The Effect of Silica Fume and Organosilane Addition on the Porosity of Cement Paste
Abstract
1. Introduction
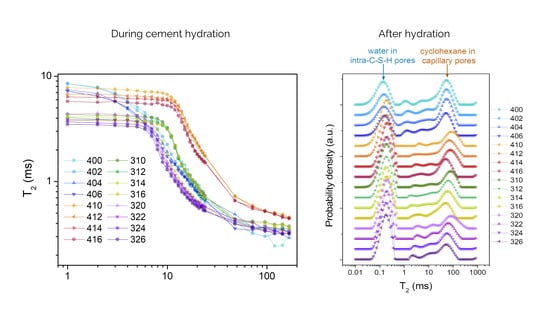
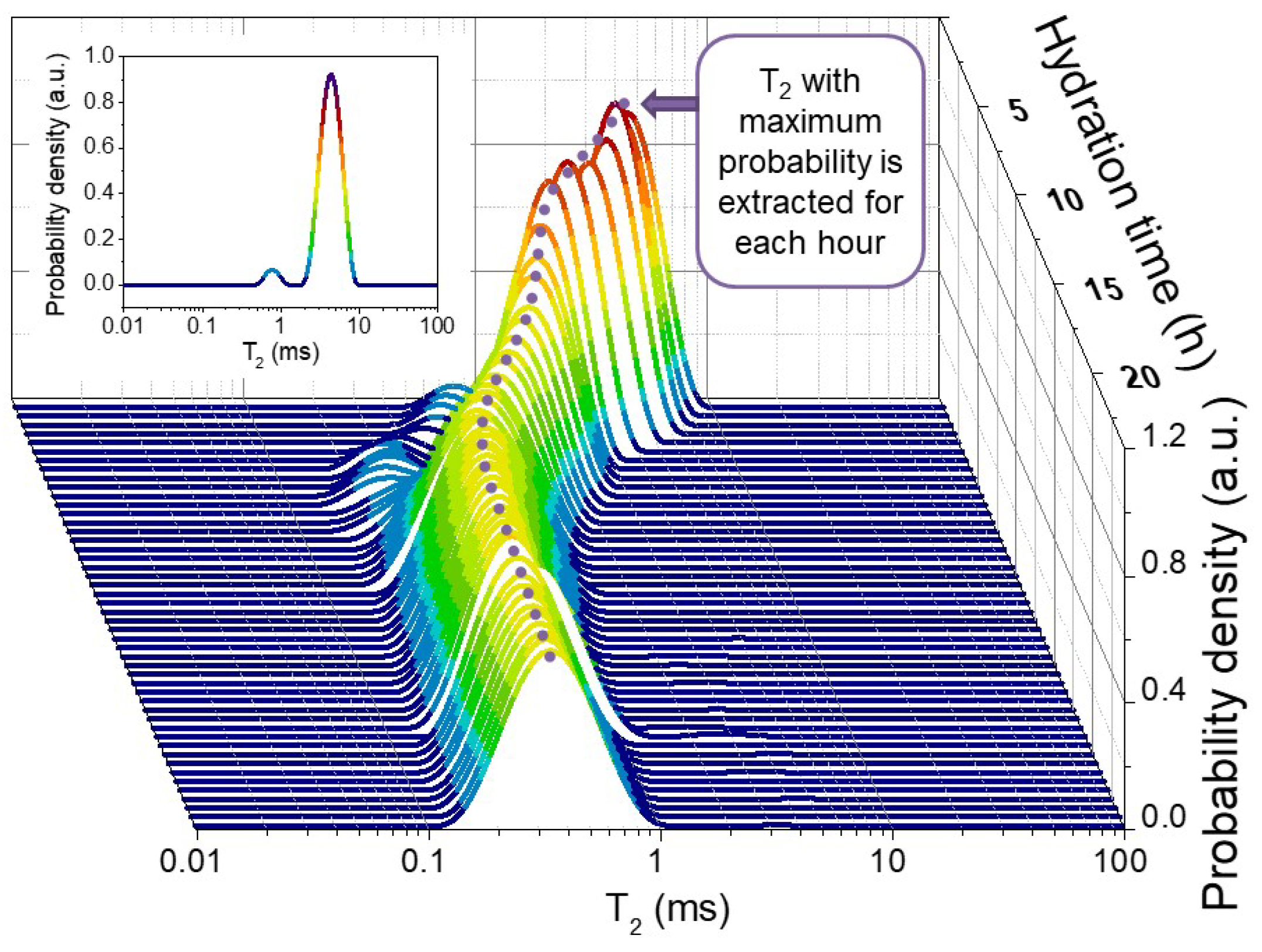
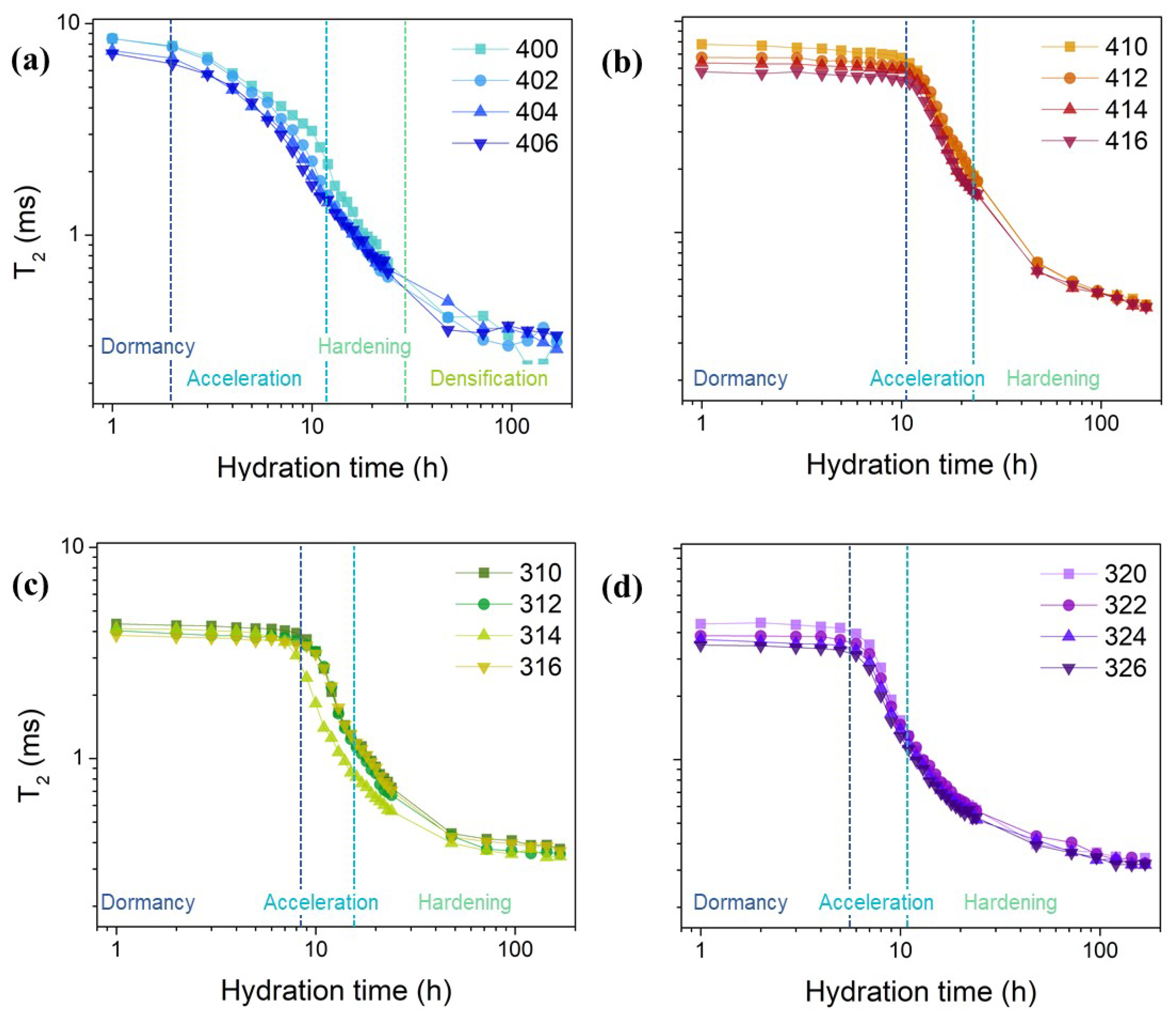
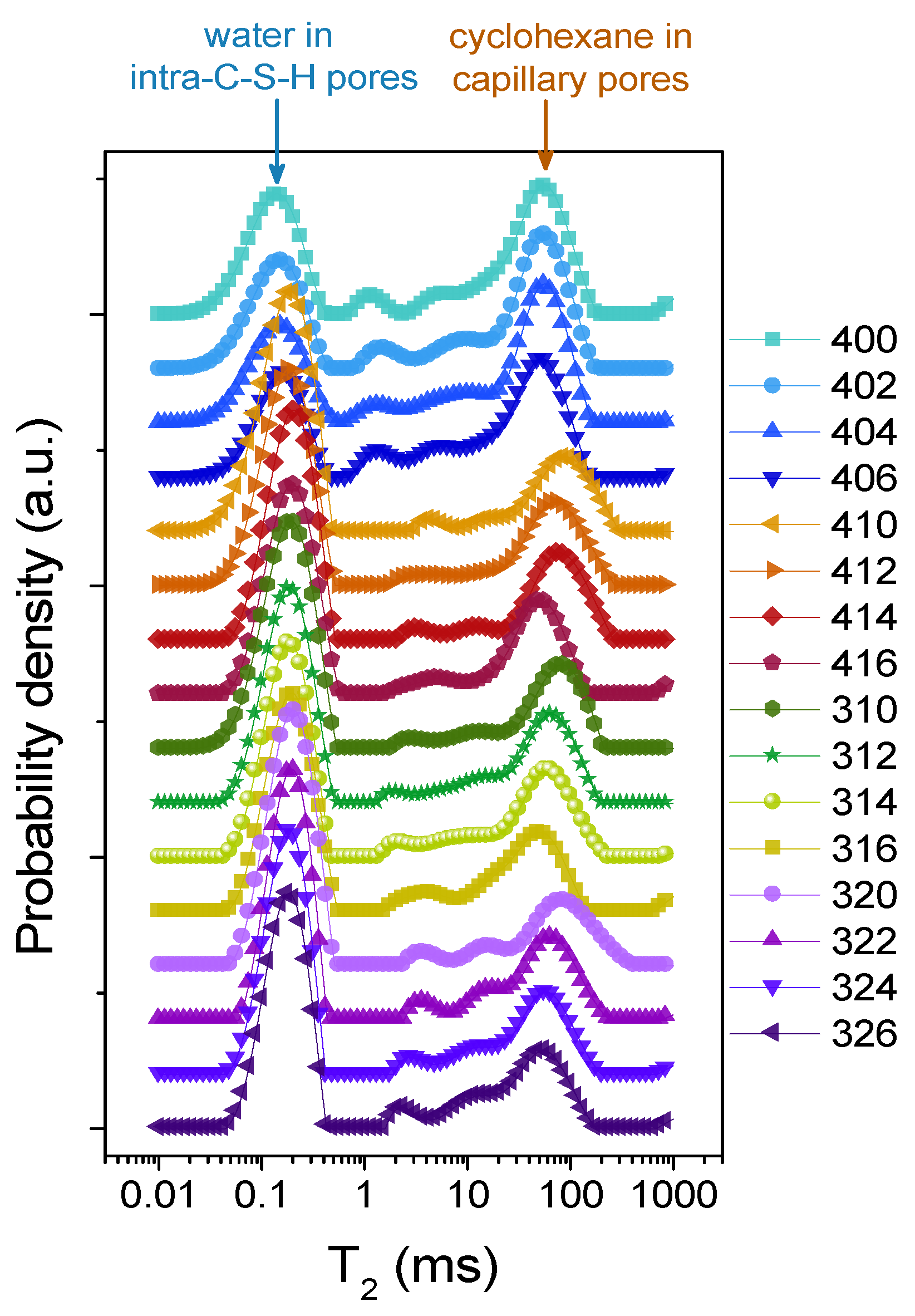
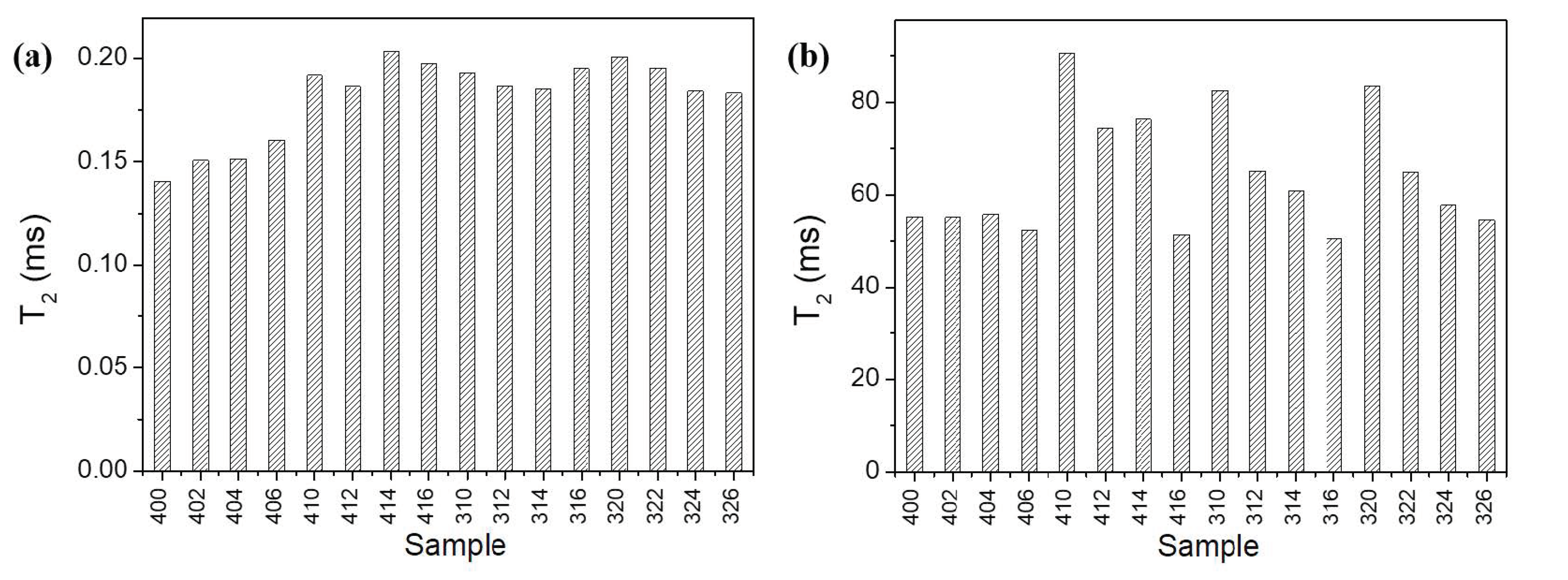
2. Results and Discussion
3. Materials and Methods
3.1. Sample Preparation
3.2. NMR Investigations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heede, P.V.D.; De Belie, N. Environmental impact and life cycle assessment (LCA) of traditional and ‘green’ concretes: Literature review and theoretical calculations. Cem. Concr. Compos. 2012, 34, 431–442. [Google Scholar] [CrossRef]
- Chen, C.; Habert, G.; Bouzidi, Y.; Jullien, A. Environmental impact of cement production: Detail of the different processes and cement plant variability evaluation. J. Clean. Prod. 2010, 18, 478–485. [Google Scholar] [CrossRef]
- Lee, J.; Mahendra, S.; Alvarez, P.J. Nanomaterials in the Construction Industry: A Review of Their Applications and Environmental Health and Safety Considerations. ACS Nano 2010, 4, 3580–3590. [Google Scholar] [CrossRef] [PubMed]
- Bribian, I.Z.; Capilla, A.V.; Uson, J.A.A. Life cycle assessment of building materials: Comparative analysis of energy and environmental impacts and evaluation of the eco-efficiency improvement potential. Build. Environ. 2011, 46, 1133–1140. [Google Scholar] [CrossRef]
- Salas, D.A.; Ramirez, A.D.; Rodriguez, C.; Petroche, D.M.; Boero, A.; Duque-Rivera, J. Environmental impacts, life cycle assessment and potential improvement measures for cement production: A literature review. J. Clean. Prod. 2016, 113, 114–122. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.; Birgisson, B.; Taylor, P.; Attoh-Okine, N.O. (Eds.) Nano-technology in civil infrastructure; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 978-3-642-16656-3. [Google Scholar]
- Oltulu, M.; Şahin, R. Pore structure analysis of hardened cement mortars containing silica fume and different nano-powders. Constr. Build. Mater. 2014, 53, 658–664. [Google Scholar] [CrossRef]
- Badea, C.; Bede, A.; Ardelean, I. The effect of silica fume on early hydration of white Portland cement via fast field cycling-NMR relaxometry. AIP Conf. Proc. 2017, 1917, 040001. [Google Scholar]
- Muller, A.; Scrivener, K.; Skibsted, J.; Gajewicz, A.; McDonald, P.J. Influence of silica fume on the microstructure of cement pastes: New insights from 1H NMR relaxometry. Cem. Concr. Res. 2015, 74, 116–125. [Google Scholar] [CrossRef]
- Hou, P.; Qian, J.; Cheng, X.; Shah, S.P. Effects of the pozzolanic reactivity of nanoSiO2 on cement-based materials. Cem. Concr. Compos. 2015, 55, 250–258. [Google Scholar] [CrossRef]
- Lilkov, V.; Petrov, O.; Tzvetanova, Y.; Savov, P. Mössbauer, DTA and XRD study of Portland cement blended with fly ash and silica fume. Constr. Build. Mater. 2012, 29, 33–41. [Google Scholar] [CrossRef]
- Yajun, J.; Cahyadi, J.H. Simulation of silica fume blended cement hydration. Mater. Struct. 2004, 37, 397–404. [Google Scholar] [CrossRef]
- Esteves, L.P. On the hydration of water-entrained cement–silica systems: Combined SEM, XRD and thermal analysis in cement pastes. Thermochim. Acta 2011, 518, 27–35. [Google Scholar] [CrossRef]
- Rossen, J.; Lothenbach, B.; Scrivener, K. Composition of C–S–H in pastes with increasing levels of silica fume addition. Cem. Concr. Res. 2015, 75, 14–22. [Google Scholar] [CrossRef]
- Qing, Y.; Zenan, Z.; Deyu, K.; Rongshen, C. Influence of nano-SiO2 addition on properties of hardened cement paste as compared with silica fume. Constr. Build. Mater. 2007, 21, 539–545. [Google Scholar] [CrossRef]
- Torii, K.; Kawamura, M. Pore structure and chloride ion permeability of mortars containing silica fume. Cem. Concr. Compos. 1994, 16, 279–286. [Google Scholar] [CrossRef]
- Cheng-Yi, H.; Feldman, R. Hydration reactions in portland cement-silica fume blends. Cem. Concr. Res. 1985, 15, 585–592. [Google Scholar] [CrossRef]
- Cheng-Yi, H.; Feldman, R. Influence of silica fume on the microstructural development in cement mortars. Cem. Concr. Res. 1985, 15, 285–294. [Google Scholar] [CrossRef]
- Khan, M.I.; Siddique, R. Utilization of silica fume in concrete: Review of durability properties. Resour. Conserv. Recycl. 2011, 57, 30–35. [Google Scholar] [CrossRef]
- Kong, X.-M.; Liu, H.; Lu, Z.-B.; Wang, D.-M. The influence of silanes on hydration and strength development of cementitious systems. Cem. Concr. Res. 2015, 67, 168–178. [Google Scholar] [CrossRef]
- Bede, A.; Pop, A.; Moldovan, M.; Ardelean, I. The influence of silanized nano-SiO2 on the hydration of cement paste: NMR investigations. AIP Conf. Proc. 2015, 1700, 60009. [Google Scholar]
- Švegl, F.; Šuput-Strupi, J.; Skrlep, L.; Kalcher, K. The influence of aminosilanes on macroscopic properties of cement paste. Cem. Concr. Res. 2008, 38, 945–954. [Google Scholar] [CrossRef]
- Pop, A.; Bede, A.; Dudescu, M.C.; Popa, F.; Ardelean, I. Monitoring the Influence of Aminosilane on Cement Hydration Via Low-field NMR Relaxometry. Appl. Magn. Reson. 2015, 47, 191–199. [Google Scholar] [CrossRef]
- Plassais, A.; Pomies, M.-P.; Lequeux, N.; Korb, J.-P.; Petit, D.; Barberon, F.; Bresson, B. Microstructure evolution of hydrated cement pastes. Phys. Rev. E 2005, 72, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Stark, J. Recent advances in the field of cement hydration and microstructure analysis. Cem. Concr. Res. 2011, 41, 666–678. [Google Scholar] [CrossRef]
- Korb, J.-P. Multiscale nuclear magnetic relaxation dispersion of complex liquids in bulk and confinement. Prog. Nucl. Magn. Reson. Spectrosc. 2018, 104, 12–55. [Google Scholar] [CrossRef]
- Pop, A.; Ardelean, I. Monitoring the size evolution of capillary pores in cement paste during the early hydration via diffusion in internal gradients. Cem. Concr. Res. 2015, 77, 76–81. [Google Scholar] [CrossRef]
- Bede, A.; Scurtu, A.-D.; Ardelean, I. NMR relaxation of molecules confined inside the cement paste pores under partially saturated conditions. Cem. Concr. Res. 2016, 89, 56–62. [Google Scholar] [CrossRef]
- Cretu, A.; Mattea, C.; Stapf, S.; Ardelean, I. The effect of silica nanoparticles on the pore structure of hydrating cement paste: A spatially resolved low-field NMR study. Mol. Phys. 2018, 117, 1006–1014. [Google Scholar] [CrossRef]
- Song, Y.-Q.; Venkataramanan, L.; Hurlimann, M.; Flaum, M.; Frulla, P.; Straley, C. T1–T2 Correlation Spectra Obtained Using a Fast Two-Dimensional Laplace Inversion. J. Magn. Reson. 2002, 154, 261–268. [Google Scholar] [CrossRef]
- Pop, A.; Badea, C.; Ardelean, I. Monitoring the ettringite formation in cement paste using low field T2-NMR. AIP Conf. Proc. 2013, 144, 141–144. [Google Scholar]
- Korb, J.-P. Nuclear magnetic relaxation of liquids in porous media. New J. Phys. 2011, 13, 35016. [Google Scholar] [CrossRef]
- Scrivener, K.; Nonat, A. Hydration of cementitious materials, present and future. Cem. Concr. Res. 2011, 41, 651–665. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, X.-M.; Lu, Z.-B.; Lu, Z.-C.; Hou, S.-S. Effects of the charge characteristics of polycarboxylate superplasticizers on the adsorption and the retardation in cement pastes. Cem. Concr. Res. 2015, 67, 184–196. [Google Scholar] [CrossRef]
- Pop, A.; Badea, C.; Ardelean, I. The Effects of Different Superplasticizers and Water-to-Cement Ratios on the Hydration of Gray Cement Using T2-NMR. Appl. Magn. Reson. 2013, 44, 1223–1234. [Google Scholar] [CrossRef]
- Bede, A.; Ardelean, I. Revealing the influence of water-cement ratio on the pore size distribution in hydrated cement paste by using cyclohexane. AIP Conf. Proc. 2017, 1917, 040002. [Google Scholar]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Sample | Water/Cement | Organosilane (%) | Silica Fume (%) |
|---|---|---|---|
| 400 | 0.4 | 0 | 0 |
| 402 | 0.4 | 0 | 2 |
| 404 | 0.4 | 0 | 4 |
| 406 | 0.4 | 0 | 6 |
| 410 | 0.4 | 1 | 0 |
| 412 | 0.4 | 1 | 2 |
| 414 | 0.4 | 1 | 4 |
| 416 | 0.4 | 1 | 6 |
| 310 | 0.3 | 1 | 0 |
| 312 | 0.3 | 1 | 2 |
| 314 | 0.3 | 1 | 4 |
| 316 | 0.3 | 1 | 6 |
| 320 | 0.3 | 2 | 0 |
| 322 | 0.3 | 2 | 2 |
| 324 | 0.3 | 2 | 4 |
| 326 | 0.3 | 2 | 6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crețu, A.; Mattea, C.; Stapf, S.; Ardelean, I. The Effect of Silica Fume and Organosilane Addition on the Porosity of Cement Paste. Molecules 2020, 25, 1762. https://doi.org/10.3390/molecules25081762
Crețu A, Mattea C, Stapf S, Ardelean I. The Effect of Silica Fume and Organosilane Addition on the Porosity of Cement Paste. Molecules. 2020; 25(8):1762. https://doi.org/10.3390/molecules25081762
Chicago/Turabian StyleCrețu, Andrea, Carlos Mattea, Siegfried Stapf, and Ioan Ardelean. 2020. "The Effect of Silica Fume and Organosilane Addition on the Porosity of Cement Paste" Molecules 25, no. 8: 1762. https://doi.org/10.3390/molecules25081762
APA StyleCrețu, A., Mattea, C., Stapf, S., & Ardelean, I. (2020). The Effect of Silica Fume and Organosilane Addition on the Porosity of Cement Paste. Molecules, 25(8), 1762. https://doi.org/10.3390/molecules25081762