Green Synthesis of an Activated Carbon-Supported Ag and ZnO Nanocomposite for Photocatalytic Degradation and Its Antibacterial Activities
Abstract
1. Introduction
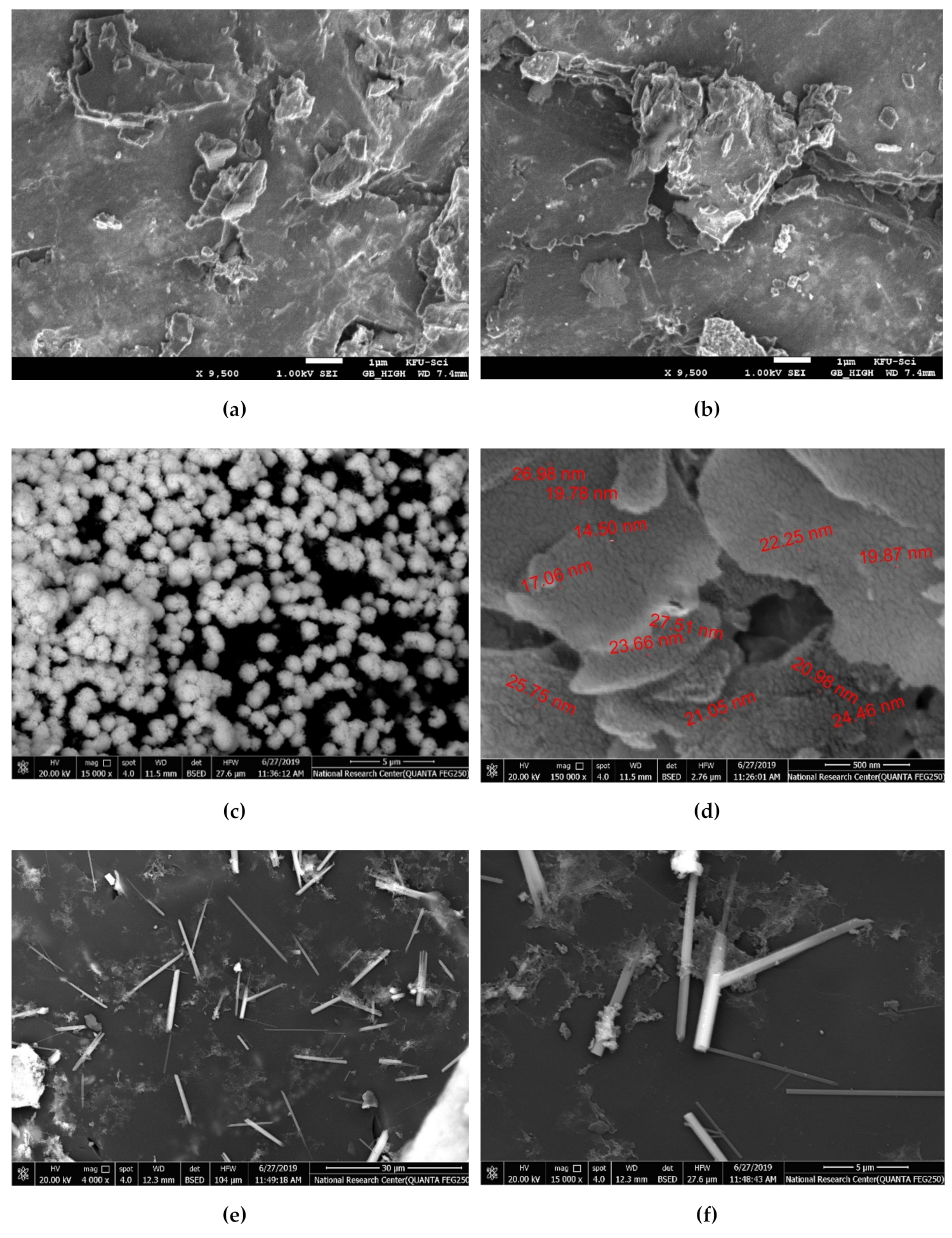
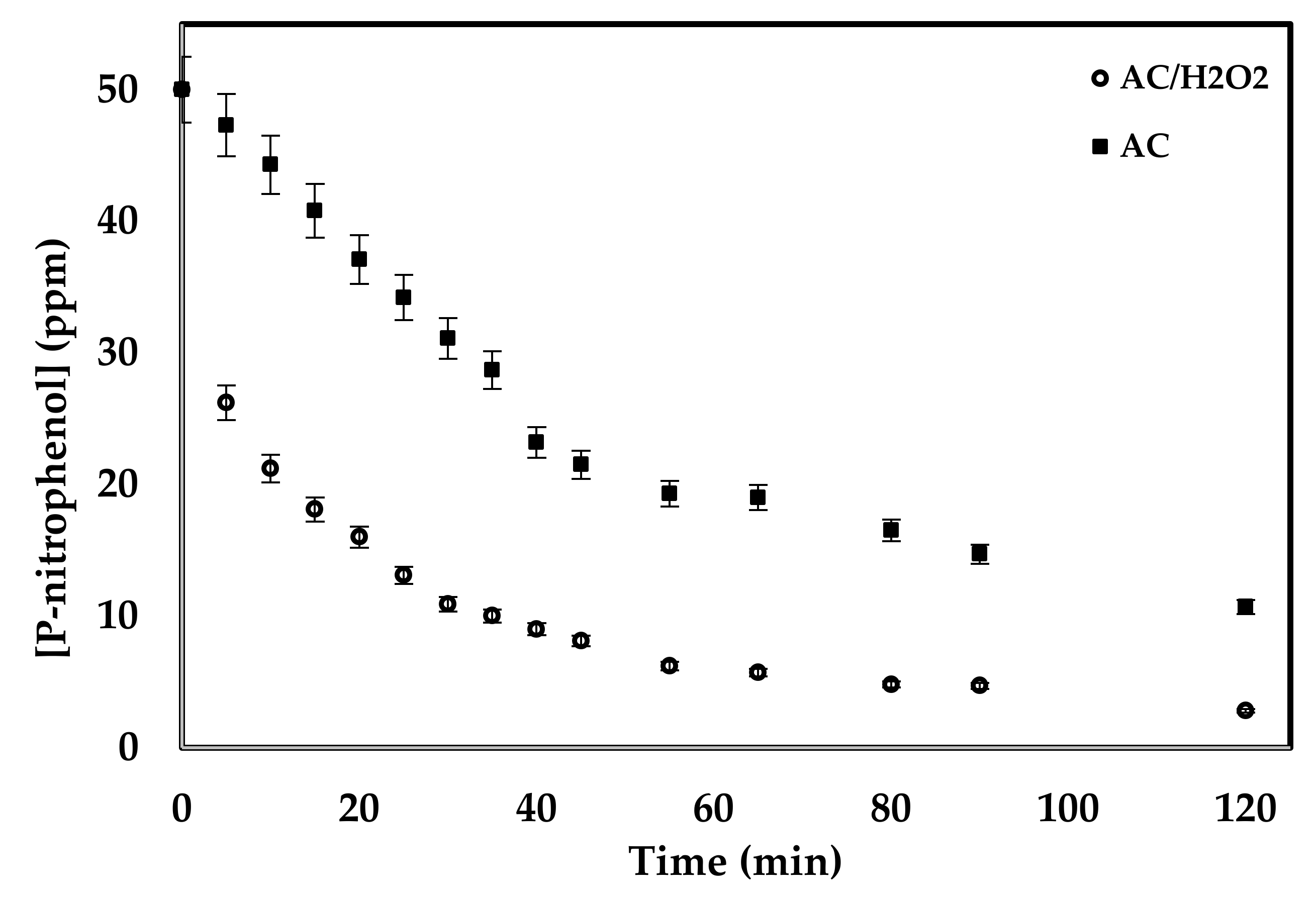
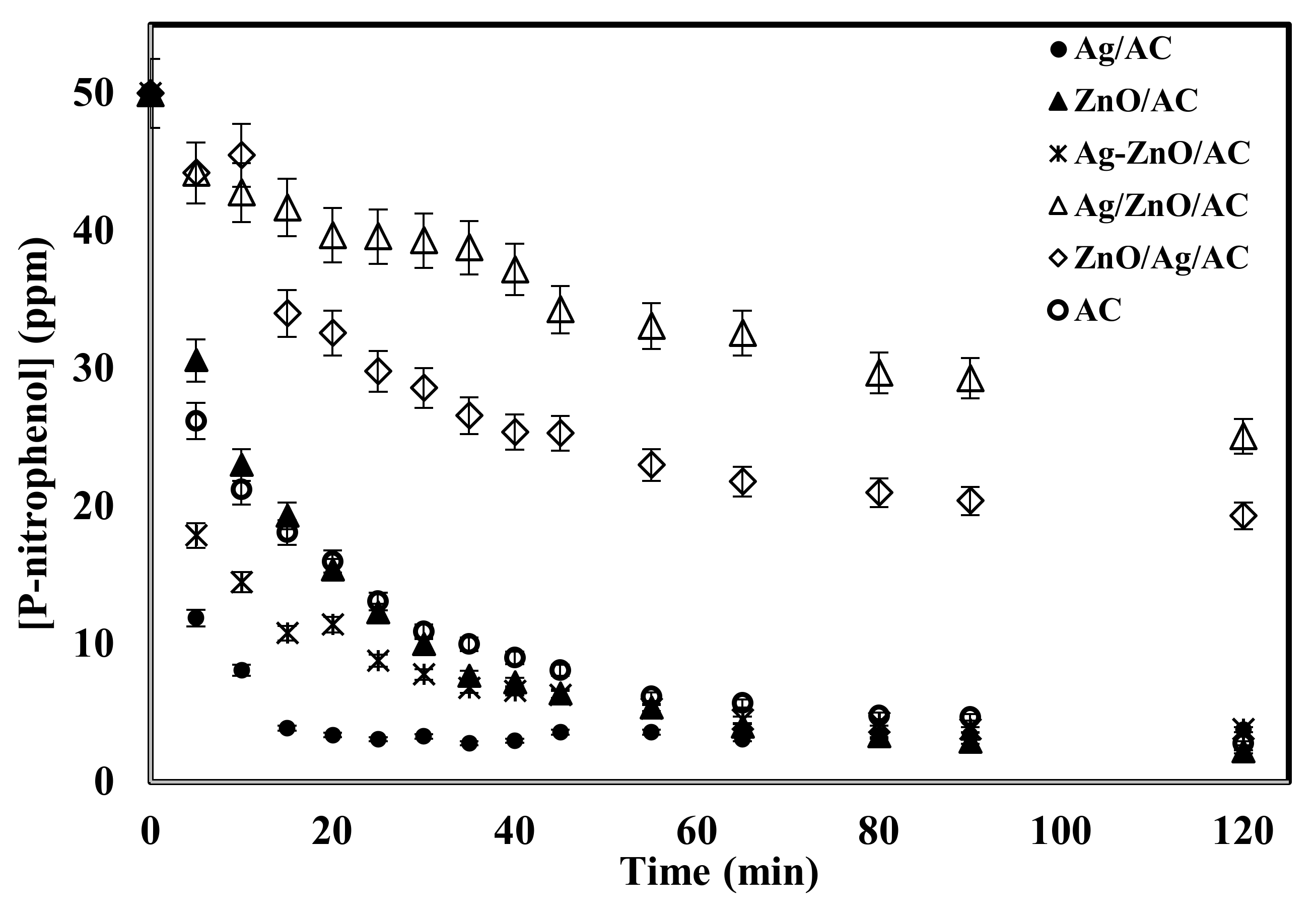
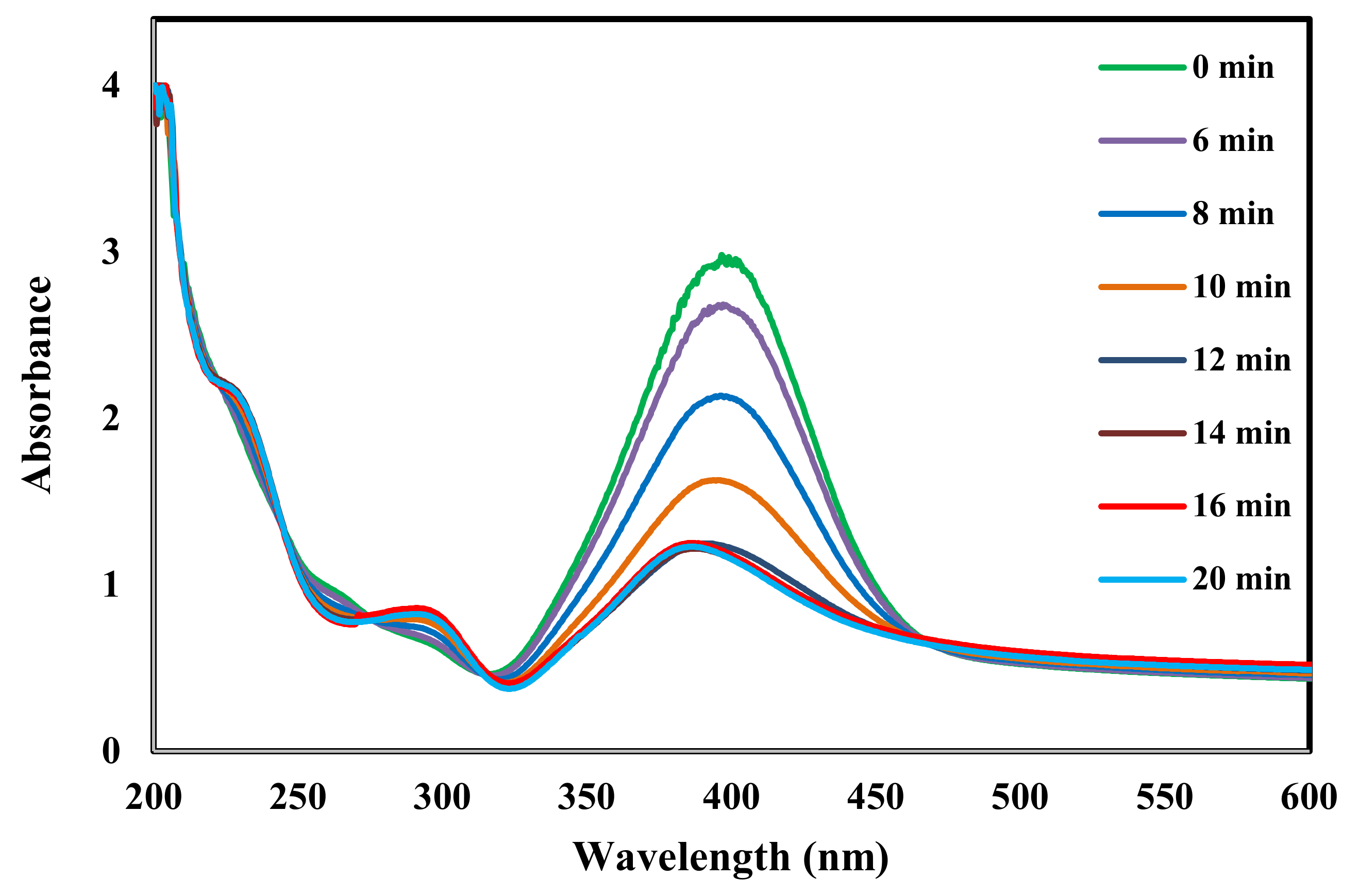
2. Results and Discussion
2.1. Catalysis Activity of the Prepared Samples
2.2. Antibacterial Activity
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of the Activated Carbon
3.3. Preparation of Neem leaf Extract
3.4. Preparation of Ag/AC
3.5. Preparation of ZnO/AC
3.6. Preparation of ZnO/Ag/AC by Successive Precipitation
3.7. Preparation of Ag/ZnO/AC by Successive Precipitation
3.8. Preparation of Ag-ZnO/AC by Co-Precipitation
3.9. Catalytic Activity
3.10. Anti-Bacterial Activity
3.11. Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dashamiri, S.; Ghaedi, M.; Dashtian, K.; Rahimi, M.R.; Goudarzi, A.; Jannesar, R. Ultrasonic enhancement of the simultaneous removal of quaternary toxic organic dyes by CuO nanoparticles loaded on activated carbon: Central composite design, kinetic and isotherm study. Ultrason. Sonochem. 2016, 31, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Eltugral, N.; Simsir, H.; Karagoz, S. Preparation of nano-silver-supported activated carbon using different ligands. Res. Chem. Intermed. 2016, 42, 1663–1676. [Google Scholar] [CrossRef]
- Karthik, C.; Radha, K.V. Silver Nanoparticle Loaded Activated Carbon: An Escalated Nanocomposite with Antimicrobial Property. Orient. J. Chem. 2016, 32, 735–741. [Google Scholar] [CrossRef]
- Acevedo, S.; Arevalo-fester, J. Efficiency Study of Silver Nanoparticles (AgNPs) Supported on Granular Activated Carbon against Escherichia coli. J. Nanomed. Res. Effic. 2014, 1, 4–8. [Google Scholar] [CrossRef]
- Nanoparticles, M.; Furlan, P.Y.; Fisher, A.J.; Furlan, A.Y.; Melcer, M.E.; Shinn, D.W.; Warren, J.B. Magnetically Recoverable and Reusable Nanoparticles for Water Disinfection. Inventions 2017, 2, 10. [Google Scholar] [CrossRef]
- Pollutants, M.; Disinfection, W.; Cairo, G. Using Silver Nanoparticles Coated on Activated Carbon Granules in Columns for Microbiological Pollutants Water Disinfection in Abu Rawash area, Great Cairo, Egypt. Aust. J. Basic Appl. Sci. 2013, 7, 422–432. [Google Scholar]
- Sadat, M.; Mojtaba, N.; Seyedeh, H.; Ghasemi, S.; Arab, M. Synthesis of ZnO nanostructure using activated carbon for photocatalytic degradation of methyl orange from aqueous solutions. Appl. Water Sci. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Marahel, F.; Ghaedi, M.; Ansari, A. Zinc Oxide Nanoparticles Loaded on Activated Carbon and Its Application for Adsorption Removal of Uric Acid. Synth. React. Inorg. Met.-Org Nano-Metal Chem. 2015, 45, 1387–1395. [Google Scholar] [CrossRef]
- Quoc, T.; Van Son, N.; Thi, H.; Dung, K.; Hoang, N.; Thu, B.; Thi, N.; Anh, V.; Dinh, N.; Hoang, N. Preparation and properties of silver nanoparticles loaded in activated carbon for biological and environmental applications. J. Hazard. Mater. 2011, 192, 1321–1329. [Google Scholar] [CrossRef]
- Karimi, M.A.; Hatefi-Mehrjardi, A.; Askarpour Kabir, A.; Zaydabadi, M. Synthesis, characterization, and application of MgO/ZnO nanocomposite supported on activated carbon for photocatalytic degradation of methylene blue. Res Chem Intermed. 2015, 41, 6157–6168. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Z.; Li, G.; Li, P.; Zhang, W.; Han, Z.; Lian, K.; Hu, J. Synthesis and characterization of Ag@ZnO nanostructures for photocatalytic degradation of rhodamine B: Influence of calcination temperature and Ag content. Appl. Phys. A. 2017, 123, 1–9. [Google Scholar] [CrossRef]
- Suresh, P.; Vijaya, J.J.; Kennedy, L.J. Synergy effect in the photocatalytic degradation of textile dyeing waste water by using microwave combustion synthesized zinc oxide supported activated carbon. React. Kinet. Mech. Catal. 2015, 114, 767–780. [Google Scholar] [CrossRef]
- Adio, S.O.; Rana, A.; Chanabsha, B.; BoAli, A.A.K.; Essa, M.; Alsaadi, A. Silver Nanoparticle-Loaded Activated Carbon as an Adsorbent for the Removal of Mercury from Arabian Gas-Condensate, Arab. J. Sci. Eng. 2019, 44, 6285–6293. [Google Scholar] [CrossRef]
- Tavakol, M.; Azar, P.A.; Saber-Tehrani, M.; Ghaedi, M. Silver Nanoparticles Loaded on Activated Carbon as a Novel Adsorbent for The Competitive Removal of Malachite Green and Methylene Blue. Int. J. Life Sci. 2015, 9, 75–92. [Google Scholar] [CrossRef]
- Altıntığ, E.; Soydan, Ö.F. Metheylene Blue Adsorption and Preparation Silver Bound to Activated Carbon with Sol-Gel Methods. Sak. Univ. J. Sci. 2018, 22, 1812–1819. [Google Scholar]
- Ghaedi, M.; Ghayedi, M.; Nasiri, S.; Sahraei, R.; Daneshfar, A. Palladium, silver, and zinc oxide nanoparticles loaded on activated carbon as adsorbent for removal of bromophenol red from aqueous solution. J. Ind. Eng. Chem. 2013, 19, 1209–1217. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, L.; Wang, F.; Cai, Y.; Du, S.; Zhang, Z. Silver nanoparticles/activated carbon composite as a facile SERS substrate for highly sensitive detection of endogenous formaldehyde in human urine by catalytic reaction. Talanta 2018, 188, 630–636. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, D. Preparation of Biomass Activated Carbon Supported Nanoscale Zero-Valent Iron (Nzvi) and Its Aqueous Solution. Water 2019, 11, 1671. [Google Scholar] [CrossRef]
- Gunasekar, V.; Ponnusami, V. Plasmonic photocatalysis and kinetics of reactive dye degradation in aqueous solution using enzymatically synthesized Ag/ZnO. J Sol-Gel Sci Technol. 2015, 74, 84–93. [Google Scholar] [CrossRef]
- Benton, O.; Apollo, S.; Naidoo, B.; Ochieng, A. Photodegradation of Molasses Wastewater Using TiO2–ZnO Nanohybrid Photocatalyst Supported on Activated Carbon. Chem. Eng. Commun. 2016, 203, 1443–1454. [Google Scholar] [CrossRef]
- Soltani, R.D.C.; Rezaee, A.; Khataee, A.R.; Safari, M. Photocatalytic process by immobilized carbon black/ZnO nanocomposite for dye removal from aqueous medium: Optimization by response surface methodology. J. Ind. Eng. Chem. 2014, 20, 1861–1868. [Google Scholar] [CrossRef]
- Raizada, P.; Singh, P.; Kumar, A.; Sharma, G.; Pare, B.; Jonnalagadda, S.B.; Thakur, P. Applied Catalysis A: General Solar photocatalytic activity of nano-ZnO supported on activated carbon or brick grain particles: Role of adsorption in dye degradation. Appl. Catal. A 2014, 486, 159–169. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Reza, M. AgPt nanoparticles supported on magnetic graphene oxide nanosheets for catalytic reduction of 4-nitrophenol: Studies of kinetics and mechanism. Appl. Organomet. Chem. 2017, 31, 1–13. [Google Scholar] [CrossRef]
- Sudhakar, P.; Soni, H. Catalytic reduction of Nitrophenols using silver nanoparticles-supported activated carbon derived from agro-waste. J. Environ. Chem. Eng. 2018, 6, 28–36. [Google Scholar] [CrossRef]
- Mortazavi, K.; Rajabi, H.; Ansari, A.; Ghaedi, M.; Dashtian, K. Preparation of silver nanoparticle loaded on activated carbon and its application for removal of malachite green from aqueous solution. Synth. React. Inorg. Met.-Org Nano-Metal Chem. 2016, 3174. [Google Scholar] [CrossRef]
- Sun, F.; Qiao, X.; Tan, F.; Wang, W.; Qiu, X. One-step microwave synthesis of Ag/ZnO nanocomposites with enhanced photocatalytic performance. J. Mater Sci. 2012, 47, 7262–7268. [Google Scholar] [CrossRef]
- Bechambi, O.; Jlaiel, L.; Najjar, W.; Sayadi, S. Photocatalytic degradation of bisphenol A in the presence of Ce–ZnO: Evolution of kinetics, toxicity and photodegradation mechanism. Mater. Chem. Phys. 2016, 173, 95–105. [Google Scholar] [CrossRef]
- Galedari, N.A.; Rahmani, M. Preparation, characterization, and application of ZnO@SiO2 core-shell structured catalyst for photocatalytic degradation of phenol. Env. Sci Pollut Res. 2017, 24, 12655–12663. [Google Scholar] [CrossRef]
- Lambert, D.; Benhebal, H.; Chaib, M.; Crine, M. Photodegradation of phenol and benzoic acid by sol-gel-synthesized alkali metal-doped ZnO. Mater. Sci. Semicond. Process. 2012, 15, 264–269. [Google Scholar] [CrossRef]
- Wu, H.; Jian, W.; Dang, H.; Zhao, X.; Zhang, L.; Li, J. Ag-ZnO Microspheres with Enhanced Photocatalytic Degradation Activities. Pol. J. Environ. Stud. 2017, 26, 871–880. [Google Scholar] [CrossRef]
- Panchal, P.; Rattan, D.; Sharma, A.; Choudhary, P.; Meena, P.; Nehra, S.P. Biogenic mediated Ag/ZnO nanocomposites for photocatalytic and antibacterial activities towards disinfection of water. J. Colloid Interface Sci. 2020, 563, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Safari, M.; Shahmoradi, B.; Zandsalimi, Y. Photocatalytic degradation of humic substances in aqueous solution using Cu-doped ZnO nanoparticles under natural sunlight irradiation. Env. Sci. Pollut. Res. 2015, 22, 16875–16880. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Karimi, H.; Yousefi, F. Silver and zinc oxide nanostructures loaded on activated carbon as new adsorbents for removal of methylene green: A comparative study. Hum. Exp. Toxicol. 2014, 33, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, R.; Karthikeyan, S.; Gupta, V.K.; Sekaran, G.; Narayanan, V.; Stephen, A. Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination. Mater. Sci. Eng. C 2013, 33, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Khan, M.M.; Lee, J.; Cho, M.H. Highly visible light active Ag@ZnO nanocomposites synthesized by gel-combustion route. J. Ind. Eng. Chem. 2014, 20, 1602–1607. [Google Scholar] [CrossRef]
- Jose, Y.J.; Manjunathan, M. Highly photocatalyst efficient in LEDs / solar active and reusable: Sm–ZnO–Ag nanoparticles for methylene blue degradation. J. Nanostruct. Chem. 2017, 7, 259–271. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Roy, P. Green synthesis of silver nanoparticles using Azadirachta indica leaf extract and its antimicrobial study. J. Radiat. Res. Appl. Sci. 2017, 7, 843–850. [Google Scholar] [CrossRef]
- Lagashetty, A.; Ganiger, S.K. Synthesis, characterization and antibacterial study of Ag-Au Bi-metallic nanocomposite by bioreduction using piper betle leaf extract. Heliyon 2019, 5, 1–6. [Google Scholar] [CrossRef]
- Djahaniani, H.; Rahimi-nasrabadi, M.; Saiedpour, M.; Nazarian, S. Facile synthesis of silver nanoparticles using Tribulus longipetalus extract and their antioxidant and antibacterial activities. Int. J. Food Prop. 2017, 20, 922–930. [Google Scholar] [CrossRef]
- Yadi, M.; Mostafavi, E.; Saleh, B.; Davaran, S.; Aliyeva, I.; Khalilov, R.; Nikzamir, M.; Nikzamir, N.; Akbarzadeh, A.; Panahi, Y.; et al. Current developments in green synthesis of metallic nanoparticles using plant extracts: A review. Artif. Cells Nanomed. Biotechnol. 2018, 46, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Gnanasangeetha, D.; Thambavani, S.D. Facile And Eco-Friendly Method For The Synthesis Of Zinc Oxide Nanoparticles Using Azadirachta And Emblica. Int. J. Pharm. Sci. Res. 2014, 5, 2866–2873. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Ortan, A.; Avramescu, S.M.; Fierascu, I. Phyto-Nanocatalysts: Green Synthesis, Characterization, and Applications. Molecules 2019, 24, 3418. [Google Scholar] [CrossRef]
- Ahmed, S.; Saifullah Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Singh, A.; Neelam Kaushik, M. Physicochemical investigations of zinc oxide nanoparticles synthesized from Azadirachta Indica (Neem)leaf extract and their interaction with Calf-Thymus DNA. Results Phys. 2019, 13, 102168. [Google Scholar] [CrossRef]
- Da’na, E.; Taha, A.; Afkar, E. Green Synthesis of Iron Nanoparticles by Acacia nilotica Pods Extract and Its Catalytic, Adsorption, and Antibacterial Activities. Appl. Sci. 2018, 8, 1922. [Google Scholar] [CrossRef]
- Meng, Y. A sustainable approach to fabricating ag nanoparticles/PVA hybrid nanofiber and its catalytic activity. Nanomaterials 2015, 5, 1124–1135. [Google Scholar] [CrossRef]
- Lalitha, A.; Subbaiya, R.; Ponmurugan, P. Green synthesis of silver nanoparticles from leaf extract Azhadirachta indica and to study its anti-bacterial and antioxidant property. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 228–235. [Google Scholar]
- Diyana, N.; Zambri, S.; Taib, N.I.; Latif, F.A.; Mohamed, Z. Utilization of Neem Leaf Extract on Biosynthesis of Iron Oxide Nanoparticles. Molecules 2019, 24, 3803–3812. [Google Scholar]
- Da’na, E.; Awad, A. Regeneration of spent activated carbon obtained from home filtration system and applying it for heavy metals adsorption. J. Environ. Chem. Eng. 2017, 5, 3091–3099. [Google Scholar] [CrossRef]
- Zare, M.; Namratha, K.; Alghamdi, S.; Mohammad, Y.H.E.; Hezam, A.; Zare, M.; Drmosh, Q.A.; Byrappa, K.; Chandrashekar, B.N.; Ramakrishna, S.; et al. Novel Green Biomimetic Approach for Synthesis of ZnO-Ag Nanocomposite; Antimicrobial Activity against Food-borne Pathogen, Biocompatibility and Solar Photocatalysis. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Mehata, M.S. Controllable synthesis of silver nanoparticles using Neem leaves and their antimicrobial activity. J. Radiat. Res. Appl. Sci. 2016, 9, 109–115. [Google Scholar] [CrossRef]
- Ali, K.; Dwivedi, S.; Azam, A.; Saquib, Q.; Al-Said, M.S.; Alkhedhairy, A.A.; Musarrat, J. Aloe vera extract functionalized zinc oxide nanoparticles as nanoantibiotics against multi-drug resistant clinical bacterial isolates. J. Colloid Interface Sci. 2016, 472, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Luque, P.A.; Soto-Robles, C.A.; Nava, O.; Gomez-Gutierrez, C.M.; Castro-Beltran, A.; Garrafa-Galvez, H.E.; Vilchis-Nestor, A.R.; Olivas, A. Green synthesis of zinc oxide nanoparticles using Citrus sinensis extract. J. Mater. Sci. Mater. Electron. 2018, 29, 9764–9770. [Google Scholar] [CrossRef]
- Parthibana, C.; Sundaramurthy, N. Biosynthesis, Characterization of ZnO Nanoparticles by Using Pyrus Pyrifolia Leaf Extract and Their Photocatalytic Activity. Int. J. Innov. Res. Sci. Eng. Technol. 2015, 4, 9710–9718. [Google Scholar] [CrossRef]
- Elmorsi, T.M.; Elsayed, M.H.; Bakr, M.F. Enhancing the removal of methylene blue by modified ZnO nanoparticles: Kinetics and equilibrium studies. Can. J. Chem. 2017, 95, 590–600. [Google Scholar] [CrossRef]
- Kumar, T.K.M.P.; Mandlimath, T.R.; Sangeetha, P.; Sakthivel, P.; Revathi, S.K.; Kumar, S.K.A.; Sahoo, S.K. Highly efficient performance of activated carbon impregnated with Ag, ZnO and Ag/ZnO nanoparticles as antimicrobial materials. RSC Adv. 2015, 5, 108034–108043. [Google Scholar] [CrossRef]
- Lepot, N.; Van Bael, M.K.; Van den Rul, H.; D’Haen, J.; Peeters, R.; Franco, D.; Mullens, J. Synthesis of ZnO nanorods from aqueous solution. Mater. Lett. 2007, 61, 2624–2627. [Google Scholar] [CrossRef]
- Shahwan, T.; Abu Sirriah, S.; Nairat, M.; Boyaci, E.; Eroĝlu, A.E.; Scott, T.B.; Hallam, K.R. Green synthesis of iron nanoparticles and their application as a Fenton-like catalyst for the degradation of aqueous cationic and anionic dyes. Chem. Eng. J. 2011, 172, 258–266. [Google Scholar] [CrossRef]
- Naseri, M.; Kamalianfar, A.; Naderi, E.; Hashemi, A. The effect of Ag nanoparticles on physical and photocatalytic properties of ZnFe2O4/SiO2 nanocomposite. J. Mol. Struct. 2020, 1206, 127706. [Google Scholar] [CrossRef]
- Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Gurav, D.D.; Jabgunde, A.M.; Kale, S.; Pardesi, K.; Shinde, V.; Bellare, J.; et al. Gnidia glauca flower extract mediated synthesis of gold nanoparticles and evaluation of its chemocatalytic potential. J. Nanobiotechnol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Kohsari, I.; Mohammad-Zadeh, M.; Minaeian, S.; Rezaee, M.; Barzegari, A.; Shariatinia, Z.; Koudehi, M.F.; Mirsadeghi, S.; Pourmortazavi, S.M. In vitro antibacterial property assessment of silver nanoparticles synthesized by Falcaria vulgaris aqueous extract against MDR bacteria. J. Sol-Gel Sci. Technol. 2019, 380–389. [Google Scholar] [CrossRef]
- Fahimmunisha, B.A.; Ishwarya, R.; AlSalhi, M.S.; Devanesan, S.; Govindarajan, M.; Vaseeharan, B. Green fabrication, characterization and antibacterial potential of zinc oxide nanoparticles using Aloe socotrina leaf extract: A novel drug delivery approach. J. Drug Deliv. Sci. Technol. 2020, 55, 101465. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
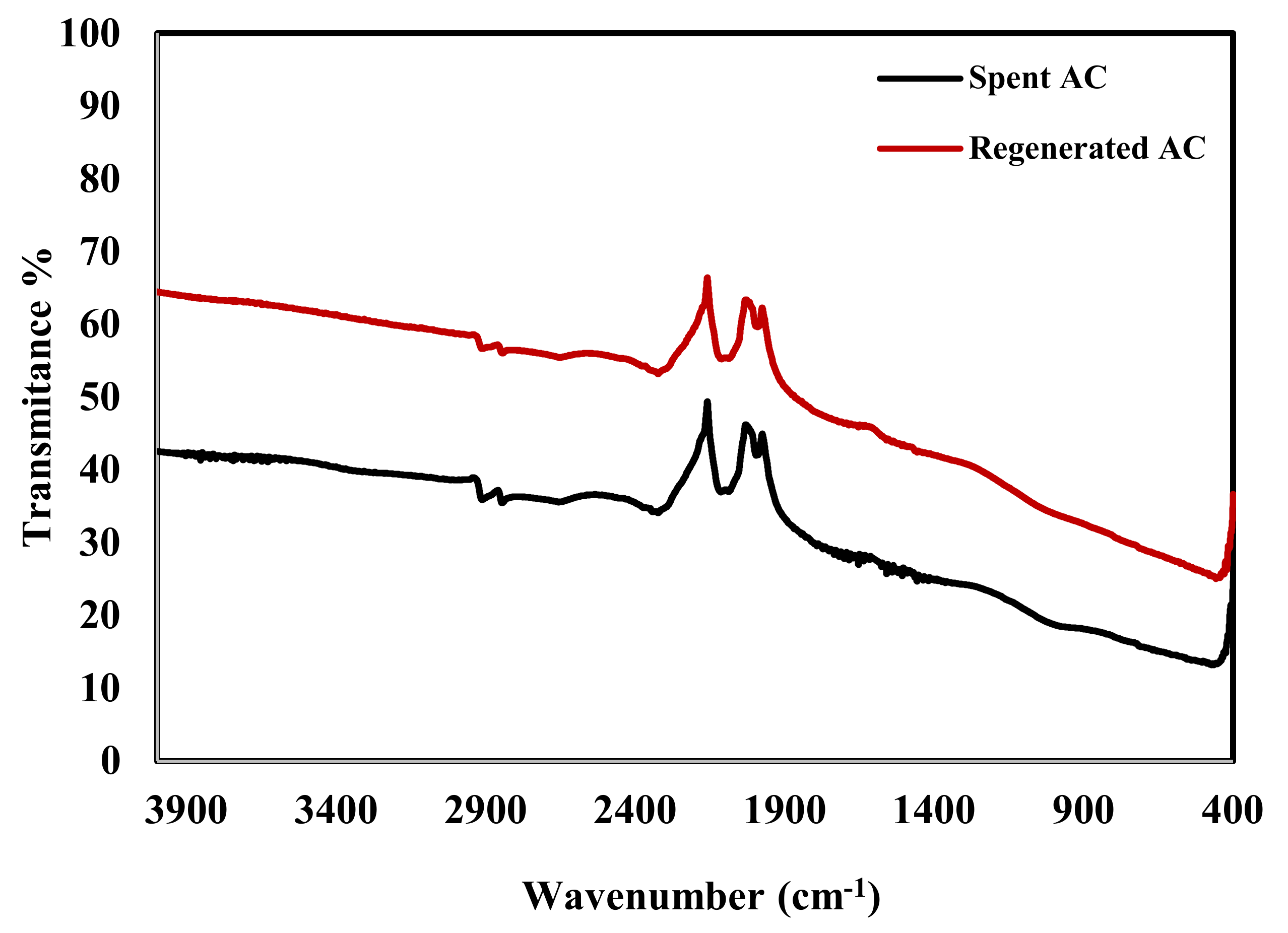
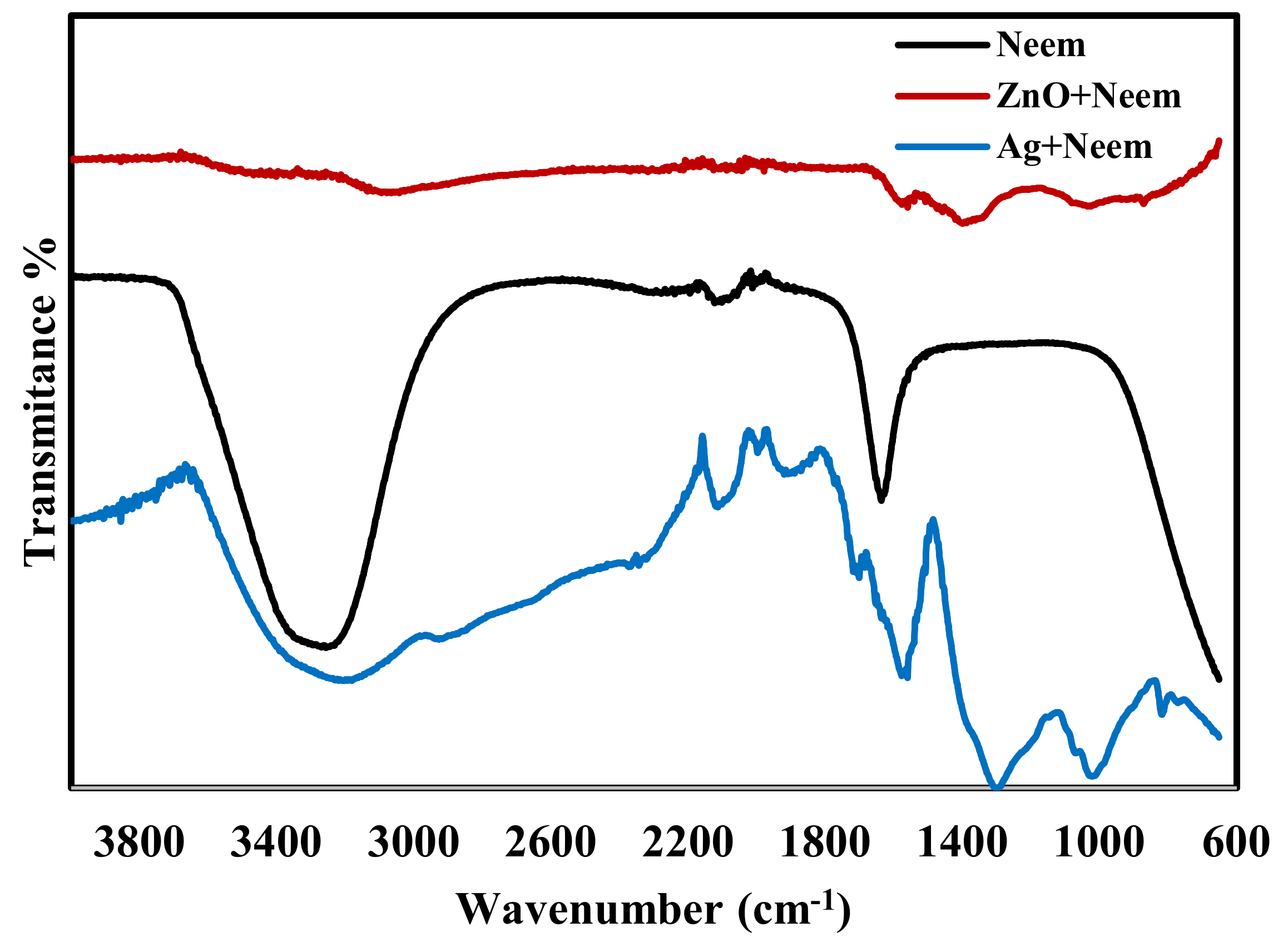

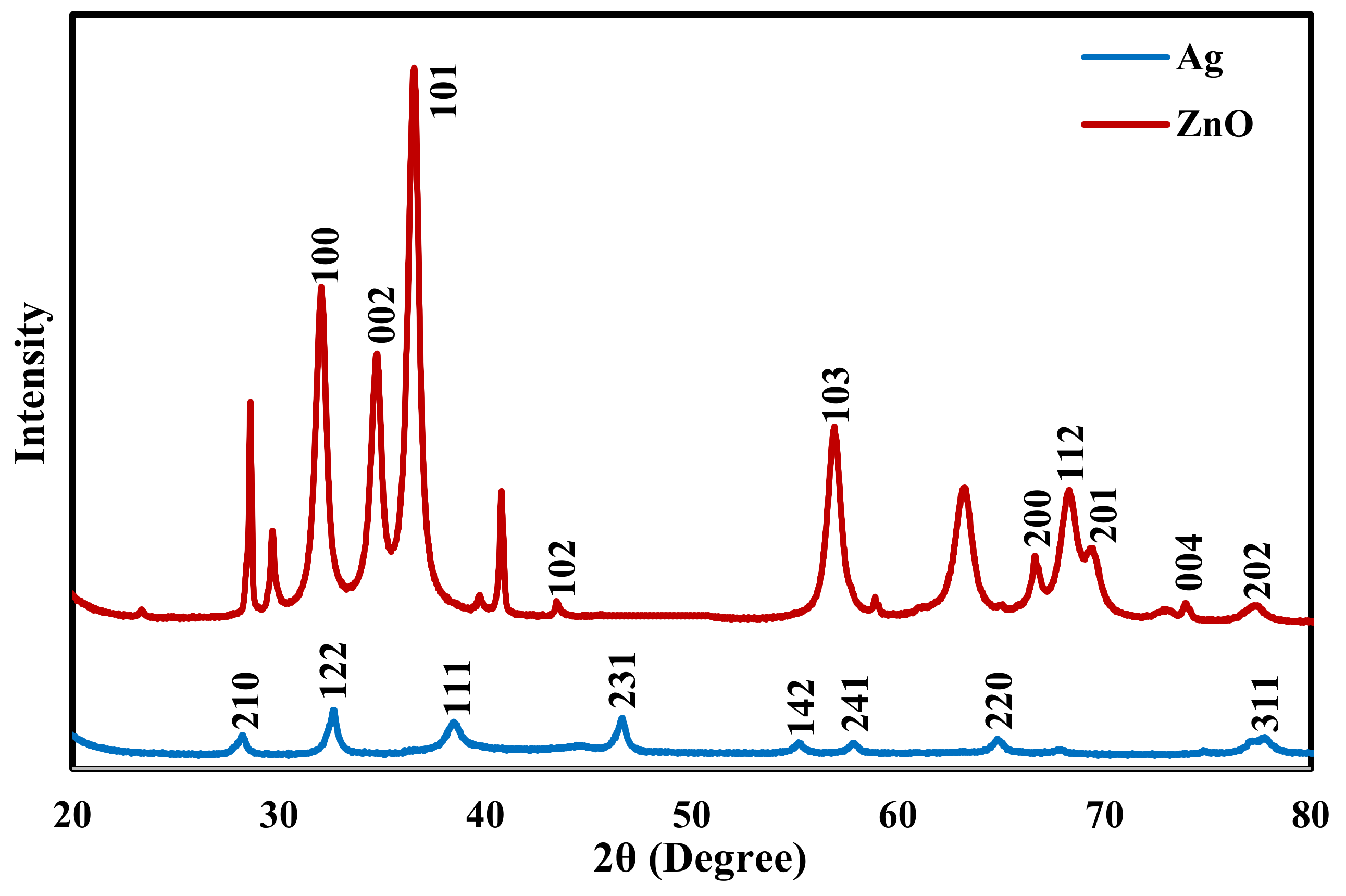
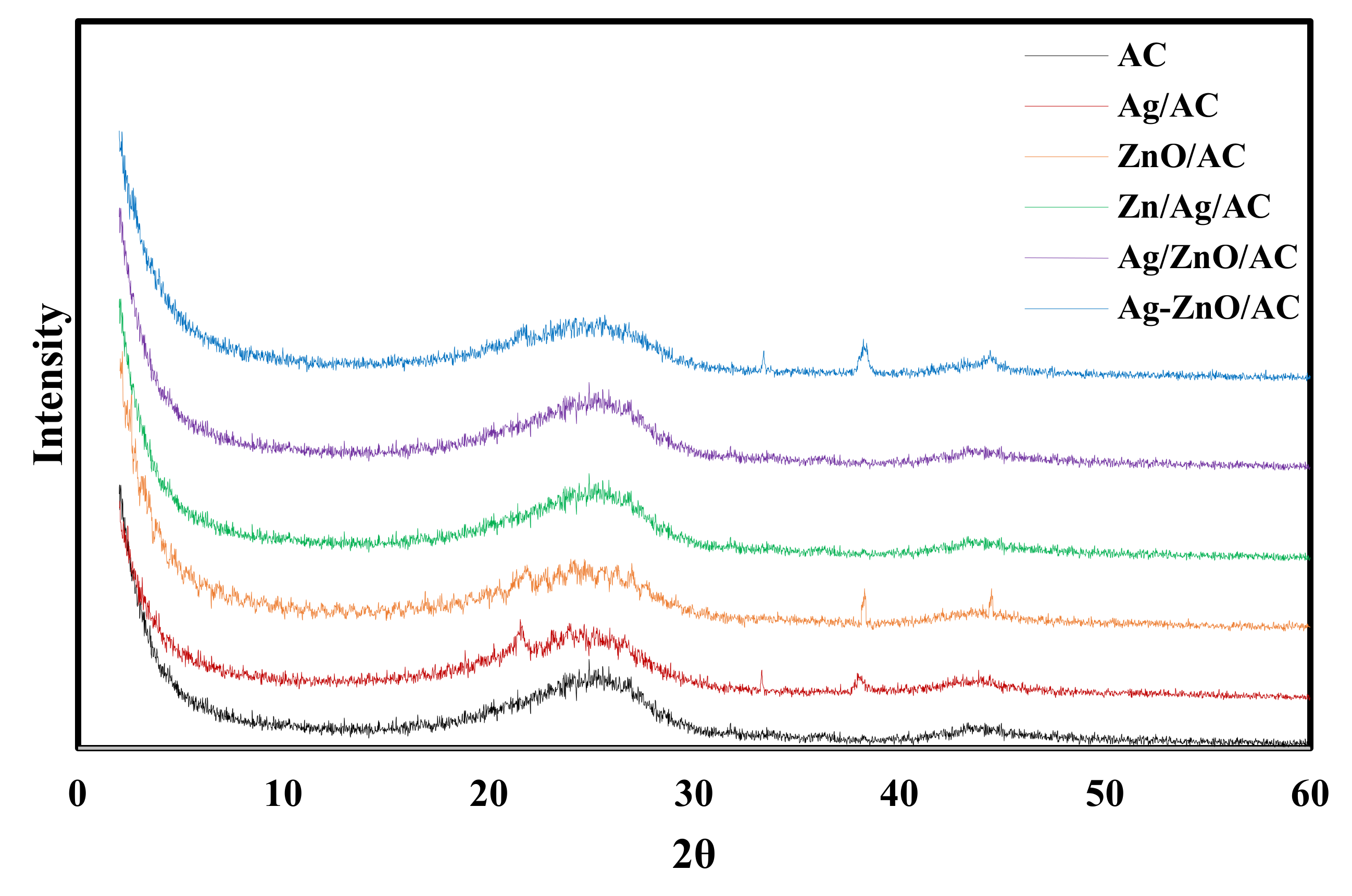


| Strains | Inhibition Zone (mm) | ||||||
|---|---|---|---|---|---|---|---|
| Ag/AC | ZnO/AC | Ag/ZnO/AC | ZnO/Ag/AC | Ag-ZnO/AC | |||
| Gram- | Esherichia coli | ATCC 25983 | 6 | 12 | 6 | 8 | 8 |
| ATCC 29212 | 6 | 6 | 6 | 6 | 8 | ||
| 37420 | 6 | 12 | 6 | 10 | 6 | ||
| Acinetobacter baumanni | 4179 | 10 | 6 | 6 | 6 | 8 | |
| 12594 | 14 | 6 | 6 | 6 | 9 | ||
| 12895 | 14 | 8 | 6 | 6 | 8 | ||
| 22497 | 6 | 6 | 6 | 6 | 8 | ||
| Pseudomonas aeruginosa | 1314a | 18 | 8 | 6 | 6 | 12 | |
| 12898 | 12 | 6 | 8 | 6 | 8 | ||
| 9381 | 12 | 8 | 8 | 6 | 8 | ||
| Klebsiella pseudomonas | 37591 | 6 | 10 | 6 | 10 | 12 | |
| 22210 | 6 | 6 | 6 | 10 | 11 | ||
| Enterobacter cloacae | 37444 | 6 | 6 | 6 | 14 | 6 | |
| Gram+ | Staphyloccocus aureus | 16678 | 19 | 13 | 18 | 12 | 12 |
| 18226 | 16 | 8 | 8 | 6 | 11 | ||
| 29213 | 6 | 6 | 6 | 6 | 8 | ||
| Nanocomposite | Pathogens | Inhibition Zone (mm) | Reference |
|---|---|---|---|
| Ag/AC | E. coli | 18 | [3] |
| Ag/AC | S. aureus | 11 | [57] |
| B. subtilis | 10 | ||
| P. aeruginosa | 13 | ||
| E. coli | 10 | ||
| ZnO/AC | S. aureus | 10 | [57] |
| B. subtilis | 11 | ||
| P. aeruginosa | 10 | ||
| E. coli | 10 | ||
| Ag/ZnO/AC | S. aureus | 14 | [57] |
| B. subtilis | 11 | ||
| P. aeruginosa | 16 | ||
| E. coli | 14 | ||
| Ag/AC | E. coli | 9–14 | [6] |
| Ag/AC | E. coli | Effective (no zone reported) | [4] |
| Gram Class | Bacteria Used in This Study | Strains Screened | Main Mechanism of Action |
|---|---|---|---|
| Gram− | Esherichia coli | ATCC 25983 ATCC 29212 37420 | Alteration of membrane permeability and respiration. |
| Klebsiella pseudomonas | 37591 22210 | Alteration of cell wall. | |
| Pseudomonas aeruginosa | 1314a 12898 9381 | Alteration of cell wall. | |
| Acinetobacter baumanni | 4179 12594 12895 22497 | ||
| Enterobacter cloacae | 37444 | ||
| Gram+ | Staphyloccocus aureus | 16678 18226 29213 | Inhibition of replication. |
| Sample | Composition | Method |
|---|---|---|
| Ag/AC | 100% AgNPs on AC | Impregnation |
| ZnO/AC | 100% ZnONPs on AC | Impregnation |
| Ag/ZnO/AC | 50% Ag and 50% ZnO on AC | Successive precipitation |
| ZnO/Ag/AC | 50% ZnO and 50% Ag on AC | Successive precipitation |
| Ag-ZnO/AC | 50% Ag and 50% ZnO on AC | Co-precipitation |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, A.; Ben Aissa, M.; Da’na, E. Green Synthesis of an Activated Carbon-Supported Ag and ZnO Nanocomposite for Photocatalytic Degradation and Its Antibacterial Activities. Molecules 2020, 25, 1586. https://doi.org/10.3390/molecules25071586
Taha A, Ben Aissa M, Da’na E. Green Synthesis of an Activated Carbon-Supported Ag and ZnO Nanocomposite for Photocatalytic Degradation and Its Antibacterial Activities. Molecules. 2020; 25(7):1586. https://doi.org/10.3390/molecules25071586
Chicago/Turabian StyleTaha, Amel, Melek Ben Aissa, and Enshirah Da’na. 2020. "Green Synthesis of an Activated Carbon-Supported Ag and ZnO Nanocomposite for Photocatalytic Degradation and Its Antibacterial Activities" Molecules 25, no. 7: 1586. https://doi.org/10.3390/molecules25071586
APA StyleTaha, A., Ben Aissa, M., & Da’na, E. (2020). Green Synthesis of an Activated Carbon-Supported Ag and ZnO Nanocomposite for Photocatalytic Degradation and Its Antibacterial Activities. Molecules, 25(7), 1586. https://doi.org/10.3390/molecules25071586






