. Hydrogen and Carbon Groups in the Structures of Rock-Forming Minerals of Rocks of the Lithospheric Mantle: FTIR and STA + QMS Data. Dokl. Earth Sci. 2018, 479, 456–459. [Google Scholar] [CrossRef]
- Fermo, P.; Piazzalunga, A.; Vecchi, R.; Valli, G.; Ceriani, M. A TGA/FT-IR study for measuring OC and EC in aerosol samples. Atmos. Chem. Phys. 2006, 6, 255–266. [Google Scholar] [CrossRef]
- Barichard, A.; Israëli, Y.; Rivaton, A. Photocrosslinking in dichromated poly(acrylic acid) during hologram recording and comparison with dichromated poly(vinyl alcohol). J. Polym. Sci. Part. A Polym. Chem. 2008, 46, 636–642. [Google Scholar] [CrossRef]
- Yogi, P.; Tanwar, M.; Saxena, S.K.; Mishra, S.; Pathak, D.K.; Chaudhary, A.; Sagdeo, P.R.; Kumar, R. Quantifying the Short-Range Order in Amorphous Silicon by Raman Scattering. Anal. Chem. 2018, 90, 8123–8129. [Google Scholar] [CrossRef]
- Balachandran, C.; Muñoz, J.F.; Arnold, T. Characterization of alkali silica reaction gels using Raman spectroscopy. Cem. Concr. Res. 2017, 92, 66–74. [Google Scholar] [CrossRef]
- Zhang, W.; Song, J.; He, Q.; Wang, H.; Lyu, W.; Feng, H.; Xiong, W.; Guo, W.; Wu, J.; Chen, L. Novel pectin based composite hydrogel derived from grapefruit peel for enhanced Cu(II) removal. J. Hazard. Mater. 2020, 384, 121445. [Google Scholar] [CrossRef]
- Trikkaliotis, D.G.; Christoforidis, A.K.; Mitropoulos, A.C.; Kyzas, G.Z. Adsorption of copper ions onto chitosan/poly(vinyl alcohol) beads functionalized with poly(ethylene glycol). Carbohydr. Polym. 2020, 234, 115890. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Wang, W.; Su, Y.; Zhao, J. Reuse of a phosphorus recovery product (struvite/palygorskite) from nutrient wastewater for copper remediation in aqueous solution and soil. Geoderma 2020, 357, 113955. [Google Scholar] [CrossRef]
- Bouabidi, Z.B.; El-Naas, M.H.; Cortes, D.; McKay, G. Steel-Making dust as a potential adsorbent for the removal of lead (II) from an aqueous solution. Chem. Eng. J. 2018, 334, 837–844. [Google Scholar] [CrossRef]
- Jawad, A.; Peng, L.; Liao, Z.; Zhou, Z.; Shahzad, A.; Ifthikar, J.; Zhao, M.; Chen, Z.; Chen, Z. Selective removal of heavy metals by hydrotalcites as adsorbents in diverse wastewater: Different intercalated anions with different mechanisms. J. Clean. Prod. 2019, 211, 1112–1126. [Google Scholar] [CrossRef]
- Yassir, A.A.; Sharon, K.H.; Mostafa, F.; Frank, C.H. The turquoise-chalcosiderite Cu(Al,Fe3+)6(PO4)4(OH)8·4H2O solid-solution series: A Mössbauer spectroscopy, XRD, EMPA, and FTIR study. Am. Mineral. 2011, 96, 1433–1442. [Google Scholar] [CrossRef]
- El-Bayaa, A.A.; Badawy, N.A.; Alkhalik, E.A. Effect of ionic strength on the adsorption of copper and chromium ions by vermiculite pure clay mineral. J. Hazard. Mater. 2009, 170, 1204–1209. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Removal of Cu(II) by natural and acid-activated clays: An insight of adsorption isotherm, kinetic and thermodynamics. Desalination 2011, 272, 66–75. [Google Scholar] [CrossRef]
- Oliveira, J.A.; Cunha, F.A.; Ruotolo, L.A.M. Synthesis of zeolite from sugarcane bagasse fly ash and its application as a low-cost adsorbent to remove heavy metals. J. Clean. Prod. 2019, 229, 956–963. [Google Scholar] [CrossRef]
- Hu, C.; Zhu, P.; Cai, M.; Hu, H.; Fu, Q. Comparative adsorption of Pb(II), Cu(II) and Cd(II) on chitosan saturated montmorillonite: Kinetic, thermodynamic and equilibrium studies. Appl. Clay Sci. 2017, 143, 320–326. [Google Scholar] [CrossRef]
- Wu, Q.; You, R.; Lv, Q.; Xu, Y.; You, W.; Yu, Y. Efficient simultaneous removal of Cu(II) and Cr2O72− from aqueous solution by a renewable amphoteric functionalized mesoporous silica. Chem. Eng. J. 2015, 281, 491–501. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds active silicate heavy metal adsorbent (ASHMA) are available from the authors. |

| 1 | 2 | 3 | 4 | 5 | 6 | Mass Fraction (%) | Atomic Ratio | Approximation Ratio | |
|---|---|---|---|---|---|---|---|---|---|
| O | 43.74 | 41.41 | 42.43 | 40.13 | 42.52 | 41.64 | 41.98 ± 1.22 | 2.62 | 3 |
| Na | 35.71 | 39.03 | 38.10 | 36.79 | 36.15 | 40.30 | 37.68 ± 1.78 | 1.64 | 2 |
| Si | 20.55 | 19.56 | 19.47 | 23.08 | 21.33 | 18.06 | 20.34 ± 1.74 | 0.73 | 1 |
| Temperature | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qmax(mg/g) | KL(L/mg) | R2 | Kf(mg/g) | n | R2 | |
| 298 K | 303.95 | 0.321 | 0.9979 | 136.38 | 6.952 | 0.9791 |
| 303 K | 326.80 | 0.106 | 0.9982 | 132.44 | 6.370 | 0.9864 |
| 308 K | 338.98 | 0.068 | 0.9972 | 130.73 | 6.000 | 0.9802 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Chen, T.; Yan, B.; Huang, Z.; Zhou, Y.; Huang, J.; Xiao, X. A Functionalized Silicate Adsorbent and Exploration of Its Adsorption Mechanism. Molecules 2020, 25, 1820. https://doi.org/10.3390/molecules25081820
Lin H, Chen T, Yan B, Huang Z, Zhou Y, Huang J, Xiao X. A Functionalized Silicate Adsorbent and Exploration of Its Adsorption Mechanism. Molecules. 2020; 25(8):1820. https://doi.org/10.3390/molecules25081820
Chicago/Turabian StyleLin, Hanzhi, Tao Chen, Bo Yan, Zulv Huang, Yang Zhou, Jian Huang, and Xianming Xiao. 2020. "A Functionalized Silicate Adsorbent and Exploration of Its Adsorption Mechanism" Molecules 25, no. 8: 1820. https://doi.org/10.3390/molecules25081820
APA StyleLin, H., Chen, T., Yan, B., Huang, Z., Zhou, Y., Huang, J., & Xiao, X. (2020). A Functionalized Silicate Adsorbent and Exploration of Its Adsorption Mechanism. Molecules, 25(8), 1820. https://doi.org/10.3390/molecules25081820







