Analysis of Nicotine Metabolites in Hair and Nails Using QuEChERS Method Followed by Liquid Chromatography–Tandem Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
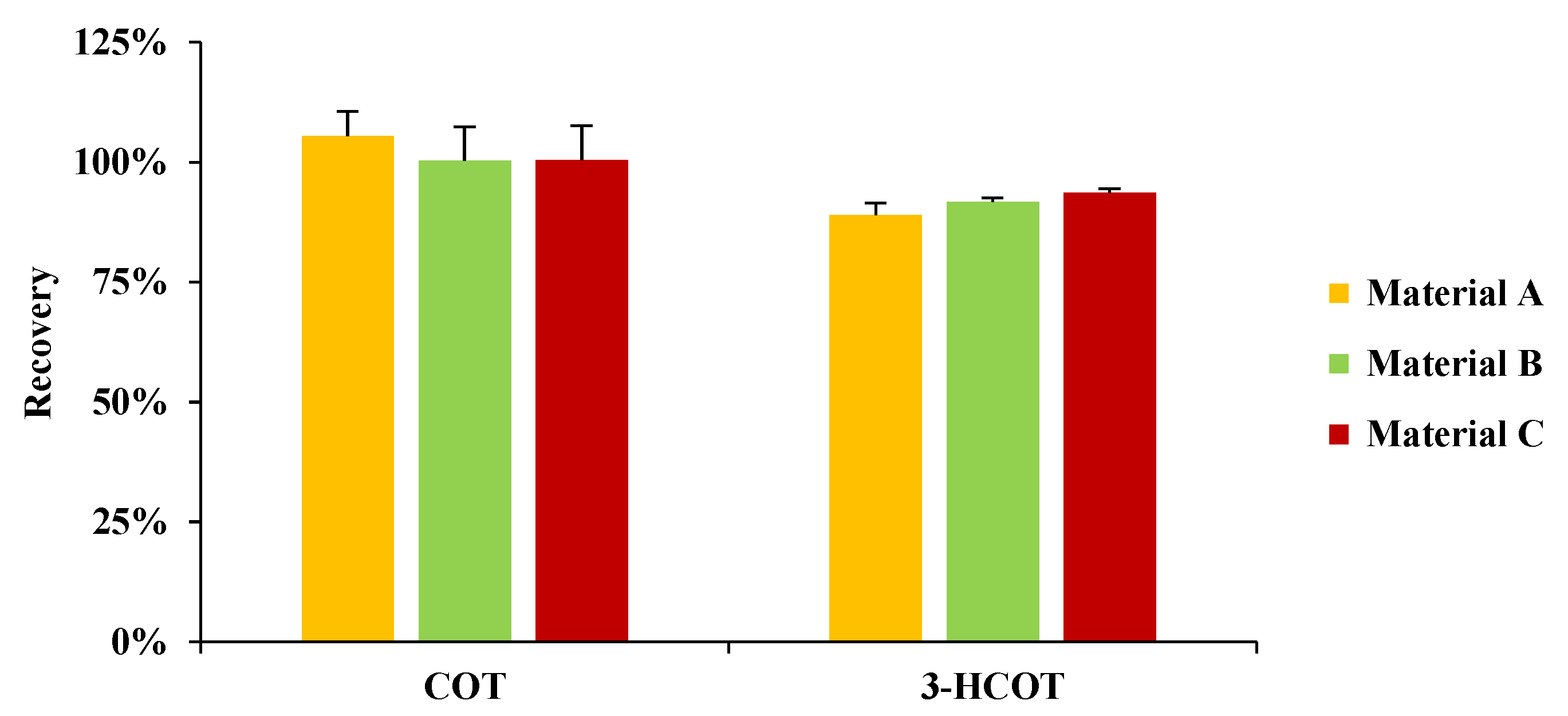
2.1. Optimization of Sample Preparation
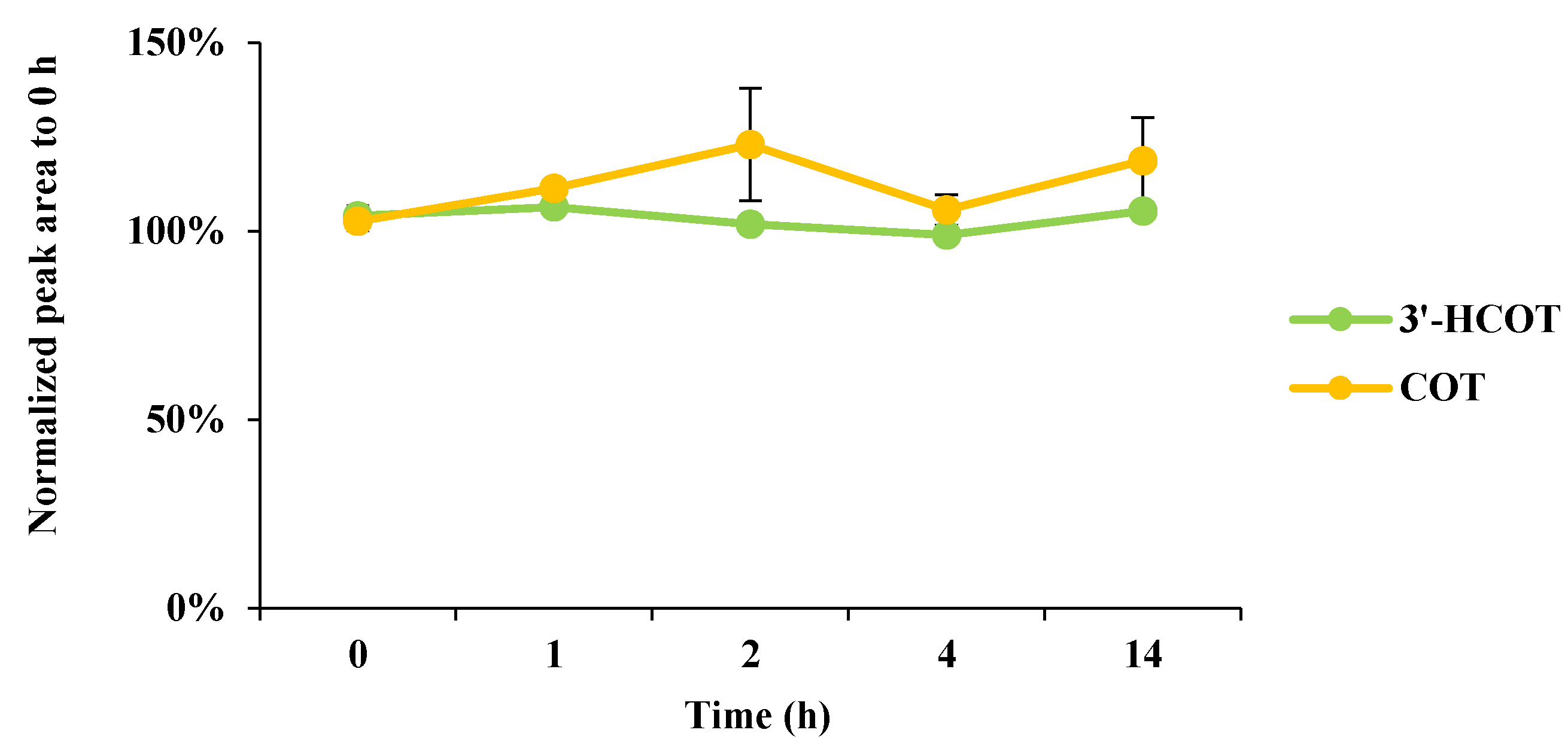
2.2. Method Validation
2.3. Method Application
2.4. Comparison with Other Methods
3. Materials and Methods
3.1. Reagents and Materials
3.2. Preparation of Standard Solutions and Human Hair and Nail Samples
3.3. Sample Preparation
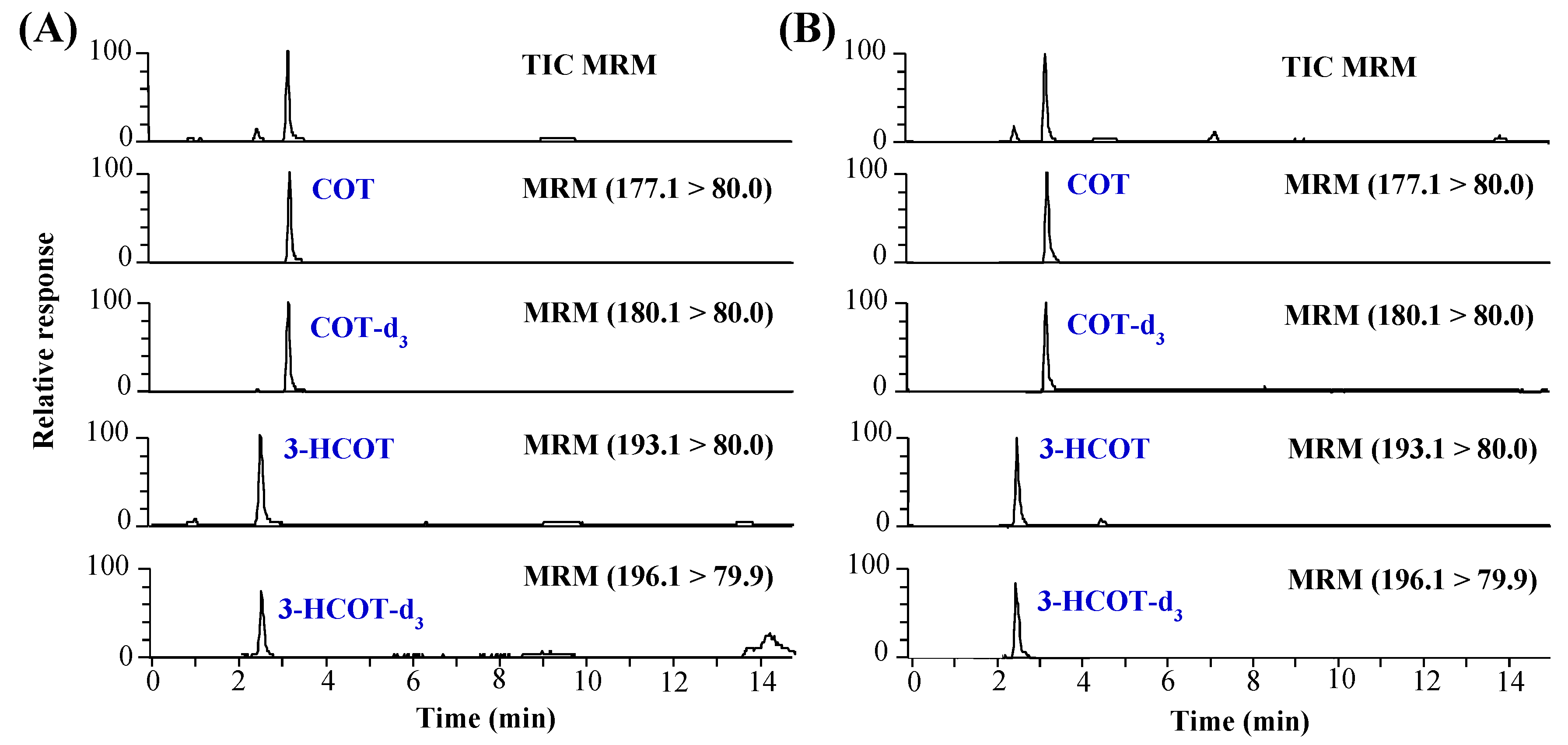
3.4. LC–MS/MS Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grana, R.; Benowitz, N.; Glantz, S.A. E-cigarettes: A scientific review. Circulation 2014, 129, 1972–1986. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Yamamoto, T.; Nunoya, K.; Yokoi, T.; Nagashima, K.; Inoue, K.; Funae, Y.; Shimada, N.; Kamataki, T.; Kuroiwa, Y. Characterization of CYP2A6 involved in 3’-hydroxylation of cotinine in human liver microsomes. J. Pharmacol. Exp. Ther. 1996, 277, 1010–1015. [Google Scholar] [PubMed]
- Dhar, P. Measuring tobacco smoke exposure: quantifying nicotine/cotinine concentration in biological samples by colorimetry, chromatography and immunoassay methods. J. Pharm. Biomed. Anal. 2004, 35, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Schutte-Borkovec, K.; Heppel, C.W.; Heling, A.K.; Richter, E. Analysis of myosmine, cotinine and nicotine in human toenail, plasma and saliva. Biomarkers 2009, 14, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Bernert, J.T.; Alexander, J.R.; Sosnoff, C.S.; McGuffey, J.E. Time course of nicotine and cotinine incorporation into samples of nonsmokers’ beard hair following a single dose of nicotine polacrilex. J. Anal. Toxicol. 2011, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.I.; Murray, G.J.; Rollins, D.E.; Tiffany, S.T.; Wilkins, D.G. Validation of a liquid chromatography–tandem mass spectrometry method for the detection of nicotine biomarkers in hair and an evaluation of wash procedures for removal of environmental nicotine. J. Anal. Toxicol. 2011, 35, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ortuno, R.; Martinez-Sanchez, J.M.; Fernandez, E.; Pascual, J.A. High-throughput wide dynamic range procedure for the simultaneous quantification of nicotine and cotinine in multiple biological matrices using hydrophilic interaction liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 8463–8473. [Google Scholar] [CrossRef][Green Version]
- Joya, X.; Pacifici, R.; Salat-Batlle, J.; Garcia-Algar, O.; Pichini, S. Maternal and neonatal hair and breast milk in the assessment of perinatal exposure to drugs of abuse. Bioanalysis 2015, 7, 1273–1297. [Google Scholar] [CrossRef]
- Perez-Ortuno, R.; Martinez-Sanchez, J.M.; Fu, M.; Fernandez, E.; Pascual, J.A. Evaluation of tobacco specific nitrosamines exposure by quantification of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in human hair of non-smokers. Sci. Rep. 2016, 6, 25043. [Google Scholar] [CrossRef]
- Hegstad, S.; Khiabani, H.; Kristoffersen, L.; Kunøe, N.; Lobmaier, P.; Christophersen, A. Drug screening of hair by liquid chromatography–tandem mass spectrometry. J. Anal. Toxicol. 2008, 32, 364–372. [Google Scholar] [CrossRef]
- Sorensen, M.; Bisgaard, H.; Stage, M.; Loft, S. Biomarkers of exposure to environmental tobacco smoke in infants. Biomarkers 2007, 12, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.R.; Piraccini, B.M.; Tosti, A. The nail and hair in forensic science. J. Am. Acad. Dermatol. 2004, 50, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Stepanov, I.; Hecht, S.S. Detection and quantitation of N′-nitrosonornicotine in human toenails by liquid chromatography–electrospray ionization-tandem mass spectrometry. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Tzatzarakis, M.N.; Vardavas, C.I.; Terzi, I.; Kavalakis, M.; Kokkinakis, M.; Liesivuori, J.; Tsatsakis, A.M. Hair nicotine/cotinine concentrations as a method of monitoring exposure to tobacco smoke among infants and adults. Hum. Exp. Toxicol. 2011, 31, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Irving, R.C.; Dickson, S.J. The detection of sedatives in hair and nail samples using tandem LC–MS–MS. Forensic Sci. Int. 2007, 166, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Mari, F.; Politi, L.; Bertol, E. Nails of newborns in monitoring drug exposure during pregnancy. Forensic Sci. Int. 2008, 179, 176–180. [Google Scholar] [CrossRef]
- Benbrahim-Tallaa, L.; Lauby-Secretan, B.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of perfluorooctanoic acid, tetrafluoroethylene, dichloromethane, 1,2-dichloropropane, and 1,3-propane sultone. Lancet Oncol. 2014, 15, 924–925. [Google Scholar] [CrossRef]
- Wilkowska, A.; Biziuk, M. Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem. 2011, 125, 803–812. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.M.; Silva, C.; Medina, S.; Câmara, J.S. QuEChERS - Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef]
- Lehmann, E.; Oltramare, C.; de Alencastro, L.F. Development of a modified QuEChERS method for multi-class pesticide analysis in human hair by GC–MS and UPLC–MS/MS. Anal. Chim. Acta 2018, 999, 87–98. [Google Scholar] [CrossRef]
- Licata, M.; Rustichelli, C.; Palazzoli, F.; Ferrari, A.; Baraldi, C.; Vandelli, D.; Verri, P.; Marchesi, F.; Silingardi, E. Hair testing in clinical setting: Simultaneous determination of 50 psychoactive drugs and metabolites in headache patients by LC tandem MS. J. Pharm. Biomed. Anal. 2016, 126, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Guideline on Bioanalytical Method Validation; Drug Evaluation Department, Ministry of Food and Drug Safety (MFDS): Chungcheongbuk-do, Korea, 2013.
- Rejczak, T.; Tuzimski, T. A review of recent developments and trends in the QuEChERS sample preparation approach. Open Chem. 2015, 13, 980–1010. [Google Scholar] [CrossRef]
- Seong, M.-W.; Hwang, J.H.; Moon, J.S.; Ryu, H.-J.; Kong, S.-Y.; Um, T.H.; Park, J.-G.; Lee, D.-H. Neonatal hair nicotine levels and fetal exposure to paternal smoking at home. Am. J. Epidemiol. 2008, 168, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Stepanov, I.; Feuer, R.; Jensen, J.; Hatsukami, D.; Hecht, S.S. Mass spectrometric quantitation of nicotine, cotinine, and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human toenails. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 2378–2383. [Google Scholar] [CrossRef] [PubMed][Green Version]
Sample Availability: Nicotine metabolites and other chemicals are available from the authors. |
| Validation Parameters | Hair | Nail | ||
|---|---|---|---|---|
| COT | 3-HCOT | COT | 3-HCOT | |
| Linearity | ||||
| Range (pg/mg) | 10–9000 | 10–9000 | 10–9000 | 10–9000 |
| r2 | 0.9999 | 0.9999 | 0.9999 | 0.9999 |
| LLOQ (pg/mg) | 10 | 10 | 10 | 10 |
| Precision (RSD %, four different levels) | ||||
| Intra-day (n = 5) | 2.1–9.6 | 0.6–8.2 | 1.1–3.1 | 1.3–4.3 |
| Inter-day (n = 3) | 1.7–4.9 | 1.3–4.1 | 0.3–12.3 | 1.0–9.7 |
| Accuracy (%, four different levels) | ||||
| Intra-day (n = 5) | 93.3–101.2 | 95.6–102.9 | 97.0–102.9 | 97.7–104.2 |
| Inter-day (n = 3) | 94.0–105.0 | 95.2–103.9 | 96.7–100.5 | 97.0–102.7 |
| Recovery (%, n = 3) | ||||
| Low level | 85.1 | 83.8 | 78.2 | 65.3 |
| Medium level | 87.2 | 71.3 | 70.4 | 57.1 |
| High level | 86.5 | 72.8 | 78.2 | 68.5 |
| Hair | Nails | |||
|---|---|---|---|---|
| COT | 3-HCOT | COT | 3-HCOT | |
| Number of detected samples | 10 | 2 | 8 | 2 |
| Median concentration (pg/mg) | 37.6 | 182.9 | 13.7 | 10.6 |
| Interquartile range (pg/mg) | 1147.0 (10.2–1157.2) | 320.8 (22.4–343.3) | 11.4 (10.0–21.4) | 0.8 (10.2–10.9) |
| Method | Organic Solvent (Volume) | Analyte | S/N ratio | Recovery (n = 3) | |
|---|---|---|---|---|---|
| Mean | RSD | ||||
| QuEChERS | Acetonitrile (5 mL) | COT | 7329.6 | 83% | 2% |
| 3-HCOT | 344.5 | 61% | 1% | ||
| LLE | Dichloromethane (2 mL) | COT | 22,227.0 | 65% | 11% |
| 3-HCOT | 68.4 | 11% | 16% | ||
| Sample (Amount) | Analyte | Sample Preparation | Extraction Step a | LOD b (pg/mg) | Ref. |
|---|---|---|---|---|---|
| Hair (10 mg) | COT | LLE | Extraction with DCM (0.5 mL) | 6.6 | [7] |
| Hair (1 mg) | COT | LLE | Extraction with diethyl ether (2 mL) | 70 | [24] |
| Hair (20 mg) | COT | LLE | Three step extraction: - Extraction with DCM (5 mL) and DCM/iPrOH (5 mL, 75/25, v/v) - Back-extraction with HCl (1 mL, 0.5 M) - Re-extraction with DCM (4 mL) and DCM/iPrOH (4 mL, 75/25, v/v) | 2.5 | [9] |
| Toenail (20–30 mg) | COT | LLE | Extraction with DCM (1 mL) | 12 | [25] |
| Toenail (20–30 mg) | COT | LLE | Repeated extraction (thrice) with DCM (0.5 mL) | 35 | [4] |
| Hair and nail (10 mg) | COT, 3-HCOT | QuEChERS | One-step extraction with acetonitrile (5 mL) | 10 c | This work |
| Analyte | tRa (min) | Precursor Ion (m/z) | Product Ions (m/z) | ||
|---|---|---|---|---|---|
| Quantitative Ion (CE b) | Qualitative Ions (CE) | ||||
| 3-HCOT | 2.6 | 193.1 | 80.0 (30) | 134.1 (20) | 106.0 (29) |
| 3-HCOT-d3 (IS) | 2.6 | 196.1 | 79.9 (32) | 134.0 (19) | 106.0 (31) |
| COT | 3.3 | 177.1 | 80.0 (29) | 98.0 (21) | 70.1 (34) |
| COT-d3 (IS) | 3.3 | 180.1 | 80.0 (28) | 101.0 (23) | 73.1 (39) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Cho, H.-D.; Suh, J.H.; Lee, J.-Y.; Lee, E.; Jin, C.H.; Wang, Y.; Cha, S.; Im, H.; Han, S.B. Analysis of Nicotine Metabolites in Hair and Nails Using QuEChERS Method Followed by Liquid Chromatography–Tandem Mass Spectrometry. Molecules 2020, 25, 1763. https://doi.org/10.3390/molecules25081763
Kim J, Cho H-D, Suh JH, Lee J-Y, Lee E, Jin CH, Wang Y, Cha S, Im H, Han SB. Analysis of Nicotine Metabolites in Hair and Nails Using QuEChERS Method Followed by Liquid Chromatography–Tandem Mass Spectrometry. Molecules. 2020; 25(8):1763. https://doi.org/10.3390/molecules25081763
Chicago/Turabian StyleKim, Junhee, Hyun-Deok Cho, Joon Hyuk Suh, Ji-Youn Lee, Eunyoung Lee, Chang Hwa Jin, Yu Wang, Sangwon Cha, Hosub Im, and Sang Beom Han. 2020. "Analysis of Nicotine Metabolites in Hair and Nails Using QuEChERS Method Followed by Liquid Chromatography–Tandem Mass Spectrometry" Molecules 25, no. 8: 1763. https://doi.org/10.3390/molecules25081763
APA StyleKim, J., Cho, H.-D., Suh, J. H., Lee, J.-Y., Lee, E., Jin, C. H., Wang, Y., Cha, S., Im, H., & Han, S. B. (2020). Analysis of Nicotine Metabolites in Hair and Nails Using QuEChERS Method Followed by Liquid Chromatography–Tandem Mass Spectrometry. Molecules, 25(8), 1763. https://doi.org/10.3390/molecules25081763






