Theoretical Investigation of Energetic Salts with Pentazolate Anion
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermal Stability
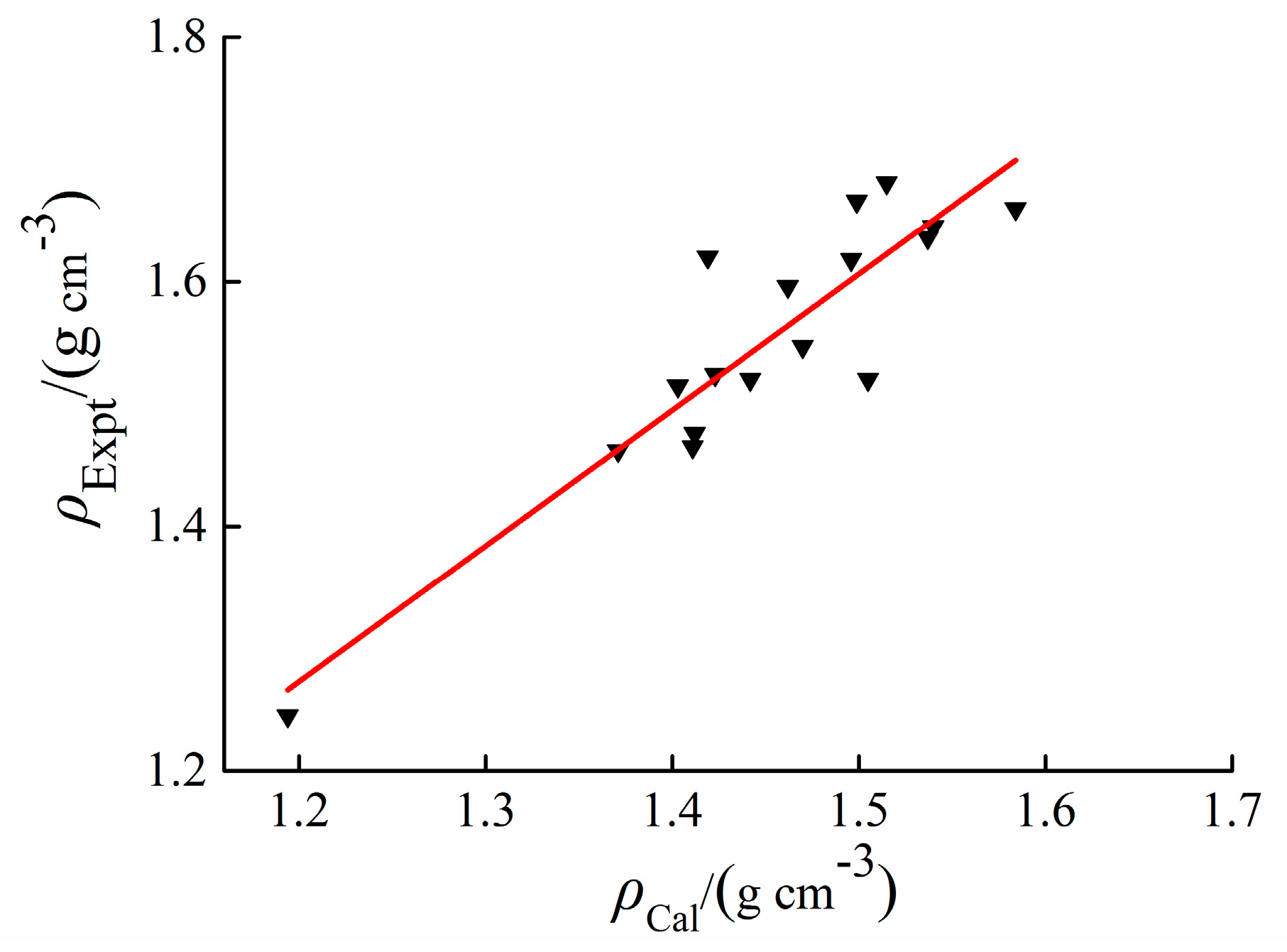
2.2. Density
3. Computational Methods
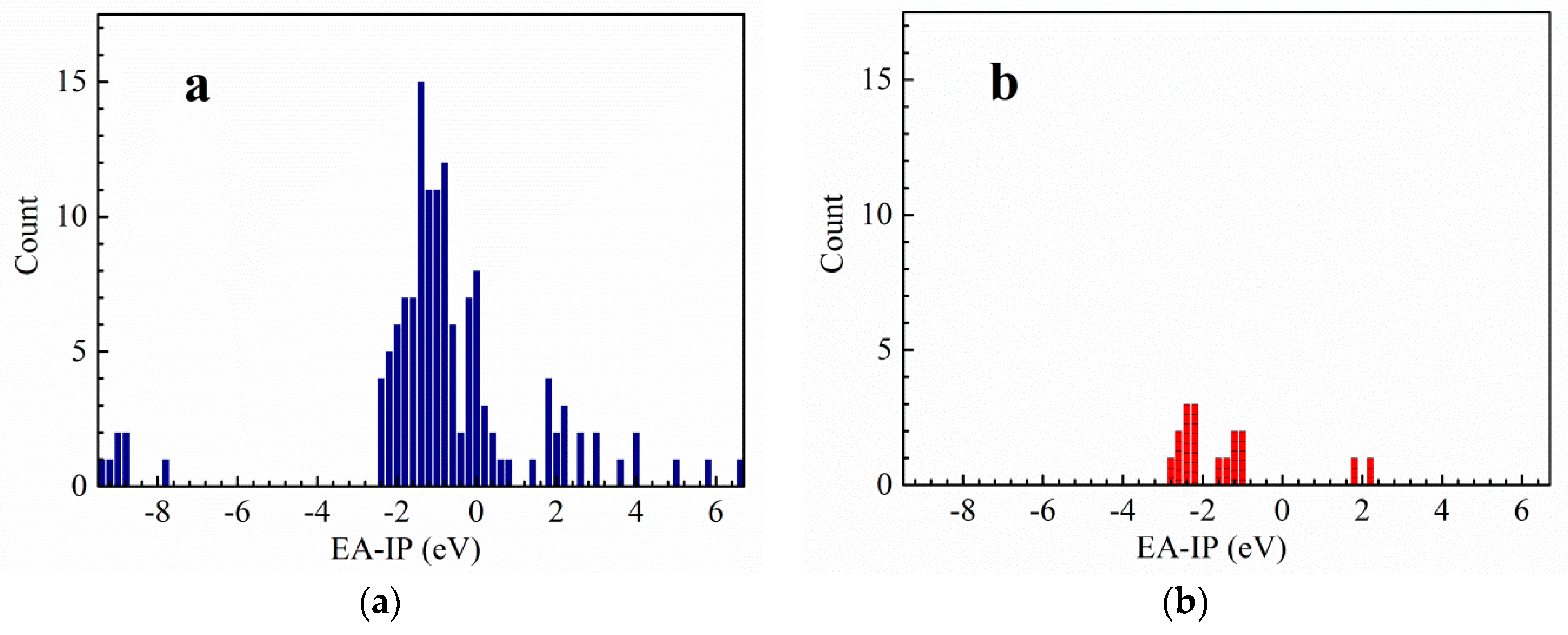
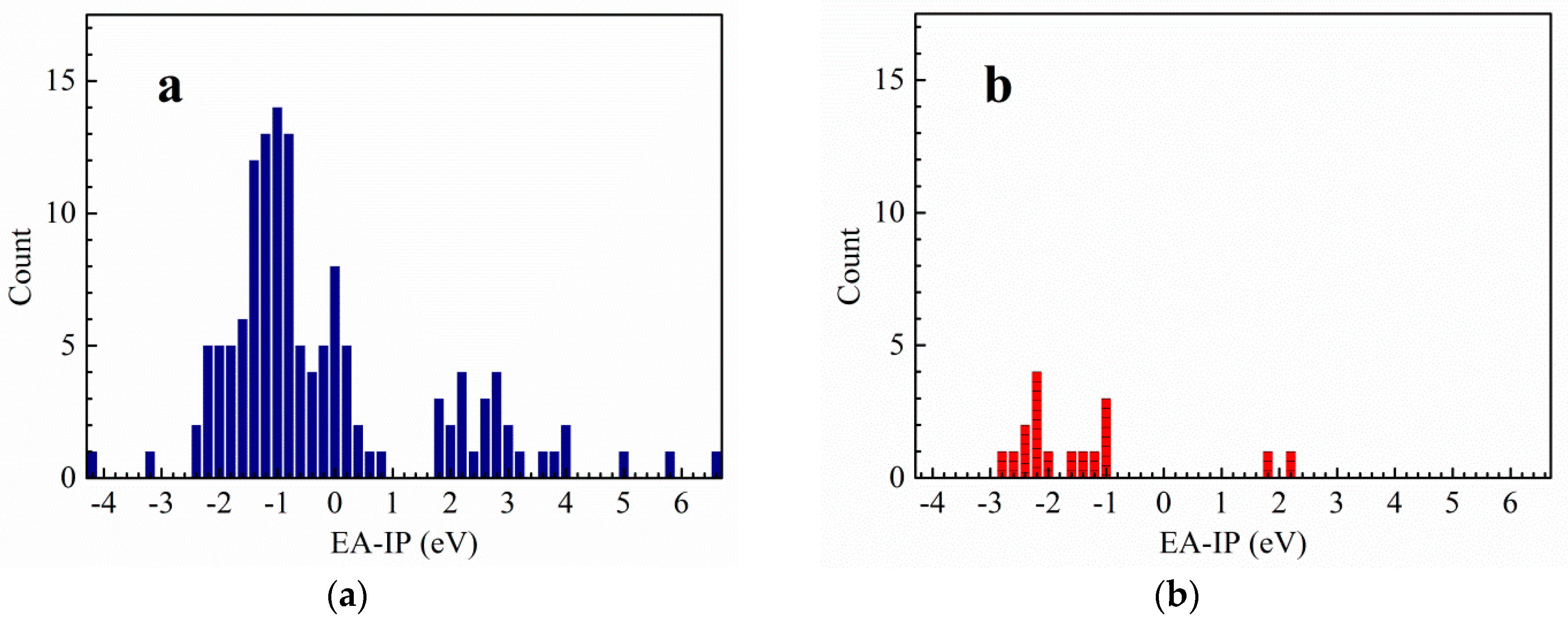
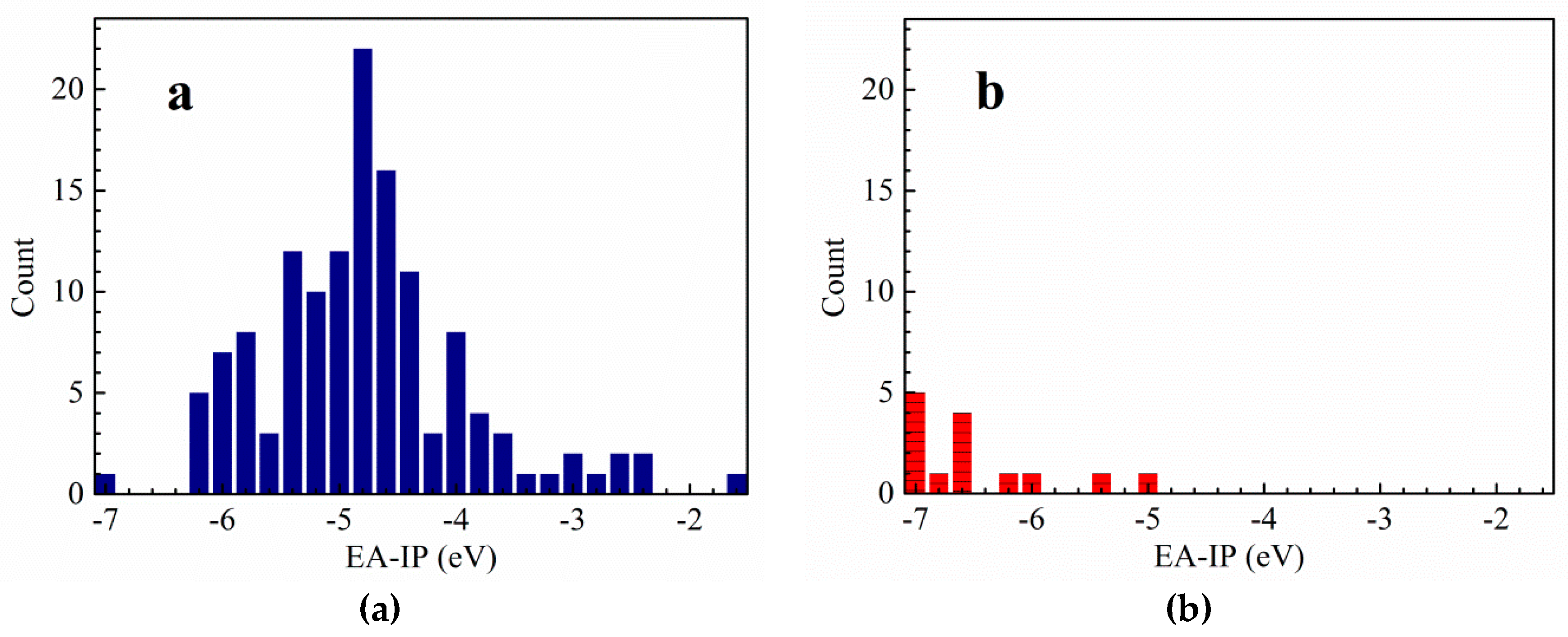
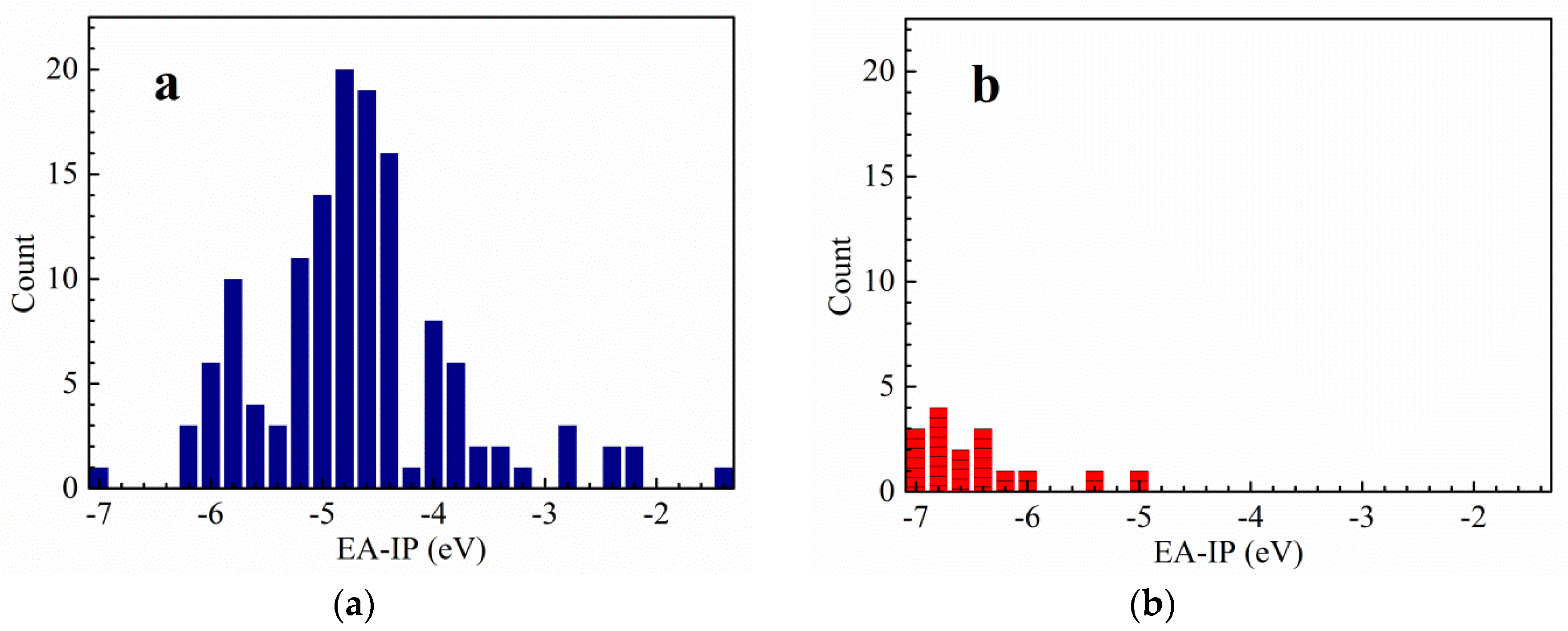
3.1. Vertical Electron Affinity and Vertical Ionization Potential
3.2. Density
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chavez, D.E.; Hanson, S.K.; Veauthier, J.M.; Parrish, D.A. Electroactive Explosives: Nitrate Ester-Functionalized 1,2,4,5-Tetrazines. Angew. Chem. Int. Edit. 2013, 52, 6876–6879. [Google Scholar] [CrossRef] [PubMed]
- Talawar, M.B.; Sivabalan, R.; Anniyappan, M.; Gore, G.M.; Asthana, S.N.; Gandhe, B.R. Emerging trends in advanced high energy materials. Combust. Explo. Shock Waves 2007, 43, 62–72. [Google Scholar] [CrossRef]
- Pagoria, P.F.; Lee, G.S.; Mitchell, A.R.; Schmidt, R.D. A review of energetic materials synthesis. Thermochim. Acta 2002, 384, 187–204. [Google Scholar] [CrossRef]
- Tsyshevsky, R.V.; Sharia, O.; Kuklja, M.M. Molecular Theory of Detonation Initiation: Insight from First Principles Modeling of the Decomposition Mechanisms of Organic Nitro Energetic Materials. Molecules 2016, 21, 236. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Sun, C.; Hu, B.; Yu, C.; Lu, M. Synthesis and characterization of the pentazolate anion cyclo-N5− in (N5)6(H3O)3(NH4)4Cl. Science 2017, 355, 374–376. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, C.; Zheng, Z.; Jiang, C.; Luo, J.; Du, Y.; Hu, B.; Sun, C.; Christe, K.O. Synthesis and Characterization of cyclo-Pentazolate Salts of NH4+, NH3OH+, N2H5+, C(NH2)3+, and N(CH3)4+. J. Am. Chem. Soc. 2018, 140, 16488–16494. [Google Scholar] [CrossRef]
- Christe, K.O.; Dixon, D.A.; Vasiliu, M.; Haiges, R.; Bingcheng, H. How Energetic are cyclo-Pentazolates? Propell. Explos. Pyrot. 2019, 44, 263–266. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. the Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Christe, K.O.; Wilson, W.W.; Sheehy, J.A.; Boatz, J.A. N-5(+): A novel homoleptic polynitrogen ion as a high energy density material (vol 38, pg 2004, 1999). Angew. Chem. Int. Edit. 2001, 40, 2947. [Google Scholar] [CrossRef]
- Gao, H.; Shreeve, J.M. Azole-Based Energetic Salts. Chem. Rev. 2011, 111, 7377–7436. [Google Scholar] [CrossRef]
- Xue, H.; Gao, Y.; Twamley, B.; Shreeve, J.M. Energetic azolium azolate salts. Inorg. Chem. 2005, 44, 5068–5072. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Shreeve, J.M. Energetic ionic liquids from azido derivatives of 1,2,4-triazole. Adv. Mater. 2005, 17, 2142–2146. [Google Scholar] [CrossRef]
- Xue, H.; Twamley, B.; Shreeve, J.M. Energetic quaternary salts containing bi(1,2,4-triazoles). Inorg. Chem. 2005, 44, 7009–7013. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.F.; Xiao, J.C.; Twamley, B.; Shreeve, J.M. Energetic salts of azotetrazolate, iminobis(5-tetrazolate) and 5,5’-bis(tetrazolate). Chem. Commun. 2005, 21, 2750–2752. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Twamley, B.; Shreeve, J.M. Energetic salts of substituted 1,2,4-triazolium and tetrazolium 3,5-dinitro-1,2,4-triazolates. J. Mater. Chem. 2005, 15, 3459–3465. [Google Scholar] [CrossRef]
- Jin, C.M.; Ye, C.F.; Piekarski, C.; Twamley, B.; Shreeve, J.M. Mono and bridged azolium pierates as energetic salts. Eur. J. Inorg. Chem. 2005, 18, 3760–3767. [Google Scholar] [CrossRef]
- Ding, P.; Wang, H.; Wen, L.; Cheng, G.; Lu, C.; Yang, H. Studies on Synthetic Technology and Reduced Sensitivity Technology of Hydrazinium Nitroformate. Ind. Eng. Chem. Res. 2014, 53, 13851–13855. [Google Scholar] [CrossRef]
- Xu, Z.; Cheng, G.; Yang, H.; Zhang, J.; Shreeve, J.M. Synthesis and Characterization of 4-(1,2,4-Triazole-5-yl)furazan Derivatives as High-Performance Insensitive Energetic Materials. Chem Eur. J. 2018, 24, 10488–10497. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Z.; Yang, H.; Lin, Q.; Cheng, G. Synthesis and characterization of a new family of energetic salts based on guanidinium cation containing furoxanyl functionality. RSC Adv. 2014, 4, 58243–58251. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Q.; Wang, P.; Lu, M. Stabilization of the Pentazolate Anion in Three Anhydrous and Metal-Free Energetic Salts. Chem. Asian J. 2018, 13, 924–928. [Google Scholar] [CrossRef]
- Tian, L.; Xu, Y.; Lin, Q.; Wang, P.; Lu, M. Syntheses of Energetic cyclo-Pentazolate Salts. Chem. Asian J. 2019, 14, 2877–2882. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tian, L.; Li, D.; Wang, P.; Lu, M. A series of energetic cyclo-pentazolate salts: Rapid synthesis, characterization, and promising performance. J. Mater. Chem. A 2019, 7, 12468–12479. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, L.; Wang, P.; Lin, Q.; Lu, M. Hydrogen Bonding Network: Stabilization of the Pentazolate Anion in Two Nonmetallic Energetic Salts. Cryst. Growth Des. 2019, 19, 1853–1859. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Jacobs, S.J. Chemistry of Detonations. I. A Simple Method for Calculating Detonation Properties of C–H–N–O Explosives. J. Chem. Phys. 2003, 48, 23–35. [Google Scholar] [CrossRef]
- Xiang, F.; Zhu, W.; Xiao, H. Theoretical studies of energetic nitrogen-rich ionic salts composed of substituted 5-nitroiminotetrazolate anions and various cations. J. Mol. Model. 2013, 19, 3103–3118. [Google Scholar] [CrossRef]
- Xiang, F.; Wu, Q.; Zhu, W.; Xiao, H. Comparative Theoretical Studies on Energetic Ionic Salts Composed of Heterocycle-Functionalized Nitraminofurazanate-Based Anions and Triaminoguanidinium Cation. J. Chem. Eng. Data 2014, 59, 295–306. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09 Revision A. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Messer, A.; Carpenter, K.; Forzley, K.; Buchanan, J.; Yang, S.; Razskazovskii, Y.; Cai, Y.L.; Sevilla, M.D. Electron spin resonance study of electron transfer rates in DNA: Determination of the tunneling constant beta for single-step excess electron transfer. J. Phys. Chem. B 2000, 104, 1128–1136. [Google Scholar] [CrossRef]
- Namazian, M. Density functional theory response to the calculation of electrode potentials of quinones in non-aqueous solution of acetonitrile. J. Mol. Struc. Theochem. 2003, 664, 273–278. [Google Scholar] [CrossRef]
- Pan, J.F.; Lee, Y.W. Crystal density prediction for cyclic and cage compounds. Phys. Chem. Chem. Phys. 2004, 6, 471–473. [Google Scholar] [CrossRef]
- Bouhmaida, N.; Ghermani, N.E. Elusive contribution of the experimental surface molecular electrostatic potential and promolecule approximation in the empirical estimate of the crystal density. J. Chem. Phys. 2005, 122, 548–553. [Google Scholar] [CrossRef]
- Rice, B.M.; Hare, J.J.; Byrd, E.F.C. Accurate predictions of crystal densities using quantum mechanical molecular volumes. J. Phys. Chem. A 2007, 111, 10874–10879. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Martinez, J.; Murray, J.S.; Concha, M.C. An electrostatic correction for improved crystal density predictions of energetic ionic compounds. Mol. Phys. 2010, 108, 1391–1396. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
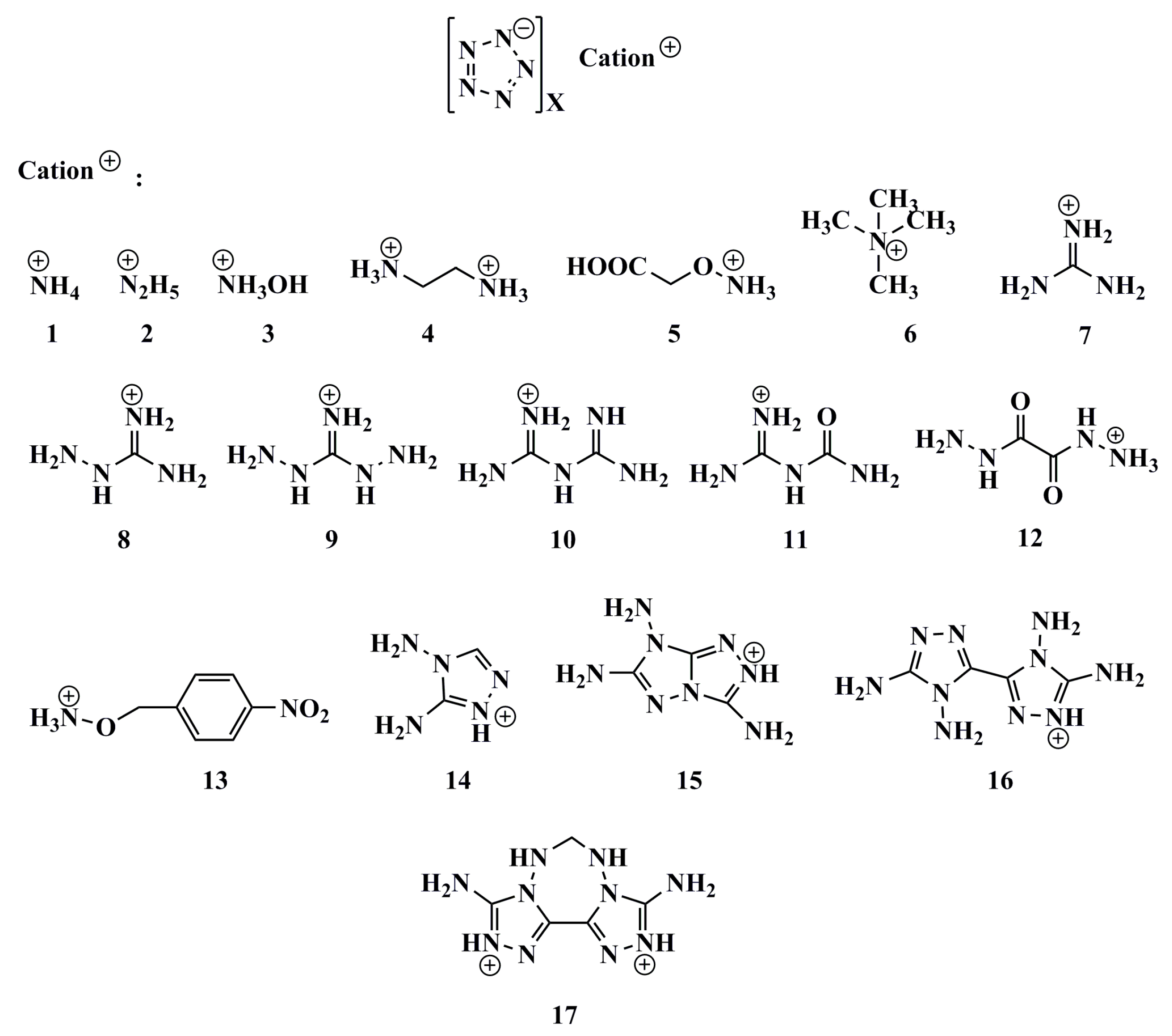
| Salts | M | V | AS+ | VS+ | AS− | VS− | M/V | ρCal | ρExpt | ρCalib | Δρ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 88 | 66.76 | 48.38 | 170.38 | 96.19 | −117.64 | 1.318 | 1.505 | 1.520 | 1.611 | 0.091 |
| 2 | 103 | 79.03 | 67.94 | 145.35 | 96.19 | −117.64 | 1.303 | 1.419 | 1.620 | 1.516 | −0.104 |
| 3 | 104 | 74.03 | 62.49 | 151.61 | 96.19 | −117.64 | 1.405 | 1.537 | 1.636 | 1.647 | 0.011 |
| 4 | 256 | 203.05 | 111.01 | 229.67 | 96.19 | −117.64 | 1.261 | 1.371 | 1.462 | 1.463 | 0.001 |
| 5 | 162 | 112.28 | 121.94 | 110.91 | 96.19 | −117.64 | 1.443 | 1.499 | 1.666 | 1.605 | −0.061 |
| 6 | 144 | 125.18 | 130.58 | 107.72 | 96.19 | −117.64 | 1.150 | 1.194 | 1.245 | 1.266 | 0.021 |
| 7 | 130 | 97.54 | 97.39 | 121.87 | 96.19 | −117.64 | 1.333 | 1.403 | 1.515 | 1.498 | −0.017 |
| 8 | 145 | 107.09 | 113.24 | 113.51 | 96.19 | −117.64 | 1.354 | 1.412 | 1.476 | 1.508 | 0.032 |
| 9 | 160 | 117.52 | 128.88 | 106.75 | 96.19 | −117.64 | 1.361 | 1.411 | 1.465 | 1.507 | 0.042 |
| 10 | 172 | 124.88 | 138.34 | 102.26 | 96.19 | −117.64 | 1.377 | 1.423 | 1.524 | 1.520 | −0.004 |
| 11 | 173 | 122.36 | 134.14 | 104.48 | 96.19 | −117.64 | 1.414 | 1.462 | 1.596 | 1.564 | −0.032 |
| 12 | 189 | 128.54 | 145.41 | 98.67 | 96.19 | −117.64 | 1.470 | 1.515 | 1.681 | 1.622 | −0.059 |
| 13 | 239 | 166.09 | 198.23 | 84.87 | 96.19 | −117.64 | 1.439 | 1.470 | 1.547 | 1.573 | 0.026 |
| 14 | 170 | 117.72 | 128.66 | 107.92 | 96.19 | −117.64 | 1.444 | 1.496 | 1.618 | 1.601 | −0.017 |
| 15 | 225 | 149.78 | 173.82 | 91.67 | 96.19 | −117.64 | 1.502 | 1.540 | 1.645 | 1.650 | 0.005 |
| 16 | 321 | 227.11 | 209.57 | 82.82 | 96.19 | −117.64 | 1.413 | 1.442 | 1.520 | 1.541 | 0.021 |
| 17 | 350 | 228.36 | 213.5 | 167.61 | 96.19 | −117.64 | 1.533 | 1.584 | 1.660 | 1.699 | 0.039 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-R.; Zhang, C.; Hu, B.-C.; Ju, X.-H. Theoretical Investigation of Energetic Salts with Pentazolate Anion. Molecules 2020, 25, 1783. https://doi.org/10.3390/molecules25081783
Wang H-R, Zhang C, Hu B-C, Ju X-H. Theoretical Investigation of Energetic Salts with Pentazolate Anion. Molecules. 2020; 25(8):1783. https://doi.org/10.3390/molecules25081783
Chicago/Turabian StyleWang, Hao-Ran, Chong Zhang, Bing-Cheng Hu, and Xue-Hai Ju. 2020. "Theoretical Investigation of Energetic Salts with Pentazolate Anion" Molecules 25, no. 8: 1783. https://doi.org/10.3390/molecules25081783





