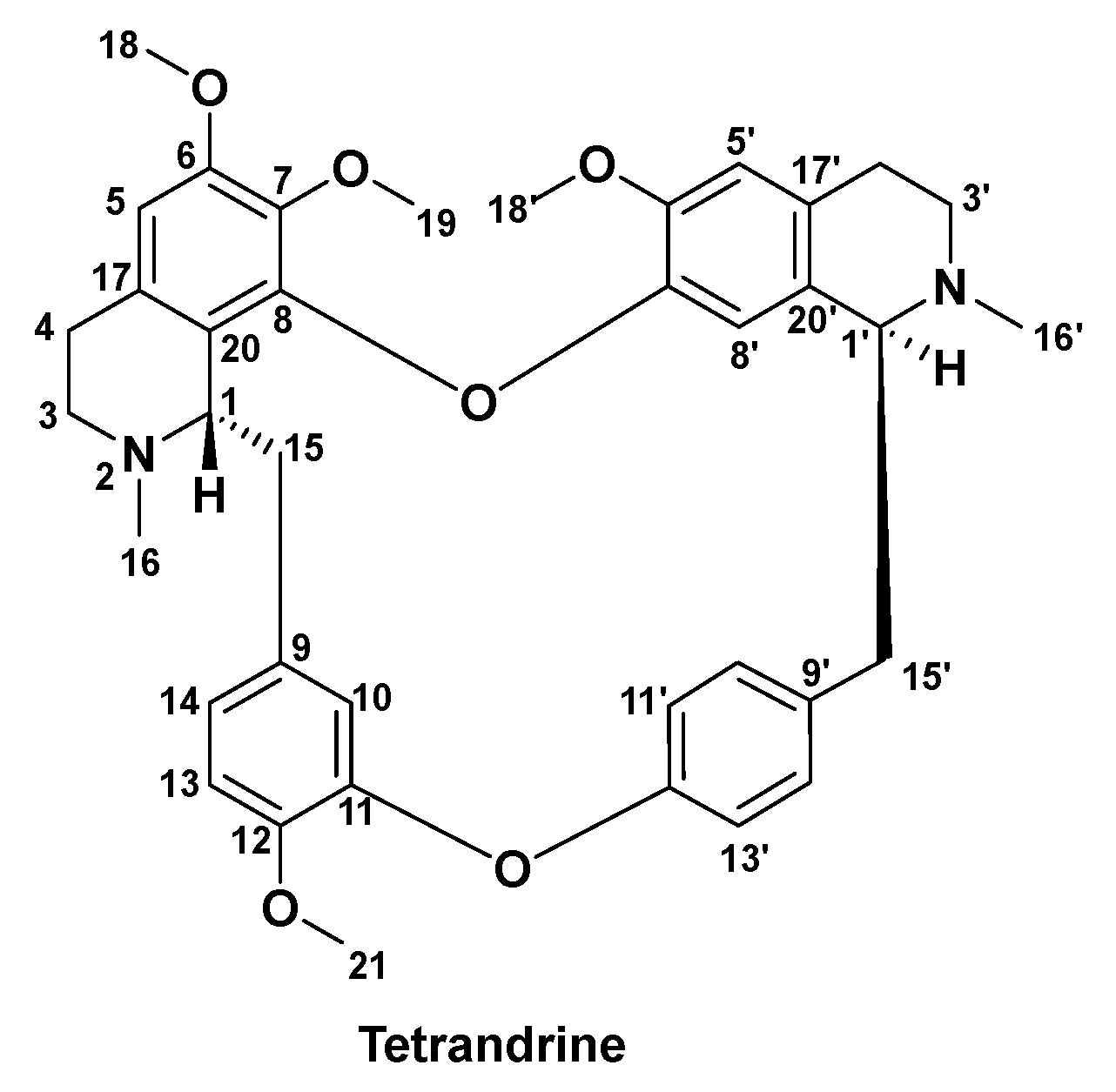
3.2. Methods of Synthesis
3.2.1. General Procedure for the Preparation of 14-Nitrotetrandrine (Tet-NO2)
Under the protection of an argon atmosphere, concentrated HNO3 (69%, 1.4 mL, 22.4 mmol) was slowly added dropwise into (CH3CO)2O (2.0 mL, 21.3 mmol) in an ice-salt bath and stirred for 10 min. Then, the tetrandrine (0.7 g, 1.12 mmol) dissolved in dry DCM (4 mL) was added dropwise into the reaction mixture and stirred in an ice-salt bath. TLC was used to monitor reaction. Upon completion, the reaction mixture was quenched with saturated aqueous solution of sodium bicarbonate, extracted with DCM (3 × 15 mL), dried over anhydrous sodium sulfate and filtered. The solvent was removed under reduced pressure. The residue was purified by silica gel chromatography from DCM/MeOH (30/1 v/v, 0.5% TEA) to afford the compound Tet-NO2. Light yellow amorphous solid, yield: 93%. Mp: 176–177 °C. 1H-NMR (400 MHz, CDCl3) δ 7.42 (1H, s), 7.37 (1H, dd, J = 2.0, 8.0 Hz), 7.12 (1H, dd, J = 2.4, 8.0 Hz), 6.77 (1H, dd, J = 2.8, 8.4 Hz), 6.54 (1H, s), 6.52 (1H, s), 6.30 (1H, s), 6.28 (1H, d, J = 2.0 Hz), 5.98 (1H, s), 3.98 (3H, s), 3.91 (1H, dd, J = 6.0, 10.8 Hz), 3.75 (3H, s), 3.69–3.63 (1H, m), 3.52–3.49 (2H, m), 3.38 (3H, s), 3.30–3.25 (1H, m), 3.18 (3H, s), 2.96–2.73 (7H, m), 2.63 (3H, s), 2.53 (1H, d, J = 12.8 Hz), 2.35 (1H, m), 2.21 (3H, s). 13C-NMR (CDCl3, 100 MHz) δ 152.3, 152.1, 151.5, 148.7, 148.2, 146.5, 144.2, 143.5, 137.5, 136.4, 133.1, 130.5, 130.4, 128.9, 128.1, 127.6, 121.6, 121.4, 121.3, 119.9, 117.2, 112.5, 108.2, 105.8, 63.6, 61.7, 60.3, 56.3, 55.8, 55.7, 45.3, 43.2, 42.8, 41.5, 37.9, 36.8, 25.3, 21.6. HRMS (ESI) calcd. for C38H42N3O8: 668.2972 [M + H]+, found: 668.2965.
3.2.2. General Procedure for the Preparation of 14-Aminotetrandrine (Tet-NH2)
To a mixture of Tet-NO2 (400.0 mg, 0.60 mmol) and palladium on carbon (5%, 40 mg) were added analytical methanol (20 mL) and hydrazine hydrate (85%, 0.18 mL, 4.80 mmol). The mixture was stirred at 65 °C for about 4 h before it was filtered by celite under reduced pressure. The filter was quenched with saturated sodium chloride solution, extracted with DCM (5 × 20 mL), dried over anhydrous sodium sulfate and filtered. The solvent was removed under reduced pressure. The crude product was recrystallized from cyclohexane and acetone (2/7, v/v) to give Tet-NH2. White amorphous solid, yield: 84%. Mp: 164–166 °C. 1H-NMR (400 MHz, CDCl3) δ 7.28 (1H, d, J = 9.6 Hz), 7.18 (1H, dd, J = 2.0, 8.0 Hz), 6.60 (1H, dd, J = 2.0, 8.4 Hz), 6.50 (1H, s), 6.46 (1H, s), 6.31 (1H, s), 6.29 (1H, s), 6.12 (1H, dd, J = 1.6, 8.0 Hz), 5.87 (1H, s), 3.94 (1H, d, J = 9.2 Hz), 3.87 (3H, s), 3.80 (1H, dd, J = 5.2, 11.2 Hz), 3.73 (3H, s), 3.64 (1H, m), 3.42 (1H, m), 3.35 (3H, s), 3.26 (1H, dd, J = 5.2, 12.4 Hz), 3.11 (3H, s), 2.88 (7H, m), 2.61 (3H, s), 2.42 (3H, s), 2.35 (2H, m). 13C-NMR (CDCl3, 100 MHz) δ 156.6, 151.6, 149.4, 148.7, 148.5, 144.2, 142.0, 140.8, 138.0, 133.2, 132.6, 129.3, 128.0, 127.6, 127.4, 122.6, 122.1, 121.3, 120.9, 120.5, 120.2, 112.3, 105.8, 100.6, 64.2, 61.5, 59.9, 56.1, 55.6, 55.5, 44.9, 43.2, 42.3, 40.8, 40.0, 38.7, 24.6, 20.6. HRMS (ESI) calcd. for C38H44N3O6: 638.3230 [M + H]+, found: 638.3233.
3.2.3. General Procedure for the Preparation of Compounds 1a–1k
To a mixture of Tet-NH2 (100 mg, 0.16 mmol), HOBT (8.47 mg, 0.63 mmol), EDCI (27.3 mg, 0.17 mmol) and Boc-l-amino acid (0.17 mmol, 1.1 eq) was added DCM (2.0 mL) under the protection of argon atmosphere, and stirred at room temperature for 1.5 to 3 h. The reaction mixture was quenched with saturated aqueous solution of sodium bicarbonate, extracted with DCM (3 × 10 mL), dried over anhydrous sodium sulfate and filtered. The solvent was removed under reduced pressure, and the residue was purified by silica gel chromatography eluated with DCM/MeOH (40/1 v/v, 0.5% TEA) to afford compounds 1a–1k.
14-((R)-2-(N-(tert-butoxycarbonyl)amino)-3-phenylpropanamido)tetrandrine (1a). White to light yellow amorphous solid, yield: 85%. Mp: 136–137 °C. 1H-NMR (600 MHz, CDCl3) δ 12.20 (s, 1H), 7.58 (s, 1H), 7.36–7.29 (m, 5H), 7.26 (t, J = 7.2 Hz, 1H), 7.23 (dd, J = 7.8, 2.4 Hz, 1H), 6.63 (dd, J = 8.4, 2.4 Hz, 1H), 6.57 (s, 1H), 6.49 (s, 1H), 6.32 (s, 1H), 6.16 (dd, J = 8.4, 1.8 Hz, 1H), 5.91 (s, 1H), 5.44 (d, J = 12.0 Hz, 1H), 4.47 (m, 1H), 3.94 (d, J = 9.0 Hz, 4H), 3.83 (dd, J = 10.8, 5.4 Hz, 1H), 3.76 (s, 3H), 3.58 (m, 1H), 3.47 (m, 1H), 3.36 (s, 3H), 3.27 (m, 2H), 3.16–3.08 (m, 5H), 3.01–2.88 (m, 4H), 2.79 (t, J = 12.0 Hz, 1H), 2.70 (dd, J = 16.2, 5.4 Hz, 1H), 2.62 (s, 3H), 2.49 (dd, J = 17.4, 4.2 Hz, 1H), 2.40 (d, J = 17.4 Hz, 4H), 1.46 (s, 9H). 13C-NMR (150 MHz, CDCl3) δ 169.2, 155.8, 155.2, 152.2, 149.4, 148.6, 148.1, 145.6, 144.2, 138.2, 136.6, 134.2, 132.9, 131.5, 129.7, 129.6, 128.6, 128.5, 127.8, 127.2, 127.0, 125.8, 121.4, 121.1, 121.1, 120.8, 120.6, 112.3, 106.9, 105.8, 79.6, 77.3, 77.1, 76.8, 64.2, 61.4, 60.1, 56.3, 56.2, 55.8, 55.6, 53.4, 45.1, 43.2, 42.5, 40.7, 40.0, 39.6, 38.9, 29.7, 28.4, 24.8, 20.7. HRMS (ESI) calcd. for C52H61N4O9: 885.4429 [M + H]+, found 885.4433.
14-((R)-2-(N-(tert-butoxycarbonyl)amino)-propanamido)tetrandrine (1b). White to light yellow amorphous solid, yield: 78%. Mp: 149–150 °C. 1H-NMR (600 MHz, CDCl3) δ 12.35 (s, 1H), 7.84 (s, 1H), 7.32 (dd, J = 7.8, 1.8 Hz, 1H), 7.23 (dd, J = 7.8, 2.4 Hz, 1H), 6.61–6.56 (m, 2H), 6.48 (s, 1H), 6.33 (s, 1H), 6.15 (dd, J = 8.4, 1.8 Hz, 1H), 5.91 (s, 1H), 5.57 (d, J = 7.8 Hz, 1H), 4.31 (m, 1H), 4.02 (d, J = 9.0 Hz, 1H), 3.97 (s, 3H), 3.82 (dd, J = 11.4, 5.4 Hz, 1H), 3.76 (s, 3H), 3.68 (m, 1H), 3.46 (m, 1H), 3.37 (s, 3H), 3.25 (dd, J = 12.0, 5.4 Hz, 1H), 3.16 (dd, J = 13.8, 5.4 Hz, 1H), 3.12 (s, 3H), 3.06 (dd, J = 15.0, 9.6 Hz, 1H), 3.02–2.86 (m, 3H), 2.78 (t, J = 11.8 Hz, 1H), 2.69 (dd, J = 16.2, 4.8 Hz, 1H), 2.62 (s, 3H), 2.55–2.49 (m, 4H), 2.45 (d, J = 15.0 Hz, 1H), 1.51 (d, J = 7.2 Hz, 12H). 13C-NMR (150 MHz, CDCl3) δ 170.8, 156.1, 155.2, 152.2, 149.5, 148.6, 148.4, 145.1, 144.2, 138.3, 134.0, 132.9, 132.2, 129.6, 128.6, 127.8, 127.1, 125.2, 121.5, 121.2, 121.1, 121.0, 120.6, 112.3, 106.2, 105.9, 79.5, 64.2, 61.2, 60.1, 56.2, 55.8, 55.5, 53.4, 50.6, 45.1, 43.1, 42.5, 40.7, 39.9, 38.9, 28.4, 24.8, 20.4. HRMS (ESI) calcd. for C46H57N4O9: 809.4117 [M + H]+, found 809.4120.
14-((R)-2-(N-(tert-butoxycarbonyl)amino)-4-methylthio-butylamido)tetrandrine (1c). White to light yellow amorphous solid, yield: 83%. Mp: 133-134 °C. 1H-NMR (600 MHz, CDCl3) δ 12.41 (s, 1H), 7.75 (s, 1H), 7.32 (dd, J = 8.1, 2.0 Hz, 1H), 7.23 (dd, J = 8.1, 2.5 Hz, 1H), 6.59 (d, J = 8.7 Hz, 2H), 6.48 (s, 1H), 6.33 (s, 1H), 6.15 (dd, J = 8.4, 2.0 Hz, 1H), 5.91 (s, 1H), 5.50 (d, J = 8.2 Hz, 1H), 4.38 (m, 1H), 4.03–3.99 (m, 1H), 3.96 (s, 3H), 3.84 (dd, J = 11.1, 5.6 Hz, 1H), 3.76 (s, 3H), 3.67 (m, 1H), 3.52–3.44 (m, 1H), 3.37 (s, 3H), 3.25 (m, 2H), 3.11 (s, 3H), 3.06 (dd, J = 14.8, 9.5 Hz, 1H), 3.03–2.88 (m, 3H), 2.78 (t, J = 11.8 Hz, 1H), 2.73–2.63 (m, 3H), 2.62 (s, 3H), 2.55–2.50 (m, 4H), 2.44 (d, J = 14.8 Hz, 1H), 2.3–2.16 (m, 1H), 2.15 (s, 3H), 2.05–2.00 (m, 1H), 1.47 (s, 9H). 13C-NMR (150 MHz, CDCl3) δ 169.6, 156.0, 155.5, 152.2, 149.4, 148.7, 148.31, 145., 4144.3, 138.3, 134.0, 132.9, 131.9, 131.9, 129.7, 128.5, 127.2, 125.7, 121.5, 121.1, 121.0, 120.7, 112.3, 106.6, 105.9, 79.7, 77.3, 77.1, 76.9, 64.2, 61.1, 60.1, 56.3, 55.8, 55.6, 54.3, 45.0, 43.1, 42.4, 40.8, 39.8, 38.8, 34.1, 30.2, 29.7, 28.4, 24.7, 20.6, 15.8. HRMS (ESI) calcd. for C48H61N4O9S: 869.4152 [M + H]+, found 869.4154.
14-((R)-3-methyl-2-(N-(tert-butoxycarbonyl)amino)-amylamido)tetrandrine (1d). White to light yellow amorphous solid, yield: 81%. Mp: 145–146 °C. 1H-NMR (600 MHz, CDCl3) δ 12.16 (s, 1H), 7.72 (s, 1H), 7.33 (dd, J = 8.4, 2.4 Hz, 1H), 7.24 (dd, J = 7.8, 2.4 Hz, 1H), 6.60 (d, J = 7.2 Hz, 2H), 6.49 (s, 1H), 6.34 (s, 1H), 6.15 (dd, J = 8.4, 1.8 Hz, 1H), 5.91 (s, 1H), 5.29 (d, J = 9.0 Hz, 1H), 4.29 (m, 1H), 4.01 (d, J = 9.6 Hz, 1H), 3.96 (s, 3H), 3.85 (dd, J = 11.4, 5.4 Hz, 1H), 3.77 (s, 3H), 3.70 (td, J = 13.8, 13.2, 4.8 Hz, 1H), 3.53–3.46 (m, 1H), 3.38 (s, 3H), 3.29 (dd, J = 12.0, 5.4 Hz, 1H), 3.22 (dd, J = 14.4, 6.0 Hz, 1H), 3.12 (s, 3H), 3.08 (dd, J = 15.0, 9.6 Hz, 1H), 3.08–2.88 (m, 3H), 2.79 (t, J = 12.0 Hz, 1H), 2.72 (dd, J = 15.6, 5.4 Hz, 1H), 2.63 (s, 3H), 2.53 (s, 4H), 2.43 (d, J = 15.0 Hz, 1H), 1.84 (m, 1H), 1.70 (t, J = 7.2 Hz, 2H), 1.46 (s, 9H), 1.07 (d, J = 6.6 Hz, 3H), 1.02 (d, J = 6.6 Hz, 3H). 13C-NMR (150 MHz, CDCl3) δ 171.1, 156.0, 155.4, 152.2, 149.4, 148.7, 148.3, 145.3, 144.3, 138.3, 133.9, 132.9, 131.9, 129.7, 128.3, 127.3, 125.8, 121.5, 121.3, 121.1, 121.0, 120.6, 112.3, 106.8, 105.9, 79.4, 64.2, 61.4, 60.1, 56.3, 55.8, 55.6, 53.8, 45.0, 43.4, 43.2, 42.3, 40.9, 39.8, 38.9, 29.7, 28.4, 24.8, 24.7, 23.3, 22.7, 20.7. HRMS (ESI) calcd. for C49H63N4O9: 851.4590 [M + H]+, found 851.4590.
14-((R)-3-(indolyl-3)-2-(N-(tert-butoxycarbonyl)amino)-propanamido)tetrandrine (1e). White to light yellow amorphous solid, yield: 84%. Mp: 152–153 °C. 1H-NMR (600 MHz, CDCl3) δ 12.06 (s, 1H), 8.16 (s, 1H), 7.72 (d, J = 7.8 Hz, 1H), 7.50 (s, 1H), 7.35 (d, J = 8.4 Hz, 1H), 7.32 (dd, J = 8.4, 2.1 Hz, 1H), 7.22 (dd, J = 8.4, 2.4 Hz, 1H), 7.21–7.18 (m, 1H), 7.17–7.11 (m, 2H), 6.64–6.61 (m, 1H), 6.55 (s, 1H), 6.48 (s, 1H), 6.30 (s, 1H), 6.16 (dd, J = 8.4, 2.4 Hz, 1H), 5.90 (s, 1H), 5.51 (d, J = 8.4 Hz, 1H), 4.59–4.52 (m, 1H), 3.92–3.81 (m, 5H), 3.75 (s, 3H), 3.46 (m, 3H), 3.33 (d, J = 18.6 Hz, 4H), 3.28 (dd, J = 12.0, 5.4 Hz, 1H), 3.11 (s, 3H), 3.02 (dd, J = 13.8, 6.0 Hz, 1H), 2.97–2.83 (m, 4H), 2.79 (t, J = 11.4 Hz, 1H), 2.71 (dd, J = 15.6, 5.4 Hz, 1H), 2.62 (s, 3H), 2.44–2.34 (m, 2H), 2.14 (s, 3H), 1.48 (s, 9H). 13C-NMR (150 MHz, CDCl3) δ 169.8, 155.8, 155.3, 155.3, 152.1, 149.3, 148.0, 145.5, 144.3, 138.1, 136.2, 132.9, 131.5, 129.7, 127.9, 127.3, 125.9, 125.9, 122.8, 122.1, 121.4, 121.2, 120.8, 120.5, 119.6, 119.1, 112.3, 111.1, 110.9, 110.8, 107.2, 105.8, 79.5, 64.2, 61.3, 60.1, 56.3, 55.8, 55.7, 55.6, 45.0, 43.0, 42.3, 40.3, 39.6, 38.9, 29.7, 29.5, 29.5, 28.4, 24.7, 20.7. HRMS (ESI) calcd. for C54H62N5O9: 924.4537 [M + H]+, found 924.4542.
14-((R)-3-hydroxy-2-(N-(tert-butoxycarbonyl)amino)-butylamido)tetrandrine (1f). White to light yellow amorphous solid, yield: 79%. Mp: 163–165 °C. 1H-NMR (600 MHz, CDCl3) δ 12.46 (s, 1H), 7.81 (s, 1H), 7.32 (dd, J = 8.4, 2.4 Hz, 1H), 7.23 (dd, J = 7.8, 2.4 Hz, 1H), 6.61 (s, 1H), 6.59 (dd, J = 8.4, 2.4 Hz, 1H), 6.49 (s, 1H), 6.33 (s, 1H), 6.15 (dd, J = 8.4, 2.4 Hz, 1H), 5.90 (s, 1H), 5.60 (d, J = 9.0 Hz, 1H), 4.25–4.19 (m, 1H), 4.15–4.11 (m, 1H), 4.01 (d, J = 9.0 Hz, 1H), 3.96 (s, 3H), 3.83 (dd, J = 11.4, 5.4 Hz, 1H), 3.76 (s, 3H), 3.66 (m, 1H), 3.50–3.45 (m, 1H), 3.38 (s, 3H), 3.26 (dd, J = 12.6, 5.4 Hz, 1H), 3.21 (dd, J = 12.6, 6.0 Hz, 1H), 3.11 (s, 4H), 3.03–2.86 (m, 4H), 2.79 (t, J = 12.0 Hz, 1H), 2.71 (dd, J = 16.2, 5.4 Hz, 1H), 2.62 (s, 3H), 2.51 (s, 4H), 2.45 (d, J = 14.8 Hz, 1H), 1.48 (s, 9H), 1.34 (d, J = 6.0 Hz, 3H). 13C-NMR (150 MHz, CDCl3) δ 169.5, 156.2, 156.1, 152.2, 149.4, 148.6, 148.3, 145.5, 144.3, 138.3, 134.0, 132.9, 131.7, 129.7, 128.5, 127.7, 127.3, 125.8, 121.6, 121.2, 121.0, 120.6, 112.3, 106.7, 105.9, 79.9, 77.3, 77.0, 76.8, 69.3, 64.2, 61.3, 60.1, 59.8, 56.3, 55.8, 55.6, 45.1, 43.3, 42.4, 40.8, 40.0, 38.8, 29.7, 28.4, 24.8, 20.8, 19.7. HRMS (ESI) calcd. for C47H59N4O10: 839.4230 [M + H]+, found 839.4226.
14-((R)-3-methyl-2-(N-(tert-butoxycarbonyl)amino)-butylamido)tetrandrine (1g). White to light yellow amorphous solid, yield: 84%. Mp: 143–144 °C. 1H-NMR (600 MHz, CDCl3) δ 12.28 (s, 1H), 7.78 (s, 1H), 7.32 (dd, J = 8.1, 1.9 Hz, 1H), 7.24 (dd, J = 7.8, 2.4 Hz, 1H), 6.60 (d, J = 9.8 Hz, 2H), 6.48 (s, 1H), 6.34 (s, 1H), 6.17–6.13 (m, 1H), 5.91 (s, 1H), 5.42 (d, J = 9.0 Hz, 1H), 4.09 (dd, J = 9.0, 6.0 Hz, 1H), 4.03 (d, J = 9.3 Hz, 1H), 3.96 (s, 3H), 3.82 (dd, J = 11.1, 5.5 Hz, 1H), 3.77 (s, 3H), 3.69 (m, 1H), 3.51–3.44 (m, 1H), 3.38 (s, 3H), 3.26 (dd, J = 12.3, 5.5 Hz, 1H), 3.21 (dd, J = 14.0, 5.9 Hz, 1H), 3.12 (s, 3H), 3.07 (dd, J = 14.8, 9.5 Hz, 1H), 3.03–2.85 (m, 3H), 2.79 (t, J = 11.8 Hz, 1H), 2.72–2.67 (m, 1H), 2.62 (s, 3H), 2.57–2.49 (m, 4H), 2.44 (d, J = 14.8 Hz, 1H), 2.12 (m, 1H), 1.47 (s, 9H), 1.11 (d, J = 6.8 Hz, 3H), 1.03 (d, J = 6.7 Hz, 3H). 13C-NMR (150 MHz, CDCl3) δ 170.1, 156.1, 156.0, 152.2, 149.5, 148.6, 148.3, 145.2, 144.2, 138.3, 134.1, 132.9, 132.0, 129.7, 128.6, 127.2, 125.6, 121.6, 121.2, 121.2, 121.0, 120.6, 112.3, 106.4, 105.9, 79.3, 64.2, 61.3, 60.3, 60.1, 56.3, 55.8, 55.5, 53.4, 45.1, 43.2, 42.5, 40.9, 39.8, 38.9, 32.9, 28.4, 24.8, 20.6, 19.9, 17.8. HRMS (ESI) calcd. for C48H61N4O9: 837.4421 [M + H]+, found 837.4433.
14-((2R,3R)-3-methyl-2-(N-(tert-butoxycarbonyl)amino)-amylamido)tetrandrine (1h). White to light yellow amorphous solid, yield: 79%. Mp: 158–159 °C. 1H-NMR (600 MHz, CDCl3) δ 12.25 (s, 1H), 7.79 (s, 1H), 7.33 (dd, J = 7.8, 1.8 Hz, 1H), 7.24 (dd, J = 7.8, 2.4 Hz, 1H), 6.60 (d, J = 8.4 Hz, 2H), 6.49 (s, 1H), 6.34 (s, 1H), 6.14 (dd, J = 8.4, 1.8 Hz, 1H), 5.91 (s, 1H), 5.38 (d, J = 9.0 Hz, 1H), 4.12–4.07 (m, 1H), 4.03 (d, J = 9.6 Hz, 1H), 3.96 (s, 3H), 3.85 (dd, J = 11.4, 5.4 Hz, 1H), 3.77 (s, 3H), 3.69 (m, 1H), 3.52–3.46 (m, 1H), 3.38 (s, 3H), 3.28 (dd, J = 12.0, 5.4 Hz, 1H), 3.21 (dd, J = 13.8, 6.0 Hz, 1H), 3.12 (s, 3H), 3.08 (dd, J = 15.0, 9.6 Hz, 1H), 3.03–2.89 (m, 3H), 2.78 (t, J = 12.0 Hz, 1H), 2.72 (dd, J = 15.6, 5.4 Hz, 1H), 2.63 (s, 3H), 2.55–2.50 (m, 4H), 2.44 (d, J = 15.0 Hz, 1H), 1.87 (m, 1H), 1.66 (m, 1H), 1.46 (s, 9H), 1.26–1.20 (m, 1H), 1.09 (d, J = 6.6 Hz, 3H), 0.97 (t, J = 7.2 Hz, 3H). 13C-NMR (150 MHz, CDCl3) δ 170.3, 156.1, 155.9, 152.2, 149.4, 148.7, 148.3, 145.2, 144.3, 138.3, 133.9, 132.9, 132.0, 129.7, 128.4, 127.4, 127.3, 125.6, 121.6, 121.3, 121.2, 121.0, 120.6, 112.3, 106.5, 105.9, 79.3, 77.3, 77.1, 76.8, 64.1, 61.3, 60.1, 59.9, 56.3, 55.8, 55.5, 45.0, 43.2, 42.3, 40.9, 39.9, 39.3, 38.9, 29.7, 28.4, 24.5, 20.6, 16.0, 11.6. HRMS (ESI) calcd. for C49H63N4O9 [M + H]+: 851.4583, found 851.4590.
14-((R)-3-(S-(tert-butoxycarbonyl)sulfydryl)-propanamido)tetrandrine (1i). White to light yellow amorphous solid, yield: 82%. Mp: 147–149 °C. 1H-NMR (600 MHz, CDCl3) δ 12.48 (s, 1H), 7.75 (s, 1H), 7.32 (dd, J = 8.4, 2.4 Hz, 1H), 7.23 (dd, J = 7.8, 2.4 Hz, 1H), 6.61–6.57 (m, 2H), 6.49 (s, 1H), 6.33 (s, 1H), 6.16 (dd, J = 8.4, 2.4 Hz, 1H), 5.91 (s, 1H), 5.58 (d, J = 8.4 Hz, 1H), 4.44 (m, 1H), 4.01 (d, J = 9.6 Hz, 1H), 3.96 (s, 3H), 3.82 (dd, J = 10.8, 5.4 Hz, 1H), 3.76 (s, 3H), 3.64 (m, 1H), 3.51–3.44 (m, 1H), 3.38 (s, 4H), 3.27–3.19 (m, 3H), 3.12 (s, 3H), 3.05 (dd, J = 15.0, 9.6 Hz, 1H), 3.02–2.86 (m, 4H), 2.79 (t, J = 12.0 Hz, 1H), 2.70 (dd, J = 16.2, 5.4 Hz, 1H), 2.62 (s, 3H), 2.55 (s, 3H), 2.51 (dd, J = 16.8, 4.8 Hz, 1H), 2.44 (d, J = 14.4 Hz, 1H), 1.50 (s, 9H), 1.47 (s, 9H). 13C-NMR (150 MHz, CDCl3) δ 168.5, 168.1, 156.0, 155.1, 152.18, 149.44, 148.61, 148.24, 145.45, 144.20, 138.25, 134.11, 132.92, 131.73, 129.66, 128.58, 127.77, 127.3, 125.7, 121.5, 121.2, 121.0, 120.9, 120.7, 112.3, 106.9, 105.8, 85.1, 79.8, 64.2, 61.3, 60.1, 56.3, 55.8, 55.6, 54.9, 53.5, 43.1, 42.5, 40.8, 39.9, 38.9, 34.3, 28.4, 28.2, 24.9, 20.7. HRMS (ESI) calcd. for C51H65N4O11S: 941.4366 [M + H]+, found 941.4365.
14-((R)-4-(C-(tert-butoxycarbonyl)carboxyl)-2-(N-(tert-butoxycarbonyl)amino)-butylamido)tetrandrine (1j) White to light yellow amorphous solid, yield: 87%. Mp: 131–132 °C. 1H-NMR (600 MHz, CDCl3) δ 12.19 (s, 1H), 7.98 (s, 1H), 7.31 (dd, J = 7.8, 1.8 Hz, 1H), 7.23 (dd, J = 7.8, 2.4 Hz, 1H), 6.59 (s, 1H), 6.58–6.55 (m, 1H), 6.49 (s, 1H), 6.33 (s, 1H), 6.13 (dd, J = 8.4, 1.8 Hz, 1H), 5.90 (s, 1H), 5.29 (d, J = 8.4 Hz, 1H), 4.25 (m, 1H), 3.99 (d, J = 9.0 Hz, 1H), 3.96 (s, 3H), 3.83 (dd, J = 11.2, 5.4 Hz, 1H), 3.77 (s, 3H), 3.62 (td, J = 12.6, 3.6 Hz, 1H), 3.53–3.47 (m, 1H), 3.38 (s, 3H), 3.29 (dd, J = 12.0, 4.8 Hz, 1H), 3.11 (s, 3H), 3.09–2.88 (m, 5H), 2.78 (t, J = 12.0 Hz, 1H), 2.72 (dd, J = 15.0, 4.8 Hz, 1H), 2.63 (s, 3H), 2.51 (s, 5H), 2.42 (dd, J = 23.6, 12.0 Hz, 2H), 2.32 (m, 1H), 2.07 (m, 1H), 1.51 (s, 9H), 1.45 (s, 9H). 13C-NMR (150 MHz, CDCl3) δ 171.6, 170.4, 156.5, 155.7, 152.2, 149.5, 148.7, 148.4, 144.6, 144.3, 138.3, 133.6, 132.9, 132.9, 129.6, 128.4, 127.1, 124.7, 121.6, 121.4, 120.9, 120.5, 112.3, 106.0, 105.9, 82.1, 79.6, 77.3, 77.1, 76.8, 64.3, 61.1, 60.1, 56.2, 55.8, 55.6, 54.2, 53.4, 45.1, 43.1, 42.4, 40.6, 40.2, 38.9, 34.0, 28.9, 28.4, 28.0, 24.7, 20.7. HRMS (ESI) calcd. for C53H67N4O13: 967.4699 [M + H]+, found 967.4671.
3.2.4. General Procedure for the Preparation of Compounds 1k and 1l
Trifluoroacetic acid (0.1 mL) was slowly added dropwise to a solution of 1k (160 mg, 0.16 mmol) or 1l (130 mg, 0.16 mmol) in DCM (2 mL) at 0 °C. After 10 min, the reaction mixture was warmed up to room temperature, and stirred for 0.5 to 1.5 h before the reaction finished. The reaction mixture was quenched with saturated aqueous solution of sodium bicarbonate, extracted with DCM (3 × 10 mL), dried over anhydrous sodium sulfate and filtered. The solvent was removed under reduced pressure. The residue was purified by silica gel chromatography from DCM/MeOH (30/1 v/v, 0.5 % TEA) to afford the pure compounds 1k and 1l.
14-((R)-2-amino-3-phenyl-propanamido)tetrandrine (1k). White amorphous solid, yield: 76%. Mp: 140–141 °C. 1H-NMR (600 MHz, CDCl3) δ 11.83 (s, 1H), 7.74 (s, 1H), 7.37–7.29 (m, 5H), 7.28–7.25 (m, 1H), 7.23 (dd, J = 8.4, 2.4 Hz, 1H), 6.63 (dd, J = 8.4, 2.4 Hz, 1H), 6.58 (s, 1H), 6.49 (s, 1H), 6.32 (s, 1H), 6.16 (dd, J = 8.4, 2.4 Hz, 1H), 5.92 (s, 1H), 4.24 (m, 1H), 4.14 (m, 1H), 3.98 (s, 3H), 3.93 (d, J = 9.6 Hz, 1H), 3.84 (dd, J = 11.2, 5.4 Hz, 1H), 3.76 (s, 3H), 3.65 (dd, J = 7.8, 6.0 Hz, 1H), 3.56–3.42 (m, 2H), 3.37 (s, 3H), 3.27 (dd, J = 12.6, 6.0 Hz, 1H), 3.21 (dd, J = 13.8, 6.0 Hz, 1H), 3.12 (s, 3H), 3.01–2.87 (m, 6H), 2.79 (t, J = 12.0 Hz, 1H), 2.71 (dd, J = 16.2, 5.4 Hz, 1H), 2.63 (s, 3H), 2.51–2.45 (m, 1H), 2.41 (d, J = 14.4 Hz, 1H), 2.37 (s, 3H). 13C-NMR (150 MHz, CDCl3) δ 173.4, 155.7, 152.1, 149.4, 148.6, 148.0, 145.4, 144.2, 138.3, 137.9, 134.1, 132.9, 131.73, 129.7, 129.4, 128.7, 128.5, 127.7, 127.2, 126.8, 125.8, 121.5, 121.4, 121.2, 120.8, 120.4, 112.4, 107.1, 105.8, 64.2, 61.44, 60.2, 58.2, 56.3, 55.8, 55.6, 45.1, 43.9, 42.5, 42.3, 41.0, 39.9, 38.8, 24.9, 21.0. HRMS (ESI) calcd. for C47H53N4O7: 785.3904 [M + H]+, found 785.3909.
14-((R)-2-amino-propanamido)tetrandrine. (1l). White to light yellow amorphous solid, yield: 83%. Mp: 137–139 °C. 1H-NMR (600 MHz, CDCl3) δ 11.96 (s, 1H), 7.88 (s, 1H), 7.32 (dd, J = 8.2, 2.2 Hz, 1H), 7.23 (dd, J = 7.8, 2.4 Hz, 1H), 6.60 (d, J = 6.0 Hz, 2H), 6.49 (s, 1H), 6.33 (s, 1H), 6.15 (dd, J = 8.4, 2.4 Hz, 1H), 5.91 (s, 1H), 4.02 (s, 1H), 3.97 (s, 3H), 3.83 (dd, J = 10.8, 5.4 Hz, 1H), 3.76 (s, 3H), 3.63 (m, 1H), 3.53 (m, 1H), 3.49–3.43 (m, 1H), 3.38 (s, 3H), 3.26 (dd, J = 12.6, 5.4 Hz, 1H), 3.11 (s, 3H), 3.06–2.87 (m, 7H), 2.78 (t, J = 11.8 Hz, 1H), 2.73–2.68 (m, 1H), 2.62 (s, 3H), 2.51 (s, 4H), 2.45 (d, J = 14.8 Hz, 1H), 1.46 (d, J = 7.2 Hz, 3H). 13C-NMR (150 MHz, CDCl3) δ 174.9, 156.0, 152.2, 149.5, 148.6, 148.3, 145.0, 144.2, 138.4, 133.9, 132.9, 132.3, 129.7, 128.5, 127.7, 127.1, 125.2, 121.6, 121.4, 121.0, 120.6, 112.3, 106.5, 105.9, 64.2, 61.2, 60.1, 56.2, 55.8, 55.6, 52.0, 45.0, 43.6, 42.4, 40.9, 40.0, 38.8, 29.7, 24.8, 22.0, 20.8. HRMS (ESI) calcd. for C41H49N4O7: 704.3589 [M + H]+, found 704.3596.
3.2.5. General Procedure for the Preparation of Compounds 2a–3k
Compound 1l (100 mg, 0.14 mmol) was dissolved in DCM (2.0 mL), triethylamine (98 %, 4.0 µL, 0.03 mmol) and isocyanate (98%, 0.16 mmol) were added into the solution in turn, and the reaction mixture was stirred for 0.5 to 1.5 h. Upon completion, the solvent was removed under reduced pressure. The residue was purified by silica gel chromatography from DCM/MeOH (50/1 v/v, 0.5 % TEA) to afford the pure compounds 2a, 2b and 2c. The compounds 3a-3k were obtained using the same method.
14-((R)-2-(3-propylureido)-3-phenylpropanamido)tetrandrine (2a). White amorphous solid, yield: 91%. Mp: 156–157 °C. 1H-NMR (600 MHz, CDCl3) δ 12.39 (s, 1H), 7.52 (s, 1H), 7.33 (d, J = 4.8 Hz, 5H), 7.28–7.19 (m, 2H), 6.63 (dd, J = 8.4, 2.4 Hz, 1H), 6.56 (s, 1H), 6.49 (s, 1H), 6.32 (s, 1H), 6.17 (dd, J = 8.4, 1.8 Hz, 1H), 5.91 (s, 1H), 5.83 (d, J = 7.4 Hz, 1H), 4.92 (s, 1H), 4.71 (m, 1H), 3.94 (s, 3H), 3.92 (d, J = 9.6 Hz, 1H), 3.84 (dd, J = 11.4, 5.4 Hz, 1H), 3.76 (s, 3H), 3.67–3.62 (m, 1H), 3.48 (dd, J = 13.8, 8.4 Hz, 1H), 3.37 (s, 3H), 3.26 (dd, J = 13.2, 7.2 Hz, 2H), 3.20 (dd, J = 13.8, 6.0 Hz, 1H), 3.11 (s, 3H), 3.11–2.87 (m, 7H), 2.80 (t, J = 12.0 Hz, 1H), 2.71 (dd, J = 15.6, 5.4 Hz, 1H), 2.63 (s, 3H), 2.51 (dd, J = 17.4, 4.8 Hz, 1H), 2.42–2.35 (m, 4H), 1.35 (m, 2H), 0.83 (t, J = 7.2 Hz, 3H). 13C-NMR (150 MHz, CDCl3) δ 170.5, 157.5, 155.8, 152.1, 149.3 148.64=, 148.1, 145.7, 144.2, 138.1, 133.0, 131.4, 129.7, 129.7, 128.4, 127.4, 126.8, 126.3, 121.4, 121.2, 120.9, 120.8, 120.7, 112.3, 106.9, 105.9, 64.2, 61.4, 60.1, 56.3, 55.7, 55.7, 55.6, 45.1, 43.1, 42.4, 42.1, 40.7, 40.7, 39.5, 39.0, 24.7, 23.4, 20.6, 11.3. HRMS (ESI) calcd. for C51H60N5O8: 870.4429 [M + H]+, found 870.4436.
14-((R)-2-(3-propylureido)propanamido)tetrandrine (3a). White amorphous solid, yield: 94%. Mp: 151–152 °C. 1H-NMR (600 MHz, CDCl3) δ 12.62 (s, 1H), 7.76 (s, 1H), 7.32 (dd, J = 8.4, 2.4 Hz, 1H), 7.23 (dd, J = 7.8, 2.4 Hz, 1H), 6.60 (s, 1H), 6.58 (dd, J = 8.4, 2.4 Hz, 1H), 6.48 (s, 1H), 6.33 (s, 1H), 6.15 (dd, J = 8.4, 2.4 Hz, 1H), 6.06–5.99 (m, 1H), 5.90 (s, 1H), 5.15–5.06 (m, 1H), 4.55 (m, 1H), 4.01 (d, J = 9.6 Hz, 1H), 3.96 (s, 3H), 3.81 (dd, J = 11.4, 5.4 Hz, 1H), 3.76 (s, 3H), 3.68 (m, 1H), 3.45 (m, 1H), 3.37 (s, 3H), 3.23 (m, 2H), 3.11 (s, 3H), 3.05 (dd, J = 15.0, 9.6 Hz, 2H), 3.01–2.86 (m, 4H), 2.78 (t, J = 12.0 Hz, 1H), 2.72–2.65 (m, 3H), 2.61 (s, 3H), 2.53 (s, 3H), 2.53–2.49 (m, 1H), 2.44 (d, J = 15.0 Hz, 1H), 1.55 (d, J = 7.2 Hz, 3H), 1.13 (t, J = 7.2 Hz, 3H). 13C-NMR (150 MHz, CDCl3) δ 172.3, 157.8, 157.7, 156.0, 152.2, 149.4, 148.6, 148.4, 145.4, 144.2, 138.2, 134.2, 133.0, 132.0, 129.7, 128.6, 127.9, 127.3, 125.8, 125.8, 121.5, 121.3, 121.0, 121.0, 120.6, 112.3, 106.3, 105.9, 77.3, 77.1, 76.8, 64.2, 61.2, 60.1, 56.3, 55.7, 55.6, 50.2, 46.1, 45.1, 43.0, 42.5, 42.1, 40.6, 39.8, 38.8, 24.9, 23.4, 22.7, 21.1, 20.5. HRMS (ESI) calcd. for C45H56N5O8: 794.4123 [M + H]+, found 794.4117.
14-((R)-2-(3-butylureido)propanamido)tetrandrine (3b). Light yellow amorphous solid, yield: 92%. Mp: 160–161 °C. 1H-NMR (600 MHz, CDCl3) δ 12.60 (s, 1H), 7.78 (s, 1H), 7.32 (dd, J = 8.4, 2.4 Hz, 1H), 7.23 (dd, J = 7.8, 2.4 Hz, 1H), 6.61 (s, 1H), 6.59 (dd, J = 8.4, 2.4 Hz, 1H), 6.49 (s, 1H), 6.33 (s, 1H), 6.15 (dd, J = 8.4, 2.4 Hz, 1H), 5.91 (s, 1H), 5.89 (d, J = 7.2 Hz, 1H), 4.89 (s, 1H), 4.55 (m, 1H), 4.02 (d, J = 9.0 Hz, 1H), 3.96 (s, 3H), 3.82 (dd, J = 11.4, 5.4 Hz, 1H), 3.76 (s, 3H), 3.71–3.66 (m, 1H), 3.47 (m, 1H), 3.38 (s, 3H), 3.27–3.20 (m, 2H), 3.12 (s, 4H), 3.09–2.86 (m, 5H), 2.79 (t, J = 12.0 Hz, 1H), 2.72–2.67 (m, 1H), 2.62 (s, 3H), 2.54 (s, 4H), 2.45 (d, J = 15.0 Hz, 1H), 1.55 (d, J = 7.2 Hz, 3H), 1.34–1.26 (m, 4H), 0.87 (t, J = 7.2 Hz, 3H). 13C-NMR (150 MHz, CDCl3) δ 172.1, 157.6, 156.1, 152.2, 149.4, 148.6, 148.4, 145.3, 144.2, 138.2, 134.2, 133.0, 132.03, 129.7, 128.6, 127.8, 127.3, 125.7, 121.5, 121.4, 121.0, 121.0, 120.6, 112.3, 106.3, 105.9, 64.2, 61.2, 60.1, 56.3, 55.7, 55.6, 50.2, 45.1, 43.1, 42.5, 40.7, 40.2, 39.8, 38.9, 32.3, 24.8, 21.1, 20.6, 20.1, 13.8. HRMS (ESI) calcd. for C46H58N5O8: 808.4280 [M + H]+, found 808.4272.
14-((R)-2-(3-tert-butylureido)propanamido)tetrandrine (3c). White to light yellow amorphous solid, yield: 90%. Mp: 215–216 °C. 1H-NMR (600 MHz, CDCl3) δ 12.40 (s, 1H), 7.82 (s, 1H), 7.33 (dd, J = 8.4, 2.4 Hz, 1H), 7.24 (dd, J = 7.8, 2.4 Hz, 1H), 6.60 (s, 1H), 6.60–6.58 (m, 1H), 6.49 (s, 1H), 6.34 (s, 1H), 6.15 (dd, J = 8.4, 2.4 Hz, 1H), 5.91 (s, 1H), 5.47 (d, J = 7.4 Hz, 1H), 4.51 (m, 1H), 4.45 (s, 1H), 4.01 (d, J = 9.0 Hz, 1H), 3.97 (s, 3H), 3.83 (dd, J = 10.8, 5.4 Hz, 1H), 3.77 (s, 3H), 3.69 (m, 1H), 3.51–3.45 (m, 1H), 3.37 (s, 3H), 3.30–3.25 (m, 1H), 3.21 (dd, J = 13.8, 6.0 Hz, 1H), 3.12 (s, 3H), 3.06 (dd, J = 15.0, 9.6 Hz, 1H), 2.95 (m, 3H), 2.79 (t, J = 12.0 Hz, 1H), 2.71 (dd, J = 15.6, 6.0 Hz, 1H), 2.63 (s, 3H), 2.53 (s, 4H), 2.45 (d, J = 15.0Hz, 1H), 1.52 (d, J = 7.2 Hz, 3H), 1.33 (s, 9H). 13C-NMR (150 MHz, CDCl3) δ 171.9, 156.7, 156.1, 152.2, 149.4, 148.6, 148.3, 145.2, 144.2, 138.2, 134.1, 132.9, 132.2, 129.7, 128.6, 127.8, 127.3, 125.5, 121.5, 121.2, 121.1, 121.0, 120.6, 112.3, 106.4, 105.9, 64.2, 61.2, 60.1, 56.2, 55.7, 55.5, 50.3, 49.9, 45.1, 43.1, 42.5, 40.7, 39.8, 38.9, 29.5, 24.8, 21.0, 20.5. HRMS (ESI) calcd. for C46H58N5O8: 808.4280 [M + H]+, found 808.4272.
14-((R)-2-(3-(p-tolyl)ureido)propanamido)tetrandrine (3d). White amorphous solid, yield: 90%. Mp: 165–167 °C. 1H-NMR (600 MHz, CDCl3) δ 12.71 (s, 1H), 7.73 (s, 1H), 7.33 (dd, J = 8.4 2.4 Hz, 1H), 7.27 (s, 1H), 7.23 (dd, J = 7.8, 2.4 Hz, 1H), 7.10–7.00 (m, 4H), 6.61 (s, 1H), 6.60–6.53 (m, 2H), 6.49 (s, 1H), 6.33 (s, 1H), 6.15 (dd, J = 8.4, 2.4 Hz, 1H), 5.92 (s, 1H), 4.66 (m, 1H), 4.03 (d, J = 9.6 Hz, 1H), 3.84 (s, 4H), 3.77 (s, 3H), 3.71 (m, 1H), 3.48 (m, 1H), 3.38 (s, 3H), 3.25 (m, 2H), 3.12 (s, 4H), 3.02–2.87 (m, 3H), 2.79 (t, J = 11.4 Hz, 1H), 2.73–2.68 (m, 1H), 2.62 (s, 3H), 2.55 (s, 3H), 2.52 (dd, J = 16.8, 4.8 Hz, 1H), 2.46 (d, J = 15.0 Hz, 1H), 2.30 (s, 3H), 1.61 (d, J = 7.2 Hz, 3H). 13C-NMR (150 MHz, CDCl3) δ 172.0, 156.0, 155.4, 152.2, 149.4, 148.6, 148.5, 145.6, 144.2, 138.2, 136.2, 134.2, 133.0, 131.8, 129.7, 129.6, 128.7, 127.9, 127.3, 125.9, 121.5, 121.3, 121.0, 120.6, 120.5, 112.3, 106.5, 105.9, 64.2, 61.3, 60.1, 56.2, 55.7, 55.6, 50.2, 46.1, 45.1, 43.1, 42.5, 40.7, 39.8, 38.9, 24.9, 21.0, 20.8, 20.5. HRMS (ESI) calcd. for C49H56N5O8: 842.4116 [M + H]+, found 842.4123.
14-((R)-2-(3-(4-methoxyphenyl)ureido)propanamido)tetrandrine (3e). White amorphous solid, yield: 90%. Mp: 183–184 °C. 1H-NMR (600 MHz, CDCl3) δ 12.69 (s, 1H), 7.71 (s, 1H), 7.32 (dd, J = 8.4, 2.4 Hz, 1H), 7.23 (dd, J = 7.8, 2.4 Hz, 1H), 7.10 (t, J = 6.0 Hz, 3H), 6.82–6.78 (m, 2H), 6.60 (s, 1H), 6.57 (dd, J = 8.4, 2.4 Hz, 1H), 6.49 (s, 1H), 6.41 (d, J = 7.8 Hz, 1H), 6.33 (s, 1H), 6.15 (dd, J = 8.4, 2.0 Hz, 1H), 5.91 (s, 1H), 4.64 (m, 1H), 4.02 (d, J = 9.6 Hz, 1H), 3.85 (s, 3H), 3.84–3.81 (m, 1H), 3.80 (s, 3H), 3.76 (s, 3H), 3.73–3.67 (m, 1H), 3.46 (m, 1H), 3.38 (s, 3H), 3.24 (m, 2H), 3.12 (s, 3H), 3.08 (dd, J = 15.0, 9.6 Hz, 1H), 3.01–2.86 (m, 3H), 2.79 (t, J = 11.4 Hz, 1H), 2.72–2.66 (m, 1H), 2.62 (s, 3H), 2.54 (s, 3H), 2.53–2.48 (m, 1H), 2.45 (d, J = 15.0 Hz, 1H), 1.60 (d, J = 6.6 Hz, 3H). 13C-NMR (150 MHz, CDCl3) δ 171.9, 156.0, 155.8, 152.3, 149.4, 148.6, 148.5, 145.5, 144.2, 138.2, 134.2, 133.0, 131.8, 131.4, 129.7, 128.7, 127.9, 127.2, 125.8, 123.3, 121.5, 121.3, 121.0, 120.6, 114.5, 114.4, 112.3, 106.5, 105.9, 64.2, 61.3, 60.1, 56.2, 55.74, 55.6, 55.5, 50.2, 45.1, 43.1, 42.5, 40.7, 39.8, 38.9, 24.9, 21.0, 20.5. HRMS (ESI) calcd. for C49H56N5O9: 858.4067 [M + H]+, found 858.4073.
14-((R)-2-(3-(4-(trifluoromethyl)phenyl)ureido)propanamido)tetrandrine (3f). Light yellow amorphous solid, yield: 95%. Mp: 156–157 °C. 1H-NMR (600 MHz, CDCl3) δ 12.93 (s, 1H), 8.09 (s, 1H), 7.62 (s, 1H), 7.36 (dd, J = 8.4, 2.4 Hz, 1H), 7.30 (s, 1H), 7.26–7.22 (m, 2H), 6.98 (d, J = 8.4 Hz, 2H), 6.66 (s, 1H), 6.59 (dd, J = 8.4, 2.4 Hz, 1H), 6.51 (s, 1H), 6.34 (s, 1H), 6.16 (dd, J = 8.4, 2.0 Hz, 1H), 5.95 (s, 1H), 4.74 (m, 1H), 4.05 (d, J = 9.6 Hz, 1H), 3.85 (dd, J = 10.8, 5.4 Hz, 1H), 3.79 (s, 3H), 3.77 (s, 3H), 3.75–3.70 (m, 1H), 3.48 (m, 1H), 3.40 (s, 3H), 3.27 (m, 2H), 3.14 (s, 4H), 3.06–2.87 (m, 4H), 2.80 (t, J = 11.4 Hz, 1H), 2.71 (dd, J = 16.6, 5.4 Hz, 1H), 2.63 (s, 3H), 2.60–2.57 (m, 3H), 2.56 (d, J = 18.0 Hz, 1H), 2.52 (d, J = 15.0 Hz, 1H), 1.70 (d, J = 7.2 Hz, 3H). 13C-NMR (150 MHz, CDCl3) δ 172.7, 155.6, 154.8, 152.3, 149.3, 148.6, 148.5, 146.5, 144.2, 142.6, 138.2, 134.5, 133.0, 131.1, 129.9, 128.8, 127.9, 127.2, 126.8, 125.8, 125.8, 121.4, 121.1, 121.0, 120.7, 120.6, 117.8, 112.3, 107.4, 105.9, 64.2, 61.6, 60.2, 56.3, 55.8, 55.6, 50.0, 46.1, 45.1, 43.3, 42.5, 40.7, 39.6, 38.9, 24.9, 21.1, 20.6. HRMS (ESI) calcd. for C49H53F3N5O8: 896.3833 [M + H]+, found 896.3841.
14-((R)-2-(3-(4-(trifluoromethoxy)phenyl)ureido)propanamido)tetrandrine (3g). Light yellow amorphous solid, yield: 91%. Mp: 173–174 °C. 1H-NMR (600 MHz, CDCl3) δ 12.90 (s, 1H), 7.86 (s, 1H), 7.65 (s, 1H), 7.35 (dd, J = 8.2, 2.2 Hz, 1H), 7.23 (dd, J = 8.1, 2.6 Hz, 1H), 7.04–6.94 (m, 5H), 6.63 (s, 1H), 6.58 (dd, J = 8.4, 2.6 Hz, 1H), 6.50 (s, 1H), 6.33 (s, 1H), 6.15 (dd, J = 8.4, 2.2 Hz, 1H), 5.93 (s, 1H), 4.71 (m, 1H), 4.04 (d, J = 9.4 Hz, 1H), 3.85 (dd, J = 11.2, 5.6 Hz, 1H), 3.80 (s, 3H), 3.77 (s, 3H), 3.72 (m, 1H), 3.48 (m, 1H), 3.39 (s, 3H), 3.29–3.21 (m, 2H), 3.13 (s, 4H), 3.03–2.88 (m, 3H), 2.80 (t, J = 11.8 Hz, 1H), 2.71 (dd, J = 16.1, 5.4 Hz, 1H), 2.63 (s, 3H), 2.57 (s, 3H), 2.54 (dd, J = 17.3, 4.7 Hz, 1H), 2.50 (s, 1H), 1.67 (d, J = 7.0 Hz, 3H). 13C-NMR (150 MHz, CDCl3) δ 172.6, 155.8, 155.1, 152.3, 149.3, 148.6, 148.5, 146.2, 144.2, 143.8, 138.2, 138.0, 134.3, 133.0, 131.3, 129.8, 128.7, 127.8, 127.2, 126.6, 121.5, 121.4, 121.2, 121.0, 120.7, 120.6, 119.9, 119.7, 112.3, 107.2, 105.9, 64.1, 61.5, 60.1, 56.3, 55.7, 55.6, 50.0, 45.8, 45.0, 43.2, 42.4, 40.7, 39.6, 38.8, 29.7, 24.8, 21.1, 20.6. HRMS (ESI) calcd. for C49H53F3N5O9: 912.3783 [M + H]+, found 912.3790.
14-((R)-2-(3-(4-fluorophenyl)ureido)propanamido)tetrandrine (3h). Light yellow amorphous solid, yield: 91%. Mp: 139–140 °C. 1H-NMR (600 MHz, CDCl3) δ 12.79 (s, 1H), 7.67 (s, 1H), 7.38 (s, 1H), 7.36–7.33 (m, 1H), 7.25 (dd, J = 8.4, 3.0 Hz, 1H), 7.03 (dd, J = 9.0, 4.8 Hz, 2H), 6.87 (t, J = 8.4 Hz, 2H), 6.68 (d, J = 7.2 Hz, 1H), 6.61 (s, 1H), 6.58 (dd, J = 8.4, 2.4 Hz, 1H), 6.50 (s, 1H), 6.33 (s, 1H), 6.15 (dd, J = 8.4, 2.4 Hz, 1H), 5.93 (s, 1H), 4.67 (m, 1H), 4.03 (d, J = 9.6 Hz, 1H), 3.89–3.85 (m, 1H), 3.84 (s, 3H), 3.77 (s, 3H), 3.69 (d, J = 15.0 Hz, 1H), 3.53–3.47 (m, 1H), 3.39 (s, 3H), 3.29 (dd, J = 7.8, 1.8 Hz, 1H), 3.24 (dd, J = 14.4, 6.0 Hz, 1H), 3.13 (s, 3H), 3.09 (dd, J = 15.0, 9.6 Hz, 1H), 3.03–2.92 (m, 3H), 2.79 (t, J = 11.4 Hz, 1H), 2.73 (d, J = 16.8 Hz, 1H), 2.64 (s, 3H), 2.56 (s, 3H), 2.53 (dd, J = 18.0, 4.2 Hz, 1H), 2.47 (d, J = 14.4 Hz, 1H), 1.64 (d, J = 7.2 Hz, 3H). 13C-NMR (150 MHz, CDCl3) δ 179.7, 170.6, 160.5, 156.1, 152.3, 149.4, 148.6, 148.4, 145.5, 144.2, 138.3, 134.2, 132.9, 131.6, 129.7, 128.7, 127.8, 127.4, 127.3, 127.2, 125.7, 121.5, 121.4, 121.0, 1209, 120.6, 117.0, 116.9, 112.3, 106.1, 105.9, 64.2, 61.2, 60.1, 56.3, 55.8, 55.6, 55.1, 46.0, 45.0, 43.3, 42.4, 40.7, 39.9, 38.9, 24.8, 20.6, 20.1. HRMS (ESI) calcd. for C48H53FN5O8: 846.3873 [M + H]+, found 846.3877.
14-((R)-2-(3-(4-chlorophenyl)ureido)propanamido)tetrandrine (3i). Light yellow amorphous solid, yield: 91%. Mp: 186–187 °C. 1H-NMR (600 MHz, CDCl3) δ 12.83 (s, 1H), 7.79 (s, 1H), 7.63 (d, J = 1.9 Hz, 1H), 7.36 (dd, J = 7.8, 2.4 Hz, 1H), 7.28–7.26 (m, 1H), 7.06 (dd, J = 9.0, 1.8 Hz, 2H), 7.00 (d, J = 7.8 Hz, 1H), 6.92 (dd, J = 9.0, 2.4 Hz, 2H), 6.63 (d, J = 1.8 Hz, 1H), 6.59 (dd, J = 8.4, 2.4 Hz, 1H), 6.50 (s, 1H), 6.34 (s, 1H), 6.16 (dd, J = 8.4, 2.4 Hz, 1H), 5.94 (d, J = 1.8 Hz, 1H), 4.70 (m, 1H), 4.03 (d, J = 9.6 Hz, 1H), 3.85–3.81 (m, 4H), 3.77 (d, J = 1.8 Hz, 3H), 3.70 (d, J = 16.8 Hz, 1H), 3.50 – 3.45 (m, 1H), 3.39 (s, 3H), 3.25 (m, 2H), 3.16–3.13 (m, 3H), 3.10 (dd, J = 13.8, 9.0 Hz, 1H), 2.96 (m, 3H), 2.80 (t, J = 12.0 Hz, 1H), 2.70 (dd, J = 16.2, 4.8 Hz, 1H), 2.62 (s, 3H), 2.57 (s, 3H), 2.55–2.52 (m, 1H), 2.49 (d, J = 15.0 Hz, 1H), 1.66 (d, J = 6.6 Hz, 3H). 13C-NMR (150 MHz, CDCl3) δ 172.7, 155.7, 155.1, 152.3, 149.3, 148.7, 148.5, 146.2, 144.2, 138.2, 137.97, 134.3, 133.0, 131.2, 129.8, 128.6, 128.6, 127.7, 127.3, 126.9, 126.6, 121.4, 121.2, 121.0, 120.7, 120.6, 120.0, 112.3, 107.2, 105.9, 64.1, 61.5, 60.1, 56.3, 55.7, 55.6, 50.0, 45.7, 45.0, 43.2, 42.4, 40.7, 39.6, 38.8, 24.8, 21.1, 20.6. HRMS (ESI) calcd. for C48H53Cl3N5O8: 852.3568 [M + H]+, found 852.3577.
14-((R)-2-(3-(4-nitrophenyl)ureido)propanamido)tetrandrine (3j). Yellow amorphous solid, yield: 94%. Mp: 175–176 °C. 1H-NMR (600 MHz, CDCl3) δ 13.03 (s, 1H), 8.43 (s, 1H), 7.93 (d, J = 9.0 Hz, 2H), 7.52 (s, 1H), 7.48 (d, J = 7.8 Hz, 1H), 7.40 (d, J = 7.4 Hz, 1H), 7.32 (dd, J = 8.4, 2.4 Hz, 1H), 6.89 (d, J = 8.4 Hz, 2H), 6.71 (s, 1H), 6.60 (dd, J = 8.4, 2.4 Hz, 1H), 6.52 (s, 1H), 6.35 (s, 1H), 6.19–6.15 (m, 1H), 5.98 (s, 1H), 4.77 (p, J = 7.2, 6.6 Hz, 1H), 4.05 (d, J = 9.6 Hz, 1H), 3.88 (dd, J = 10.4, 5.4 Hz, 1H), 3.79 (s, 3H), 3.77 (s, 3H), 3.73 (dd, J = 12.6, 3.6 Hz, 1H), 3.50 (m, 1H), 3.41 (s, 3H), 3.28 (m, 2H), 3.18–3.11 (m, 4H), 3.08–3.01 (m, 1H), 2.95 (m, 2H), 2.81 (t, J = 11.4 Hz, 1H), 2.73 (dd, J = 16.2, 5.4 Hz, 1H), 2.65 (s, 3H), 2.60 (s, 3H), 2.56 (d, J = 14.4 Hz, 2H), 1.72 (d, J = 7.2 Hz, 3H). 13C-NMR (150 MHz, CDCl3) δ 172.8, 155.2, 154.2, 152.4, 149.3, 148.6, 148.5, 147.0, 145.8, 144.1, 141.7, 138.2, 134.8, 133.1, 130.6, 130.1, 128.8, 127.9, 127.3, 127.1, 124.9, 121.4, 121.3, 120.7, 120.7, 120.2, 117.1, 112.3, 107.7, 105.8, 77.3, 77.1, 76.8, 64.1, 61.7, 60.3, 56.3, 55.8, 55.6, 49.9, 46.0, 45.1, 43.3, 42.5, 40.8, 39.4, 38.8, 24.9, 21.2, 20.6. HRMS (ESI) calcd. for C48H53N6O10: 873.3811[M + H]+, found 873.3818.
14-((R)-2-(3-(1-naphthalenyl)ureido)propanamido)tetrandrine (3k). Light yellow amorphous solid, yield: 90%. Mp: 164–166 °C. 1H-NMR (600 MHz, CDCl3) δ 12.63 (s, 1H), 8.09 (d, J = 8.4 Hz, 1H), 7.89–7.84 (m, 2H), 7.76–7.71 (m, 2H), 7.59 (s, 1H), 7.50 (t, J = 7.8 Hz, 1H), 7.48–7.45 (m, 1H), 7.40 (t, J = 7.8 Hz, 1H), 7.31 (dd, J = 8.4, 2.4 Hz, 1H), 7.20 (dd, J = 8.4, 2.4 Hz, 1H), 6.81–6.69 (m, 1H), 6.51 (dd, J = 8.4, 2.4 Hz, 1H), 6.47 (d, J = 5.4 Hz, 2H), 6.28 (s, 1H), 6.11 (dd, J = 8.4, 2.4 Hz, 1H), 5.88 (s, 1H), 4.67 (m, 1H), 3.95 (d, J = 9.6 Hz, 1H), 3.81 (dd, J = 11.4, 5.4 Hz, 1H), 3.76 (s, 3H), 3.64 (s, 3H), 3.60 (m, 1H), 3.46 (m, 1H), 3.36 (s, 3H), 3.24 (dd, J = 12.5, 5.4 Hz, 1H), 3.10 (s, 3H), 3.07 (dd, J = 14.4, 6.0 Hz, 1H), 2.99 (dd, J = 14.4, 9.6 Hz, 1H), 2.95–2.85 (m, 2H), 2.81–2.74 (m, 2H), 2.68 (dd, J = 15.6, 5.4 Hz, 1H), 2.61 (s, 3H), 2.41 (s, 3H), 2.38–2.32 (m, 2H), 1.61 (d, J = 7.2 Hz, 3H). 13C-NMR (150 MHz, CDCl3) δ 171.7, 156.4, 156.4, 156.1, 152.2, 149.4, 148.6, 148.2, 145.2, 144.2, 138.2, 134.4, 134.0, 133.7, 132.9, 131.8, 129.6, 128.6, 128.3, 127.,8 127.2, 126.1, 126.1, 125.9, 125.6, 125.4, 122.0, 121.6, 121.2, 121.0, 120.9, 120.6, 112.3, 106.1, 105.9, 64.2, 61.2, 60.0, 55.9, 55.7, 55.5, 50.4, 46.0, 45.1, 43.0, 42.5, 40.5, 39.8, 38.9, 24.8, 21.0, 20.4. HRMS (ESI) calcd. for C52H56N5O8: 878.4115 [M + H]+, found 878.4123.