Silacyclohexanes, Sila(hetero)cyclohexanes and Related Compounds: Structure and Conformational Analysis
Abstract
:1. Introduction
2. General Features of Sila(hetero)cyclohexanes
3. Silacyclohexanes
4. Thiasilacyclohexanes
5. Azasilacyclohexanes (azasilinanes) and Related Compounds
6. Oxasilacyclohexanes (Silatetrahydropyrans)
7. Si-X-silacyclohexanes (X = Hlg, CN, OMe)
8. Solution vs. Gas Conformational Preferences in Miscellaneous Silacyclohexanes
9. Miscellaneous Silacyclohexanes and Related Compounds
Funding
Acknowledgments
Conflicts of Interest
References
- Kleinpeter, E. Conformational Analysis of Saturated Heterocyclic Six-Membered Rings. Adv. Heterocycl. Chem. 2004, 86, 41–127. [Google Scholar]
- Shainyan, B.A.; Kleinpeter, E. Silacyclohexanes and silaheterocyclohexanes: Why are they so different from other heterocyclohexanes? Tetrahedron 2013, 69, 5927–5936. [Google Scholar] [CrossRef]
- Shainyan, B.A. Structure and conformational analysis of silacyclohexanes and 1,3-silaheterocyclohexanes. Tetrahedron 2016, 72, 5027–5035. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Suslova, E.N.; Schilde, U. Crystal structures and theoretical calculations of trans-2,4,4-trimethyl-4-silathiane 1-oxide and 4,4-dimethyl-4-silathiane 1,1-dioxide. Struct. Chem. 2008, 19, 889–894. [Google Scholar] [CrossRef]
- Arnason, I.; Kvaran, Á.; Jonsdottir, S.; Gudnason, P.I.; Oberhammer, H. Conformations of Silicon-Containing Rings. 5., Conformational Properties of 1-Methyl-1-silacyclohexane: Gas Electron Diffraction, Low-Temperature NMR, and Quantum Chemical Calculations. J. Org. Chem. 2002, 67, 3827–3831. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Kleinpeter, E. Conformational preferences of Si-Ph,H and Si-Ph,Me silacyclohexanes and 1,3-thiasilacyclohexanes. Additivity of conformational energies in 1,1-disubstituted heterocyclohexanes. Tetrahedron 2012, 68, 114–125. [Google Scholar] [CrossRef]
- Bushweller, C.H. Conformational Behaviour of Six-Membered Rings: Analysis, Dynamics and Stereoelectronic Effects; Juaristi, E., Ed.; VCH Publisher: New York, NY, USA, 1995; Chapter 2; pp. 25–58. [Google Scholar]
- Eliel, E.L.; Wilen, S.H.; Mander, L.N. Stereochemistry of organic compounds; Wiley: New York, NY, USA, 1994; Chapter 11; pp. 665–834. [Google Scholar]
- Girichev, G.V.; Giricheva, N.I.; Bodi, A.; Gudnason, P.I.; Jonsdottir, S.; Kvaran, Á.; Arnason, I.; Oberhammer, H. Unexpected conformational properties of 1-trifluoromethyl-1-silacyclohexane C5H10SiHCF3: Gas electron diffraction, low-temperature NMR spectroscopic studies, and quantum chemical calculations. Chem. Eur. J. 2007, 13, 1776–1783. [Google Scholar] [CrossRef]
- Bodi, A.; Kvaran, Á.; Jonsdottir, S.; Antonsson, E.; Wallevik, S.Ó.; Arnason, I.; Belyakov, A.V.; Baskakov, A.A.; Hölbling, M.; Oberhammer, H. Conformational Properties of 1-Fluoro-1-silacyclohexane, C5H10SiHF: Gas Electron Diffraction, Low-Temperature NMR, Temperature-Dependent Raman Spectroscopy, and Quantum Chemical Calculations. Organometallics 2007, 26, 6544–6550. [Google Scholar] [CrossRef]
- Belyakov, A.V.; Baskakov, A.A.; Naraev, V.N.; Rykov, A.N.; Oberhammer, H.; Arnason, I.; Wallevik, S.Ó. Molecular structure and conformational preferences of 1-chloro-1-silacyclohexane, CH2(CH2CH2)2SiH-Cl, as studies by gas-phase electron diffraction and quantum chemistry. Russ. J. Gen. Chem. 2011, 81, 2257–2261. [Google Scholar] [CrossRef]
- Belyakov, A.V.; Baskakov, A.A.; Naraev, V.N.; Rykov, A.N.; Oberhammer, H.; Arnason, I.; Wallevik, S.Ó. Molecular structure and conformational preferences of 1-bromo-1-silacyclohexane, CH2(CH2CH2)2SiH-Br, as studies by gas-phase electron diffraction and quantum chemistry. Russ. J. Phys. Chem. A. 2011, 86, 1563–1566. [Google Scholar] [CrossRef]
- Belyakov, A.V.; Baskakov, A.A.; Berger, R.J.F.; Mitzel, N.W.; Oberhammer, H.; Arnason, I.; Wallevik, S.Ó. Molecular structure and conformational preferences of gaseous 1-iodo-1-silacyclohexane. J. Mol. Struct. 2012, 1012, 126–130. [Google Scholar] [CrossRef]
- Weldon, A.; Tschumper, G.S. Intrinsic conformational preferences of and an anomeric-like effect in 1-substituted silacyclohexanes. Int. J. Quant. Chem. 2007, 107, 2261–2265. [Google Scholar] [CrossRef]
- Wallevik, S.Ó.; Bjornsson, R.; Kvaran, Á.; Jonsdottir, S.; Arnason, I.; Belyakov, A.V.; Baskakov, A.A.; Hassler, K.; Oberhammer, H. Conformational Properties of 1-Silyl-1-Silacyclohexane, C5H10SiHSiH3: Gas Electron Diffraction, Low-Temperature NMR, Temperature-Dependent Raman Spectroscopy, and Quantum Chemical Calculations. J. Phys. Chem. A 2010, 114, 2127–2135. [Google Scholar] [CrossRef]
- Belyakov, A.V.; Sigolaev, Y.; Shlykov, S.A.; Wallevik, S.Ó.; Jonsdottir, N.R.; Bjornsson, R.; Jonsdottir, S.; Kvaran, Á.; Kern, T.; Hassler, K.; et al. Conformational properties of 1-tert-butyl-1-silacyclohexane, C5H10SiH(t-Bu): Gas-phase electron diffraction, temperature dependent Raman spectroscopy, and quantum chemical calculations. Struct. Chem. 2015, 26, 445–453. [Google Scholar] [CrossRef]
- Shen, Q.; Rhodes, S.; Cochran, J.C. Molecular structure and conformation of cyclohexylsilane as determined by gas-phase electron diffraction. Organometallics 1992, 11, 485–486. [Google Scholar] [CrossRef]
- Wallevik, S.Ó.; Bjornsson, R.; Kvaran, Á.; Jonsdottir, S.; Girichev, G.V.; Giricheva, N.L.; Hassler, K.; Arnason, I. Conformational properties of 1-fluoro-1-methyl-silacyclohexane and 1-methyl-1-trifluoromethyl-1-silacyclohexane: Gas electron diffraction, low-temperature NMR, temperature-dependent Raman spectroscopy, and quantum chemical calculations. J. Mol. Struct. 2010, 978, 209–219. [Google Scholar] [CrossRef]
- Allinger, N.L.; Tribble, M.T. Conformational analysis—LXXVIII. The conformation of phenyl-cyclohexane, and related molecules. Tetrahedron Lett. 1971, 12, 3259–3262. [Google Scholar] [CrossRef]
- Wiberg, K.B.; Castejon, H.; Bailey, W.F.; Ochterski, J. Conformational studies in the cyclohexane series. 2. Phenylcyclohexane and 1-methyl-1-phenylcyclohexane. J. Org. Chem. 2000, 65, 1181–1187. [Google Scholar] [CrossRef]
- Mazaleyrat, J.P.; Welvart, Z. Non-additivity of the conformational free-energy differences of 1-substituted cyclohexylamines. J. Chem. Soc. D Chem. Comm. 1969, 485–486. [Google Scholar] [CrossRef]
- Sicsic, S.; Welvart, Z. Conformational gem-effect in disubstituted cyclohexanes. Chem. Comm. 1966, 499–500. [Google Scholar] [CrossRef]
- Uebel, J.J.; Goodwin, H.W. Conformational analysis of 1-methylcyclohexanol. J. Org. Chem. 1968, 33, 3317–3319. [Google Scholar] [CrossRef]
- Allinger, N.L.; Liang, C.D. Conformational analysis. LXIII. The 1-methylcyclohexanol system. J. Org. Chem. 1968, 33, 3319–3321. [Google Scholar] [CrossRef]
- Eliel, E.L.; Enanoza, R.M. Conformational analysis. XXVI. Conformational equilibria in 5,5-disubstituted 1,3-dioxanes. J. Am. Chem. Soc. 1972, 94, 8072–8081. [Google Scholar] [CrossRef]
- Eliel, E.I.; Manoharan, M. Conformational analysis. 40. Conformation of 1-methyl-1-phenylcyclohexane and conformational energies of the phenyl and vinyl groups. J. Org. Chem. 1981, 46, 1959–1962. [Google Scholar] [CrossRef]
- Allinger, N.L.; Liang, C.D. Conformational analysis. LVI. Chlorocyclohexane and 1-chloro-1-methylcyclohexane. J. Org. Chem. 1967, 32, 2391–2394. [Google Scholar] [CrossRef]
- Schneider, H.-J.; Hoppen, V. Carbon-13 nuclear magnetic resonance substituent-induced shieldings and conformational equilibriums in cyclohexanes. J. Org. Chem. 1978, 43, 3866–3873. [Google Scholar] [CrossRef]
- Klaeboe, P.; Nielsen, C.J.; Horn, A.; Guirgis, G.A.; Overby, J.S.; Aleksa, V. Raman and infrared spectra, quantum chemical calculations, conformations and spectral assignments of 1-chloro-1-methyl-1-silacyclohexane. J. Mol. Struct. 2013, 1047, 282–291. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Kirpichenko, S.V.; Kleinpeter, E. Synthesis and conformational properties of 1,3-dimethyl-3-phenyl-1,3-azasilinane. Low temperature dynamic NMR and computational study. Arkivoc 2012, 2012, 175–185. [Google Scholar]
- Kirpichenko, S.V.; Shainyan, B.A.; Kleinpeter, E. Unusual conformational preferences of 1,3-dimethyl-3-isopropoxy-3-silapiperidine. J. Phys. Org. Chem. 2012, 25, 1321–1327. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Kirpichenko, S.V.; Kleinpeter, E.; Shlykov, S.A.; Osadchiy, D.Y. Molecular structure and conformational analysis of 3-methyl-3-phenyl-3-silatetrahydropyran. Gas-phase electron diffraction, low temperature NMR and quantum chemical calculations. Tetrahedron 2015, 71, 3810–3818. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Kirpichenko, S.V.; Kleinpeter, E. Stereochemistry of 3-isopropoxy-3-methyl-1,3-oxasilinane—The first 3-silatetrahedropyran with an exocyclic RO–Si bond. Tetrahedron 2015, 71, 6720–6726. [Google Scholar] [CrossRef]
- Kirpichenko, S.V.; Shainyan, B.A.; Kleinpeter, E.; Shlykov, S.A.; Phien, T.D.; Albanov, A.I. Synthesis of 3-fluoro-3-methyl-3-silatetrahydropyran and its conformational preferences in gas and solution by GED, NMR and theoretical calculations. Tetrahedron 2018, 74, 1859–1867. [Google Scholar] [CrossRef]
- Kirpichenko, S.V.; Kleinpeter, E.; Ushakov, I.A.; Shainyan, B.A. Conformational Analysis of 3-Methyl-3-silathiane and 3-Fluoro-3-methyl-3-silathiane. J. Phys. Org. Chem. 2011, 24, 320–326. [Google Scholar] [CrossRef]
- Noll, J.E.; Daubert, B.F.; Speier, J.L. Effect of organic Si substituents on the basic strength of amines. J. Am. Chem. Soc. 1951, 73, 3871–3873. [Google Scholar] [CrossRef]
- Sommer, L.H.; Rockett, J. Polar effects of organic Si substituents in aliphatic amines. J. Am. Chem. Soc. 1951, 73, 5130–5134. [Google Scholar] [CrossRef]
- Zingler, G.; Kelling, H.; Popowski, E. Untersuchungen zur Basizitat von Alkyl- and Silylalkylaminen IIZ. Z. Anorg. Allg. Chem. 1981, 476, 41–54. [Google Scholar] [CrossRef]
- Vashchenko, A.V.; Abramov, A.V.; Frolov, Y.L. The fine difference in electronic structure of heteroatoms in methyl vinyl ether and sulfide. J. Mol. Struct. Theochem 2002, 594, 107–111. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Suslova, E.N.; Phien, T.D.; Shlykov, S.A.; Heydenreich, M.; Kleinpeter, E. 1-Methylthio-1-phenyl-1-silacyclohexane: Synthesis, conformational preferences in gas and solution by GED, NMR and theoretical calculations. Tetrahedron 2019, 75, 130677. [Google Scholar] [CrossRef]
- Kirpichenko, S.V.; Kleinpeter, E.; Shainyan, B.A. Conformational analysis of 3,3-dimethyl-3-silathiane, 2,3,3-trimethyl-3-silathiane and 2-trimethylsilyl-3,3-dimethyl-3-silathiane − Preferred conformers, barriers to ring inversion and substituent effects. J. Phys. Org. Chem. 2010, 23, 859–865. [Google Scholar] [CrossRef]
- Anteunis, M.J.O.; Dedeyne, R. 1H nmr study and conformation of 3,3-dimethyl-3-sila-1-heterocyclo-hexanes and derivatives (heteroatom SiCl2, SiMe2, O, NMe, S, Se, Te). Org. Magn. Res. 1977, 9, 127–132. [Google Scholar] [CrossRef]
- Kitching, W.; Olszowy, H.A.; Drew, G.M.; Adcock, W. Conformational preference of the trimethylsilyl group. J. Org. Chem. 1982, 47, 5153–5156. [Google Scholar] [CrossRef]
- Freeman, F.; Phornvoranunt, A.; Hehre, W.J. Molecular orbital study of the conformational energies (-ΔG degrees or A values) of 2- alkyltetrahydro-2H-thiopyrans (tetrahydrothiopyrans, thiacyclohexanes, thianes). J. Phys. Org. Chem. 1999, 12, 176–186. [Google Scholar] [CrossRef]
- Juaristi, E.; Ordonez, M. Conformational preference of the sulfinyl group in six-membered heterocycles. In Organosulfur Chemistry; Page, P., Ed.; Springer: Berlin, Germany, 1998; Chapter 3; pp. 63–95. [Google Scholar]
- Lambert, J.B.; Bailey, D.S.; Mixan, C.E. The persistence of the 1-axial preference in thianes. J. Org. Chem. 1972, 37, 377–382. [Google Scholar] [CrossRef]
- Barbarella, G.; Dembech, P.; Tugnoli, V. 13C and 17O chemical shifts and conformational analysis of mono- and di-methyl-substituted thiane 1-oxide and thiane 1,1-dioxide. Org. Magn. Reson. 1984, 22, 402–407. [Google Scholar] [CrossRef]
- Kirpichenko, S.V.; Albanov, A.I.; Pestunovich, V.A. Conformational Behavior of 3,3-dimethyl-3-silathiane 1-oxide and its diastereomeric 2-methyl derivatives. J. Sulf. Chem. 2004, 25, 21–27. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Suslova, E.N.; Kleinpeter, E. Conformational Analysis of 4,4-dimethyl-4-silathiane and its S-oxides. J. Phys. Org. Chem. 2011, 24, 1188–1192. [Google Scholar] [CrossRef]
- Shainyan, B.A. Computational Study of 4-Fluoro-4-Chloro- and 4-Fluoro-4-Bromo-4-silathiacyclohexane S-Oxides: Effect of Halogen on the S=O→Si Intra-molecular Coordination in the Boat and Twist Conformers. Int. J. Quant. Chem. 2007, 107, 189–199. [Google Scholar] [CrossRef]
- Pestunovich, V.A.; Larin, M.F.; Sorokin, M.S.; Albanov, A.I.; Voronkov, M.G. Diastereotopy of equatorial fluorines at the trigonal-bipyramidal silicon atom in organyl [β-(trifluorosilyl)ethyl]sulfoxide molecules. J. Organomet. Chem. 1985, 280, C17–C20. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Suslova, E.N.; Kleinpeter, E. Conformational Analysis of N-phenyl- and N-trifyl-4,4-dimethyl-4-silathiane 1-sulfimides. J. Phys. Org. Chem. 2011, 24, 698–704. [Google Scholar] [CrossRef]
- Jalsovszky, I.; Kucsman, Á.; Ruff, F.; Koritsánszky, T.; Argay, G.; Kálmán, A. Conformational analysis of thiane-1 imides: An x-ray study of thiane-1-tosylimide and diastereoisomeric 2-alkyl- and 4-phenylthiane-1-tosylimides. J. Mol. Struct. 1987, 156, 165–192. [Google Scholar] [CrossRef]
- Lazareva, N.F.; Shainyan, B.A.; Kleinpeter, E. 4-Alkyl-2,2,6,6-tetramethyl-1,4,2,6-oxaazadisilinanes: Synthesis, structure, and conformational analysis. J. Phys. Org. Chem. 2010, 23, 84–89. [Google Scholar] [CrossRef]
- LeMaster, C.B.; LeMaster, C.L.; Tafazzoli, M.; Suarez, C.; True, N.S. Pressure- and temperature-dependent proton NMR studies of N-methylmorpholine ring inversion. J. Phys. Chem. 1990, 94, 3461–3466. [Google Scholar] [CrossRef]
- Lunazzi, L.; Casarini, D.; Cremonini, M.A.; Anderson, J.E. The effect of exocyclic conjugation on the inversion of a saturated sox-membered ring. A dynamic NMR study of N-substituted morpholines. Tetrahedron 1991, 47, 7465–7470. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Suslova, E.N.; Kleinpeter, E. Conformational Analysis of 4,4-dimethyl-1-(trifluoromethylsulfonyl)-1,4-azasilinane and 2,2,6,6-tetramethyl-4-(trifluoromethylsulfonyl)-1,4,2,6-oxazadisilinane. J. Phys. Org. Chem. 2012, 25, 83–90. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Ushakov, I.A.; Koch, A.; Kleinpeter, E. Stereodynamics of 1-(Methylsulfonyl)-3,5-Bis(trifluoromethylsulfonyl)-1,3,5-Triazinane: Experimental and Theoretical Analysis. J. Org. Chem. 2006, 71, 7638–7642. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Ushakov, I.A.; Mescheryakov, V.I.; Schilde, U.; Koch, A.; Kleinpeter, E. The stereodynamics of 3,5-bis(trifluoromethylsulfonyl)-1,3,5-oxadiazi nane and 1,3,5-tris(trifluoromethylsulfonyl)-1,3,5-triazinane—An Experimental and Theoretical Study. Tetrahedron 2007, 63, 11828–11837. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Ushakov, I.A.; Mescheryakov, V.I.; Koch, A.; Kleinpeter, E. Variable temperature NMR and theoretical study of the stereodynamics of 5-trifluoromethylsulfonyl-1,3,5-dioxaazinane: Perlin effect subject to heteroatom substitution. Tetrahedron 2008, 64, 5379–5383. [Google Scholar] [CrossRef]
- Tacke, R.; Heinrich, T.; Bertermann, R.; Burschka, C.; Hamacher, A.; Kassack, M.U. Sila-haloperidol: A Silicon Analogue of the Dopamine (D2) Receptor Antagonist Haloperidol. Organometallics 2004, 23, 4468–4477. [Google Scholar] [CrossRef]
- Heinrich, T.; Burschka, C.; Penka, M.; Wagner, B.; Tacke, R. 4-Silapiperidine and 4-silapiperidinium derivatives: Syntheses and structural characterization. J. Organometal. Chem. 2005, 690, 33–47. [Google Scholar] [CrossRef]
- Ilg, R.; Burschka, C.; Schepmann, D.; Wünsch, B.; Tacke, R. Synthesis and Pharmacological Characterization of Sila-panamesine, a Sila-Analogue of the σ Receptor Ligand Panamesine (EMD 57445). Organometallics 2006, 25, 5396–5408. [Google Scholar] [CrossRef]
- Tacke, R.; Nguyen, B.; Burschka, C.; Lippert, W.P.; Hamacher, A.; Urban, C.; Kassack, M.U. Sila-Trifluperidol, a Silicon Analogue of the Dopamine (D2) Receptor Antagonist Trifluperidol: Synthesis and Pharmacological Characterization. Organometallics 2010, 29, 1652–1660. [Google Scholar] [CrossRef]
- Fischer, M.; Tacke, R. Synthesis of 4-Silapiperidine Building Blocks with N–H Groups Using the Staudinger Reaction. Organometallics 2013, 32, 7181–7185. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Kirpichenko, S.V.; Kleinpeter, E.; Shlykov, S.A.; Osadchiy, D.Y.; Chipanina, N.N.; Oznobikhina, L.P. 1,3-Dimethyl-3-silapiperidine: Synthesis, Molecular Structure, and Conformational Analysis by Gas-Phase Electron Diffraction, Low Temperature NMR, IR and Raman Spectroscopy, and Quantum Chemical Calculations. J. Org. Chem. 2013, 78, 3939–3947. [Google Scholar] [CrossRef] [PubMed]
- Eliel, E.L.; Kandasamy, D.; Yen, C.-Y.; Hargrave, K.D. Conformational analysis. 39. Carbon-13 NMR spectra of saturated heterocycles. 9. Piperidine and N-methylpiperidine. J. Am. Chem. Soc. 1980, 102, 3698–3707. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Kirpichenko, S.V.; Shlykov, S.A.; Kleinpeter, E. Structure and Conformational Properties of 1,3,3-trimethyl-1,3-Azasilinane: Gas Electron Diffraction, Dynamic NMR and Theoretical Study. J. Phys. Chem. A 2012, 116, 784–789. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Kirpichenko, S.V.; Kleinpeter, E. Synthesis and conformational analysis of 1,3-azasilinanes. Tetrahedron 2012, 26, 7494–7501. [Google Scholar] [CrossRef]
- Lazareva, N.F.; Shainyan, B.A.; Schilde, U.; Chipanina, N.N.; Oznobikhina, L.P.; Albanov, A.I.; Kleinpeter, E. Synthesis, Molecular Structure, Conformational Analysis, and Chemical Properties of Silicon-Containing Derivatives of Quinolizidine. J. Org. Chem. 2012, 77, 2382–2388. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Kirpichenko, S.V.; Chipanina, N.N.; Oznobikhina, L.P.; Kleinpeter, E.; Shlykov, S.A.; Osadchiy, D.Y. Synthesis and Conformational Analysis of 3-Methyl-3-silatetrahydropyran by GED, FTIR, NMR, and Theoretical Calculations: Comparative Analysis of 1-Hetero-3-methyl-3-silacyclohexanes. J. Org. Chem. 2015, 80, 12492–12500. [Google Scholar] [CrossRef]
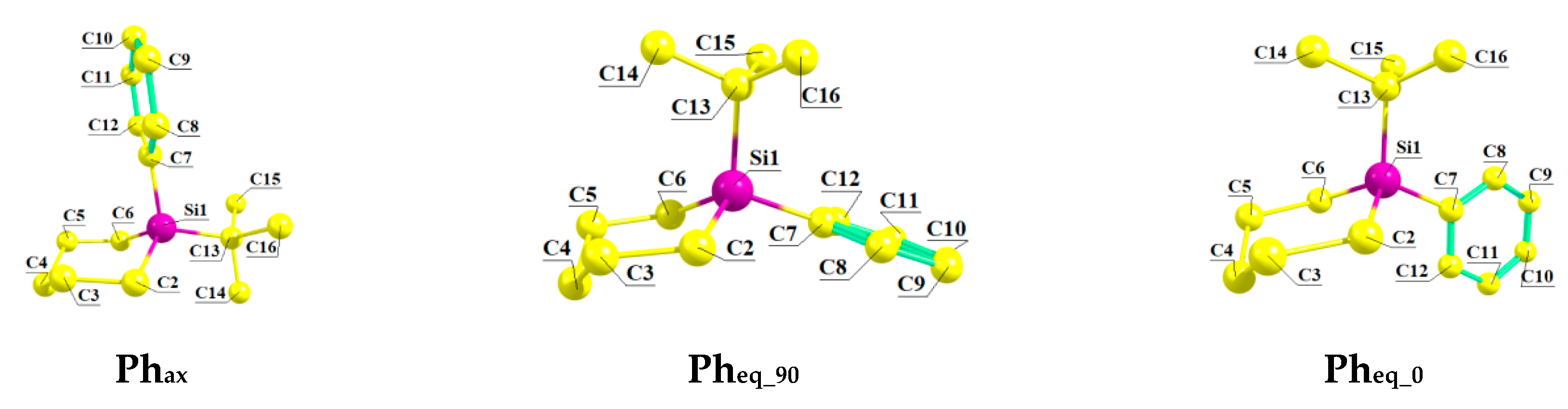
- Shainyan, B.A.; Kirpichenko, S.V.; Kleinpeter, E. Conformational preferences of the Ph Group in 1-Phenyl-1-X-1-silacyclo-hexanes (X = MeO, HO) and 3-Ph-3-X-3-silatetrahydropyrans (X=HO, H) by LTe 13C-NMR Spectroscopy and Theoretical Calculations. J. Org. Chem. 2017, 82, 13414–13422. [Google Scholar] [CrossRef]
- Wallevik, S.Ó.; Bjornsson, R.; Kvaran, Á.; Jonsdottir, S.; Arnason, I.; Belyakov, A.V.; Kern, T.; Hassler, K. Conformational Properties of 1-Halogenated-1-Silacyclohexanes, C5H10SiHX (X = Cl, Br, I): Gas Electron Diffraction, Low-Temperature NMR, Temperature-Dependent Raman Spectroscopy, and Quantum-Chemical Calculations. Organometallics 2013, 32, 6996–7005. [Google Scholar] [CrossRef]
- Belyakov, A.V.; Sigolaev, Y.F.; Shlykov, S.A.; Wallevik, S.Ó.; Jonsdottir, N.R.; Jonsdottir, S.; Kvaran, Á.; Bjornsson, R.; Arnason, I. Conformational properties of 1-cyano-1-silacyclohexane, C5H10SiHCN: Gas electron diffraction, low-temperature NMR and quantum chemical calculations. J. Mol. Struct. 2017, 1132, 149–156. [Google Scholar] [CrossRef]
- Shlykov, S.A.; Puchkov, B.V.; Arnason, I.; Wallevik, S.Ó.; Giricheva, N.I.; Girichev, G.V.; Zhabanov, Y.A. 1-Methoxy-1-silacyclohexane: Synthesis, molecular structure and conformational behavior by gas electron diffraction, Raman spectroscopy and quantum chemical calculations. J. Mol. Struct. 2018, 1154, 156–570. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Belyakov, A.V.; Sigolaev, Y.F.; Khramov, A.N.; Kleinpeter, E. Molecular Structure and Conformational Analysis of 1-Phenyl-1-X-1-Silacyclohexanes (X = F, Cl) by Electron Diffraction, Low-Temperature NMR, and Quantum Chemical Calculations. J. Org. Chem. 2017, 82, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Suslova, E.N.; Shainyan, B.A. 1-Phenyl-1-halo-1-silacyclohexanes. Russ. J. Gen. Chem. 2016, 86, 1854–1858. [Google Scholar] [CrossRef]
- Phien, T.D.; Kuzmina, L.E.; Kvaran, Á.; Jonsdottir, S.; Arnason, I.; Shlykov, S.A. Cyanocyclohexane: Axial-to-equatorial “seesaw” parity in gas and condensed phases. J. Mol. Struct. 2018, 1168, 127–134. [Google Scholar] [CrossRef]
- Shlykov, S.A.; Osadchiy, D.Y.; Chipanina, N.N.; Oznobikhina, L.P.; Shainyan, B.A. Molecular structure and conformational analysis of 3-methyl-3-silathiane by gas phase electron diffraction, FT-IR spectroscopy and quantum chemical calculations. J. Mol. Struct. 2015, 1100, 555–561. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Kirpichenko, S.V.; Osadchiy, D.Y.; Shlykov, S.A. Molecular structure and conformations of 1-phenyl-1-silacyclohexane from gas-phase electron diffraction and quantum chemical calculations. Struct. Chem. 2014, 25, 1677–1685. [Google Scholar] [CrossRef]
- Phien, T.D.; Shlykov, S.A.; Shainyan, B.A. Molecular structure and conformational behavior of 1-methyl-1-phenylsilacyclohexane studied by gas electron diffraction, IR spectroscopy and quantum chemical calculations. Tetrahedron 2017, 73, 1127–1134. [Google Scholar] [CrossRef]
- Wiberg, K.B.; Lambert, K.M.; Bailey, W.F. The Role of CH···O Coulombic Interactions in Determining Rotameric Conformations of Phenyl Substituted 1,3-Dioxanes and Tetrahydropyrans. J. Org. Chem. 2015, 80, 7884–7889. [Google Scholar] [CrossRef]
- Phien, T.D.; Kuzmina, L.E.; Suslova, E.N.; Shainyan, B.A.; Shlykov, S.A. Conformational rivalry of geminal sub-stituents in silacyclohexane derivatives: 1-phenyl vs. 1-OR, R=H or Me. Tetrahedron 2019, 75, 3038–3045. [Google Scholar] [CrossRef]
- Suslova, E.N.; Shainyan, B.A. 1-Phenyl-1-X-1-silacyclohexanes (X = MeO, OH, Me2N). Russ. J. Gen. Chem. 2017, 87, 1645–1648. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Suslova, E.N.; Phien, T.D.; Shlykov, S.A.; Kleinpeter, E. Synthesis, conformational preferences in gas and solution, and molecular gear rotation in 1-(dimethylamino)-1-phenyl-1-silacyclohexane by gas phase electron diffraction (GED), LT NMR and theoretical calculations. Tetrahedron 2018, 74, 4299–4307. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Kleinpeter, E.; Suslova, E.N. Conformational Analysis of (1,1′-Phenyl-1,1′-silacyclohex-1-yl)disiloxane. DFT and Low-Temperature 13C NMR Spectroscopy Study. Russ. J. Gen. Chem. 2019, 89, 713–716. [Google Scholar] [CrossRef]
- Kleinpeter, E.; Shainyan, B.A. Very low temperature dynamic 29Si NMR study of the conformational equilibrium of (1,1’-phenyl-1,1’-silacyclohex-1-yl)disiloxane. Magn. Res. Chem. 2019, 89, 713–716. [Google Scholar]
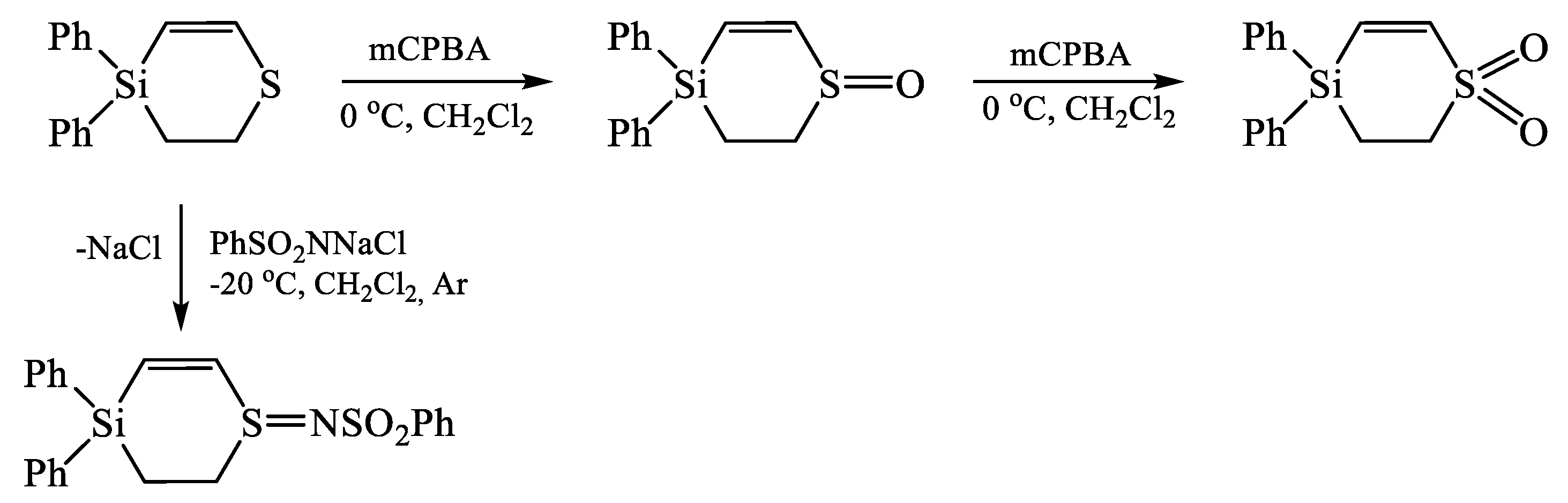
- Suslova, E.N.; Shainyan, B.A. 4,4-Dimethyl-3,4-dihydro-2H-1,4-thiasiline – the first cyclic organosilicon vinyl sulfide. Mendeleev Commun. 2013, 23, 255–256. [Google Scholar] [CrossRef]
- Jensen, F.R.; Bushweller, C.H. Conformational preferences and interconversion barriers in cyclohexene and derivatives. J. Am. Chem. Soc. 1969, 91, 5774–5782. [Google Scholar] [CrossRef]
- Anet, F.A.L.; Bourn, A.J.R. Nuclear Magnetic Resonance Line-Shape and Double-Resonance Studies of Ring Inversion in Cyclohexane-d11. J. Am. Chem. Soc. 1967, 89, 760–768. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Kleinpeter, E. Conformational flexibility of 4,4-dimethyl-3,4-dihydro-2H-1,4-thiasiline and its monoheterocyclic analogs. Russ. J. Gen. Chem. 2014, 84, 1129–1133. [Google Scholar] [CrossRef]
- Suslova, E.N.; Shainyan, B.A. Synthesis of 4, 4-diphenyl-3, 4-dihydro-2H-1, 4-thiasiline. Sulfur Chem. 2014, 35, 641–648. [Google Scholar] [CrossRef]
- Suslova, E.N.; Shainyan, B.A. S-functional derivatives of 3,4-dihydro-2H-1,4-thiasilines. Russ. J. Gen. Chem. 2015, 85, 2743–2747. [Google Scholar] [CrossRef]

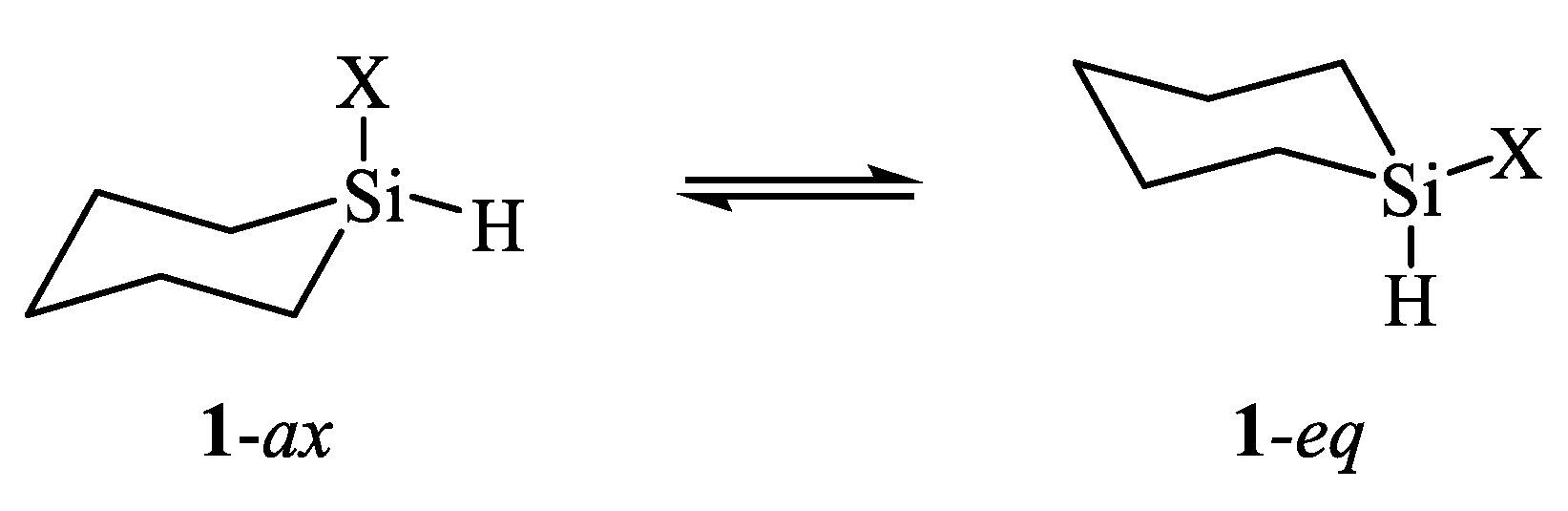
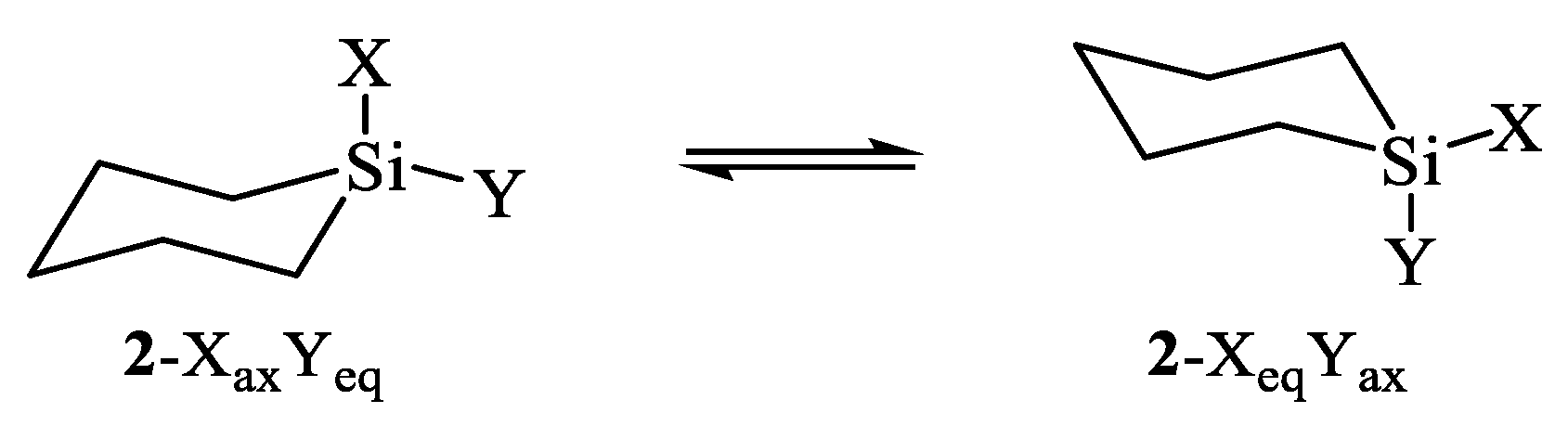
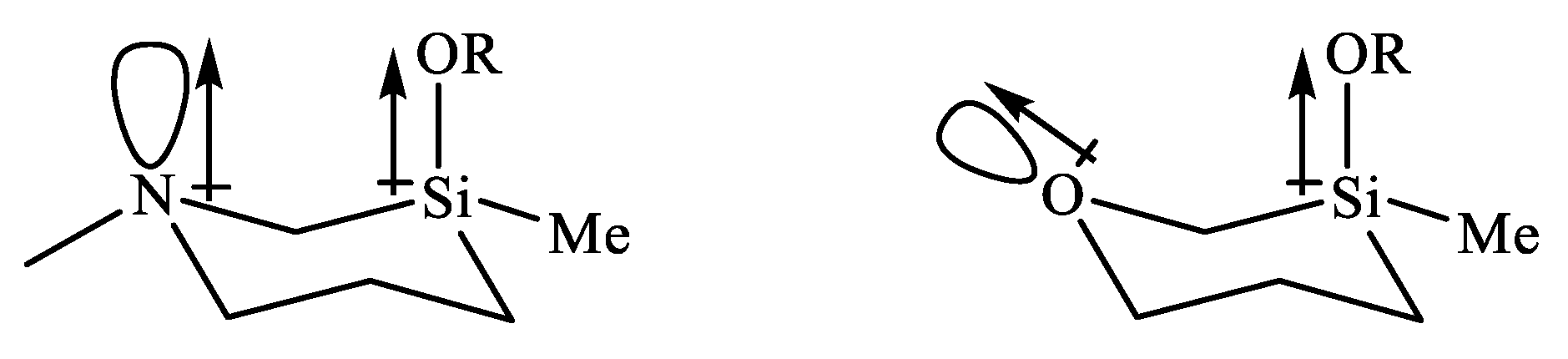
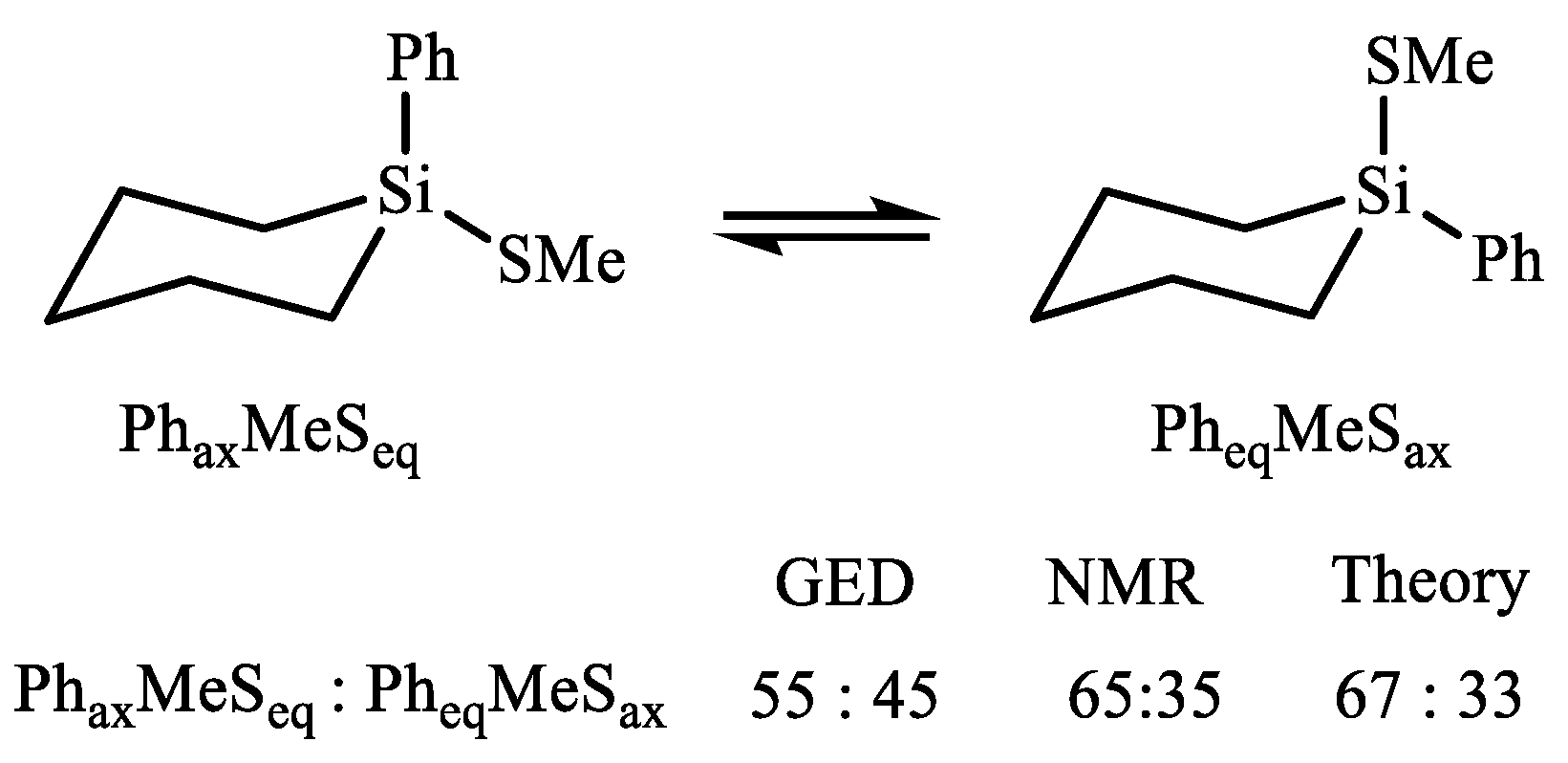
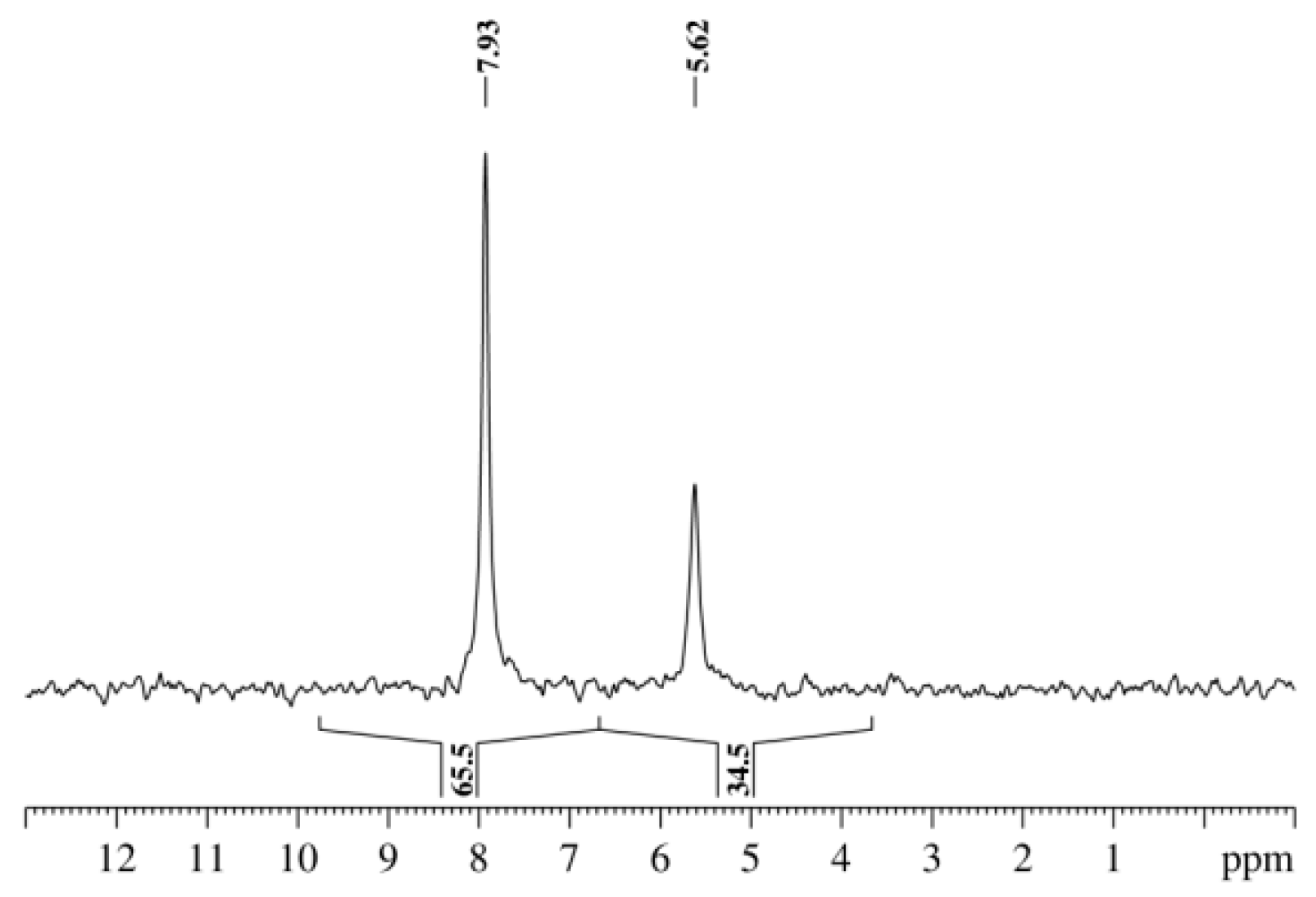

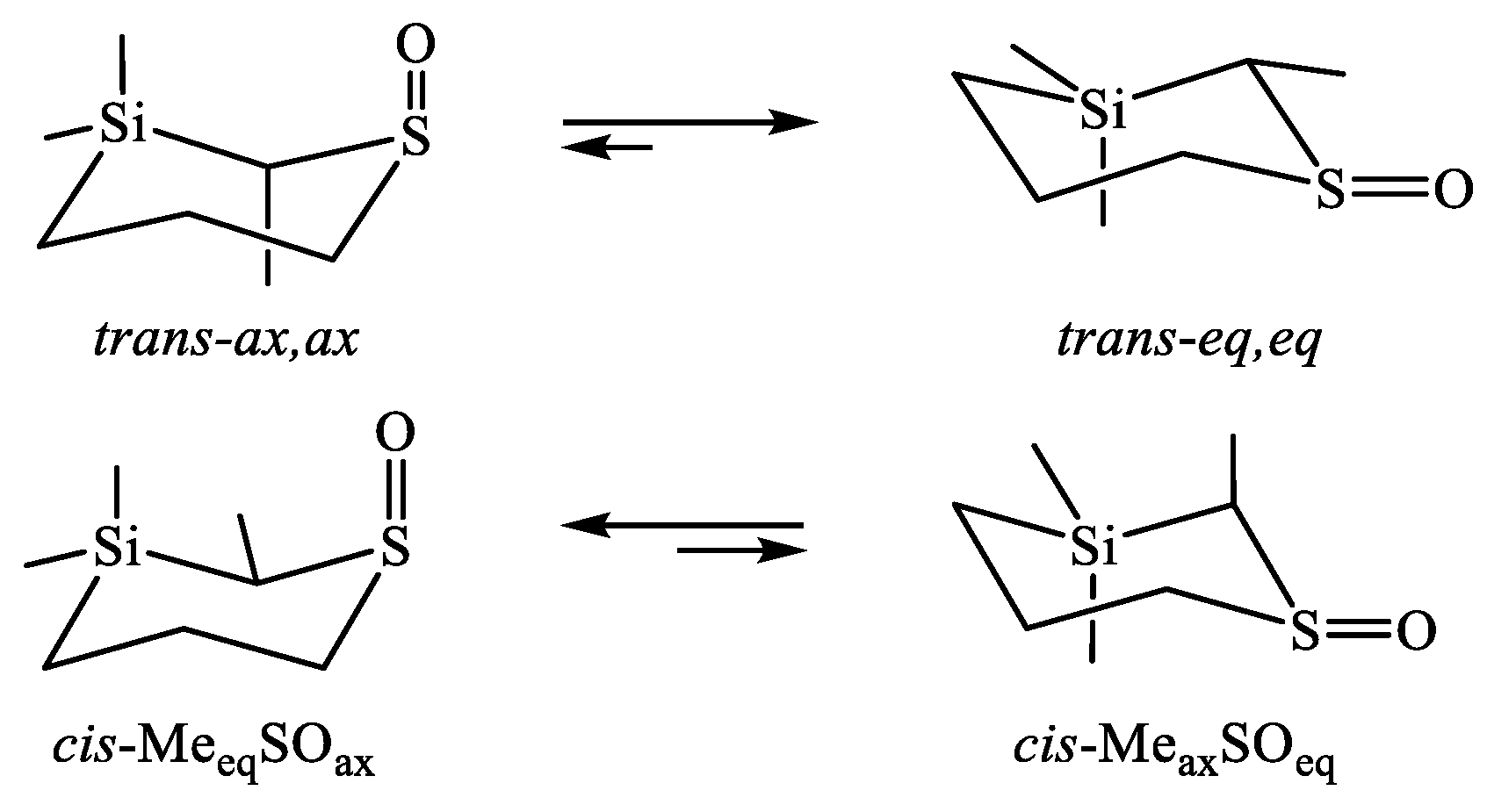
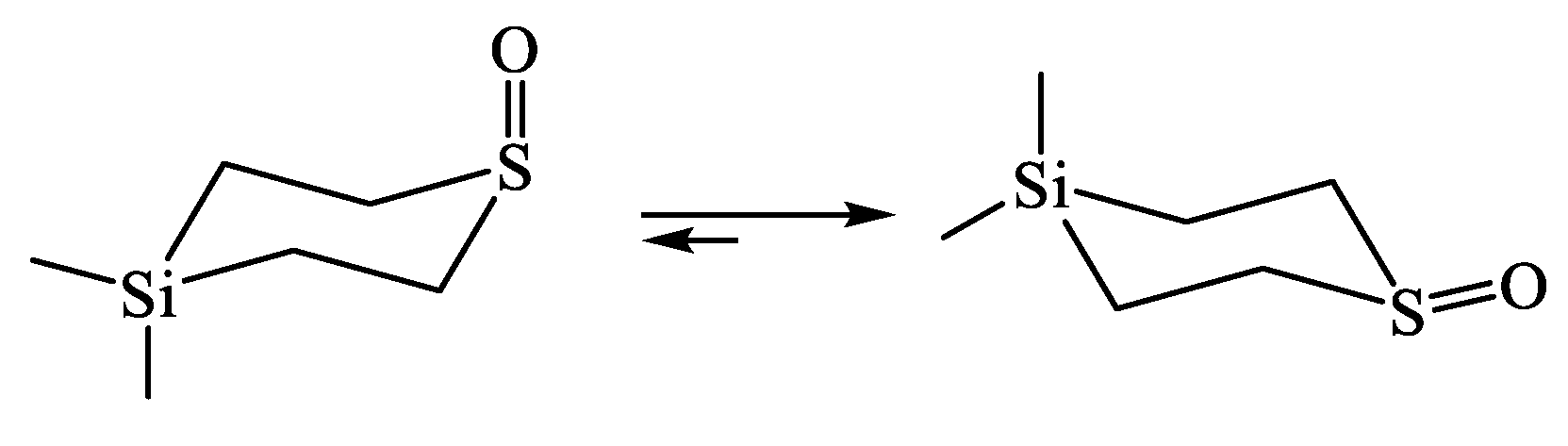
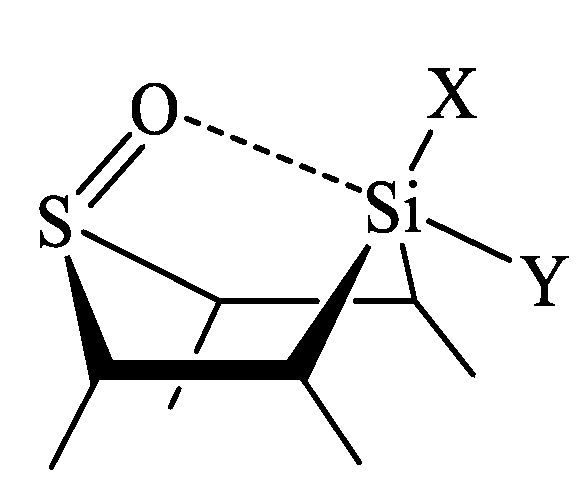
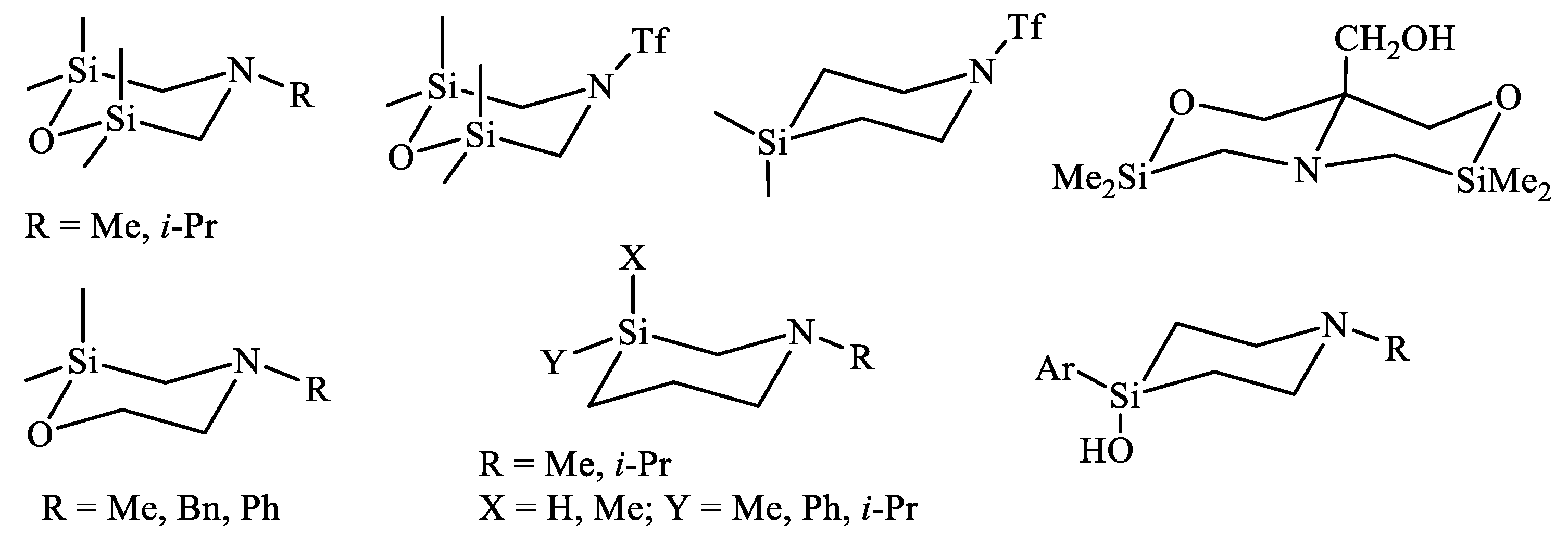

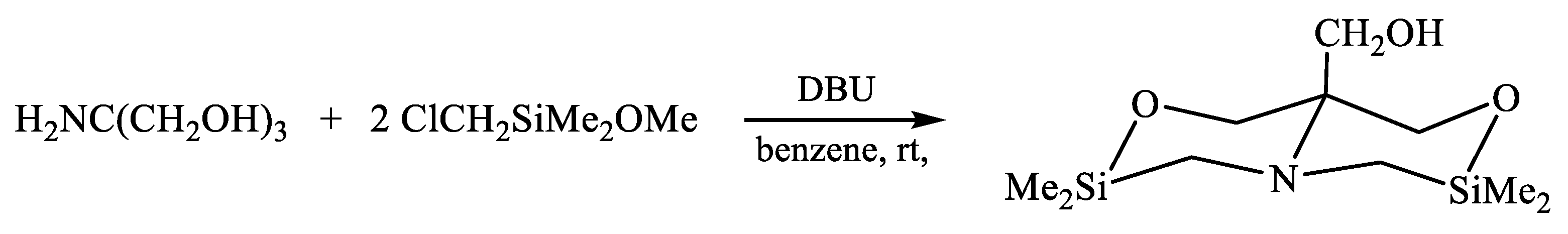
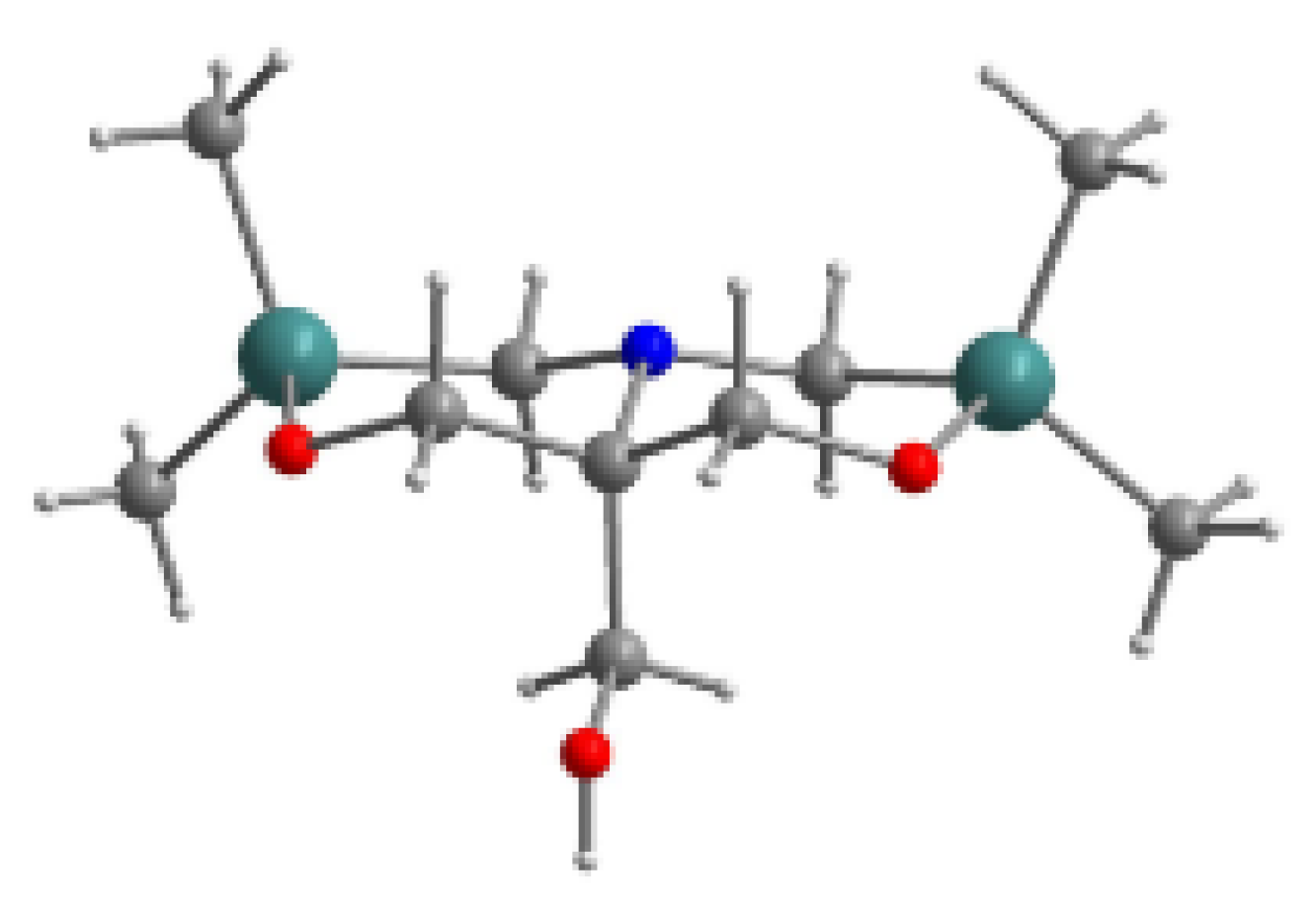
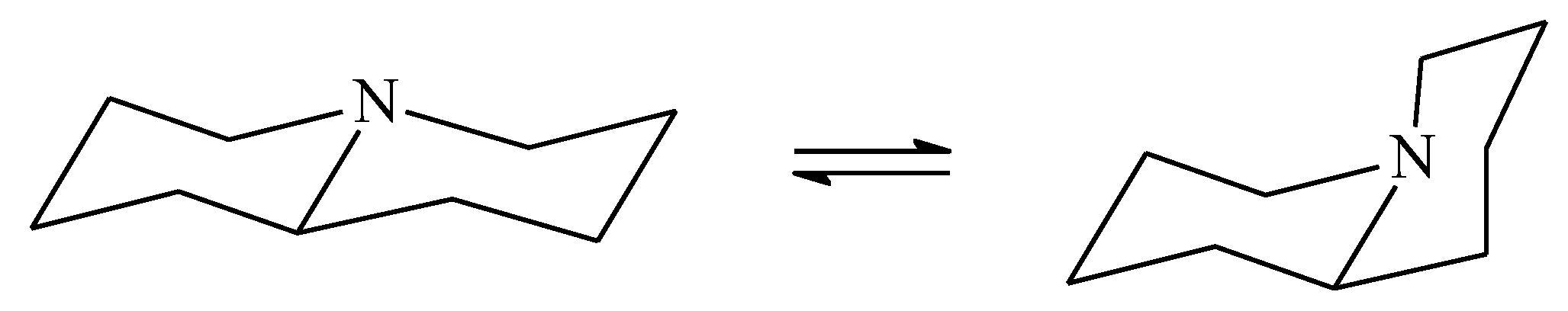
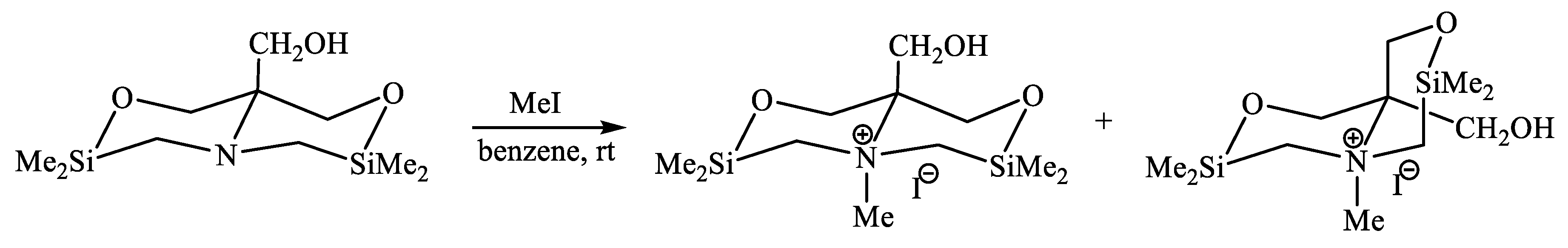


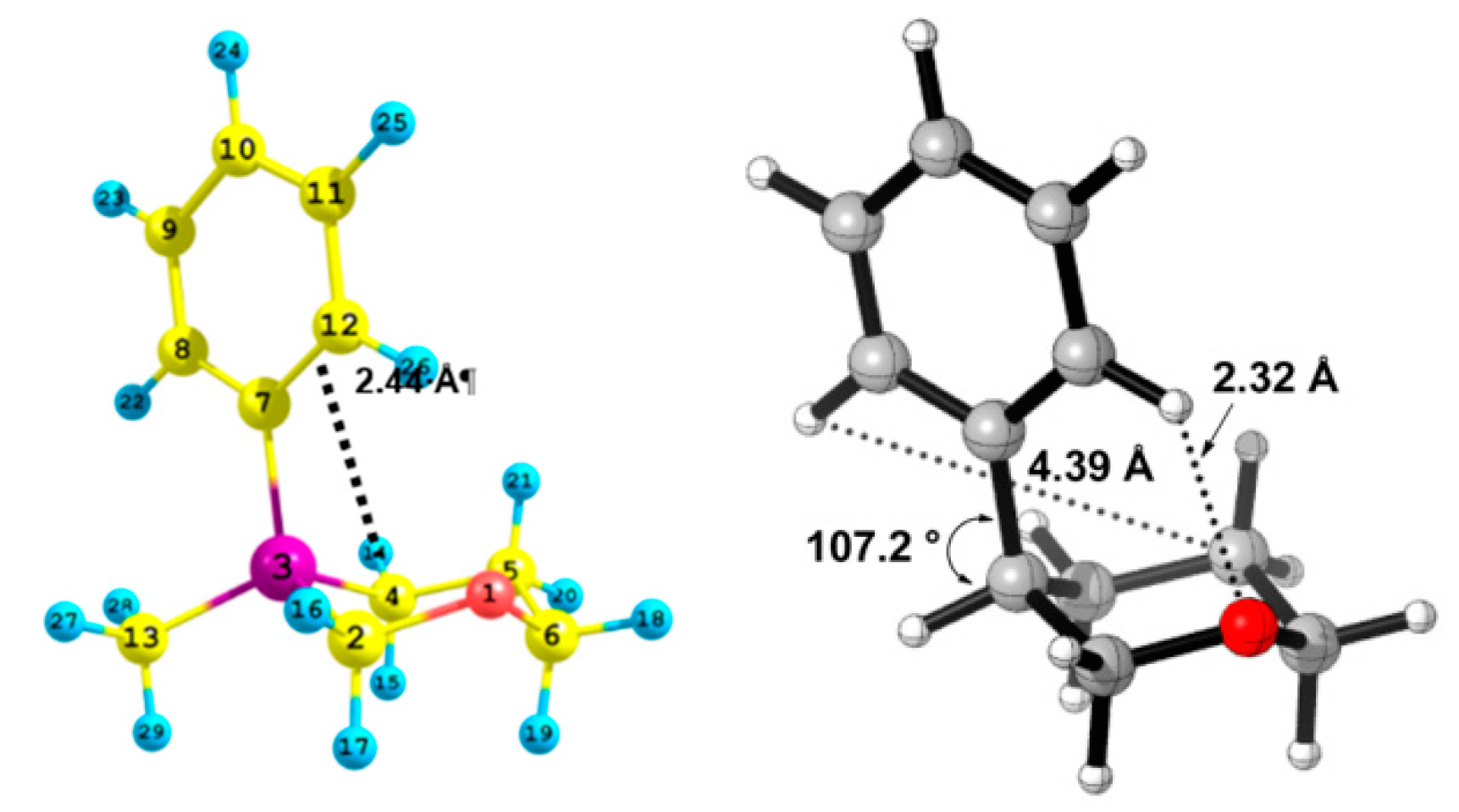
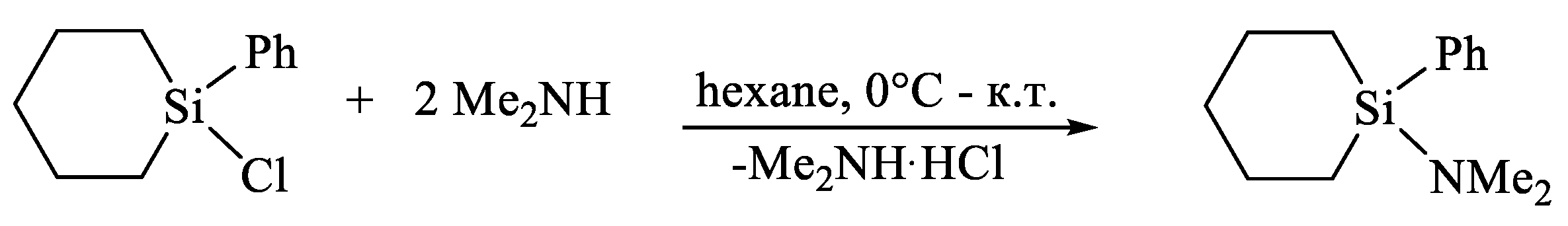
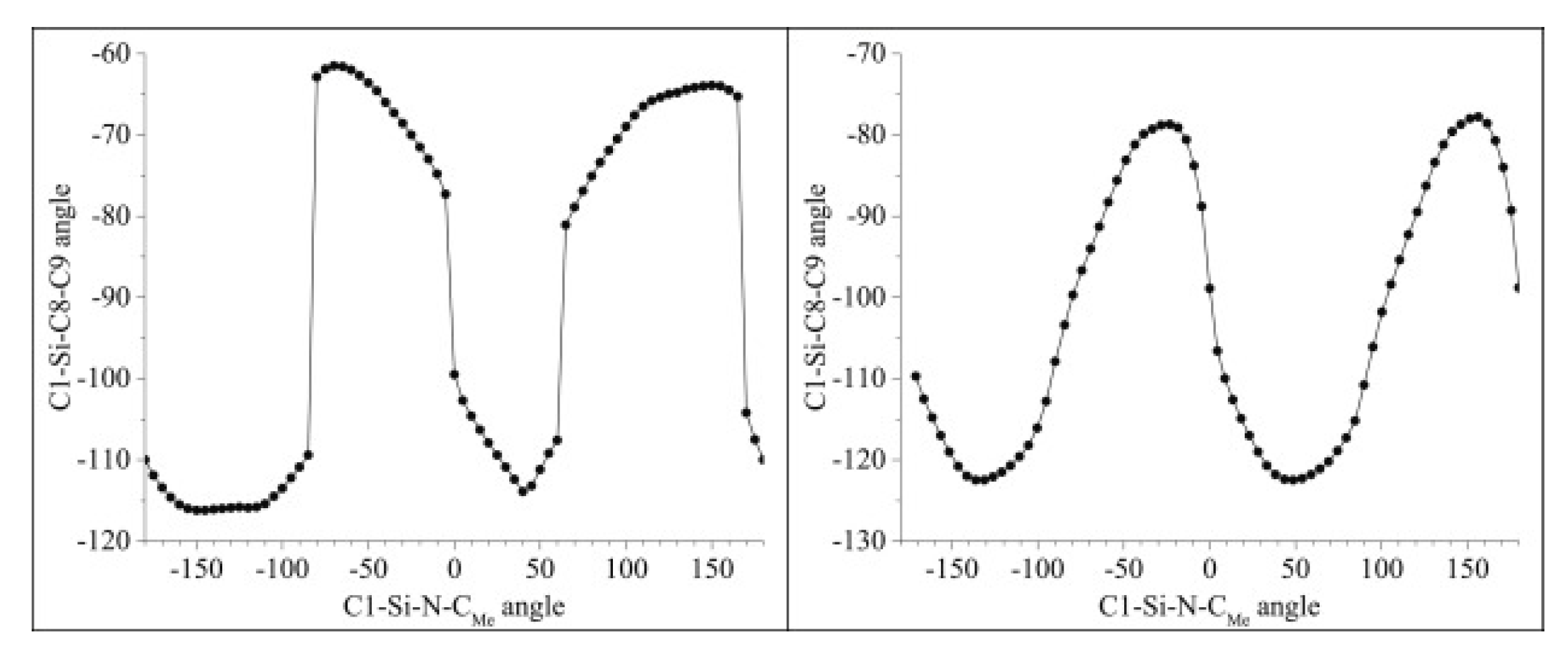
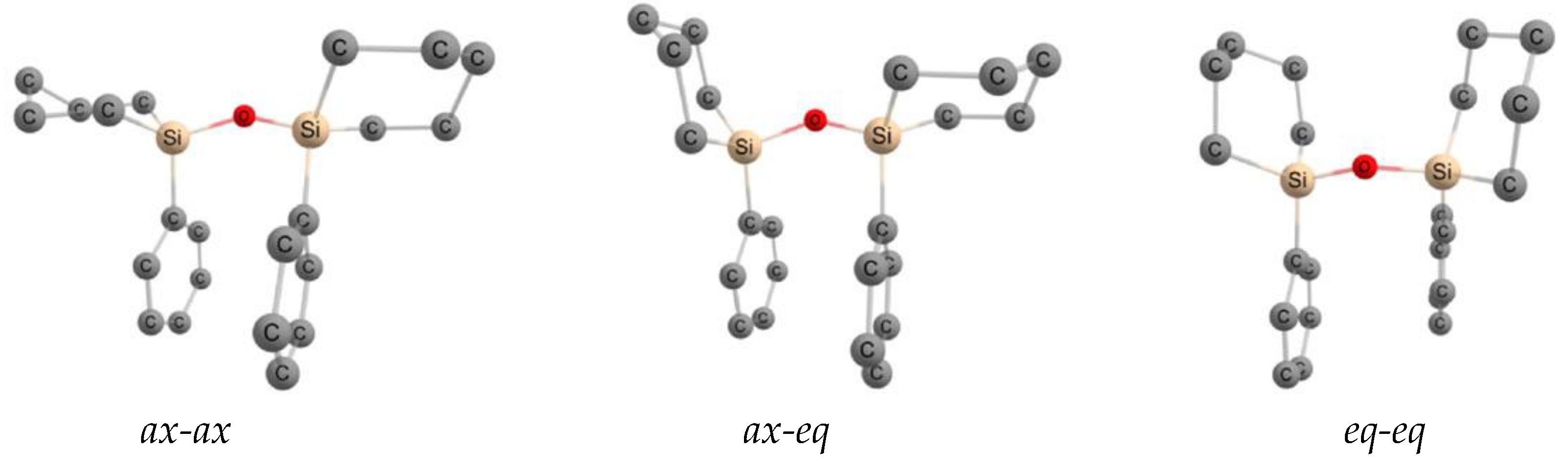
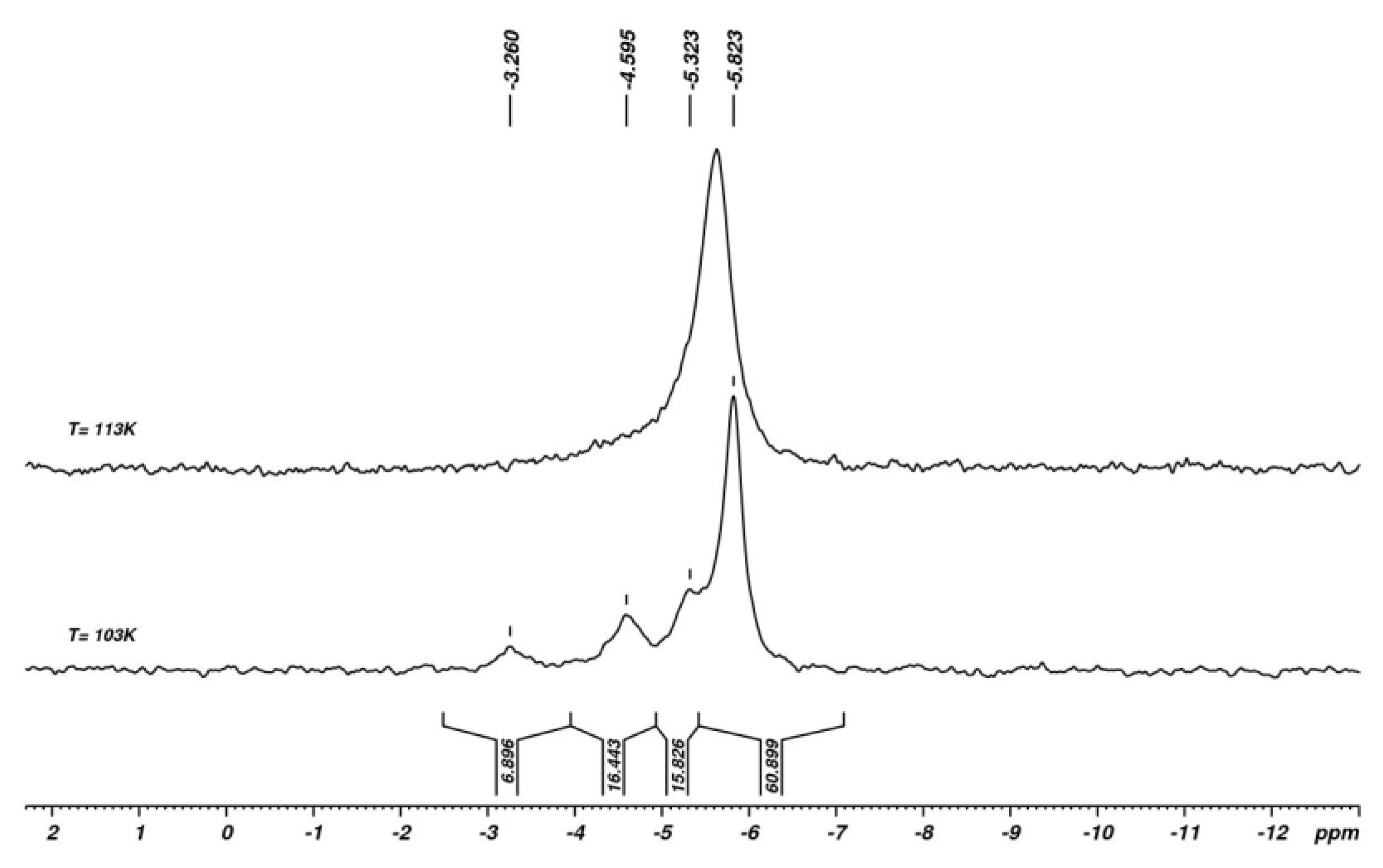
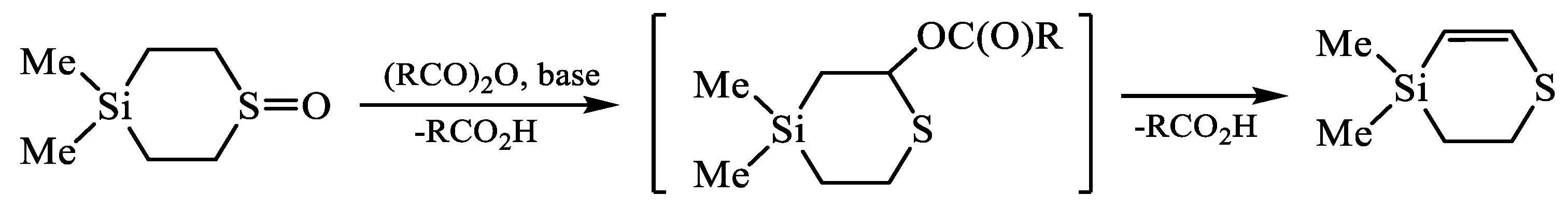
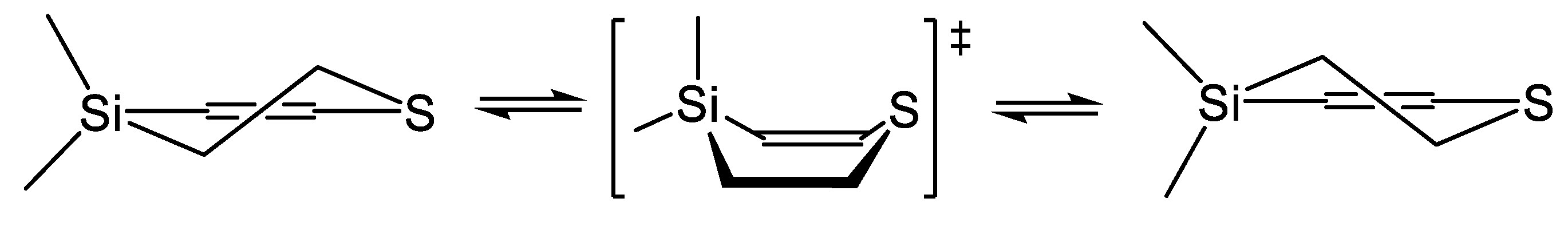

| X | A(X)C | A(X)Si |
|---|---|---|
| F | 0.36 | −0.28 [10] |
| Cl | 0.53 | −0.43 [11] |
| Br | 0.48 | −0.82 [12] |
| I | 0.49 | −0.59 [13] |
| OH | 0.6–1.0 | 0.03 [14] |
| SiH3 | 0.33 a | 0.05 [15] |
| Me | 1.76 | 0.23 [5] |
| CF3 | 2.50 | 0.40 [9] |
| Ph | 2.87 | 0.25 [6] |
| t-Bu | 4.7 | 1.30 [16] |

| X | Y | ΔG°ax–eq | ΔG°add | ΔΔG° | Ref. |
|---|---|---|---|---|---|
| Me | F | 0.86 | 1.60 | −0.74 | [18] |
| Me | CF3 | 0.53 | 1.31 | −0.78 | [18] |
| Me | Ph | 0.32 | −1.11 | 1.43 | [19,20] |
| Me | NMe2 | −0.4 | 0.21 | −0.61 | [21] |
| Ph | NMe2 | −0.5 | 1.34 | −1.84 | [22] |
| Me | OH | 0.31 | 0.73 | −0.42 | [23,24] |
| Ph | OH | 0.5 | 1.86 | −1.36 | [25] |

| No. | X | Y | Z | ΔG°ax–eq | ΔG°add | ΔΔG° | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | CH2 | Me | F | 0.32 | 0.51 | −0.19 | [18] |
| 2 | CH2 | Me | CF3 | 0.61 | 0.49 | 0.12 | [18] |
| 3 | CH2 | Me | Cl | 0.36 | 0.36 | 0.00 | [29] |
| 4 | CH2 | Me | Ph | 0.11 | 0.02 | 0.09 | [6] |
| 5 | CH2 | Ph | F | 0.46 | 0.53 | −0.07 | [8] |
| 6 | CH2 | Ph | Cl | 0.53 | 0.68 | −0.15 | [8] |
| 7 | CH2 | Ph | OR | 0.20 | (0.20) | 0.00 | [9] |
| 8 | CH2 | Ph | t-Bu | 1.1 a | 1.05 | 0.05 | a |
| 9 | NR | Me | Ph | 0.10–0.24 | (0.02) | (−0.08–(−0.22)) | [30] |
| 10 | NR | Me | OR | −0.21 | (0.20) | (−0.41) | [31] |
| 11 | O | Me | Ph | 0.16 | (0.02) | (0.14) | [32] |
| 12 | O | Me | OR | 0.02 | (0.20) | (−0.18) | [33] |
| 13 | O | Me | F | 0.37(gas) >1.4(solution) | 0.51 0.51 | 0.14 >0.9 | [34] [34] |
| 14 | S | Me | Ph | 0.15 | 0.25 | −0.10 | [6] |
| 15 | S | Me | F | −0.78 | −0.79 | 0.01 | [35] |
| R | X | Xax Conformer | ||
|---|---|---|---|---|
| Gas | Solution | Ref. | ||
| H | F | 63.0 | 64.2 | [10] |
| Cl | 62.5 | 83.0 | [73] | |
| Br | 70.5 | 85.7 | [73] | |
| I | 54.0 | 85.7 | [73] | |
| CN | 84 | 35 | [74] | |
| OMe | 59 | − * | [75] | |
| Ph | F | 40 | 76 | [76] |
| Cl | 79 | 82 | [76] | |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shainyan, B.A. Silacyclohexanes, Sila(hetero)cyclohexanes and Related Compounds: Structure and Conformational Analysis. Molecules 2020, 25, 1624. https://doi.org/10.3390/molecules25071624
Shainyan BA. Silacyclohexanes, Sila(hetero)cyclohexanes and Related Compounds: Structure and Conformational Analysis. Molecules. 2020; 25(7):1624. https://doi.org/10.3390/molecules25071624
Chicago/Turabian StyleShainyan, Bagrat A. 2020. "Silacyclohexanes, Sila(hetero)cyclohexanes and Related Compounds: Structure and Conformational Analysis" Molecules 25, no. 7: 1624. https://doi.org/10.3390/molecules25071624
APA StyleShainyan, B. A. (2020). Silacyclohexanes, Sila(hetero)cyclohexanes and Related Compounds: Structure and Conformational Analysis. Molecules, 25(7), 1624. https://doi.org/10.3390/molecules25071624






