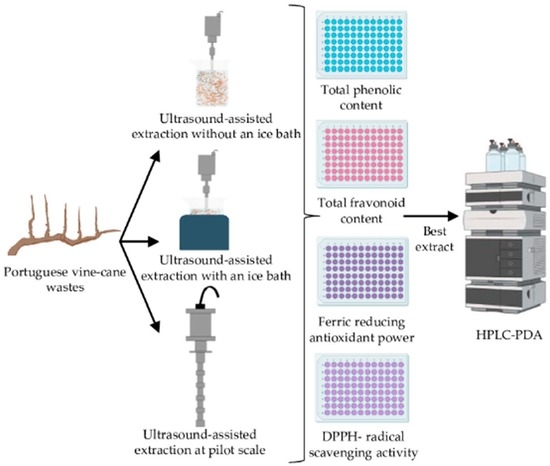
Vine-Canes Valorisation: Ultrasound-Assisted Extraction from Lab to Pilot Scale
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Phenolic and Flavonoid Content and Antioxidant Activity
2.1.1. Total Phenolic and Flavonoid Content
2.1.2. DPPH-Radical Scavenging Activity Assay
2.1.3. Ferric Reducing Antioxidant Power assay
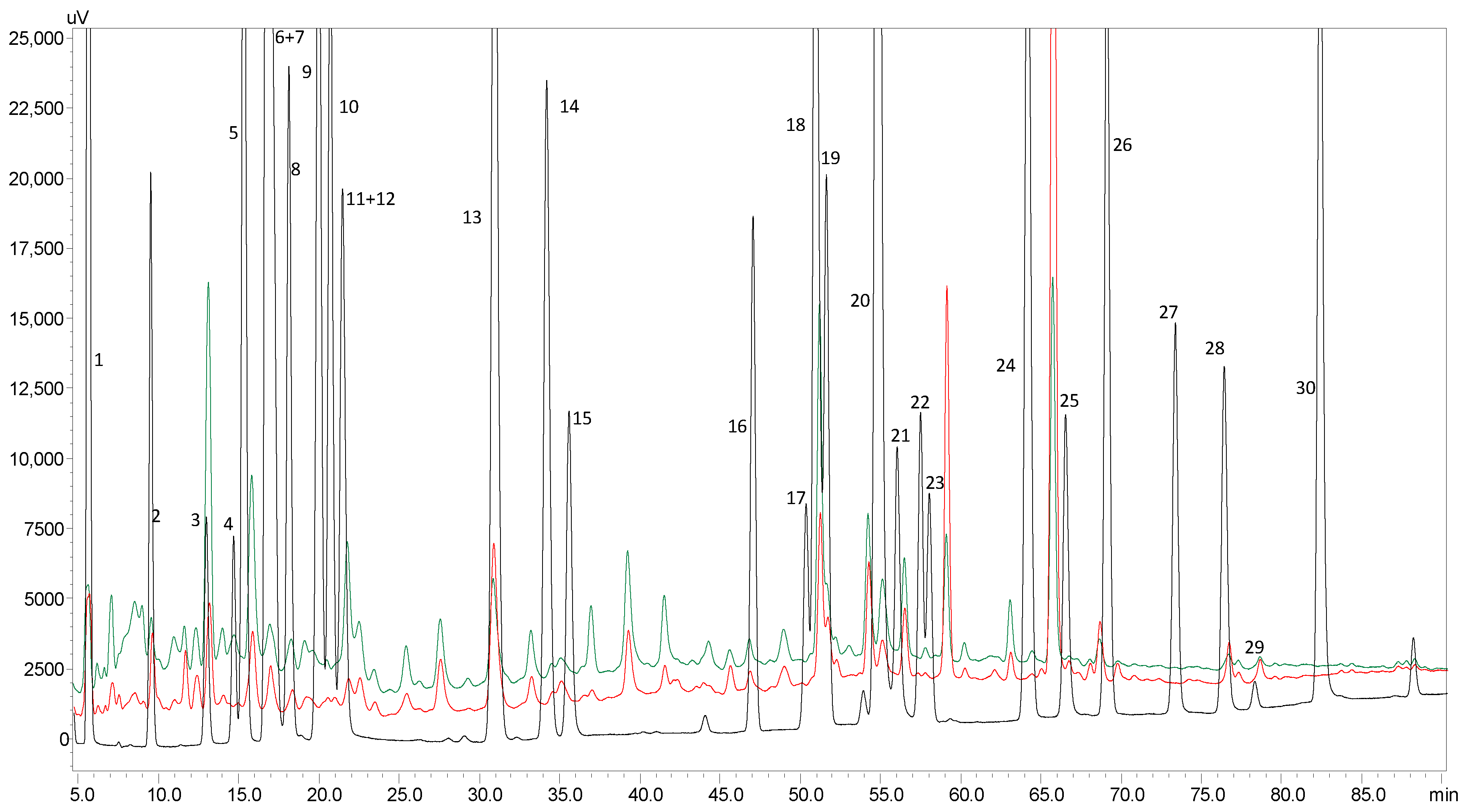
2.2. Identification of Individual Phenolic Compounds by HPLC-PDA
3. Materials and Methods
3.1. Chemicals
3.2. Vine-Cane Samples
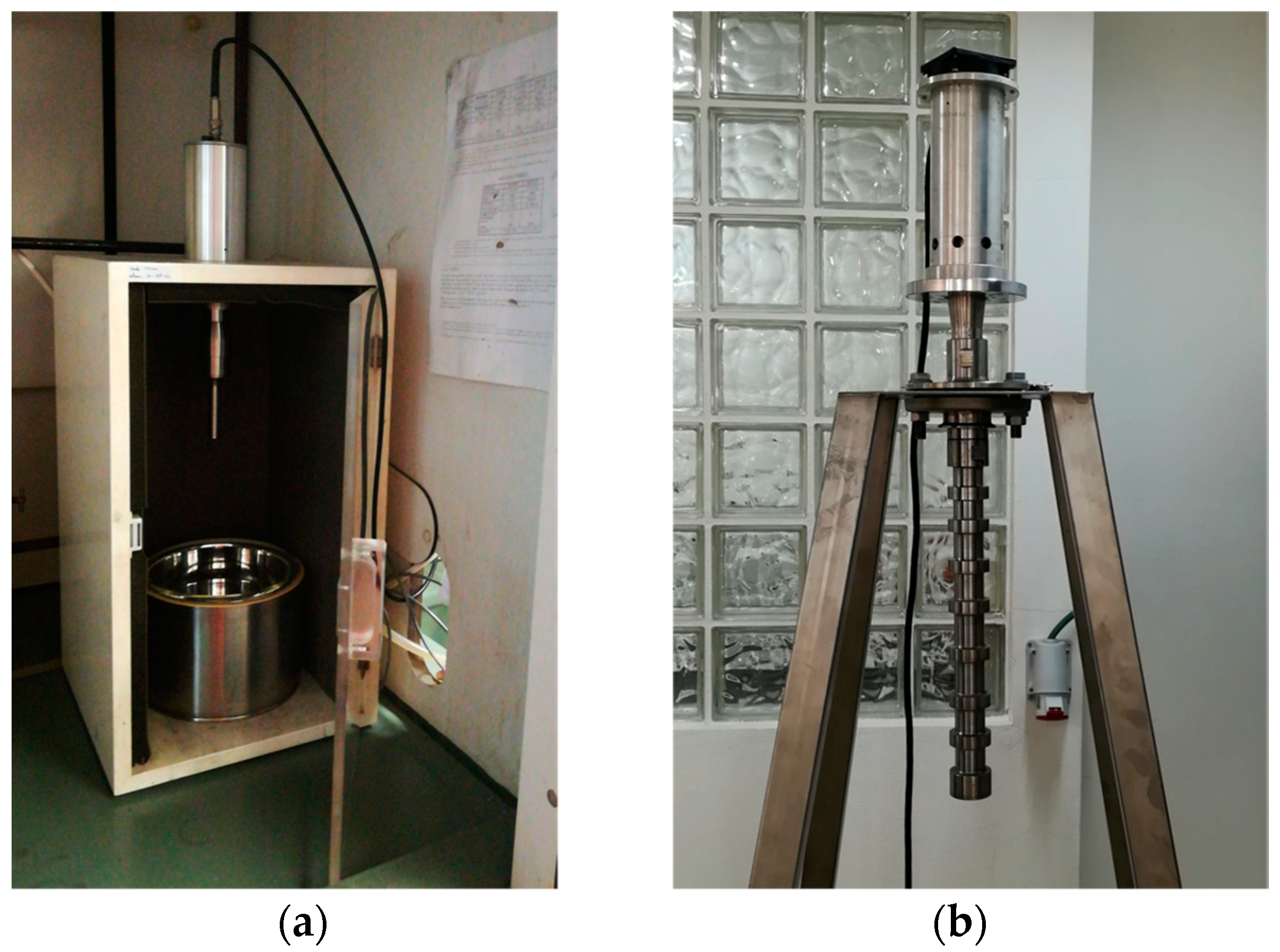
3.3. Ultrasound-Assisted Extraction: Laboratorial and Pilot Scale
3.4. Determination of Total Phenolic and Flavonoid Content and Antioxidant Activity
3.4.1. Total Phenolic and Flavonoid Content
3.4.2. DPPH-Radical Scavenging Activity Assay
3.4.3. Ferric Reducing Antioxidant Power Assay
3.5. High Performance Liquid Chromatography Analysis
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Devesa-Rey, R.; Vecino, X.; Varela-Alende, J.L.; Barral, M.T.; Cruz, J.M.; Moldes, A.B. Valorization of winery waste vs. the costs of not recycling. Waste Manag. 2011, 31, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- International Organisation of Vine and Wine. 2019 Statistical Report on World Vitiviniculture. 2019. Available online: http://www.oiv.int/public/medias/6782/oiv-2019-statistical-report-on-world-vitiviniculture.pdf. (accessed on 10 January 2020).
- Florestal, I.M. Estudo do Impacto na Economia Local/Regional Autárquica pelo Aproveitamento e Valorização Energética e Económica das Podas 2014. Available online: https://www.imflorestal.com/docs/1.3.-Estudo-do-impacto-na-economia-local.pdf. (accessed on 30 May 2019).
- International Organization of Vine and Wine. Country Profile. Available online: http://oiv.int/en/statistiques/?year=2016&countryCode=oiv (accessed on 10 January 2020).
- Piñeiro, Z.; Marrufo-Curtido, A.; Serrano, M.J.; Palma, M. Ultrasound-assisted extraction of stilbenes from grape canes. Molecules 2016, 21, 1–12. [Google Scholar]
- Gómez, S.J.; Causapé, M.C.C.; Martínez, A.A. Distribution of nutrients in anaerobic digestion of vine shoots. Bioresour. Technol. 1993, 45, 93–97. [Google Scholar] [CrossRef]
- Tag, A.T.; Duman, G.; Ucar, S.; Yanik, J. Effects of feedstock type and pyrolysis temperature on potential applications of biochar. J. Anal. Appl. Pyrolysis 2016, 120, 200–206. [Google Scholar] [CrossRef]
- Dávila, I.; Gullón, B.; Labidi, J.; Gullón, P. Multiproduct biorefinery from vine shoots: Bio-Ethanol and lignin production. Renew. Energy 2019, 142, 612–623. [Google Scholar] [CrossRef]
- Jiménez, L.; Angulo, V.; Ramos, E.; De La Toerre, M.J.; Ferrer, J.L. Comparison of various pulping processes for producing pulp from vine shoots. Ind. Crops Prod. 2006, 23, 122–130. [Google Scholar] [CrossRef]
- Ntalos, G.A.; Grigoriou, A.H. Characterization and utilisation of vine prunings as a wood substitute for particleboard production. Ind. Crops Prod. 2002, 16, 59–68. [Google Scholar] [CrossRef]
- Fernandes, M.J.; Moreira, M.M.; Paíga, P.; Dias, D.; Bernardo, M.; Carvalho, M.; Lapa, N.; Fonseca, I.; Morais, S.; Figueiredo, S.; et al. Evaluation of the adsorption potential of biochars prepared from forest and agri-food wastes for the removal of fluoxetine. Bioresour. Technol. 2019, 292, 121973. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Vine-shoot waste aqueous extracts for re-use in agriculture obtained by different extraction techniques: Phenolic, volatile, and mineral compounds. J. Agric. Food Chem. 2014, 62, 10861–10872. [Google Scholar] [CrossRef]
- Gullón, B.; Eibes, G.; Moreira, M.T.; Dávila, I.; Labidi, J.; Gullón, P. Antioxidant and antimicrobial activities of extracts obtained from the refining of autohydrolysis liquors of vine shoots. Ind. Crops Prod. 2017, 107, 105–113. [Google Scholar] [CrossRef]
- Delgado-Torre, M.P.; Ferreiro-Vera, C.; Priego-Capote, F.; Pérez-Juan, P.M.; Luque De Castro, M.D. Comparison of accelerated methods for the extraction of phenolic compounds from different vine-shoot cultivars. J. Agric. Food Chem. 2012, 60, 3051–3060. [Google Scholar] [CrossRef] [PubMed]
- Rajha, H.N.; Boussetta, N.; Louka, N.; Maroun, R.G.; Vorobiev, E. A comparative study of physical pretreatments for the extraction of polyphenols and proteins from vine shoots. Food Res. Int. 2014, 65, 462–468. [Google Scholar] [CrossRef]
- Karacabey, E.; Mazza, G.; Bayindirli, L.; Artik, N. Extraction of Bioactive Compounds from Milled Grape Canes (Vitis vinifera) Using a Pressurized Low-Polarity Water Extractor. Food Bioprocess. Technol. 2012, 5, 359–371. [Google Scholar] [CrossRef]
- Moreira, M.M.; Barroso, M.F.; Porto, J.V.; Ramalhosa, M.J.; Švarc-Gajić, J.; Estevinho, L.; Moreias, S.; Delerue-Matos, C. Potential of Portuguese vine shoot wastes as natural resources of bioactive compounds. Sci. Total Environ. 2018, 634, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Luque-Rodríguez, J.M.; Pérez-Juan, P.; Luque De Castro, M.D. Extraction of polyphenols from vine shoots of Vitis vinifera by superheated ethanol-water mixtures. J. Agric. Food Chem. 2006, 54, 8775–8781. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, C.; Natolino, A.; Scalet, M. Ultrasound-assisted extraction of proanthocyanidins from vine shoots of Vitis vinifera. J. Wine Res. 2018, 29, 290–301. [Google Scholar] [CrossRef]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. Solvent-free microwave extraction of essential oil from aromatic herbs: Comparison with conventional hydro-distillation. J. Chromatogr. A 2004, 1043, 323–327. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Fathi, A.; Perego, P.; Dehghani, F. Extraction of antioxidants from winery wastes using subcritical water. J. Supercrit. Fluids 2012, 65, 18–24. [Google Scholar] [CrossRef]
- Alexandru, L.; Binello, A.; Mantegna, S.; Boffa, L.; Chemat, F.; Cravotto, G. Efficient green extraction of polyphenols from post-harvested agro-industry vegetal sources in Piedmont. Comptes Rendus Chim. 2014, 17, 212–217. [Google Scholar] [CrossRef]
- Tadeo, J.L.; Sánchez-Brunete, C.; Albero, B.; García-Valcárcel, A.I. Application of ultrasound-assisted extraction to the determination of contaminants in food and soil samples. J. Chromatogr. A 2010, 1217, 2415–2440. [Google Scholar] [CrossRef]
- Vardanega, R.; Santos, D.; De Almeida, M.A. Intensification of bioactive compounds extraction from medicinal plants using ultrasonic irradiation. Pharmacogn. Rev. 2014, 8, 88. [Google Scholar] [PubMed]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Li, X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Samaram, S.; Mirhosseini, H.; Tan, C.P.; Ghazali, H.M. Ultrasound-assisted extraction and solvent extraction of papaya seed oil: Crystallization and thermal behavior, saturation degree, color and oxidative stability. Ind. Crops Prod. 2014, 52, 702–708. [Google Scholar] [CrossRef]
- Barros, A.; Gironés-Vilaplana, A.; Teixeira, A.; Collado-González, J.; Moreno, D.A.; Gil-Izquierdo, A.; Rosa, E.; Domíngues-Perles, R. Evaluation of grape (Vitis vinifera L.) stems from Portuguese varieties as a resource of (poly)phenolic compounds: A comparative study. Food Res. Int. 2014, 65, 375–384. [Google Scholar] [CrossRef]
- Farhadi, K.; Esmaeilzadeh, F.; Hatami, M.; Forough, M.; Molaie, R. Determination of phenolic compounds content and antioxidant activity in skin, pulp, seed, cane and leaf of five native grape cultivars in West Azerbaijan province, Iran. Food Chem. 2016, 199, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Sen, I.; Tokatli, F. Authenticity of wines made with economically important grape varieties grown in Anatolia by their phenolic profiles. Food Control 2014, 46, 446–454. [Google Scholar] [CrossRef]
- Pimpão, R.C.; Dew, T.; Oliveira, P.B.; Williamson, G.; Ferreira, R.B.; Santos, C.N. Analysis of Phenolic Compounds in Portuguese Wild and Commercial Berries after Multienzyme Hydrolysis. J. Agric. Food Chem. 2013, 61, 4053–4062. [Google Scholar] [CrossRef]
- Karacabey, E.; Mazza, G. Optimization of solid-liquid extraction of resveratrol and other phenolic compounds from milled grape canes (Vitis vinifera). J. Agric. Food Chem. 2008, 56, 6318–6325. [Google Scholar] [CrossRef]
- Rajha, H.N.; Jaoude, N.A.; Louka, N.; Maroun, R.G.; Vorobiev, E. Industrial Byproducts Valorization Through Energy Saving Processes. Alkaline Extraction of Polyphenols from Vine Shoots. In Proceedings of the International Conference on Renewable Energies for Developing Countries (REDEC), Beirut, Lebanon, 26–27 November 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 89–94. [Google Scholar]
- Pérez-Serradilla, J.A.; Luque de Castro, M.D. Microwave-assisted extraction of phenolic compounds from wine lees and spray-drying of the extract. Food Chem. 2011, 124, 1652–1659. [Google Scholar] [CrossRef]
- Liazid, A.; Palma, M.; Brigui, J.; Barroso, C.G. Investigation on phenolic compounds stability during microwave-assisted extraction. J. Chromatogr. A 2007, 1140, 29–34. [Google Scholar] [CrossRef]
- Rayne, S.; Karacabey, E.; Mazza, G. Grape cane waste as a source of trans-resveratrol and trans-viniferin: High-value phytochemicals with medicinal and anti-phytopathogenic applications. Ind. Crops Prod. 2008, 27, 335–340. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alexandru, L.; Cravotto, G.; Giordana, L.; Binello, A.; Chemat, F. Ultrasound-assisted extraction of clove buds using batch- and flow-reactors: A comparative study on a pilot scale. Innov. Food Sci. Emerg. Technol. 2013, 20, 167–172. [Google Scholar] [CrossRef]
- Wu, Z.; Ferreira, D.F.; Crudo, D.; Bosco, V.; Stevanato, L.; Costale, A.; Cravotto, G. Plant and Biomass Extraction and Valorisation under Hydrodynamic Cavitation. Processes 2019, 7, 965. [Google Scholar] [CrossRef]
- Incbio. Available online: http://incbio.com/pt/index.html (accessed on 11 January 2020).
- Singleton, V.L.; Rossi, J.A. Colorimetry to total phenolics with phosphomolybdic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Paz, M.; Gúllon, P.; Barroso, M.F.; Carvalho, A.P.; Domingues, V.F.; Gomes, A.M.; Becker, H.; Longhinotti, E.; Delerue-Matos, C. Brazilian fruit pulps as functional foods and additives: Evaluation of bioactive compounds. Food Chem. 2015, 172, 462–468. [Google Scholar] [CrossRef]
- Rodrigues, F.; Palmeira-de-Oliveira, A.; das Neves, J.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B. Medicago spp. extracts as promising ingredients for skin care products. Ind. Crops Prod. 2013, 49, 634–644. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma as a measure of antioxodant. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Moreira, M.M.; Barroso, M.F.; Boeykens, A.; Withouck, H.; Morais, S.; Delerue-Matos, C. Valorization of apple tree wood residues by polyphenols extraction: Comparison between conventional and microwave-assisted extraction. Ind. Crops Prod. 2017, 104, 210–220. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
| Time (min.) | UAE Technique | TPC (mg GAE/g DW) | TFC (mg EE/g DW) | DPPH-RSA (mg TE/g DW) | FRAP (mg AAE/g DW) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| TN | TR | TN | TR | TN | TR | TN | TR | |||
| 15 | Lab scale | With ice bath | 9.3 ± 0.3 c,3 | 14.9 ± 0.7 b,3,* | 2.32 ± 0.19 c,3 | 4.5 ± 0.26 c,2,* | 6.1 ± 0.3 c,2 | 15.7 ± 0.7 c,3,* | 5.2 ± 0.5 c | 10.2 ± 0.6 2,* |
| Without ice bath | 15.5 ± 0.5 c,1 | 18.7 ± 1.2 c,2,* | 3.78 ± 0.44 b,1 | 6.9 ± 0.6 c,1,* | 12.5 ± 1.4 c,1 | 29.2 ± 1.6 b,1,* | 5.2 ± 0.4 c | 12.4 ± 0.9 b,1,* | ||
| Pilot scale | 10.6 ± 1.2 b,2 | 20.6 ± 1.1 b,1,* | 2.87 ± 0.25 b,2 | 6.5 ± 0.5 c,1,* | 14.0 ± 1.4 b,1 | 22.7 ± 0.4 b,2,* | 6.0 ± 0.5 b | 11.8 ± 0.9 c,1,* | ||
| 30 | Lab scale | With ice bath | 13.5 ± 1.6 b,2 | 17.3 ± 0.8 a,2,* | 3.44 ± 0.12 b,2 | 6.0 ± 0.5 b,3,* | 12.1 ± 1.0 b,2 | 20.1 ± 1.2 b,3,* | 8.1 ± 0.7 b,1 | 10.6 ± 0.4 2,* |
| Without ice bath | 18.6 ± 1.3 b,1 | 23.3 ± 2.0 b,1,* | 4.79 ± 0.46 a,1 | 8.1 ± 0.7 b,1,* | 16.5 ± 0.6 a,1 | 34.2 ± 2.4 a,1,* | 6.5 ± 0.5 b,2 | 13.5 ± 1.2 b,1,* | ||
| Pilot scale | 14.2 ± 1.4 a,2 | 23.9 ± 0.8 b,1,* | 3.62 ± 0.24 b,2 | 7.3 ± 0.6 b,2,* | 16.0 ± 0.9 a,1 | 23.3 ± 2.1 b,2,* | 6.6 ± 0.6 a,b,2 | 13.5 ± 1.1 b,1,* | ||
| 60 | Lab scale | With ice bath | 16.2 ± 2.1 a,2 | 17.1 ± 1.3 a,3 | 4.11 ± 0.20 a,2 | 6.8 ± 0.6 a,3,* | 17.6 ± 2.0 a,1 | 22.5 ± 1.3 a,3,* | 10.5 ± 0.6 a,1 | 10.6 ± 0.7 3 |
| Without ice bath | 20.1 ± 0.6 a,1 | 32.6 ± 2.1 a,1,* | 5.20 ± 0.20 a,1 | 9.5 ± 0.6 a,1,* | 14.8 ± 0.6 b,2 | 26.3 ± 1.5 c,2,* | 8.9 ± 0.4 a,2 | 20.1 ± 1.5 a,1,* | ||
| Pilot scale | 15.0 ± 1.4 a,2 | 26.0 ± 1.5 a,2,* | 4.62 ± 0.52 a,1,2 | 8.3 ± 0.8 a,2,* | 14.9 ± 1.5 a,b,2 | 33.4 ± 2.1 a,1,* | 7.3 ± 0.6 a,3 | 15.1 ± 1.5 a,2,* | ||
| Compound | TN | TR |
|---|---|---|
| Phenolic acids | ||
| Gallic acid | 13.8 ± 0.7 | 15.1 ± 0.8 |
| Protocatechuic acid | 13.7 ± 0.7 | 10.7 ± 0.5 |
| 4-hydroxyphenilacetic acid | 4.4 ± 0.2 | 8.3 ± 0.4 |
| 4-hydroxybenzoic acid | 6.8 ± 0.3 | 5.3 ± 0.3 |
| 4-hydroxybenzaldehyde | 8.6 ± 0.4 | 9.4 ± 0.5 |
| Chlorogenic acid | 11.1 ± 0.6 | 11.5 ± 0.6 |
| Vanillic acid | 4.8 ± 0.2 | 6.5 ± 0.3 |
| Caffeic acid | 6.4 ± 0.3 | 6.3 ± 0.3 |
| Syringic acid | <LOQ a | <LOQ |
| p-coumaric acid | 10.8 ± 0.5 | 8.9 ± 0.4 |
| Ferulic acid | 4.3 ± 0.2 | 4.4 ± 0.2 |
| Sinapic acid | 6.6 ± 0.3 | 6.6 ± 0.3 |
| Ellagic acid | <LOD b | <LOD |
| Cinnamic acid | 6.5 ± 0.3 | 7.6 ± 0.4 |
| ∑Phenolic acids | 97.7 ± 4.9 | 100.4 ± 5.0 |
| Flavanols | ||
| Catechin | 35.5 ± 1.8 | 131.4 ± 6.6 |
| Epicatechin | 7.5 ± 0.4 | 18.1 ± 0.9 |
| ∑Flavanols | 43.0 ± 2.1 | 149.5 ± 7.5 |
| Flavanones | ||
| Naringin | 4.4 ± 0.2 | 4.9 ± 0.2 |
| Naringenin | 2.6 ± 0.1 | 3.0 ± 0.1 |
| Pinocenbrin | 10.3 ± 0.5 | 10.3 ± 0.5 |
| ∑Flavanones | 17.3 ± 0.9 | 18.2 ± 0.9 |
| Flavonols | ||
| Rutin | 23.3 ± 1.2 | 21.8 ± 1.1 |
| Quercetin-3-O-glucopyranoside | 12.8 ± 0.6 | 14.2 ± 0.7 |
| Myricetin | 74.9 ± 3.7 | 74.3 ± 3.7 |
| Kaempferol-3-O-glucoside | 9.9 ± 0.5 | 9.7 ± 0.5 |
| Kaempferol-3-O-rutinoside | 10.8 ± 0.5 | 12.4 ± 0.6 |
| Quercetin | 20.1 ± 1.0 | 19.7 ± 1.0 |
| Tiliroside | 8.2 ± 0.4 | 8.0 ± 0.4 |
| Kaempferol | 23.9 ± 1.2 | 22.8 ± 1.1 |
| ∑Flavonols | 183.8 ± 9.2 | 173.4 ± 8.7 |
| Stilbenes | ||
| Resveratrol | 84.0 ± 4.2 | 153.4 ± 7.7 |
| ∑Stilbenes | 84.0 ± 4.2 | 153.4 ± 7.7 |
| Others | ||
| Phloridzin | 7.9 ± 0.4 | 8.1 ± 0.4 |
| Phloretin | 5.6 ± 0.3 | 3.9 ± 0.2 |
| ∑Others | 13.5 ± 0.7 | 12.0 ± 0.6 |
| ∑All phenolic compounds | 439 | 617 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorosh, O.; Moreira, M.M.; Rodrigues, F.; Peixoto, A.F.; Freire, C.; Morais, S.; Delerue-Matos, C. Vine-Canes Valorisation: Ultrasound-Assisted Extraction from Lab to Pilot Scale. Molecules 2020, 25, 1739. https://doi.org/10.3390/molecules25071739
Dorosh O, Moreira MM, Rodrigues F, Peixoto AF, Freire C, Morais S, Delerue-Matos C. Vine-Canes Valorisation: Ultrasound-Assisted Extraction from Lab to Pilot Scale. Molecules. 2020; 25(7):1739. https://doi.org/10.3390/molecules25071739
Chicago/Turabian StyleDorosh, Olena, Manuela M. Moreira, Francisca Rodrigues, Andreia F. Peixoto, Cristina Freire, Simone Morais, and Cristina Delerue-Matos. 2020. "Vine-Canes Valorisation: Ultrasound-Assisted Extraction from Lab to Pilot Scale" Molecules 25, no. 7: 1739. https://doi.org/10.3390/molecules25071739
APA StyleDorosh, O., Moreira, M. M., Rodrigues, F., Peixoto, A. F., Freire, C., Morais, S., & Delerue-Matos, C. (2020). Vine-Canes Valorisation: Ultrasound-Assisted Extraction from Lab to Pilot Scale. Molecules, 25(7), 1739. https://doi.org/10.3390/molecules25071739