Sedative Effects of Latexes Obtained from Some Lactuca L. Species Growing in Turkey
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Collection of Latex
4.2. HPLC Analysis
4.3. Bioactivity Tests
4.3.1. Animals
4.3.2. Administration of Test Materials
4.3.3. In Vivo Tests in Mice
Traction Test
Fireplace Test
Holeboard Test
Thiopental-Induced Sleeping Test
Statistical Analysis of the Data
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghorbani, A.; Rakhshandeh, H.; Sadeghnia, H.R. Potentiating effects of Lactuca sativa on pentobarbital-induced sleep. Iran. J. Pharm. 2013, 12, 401–406. [Google Scholar]
- Oha, D.R.; Kima, Y.; Joa, A.; Choia, E.J.; Oha, K.N.; Kima, J.; Kanga, H.; Kimb, R.Y.; Choia, V.C. Sedative and hypnotic effects of Vaccinium bracteatum Thunb. through the regulation of serotonegic and GABAA-ergic systems: Involvement of 5-HT1A receptor agonistic activity. Biomed. Pharmacother. 2019, 109, 2218–2227. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.S.; Hao, J.F.; Zhou, H.Y.; Zhu, L.X.; Wang, J.H.; Song, F.Q. Pharmacologica lstudies on the sedative-hypnotic effect of Semen Ziziphi spinosae (Suanzaoren) and Radix et Rhizoma Salviae miltiorrhizae (Danshen) extracts and the synergistic effect of their combinations. Phytomedicine 2010, 17, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Hong, K.B.; Noh, D.O.; Suh, H.J. Sleep-inducing effect of lettuce (Lactuca sativa) varieties on pentobarbital-induced sleep. Food Sci. Biotechnol. 2017, 26, 807–814. [Google Scholar] [CrossRef]
- Johnson, T. CRC Ethnobotany Desk Reference; CRC Press: Boca Raton, FL, USA, 1998; pp. 452–454. [Google Scholar]
- Michalska, K.; Szneler, E.; Kisiel, W. Sesquiterpene lactones from Lactuca canadensis and their chemotaxonomic significance. Phytochemistry 2013, 90, 90–94. [Google Scholar] [CrossRef]
- Postu, P.A.; Noumedem, J.A.; Cioanca, O.; Hancianu, M.; Mihasan, M.; Ciorpac, M.; Gorgan, D.L.; Petre, B.A.; Hritcu, L. Lactuca capensis reverses memory deficits in Aβ1-42-induced an animal model of Alzheimer’s disease. J. Cell. Mol. Med. 2018, 22, 111–122. [Google Scholar] [CrossRef]
- Gopal, S.S.; Lakshmi, M.J.; Sharavana, G.; Sathaiah, G.; Sreerama, Y.N.; Baskaran, V. Lactucaxanthin–A potential anti-diabetic carotenoid from lettuce (Lactuca sativa) inhibits α-amylase and α-glucosidase activity in vitro and in diabetic rats. Food Funct. 2017, 8, 1124–1131. [Google Scholar] [CrossRef]
- KanthaL, L.K.; Satyavathi, K.; Bhojaraju, P.; Kumar, M.P. Hepatoprotective activity of methanolic extracts of Lactuca runcinata DC and Gyrocarpus asiaticus Willd. Beni-Suef Univ. J. Basic Appl. Sci. 2017, 6, 321–325. [Google Scholar] [CrossRef]
- Sadeghnia, H.R.; Farahm, S.K.; Asadpour, E.; Rakhsh, H.; Ghorbani, A. Neuroprotective effect of Lactuca sativa on glucose/serum deprivation-induced cell death. Afr. J. Pharm. Pharmacol. 2012, 6, 2464–2471. [Google Scholar] [CrossRef]
- Sayyah, M.; Hadidi, N.; Kamalinejad, M. Analgesic and anti-inflammatory activity of Lactuca sativa seed extract in rats. J. Ethnopharmacol. 2004, 92, 325–329. [Google Scholar] [CrossRef]
- Harsha, S.N.; Anilakumar, K.R. Effects of Lactuca sativa extract on exploratory behavior pattern, locomotor activity and anxiety in mice. Asian Pac. J. Trop. Dis. 2012, 2, S475–S479. [Google Scholar] [CrossRef]
- Chu, Y.-F.; Sun, J.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common vegetables. J. Agric. Food Chem. 2002, 50, 6910–6916. [Google Scholar] [CrossRef] [PubMed]
- Michalska, K.; Stojakowska, A.; Malarz, J.; Doležalová, I.; Lebeda, A.; Kisiel, W. Systematic implications of sesquiterpene lactones in Lactuca species. Biochem. Syst. Ecol. 2009, 37, 174–179. [Google Scholar] [CrossRef]
- Wesołowska, A.; Nikiforuk, A.; Michalska, K.; Kisiel, W.; Chojnacka-Wójcik, E. Analgesic and sedative activities of lactucin and some lactucin-like guaianolides. J. Ethnopharmacol. 2006, 107, 254–258. [Google Scholar] [CrossRef]
- Kim, H.W.; Suh, H.J.; Choi, H.S.; Hong, K.B.; Jo, K. Effectiveness of the sleep enhancement by green romaine lettuce (Lactuca sativa) in a rodent model. Biol. Pharm. Bull. 2019, 42, 1726–1732. [Google Scholar] [CrossRef]
- Baytop, T. Turkiye’de Bitkiler ile Tedavi (Gecmiste ve Bugun); Nobel Tip Kitabevleri: Istanbul, Turkey, 1998. [Google Scholar]
- Ismail, H.; Mirza, B. Evaluation of analgesic, anti-inflammatory, anti-depressant and anti-coagulant properties of Lactuca sativa (CV. Grand Rapids) plant tissues and cell suspension in rats. BMC Complement. Altern. Med. 2015, 15, 199. [Google Scholar] [CrossRef]
- Sutrisna, E.; Azizah, T.; Wuryaningrum, A.; Sari, M.P. The potency of Lactuca sativa Linn. and Apium graveolens L. from Indonesia as tranquilizer. Int. J. Ayurveda Pharm. Res. 2015, 3, 6–11. [Google Scholar]
- Sessa, R.A.; Bennett, M.H.; Lewis, M.J.; Mansfield, J.W.; Beale, M.H. Metabolite profiling of sesquiterpene lactones from Lactuca species major latex components are novel oxalate and sulfate conjugates of lactucin and its derivatives. J. Biol. Chem. 2000, 275, 26877–26884. [Google Scholar]
- Courvoisier, S.; Ducrot, R.; Julou, L. Psychotropic Drugs; Garattini, S., Ghetti, V., Eds.; Elsevier: Amsterdam, The Netherlands, 1957. [Google Scholar]
- Laroche, M.-J.; Rousselet, F. Les Animaux de Laboratoire: Éthique et Bonnes Pratiques; Editions Masson: Paris, France, 1990. [Google Scholar]
- Srikanth, J.; Muralidharan, P. CNS activity of the methanol extracts of Sapindus emarginatus Vahl in experimental animal models. J. Sci. Res. 2009, 1, 583–593. [Google Scholar] [CrossRef]
- Jänicke, B.; Coper, H. Tests in rodents for assessing sensorimotor performance during aging. In Advances in Psychology; Ferrandez, A.-M., Teasdale, N., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 114, pp. 201–233. [Google Scholar]
- Hoffmann, G. Les Animaux de Laboratoire: Précis; Editions Masson: Paris, France, 1963. [Google Scholar]
- Alnamer, R.; Alaoui, K.; Bouidida, E.H.; Benjouad, A.; Cherrah, Y. Sedative and hypnotic activities of the methanolic and aqueous extracts of Lavandula officinalis from Morocco. Adv. Pharmacol. Sci. 2012, 2012, 270824. [Google Scholar]
- Clark, G.; Koester, A.G.; Pearson, D.W. Exploratory behavior in chronic disulfoton poisoning in mice. Psychopharmacology 1971, 20, 169–171. [Google Scholar] [CrossRef]
- File, S.E.; Wardill, A.G. Validity of head-dipping as a measure of exploration in a modified hole-board. Psychopharmacology 1975, 44, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Khan, I. Pharmacological evaluation of sedative and hypnotic activities of methanolic extract of Lycopus europaeus in mice. Int. J. Phytopharm. 2013, 2, 8–12. [Google Scholar]
- Williamson, E.M.; Okpako, D.T.; Evans, F.J. Selection, Preparation and Pharmacological Evaluation of Plant Material; John Wiley & Sons: Hoboken, NJ, USA, 1996; Volume 1. [Google Scholar]
- Herrera-Ruiz, M.; Gutiérrez, C.; Jiménez-Ferrer, J.E.; Tortoriello, J.; Mirón, G.; León, I. Central nervous system depressant activity of an ethyl acetate extract from Ipomoea stans roots. J. Ethnopharmacol. 2007, 112, 243–247. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
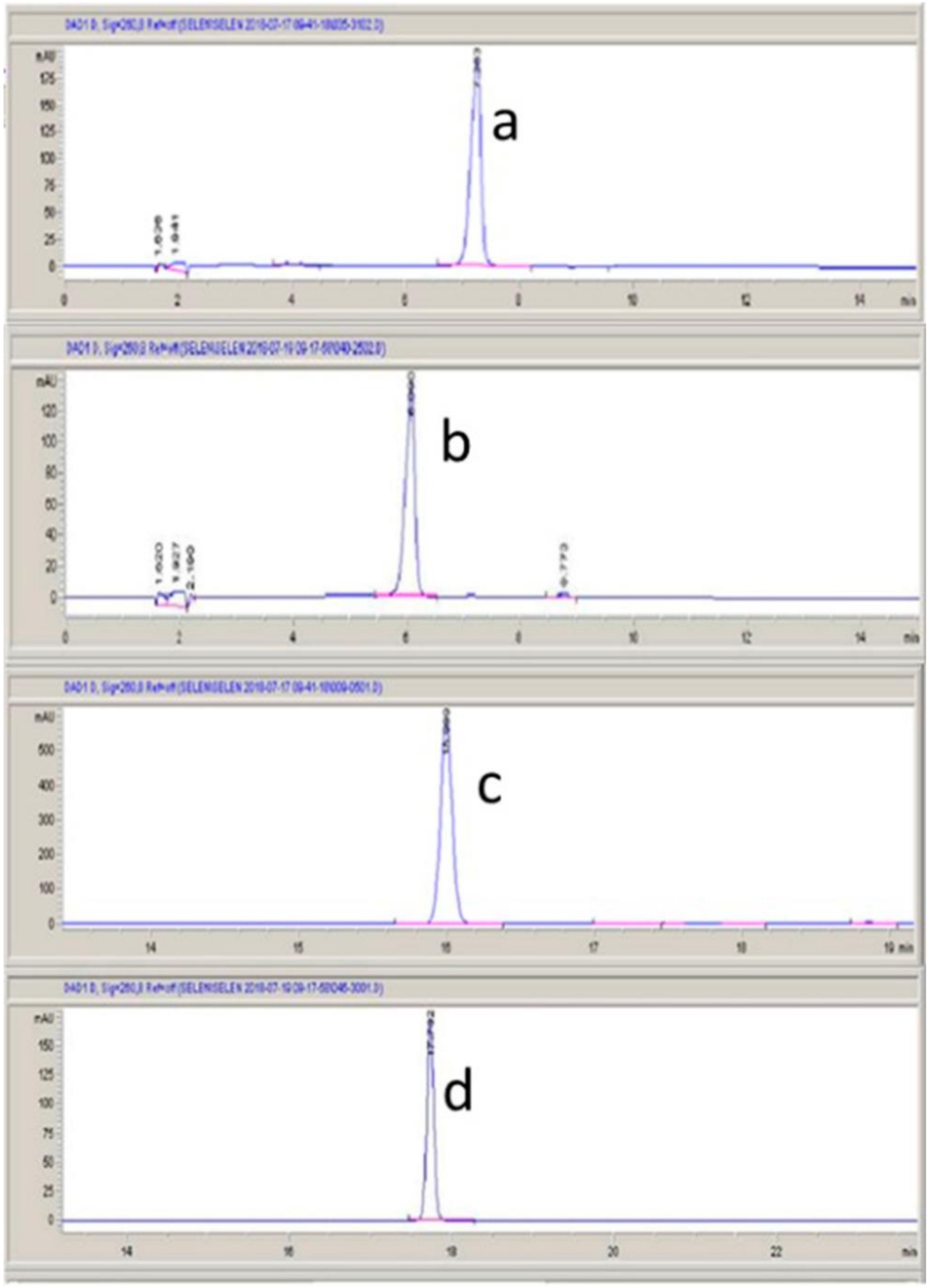

| Test Material | Dose (mg/kg) | Traction Test (s) (Re-Establishment Time) Mean ± SEM | Fireplace Test (s) (Time to Go Back the Tube in Seconds) Mean ± SEM | Holeboard Test (Explored Holes during 5 min) Mean ± SEM |
|---|---|---|---|---|
| Control | - | 0.11 ± 0.00 | 9.14 ± 0.87 | 34.11 ± 1.86 |
| LG | 100 | 2.15 ± 0.19 | 12.56 ± 1.22 | 33.47 ± 1.18 |
| LM | 100 | 1.05 ± 0.06 | 10.28 ± 0.93 | 31.06 ± 1.93 |
| LSA | 100 | 7.83 ± 0.29 ** | 131.84 ± 1.41 *** | 6.21 ± 0.52 ** |
| LSE | 100 | 5.34 ± 0.37 * | 93.99 ± 0.98 ** | 15.64 ± 0.97 * |
| LV | 100 | 4.21 ± 0.14 * | 75.99 ± 1.04 * | 27.58 ± 1.10 |
| Lorazepam | 1 | 10.27 ± 0.43 *** | 168.39 ± 1.35 *** | 0.00 ± 0.00 *** |
| Test Material | Dose (mg/kg) | Onset of Sleeping (min) Mean ± SEM | Sleeping Duration (min) Mean ± SEM |
|---|---|---|---|
| Control | - | 54.11 ± 1.98 | 60.28 ± 2.47 |
| LG | 100 | 49.53 ± 2.75 | 78.61 ± 2.80 |
| LM | 100 | 52.81 ± 2.90 | 66.49 ± 2.64 |
| LSA | 100 | 20.13 ± 1.71 *** | 224.26 ± 2.52 *** |
| LSE | 100 | 24.77 ± 1.93 * | 205.91 ± 2.87 ** |
| LV | 100 | 36.15 ± 2.01 * | 173.66 ± 2.98 * |
| Lorazepam | 1 | 17.43 ± 1.54 *** | 297.15 ± 2.83 *** |
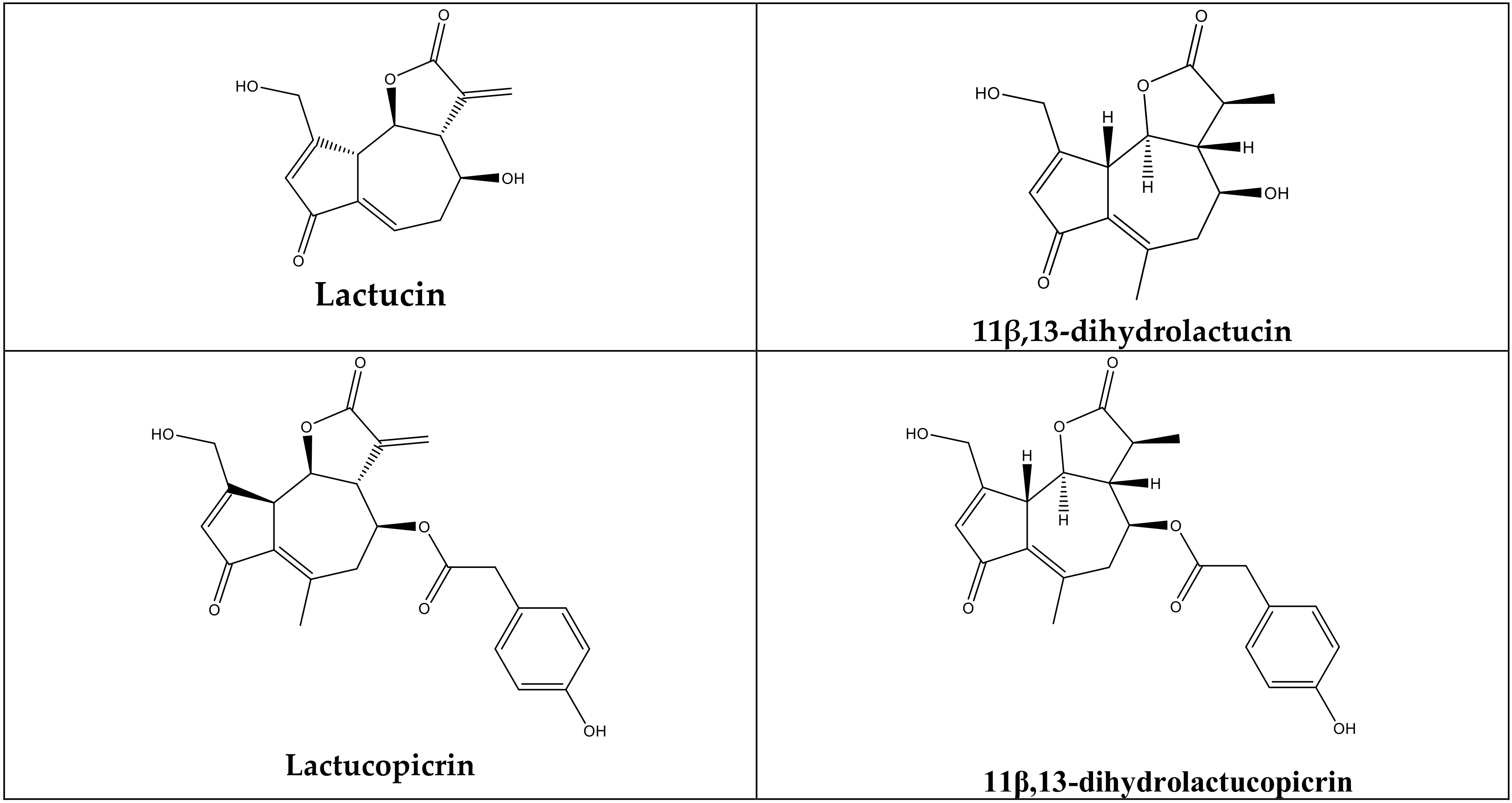
| Materials | Lactucin (mg std/glatex) | Lactucopicrin (mg std/glatex) | 11β,13 Dihydrolactucopicrin (mg std/glatex) | 11β,13 Dihydrolactucin (mg std/glatex) |
|---|---|---|---|---|
| LG | 0.45551 ± 0.01 * | - | 0.75238 ± 0.01 * | 0.55975 ± 0.1 * |
| LM | - | - | - | - |
| LSA | 13.94970 ± 0.24 * | 0.61729 ± 0.02 * | 0.49266 ± 0.01 * | 0.47723 ± 0.01 * |
| LSE | 57.53249 ± 0.27 * | - | - | 2.35356 ± 0.03 * |
| LV | 1.40352 ± 0.01 * | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilgün, S.; Küpeli Akkol, E.; Ilhan, M.; Çiçek Polat, D.; Baldemir Kılıç, A.; Coşkun, M.; Sobarzo-Sánchez, E. Sedative Effects of Latexes Obtained from Some Lactuca L. Species Growing in Turkey. Molecules 2020, 25, 1587. https://doi.org/10.3390/molecules25071587
Ilgün S, Küpeli Akkol E, Ilhan M, Çiçek Polat D, Baldemir Kılıç A, Coşkun M, Sobarzo-Sánchez E. Sedative Effects of Latexes Obtained from Some Lactuca L. Species Growing in Turkey. Molecules. 2020; 25(7):1587. https://doi.org/10.3390/molecules25071587
Chicago/Turabian StyleIlgün, Selen, Esra Küpeli Akkol, Mert Ilhan, Derya Çiçek Polat, Ayse Baldemir Kılıç, Maksut Coşkun, and Eduardo Sobarzo-Sánchez. 2020. "Sedative Effects of Latexes Obtained from Some Lactuca L. Species Growing in Turkey" Molecules 25, no. 7: 1587. https://doi.org/10.3390/molecules25071587
APA StyleIlgün, S., Küpeli Akkol, E., Ilhan, M., Çiçek Polat, D., Baldemir Kılıç, A., Coşkun, M., & Sobarzo-Sánchez, E. (2020). Sedative Effects of Latexes Obtained from Some Lactuca L. Species Growing in Turkey. Molecules, 25(7), 1587. https://doi.org/10.3390/molecules25071587








