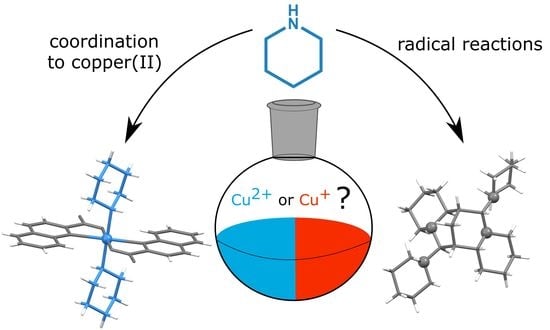
Beyond the Simple Copper(II) Coordination Chemistry with Quinaldinate and Secondary Amines
Abstract
1. Introduction
2. Results and Discussion
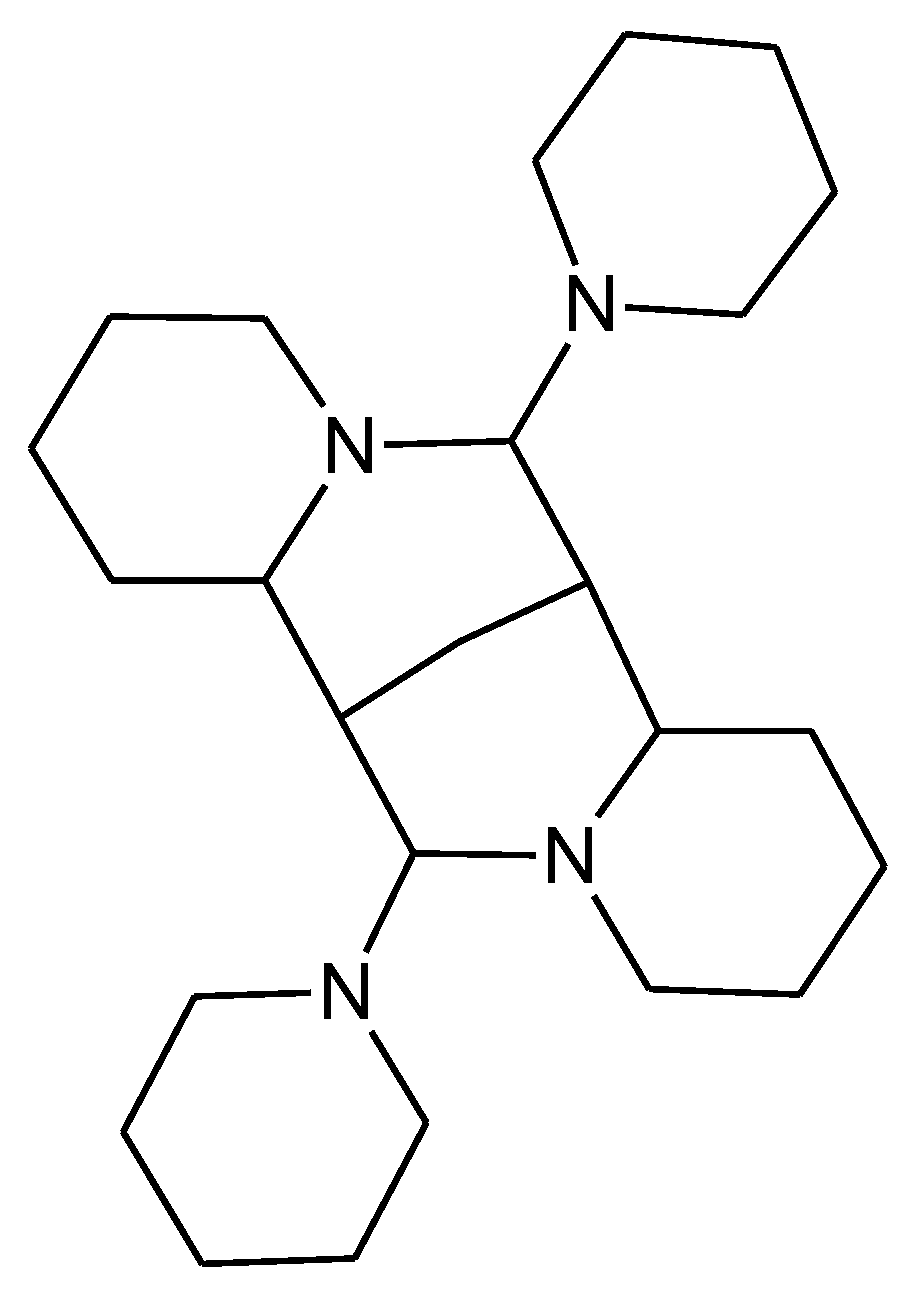
2.1. Synthetic Considerations
2.2. Solid State Structures
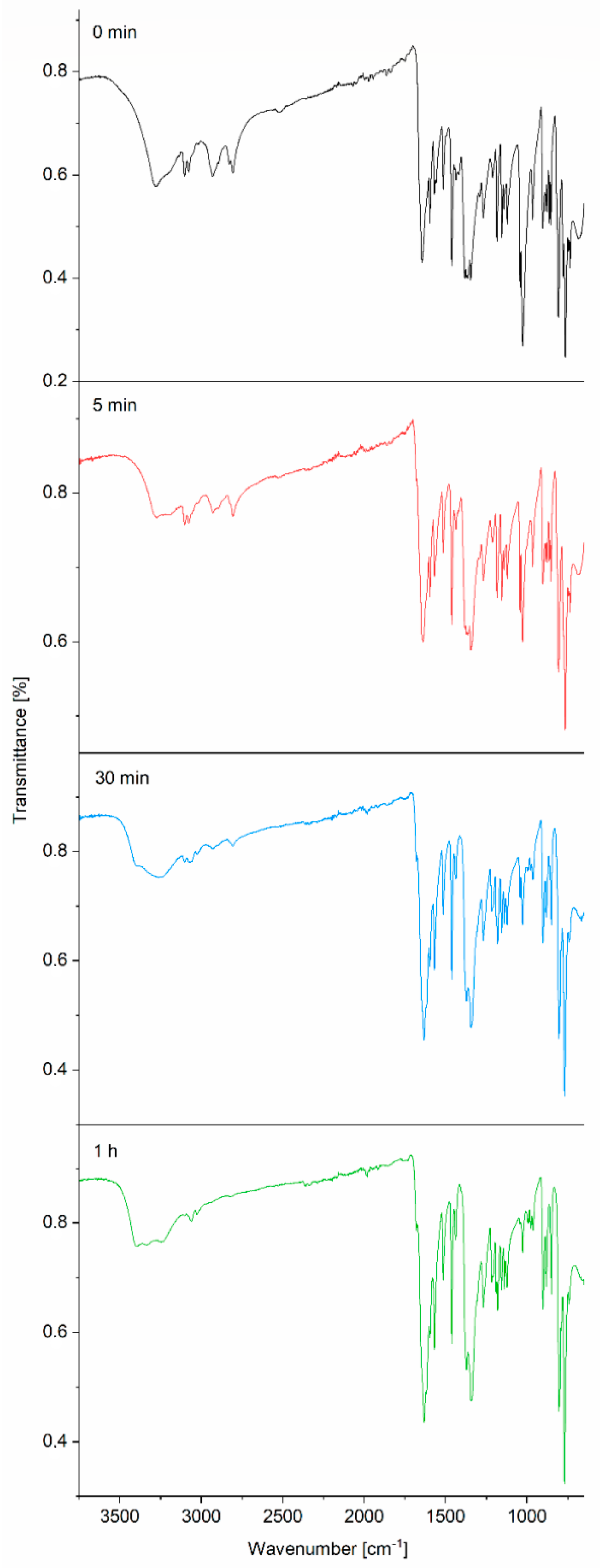
2.3. Infrared Spectra
2.4. Conversion of [Cu(quin)2(CH3OH)]∙CH3OH (1) into the Aqua Complex
3. Materials and Methods
3.1. General
3.2. Synthetic Procedures
3.2.1. Synthesis of the Copper(II) Starting Material
3.2.2. Synthesis of [Cu(quin)2(pyro)2] (2)
3.2.3. Synthesis of (pyroH)[Cu(quin)2Cl] (3)
3.2.4. Synthesis of [Cu(quin)2(morph)2] (4)
3.2.5. Preparation of Single Crystals of [Cu(quin)2(morph)2] (4) and trans-[Cu(en)2(H2O)2](morphCOO)2 (5)
3.2.6. Synthesis of [Cu(quin)2(pipe)] (6)
3.2.7. Reaction of 6 with Piperidine
3.2.8. Synthesis of [Cu(quin)2(pipe)2] (7)
3.2.9. Reaction of [Cu(quin)2(H2O)] with Piperidine at Ambient Conditions
3.2.10. Synthesis of [Cu(quin)2(pipe)2]∙CH3CH2CN (9)
3.2.11. Synthesis of (pipeH)[Cu(quin)2Cl] (11)
3.3. X-ray Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holm, R.H.; Kennepohl, P.; Solomon, E.I. Structural and functional aspects of metal sites in biology. Chem. Rev. 1996, 96, 2239–2314. [Google Scholar] [CrossRef] [PubMed]
- Worrall, J.A.R.; Machczynski, M.C.; Keijser, B.J.F.; di Rocco, G.; Ceola, S.; Ubbink, M.; Vijgenboom, E.; Canters, G.W. Spectroscopic characterization of a high-potential lipo-cupredoxin found in Streptomyces coelicolor. J. Am. Chem. Soc. 2006, 128, 14579–14589. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, A.C. Copper delivery by metallochaperone proteins. Acc. Chem. Res. 2001, 34, 119–128. [Google Scholar] [CrossRef]
- Conry, R.R. Copper: Inorganic & Coordination Chemistry. In Encyclopedia of Inorganic Chemistry, 2nd ed.; King, R.B., Ed.; Wiley: Hoboken, NJ, USA, 2005; Volume 1, pp. 1–19. [Google Scholar]
- Cotton, F.A.; Wilkinson, G. Advanced Inorganic Chemistry, 4th ed.; Wiley: New York, NY, USA, 1980; pp. 79, 798–821. [Google Scholar]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Sharpe, A.G. Inorganic Chemistry, 5th ed.; Pearson: Harlow, UK, 2018; p. 693. [Google Scholar]
- Haas, K.L.; Franz, K.J. Application of metal coordination chemistry to explore and manipulate cell biology. Chem. Rev. 2009, 109, 4921–4960. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; O’Hagan, D.; Mocek, U.; Zeng, Z.; Yuen, L.D.; Frenzel, T.; Unkefer, C.J.; Beale, J.M.; Floss, H.G. Biosynthesis of the antibiotic thiostrepton. Methylation of tryptophan in the formation of the quinaldic acid moiety by transfer of the methionine methyl group with net retention of configuration. J. Am. Chem. Soc. 1989, 111, 7274–7276. [Google Scholar] [CrossRef]
- Vogel, A.I. Vogel’s Textbook of Quantitative Inorganic Analysis, Including Elementary Instrumental Analysis, 4th ed.; Longman: London, UK, 1978. [Google Scholar]
- Kolthoff, I.M.; Sandell, E.B. Textbook of Quantitative Inorganic Analysis, 3rd ed.; Macmillan: New York, NY, USA, 1952. [Google Scholar]
- Lamprecht, G.J.; Beetge, J.H.; Leipoldt, J.G.; De Waal, D.R. The structure of 2-carboxyquinolinatobis(triphenylphosphite)rhodium(I). Inorg. Chim. Acta 1986, 113, 157–160. [Google Scholar] [CrossRef]
- Cano, M.; Heras, J.V.; Lobo, M.A.; Martinez, M.; Pinilla, E.; Gutierrez, E.; Monge, M.A. Reactivity of pyridinecarboxylate complexes of rhodium(I) towards Hg(OOCCF3)2 and Hg(Ph)(OOCCF3). Crystal structure of Rh(C10H6NO2)(OOCCF3)2(H2O)[P(4-CH3-C6H4)3]–III. Polyhedron 1991, 10, 187–196. [Google Scholar] [CrossRef]
- Lamprecht, G.J.; Leipoldt, J.G.; Roodt, A. Structure of carbonyl(2-quinolinecarboxylato-κN,κO)(triphenylphosphite-κP)rhodium(I). Acta Crystallogr. Sect. C 1991, 47, 2209–2211. [Google Scholar] [CrossRef]
- Cano, M.; Heras, J.V.; Lobo, M.A.; Pinilla, E.; Monge, M.A. Mono- and binuclear quinaldinate complexes of rhodium with (P–P) donor ligands. Crystal structure of [Rh2(quin)2(CO)2(μ-dppm)]. Oxidative addition reactions–V. Polyhedron 1994, 13, 1563–1573. [Google Scholar] [CrossRef]
- Brand, U.; Vahrenkamp, H. A new tridentate N,N,S ligand and its zinc complexes. Inorg. Chem. 1995, 34, 3285–3293. [Google Scholar] [CrossRef]
- Okabe, N.; Makino, T. trans-Diaquabis(2-quinolinecarboxylato-N,O)iron(II)–ethanol–water (1/2/2). Acta Crystallogr. Sect. C 1998, 54, 1279–1280. [Google Scholar] [CrossRef]
- Dobrzyńska, D.; Duczmal, M.; Jerzykiewicz, L.B.; Warchulska, J.; Drabent, K. Synthesis, spectroscopy, and magnetic properties of FeII and CoII quinoline-2-carboxylates—Crystal structure of trans-bis(quinoline-2-carboxylato)bis(propanol)iron(II). Eur. J. Inorg. Chem. 2004, 2004, 110–117. [Google Scholar] [CrossRef]
- Dobrzyńska, D.; Jerzykiewicz, L.B. Adenine ribbon with Watson−Crick and Hoogsteen motifs as the “double-sided adhesive tape” in the supramolecular structure of adenine and metal carboxylate. J. Am. Chem. Soc. 2004, 126, 11118–11119. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyńska, D.; Jerzykiewicz, L.B.; Duczmal, M. Synthesis and properties of iron (II) quinoline-2-carboxylates, crystal structure of trans-diaquabis(quinoline-2-carboxylato)iron (II) bis(dichloromethane) solvate. Polyhedron 2005, 24, 407–412. [Google Scholar] [CrossRef]
- Jain, S.L.; Slawin, A.M.Z.; Woollins, J.D.; Bhattacharyya, P. The reactions of elemental iron with dipicolinic acid (H2dipic) and quinaldic acid (Hquin)—X-ray crystal structures of [C5H5NH][Fe(Dipic)(Hdipic)(C5H5N)2]·3C5H5N, [Fe2(µ-O)(Dipic)2(C5H5N)4]·2C5H5N·2H2O and trans-[Fe(Quin)2(MeOH)2]. Eur. J. Inorg. Chem. 2005, 2005, 721–726. [Google Scholar] [CrossRef]
- Dobrzyńska, D.; Jerzykiewicz, L.B.; Jezierska, J.; Duczmal, M. Crystal structure and characterization of manganese(II) carboxylates: 3D metal−organic frameworks. Cryst. Growth Des. 2005, 5, 1945–1951. [Google Scholar] [CrossRef]
- Starynowicz, P. Structure of bis-μ-(2-quinolinecarboxylato-O,O,O′)bis[triaqua(2-quinolinecarboxylato-N,O)(2-quinolinecarboxylato-O)neodymium(III)] trihydrate. Acta Crystallogr. Sect. C 1990, 46, 2068–2070. [Google Scholar] [CrossRef]
- Żurowska, B.; Mroziński, J.; Ciunik, Z. Structure and magnetic properties of a copper(II) compound with syn–anti carboxylato- and linear Cu–Cl–Cu chloro-bridges. Polyhedron 2007, 26, 3085–3091. [Google Scholar] [CrossRef]
- Żurowska, B.; Ślepokura, K. Structure and magnetic properties of polynuclear copper(II) compounds with syn–anti carboxylato- and bromo-bridges. Inorg. Chim. Acta 2008, 361, 1213–1221. [Google Scholar] [CrossRef]
- Houghton, D.T.; Gydesen, N.W.; Arulsamy, N.; Mehn, M.P. Synthesis and characterization of iron(II) quinaldate complexes. Inorg. Chem. 2010, 49, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Haendler, H.M. Copper quinaldinate monohydrate [aquabis(2-quinolinecarboxylato)copper(II)]; pentacoordinate copper. Acta Crystallogr. Sect. C 1986, 42, 147–149. [Google Scholar] [CrossRef]
- Albinati, A.; Carraro, M.L.; Gross, S.; Rancan, M.; Rizzato, S.; Tondello, E.; Venzo, A. Synthesis and characterisation of a new Cu(O2CNAllyl2)2 carbamato complex and an unusual polymeric CuI Complex [CuI4Cl4(NHAllyl2)4]n: New insights into metal carbamato chemistry. Eur. J. Inorg. Chem. 2009, 2009, 5346–5351. [Google Scholar] [CrossRef]
- Kitos, A.A.; Efthymiou, C.G.; Manos, M.J.; Tasiopoulos, A.J.; Nastopoulos, V.; Escuer, A.; Perlepes, S.P. Interesting copper(II)-assisted transformations of 2-acetylpyridine and 2-benzoylpyridine. Dalton Trans. 2016, 45, 1063–1077. [Google Scholar] [CrossRef]
- Tessarolo, J.; Venzo, A.; Bottaro, G.; Armelao, L.; Rancan, M. Hampered subcomponent self-assembly leads to an aminal ligand: Reactivity with silver(I) and copper(II). Eur. J. Inorg. Chem. 2017, 2017, 30–34. [Google Scholar] [CrossRef]
- Lada, Z.G.; Soto Beobide, A.; Savvidou, A.; Raptopoulou, C.P.; Psycharis, V.; Voyiatzis, G.A.; Turnbull, M.M.; Perlepes, S.P. A unique copper(II)-assisted transformation of acetylacetone dioxime in acetone that leads to one-dimensional, quinoxaline-bridged coordination polymers. Dalton Trans. 2017, 46, 260–274. [Google Scholar] [CrossRef]
- Balewski, Ł.; Gdaniec, M.; Sączewski, J.; Wicher, B.; Sączewski, F. Copper(II)-assisted hydrolysis of cyclic ureas: Transformation of 1-(pyridin-2-yl)-2,3,7,8-tetrahydro-1H-imidazo[2,1-b][1,3,5]triazepin-5(6H)-ones into N1-[1-(pyridin-2-yl)imidazolidin-2-ylidene]-ethane-1,2-diamine ligands. Inorg. Chim. Acta 2017, 467, 287–296. [Google Scholar] [CrossRef]
- Kamat, V.; Revankar, V. 1,10-Phenanthroline-copper mediated ligand transformations: Synthesis and characterization of unusual mixed ligand complexes of copper (II). Inorg. Chim. Acta 2018, 476, 77–82. [Google Scholar] [CrossRef]
- Voitekhovich, S.V.; Grigoriev, Y.V.; Lyakhov, A.S.; Matulis, V.E.; Ivashkevich, L.S.; Ivashkevich, O.A. 2-(1H-Tetrazol-1-yl)thiazole: Complexation and copper-assisted tetrazole ring transformation. Polyhedron 2019, 171, 423–432. [Google Scholar] [CrossRef]
- Gusev, A.; Nemec, I.; Herchel, R.; Shul’gin, V.; Ryush, I.; Kiskin, M.; Efimov, N.; Ugolkova, E.; Minin, V.; Lyssenko, K.; et al. Copper(II) self-assembled clusters of bis((pyridin-2-yl)-1,2,4-triazol-3-yl)alkanes. Unusual rearrangement of ligands under reaction conditions. Dalton Trans. 2019, 48, 3052–3060. [Google Scholar] [CrossRef]
- Modec, B. Crystal chemistry of zinc quinaldinate complexes with pyridine-based ligands. Crystals 2018, 8, 52. [Google Scholar] [CrossRef]
- Brown, C.J.; Gray, L.R. Morpholinium morpholinoformate. Acta Crystallogr. Sect. B 1982, 38, 2307–2308. [Google Scholar] [CrossRef]
- Von Dreele, R.B.; Bradshaw, R.L.; Burke, W.J. Structure of morpholinium 4-morpholinecarboxylate, C4H10NO+.C5H8NO3−. Acta Crystallogr. Sect. C 1983, 39, 253–255. [Google Scholar] [CrossRef]
- Dell’Amico, D.B.; Calderazzo, F.; Labella, L.; Marchetti, F.; Pampaloni, G. Converting carbon dioxide into carbamato derivatives. Chem. Rev. 2003, 103, 3857–3898. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, S.; Xu, Y. Molecular complex morpholine–CO2–H2O. J. Mol. Struct. 2009, 919, 21–25. [Google Scholar] [CrossRef]
- Lane, E.M.; Zhang, Y.; Hazari, N.; Bernskoetter, W.H. Sequential hydrogenation of CO2 to methanol using a pincer iron catalyst. Organometallics 2019, 38, 3084–3091. [Google Scholar] [CrossRef]
- Sajan, P.G.; Rohith, T.; Patil, S.; Kumara, M.N. Detection and quantification of trace level of ethylenediamine in morpholine and its impact on the quality of a pharmaceutical candidate. Int. J. Pharm. Sci. Rev. Res. 2015, 33, 242–247. [Google Scholar]
- Mills, J.E.; Maryanoff, C.A.; McComsey, D.F.; Stanzione, R.C.; Scott, L. The reaction of amines with methylene chloride. Evidence for rapid aminal formation from N-methylenepyrrolidinium chloride and pyrrolidine. J. Org. Chem. 1987, 52, 1857–1859. [Google Scholar] [CrossRef]
- Federsel, H.J.; Koenberg, E.; Lilljequist, L.; Swahn, B.M. Dichloromethane as reactant in synthesis: An expedient transformation of prolinamide to a novel pyrrolo[1,2-c]imidazolone. J. Org. Chem. 1990, 55, 2254–2256. [Google Scholar] [CrossRef]
- Rawat, V.S.; Bathini, T.; Govardan, S.; Sreedhar, B. Catalyst-free activation of methylene chloride and alkynes by amines in a three-component coupling reaction to synthesize propargylamines. Org. Biomol. Chem. 2014, 12, 6725–6729. [Google Scholar] [CrossRef]
- Williams, H. The reaction of piperidine with commercial chloroform and other halomethanes. J. Org. Chem. 1964, 29, 2046–2047. [Google Scholar] [CrossRef]
- Riley, M.J.; Neill, D.; Bernhardt, P.V.; Byriel, K.A.; Kennard, C.H.L. Thermochromism and structure of piperazinium tetrachlorocuprate(II) complexes. Inorg. Chem. 1998, 37, 3635–3639. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, A.K.; Kieslich, G.; Yeung, H.H.-M. Thermodynamic and kinetic effects in the crystallization of metal–organic frameworks. Acc. Chem. Res. 2018, 51, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Sheludyakov, Y.L.; Golodkov, V.A. Reduction of copper(II) complexes by amines. Zh. Prikl. Khim. 1986, 59, 1328–1330. [Google Scholar]
- Yokoi, H.; Isobe, T. An ESR study of the oxidation-reduction reaction of piperidine with a copper(II) bis-dithiocabamate complex. Bull. Chem. Soc. Jpn. 1968, 41, 1489. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Nguyen, T.H.; Zheng, N. The chemistry of amine radical cations produced by visible light photoredox catalysis. Beilstein J. Org. Chem. 2013, 9, 1977–2001. [Google Scholar] [CrossRef]
- Näther, C.; Beck, A. Chlorobis(piperidine-κN)copper(I). Acta Crystallogr. Sect. E 2004, 60, m1008–m1009. [Google Scholar] [CrossRef]
- Bowmaker, G.A.; Hanna, J.V.; Hart, R.D.; Healy, P.C.; White, A.H. Structural and solid-state 31P nuclear magnetic resonance studies on 1:1:1 mixed nitrogen and phosphine base complexes with copper(I) halides. J. Chem. Soc. Dalton Trans. 1994, 2621–2629. [Google Scholar] [CrossRef]
- Schramm, V. Crystal and molecular structure of tetrameric copper(I) iodide-piperidine, a complex with a tetrahedral tetrakis[copper(I) iodide] core. Inorg. Chem. 1978, 17, 714–718. [Google Scholar] [CrossRef]
- Zhu, M.; Fujita, K.-I.; Yamaguchi, R. Aerobic oxidative amidation of aromatic and cinnamic aldehydes with secondary amines by CuI/2-pyridonate catalytic system. J. Org. Chem. 2012, 77, 9102–9109. [Google Scholar] [CrossRef]
- Goher, M.A.S.; Hafez, A.K.; Mak, T.C.W. A copper(I) complex containing a new structure of the [Cu2I3]− anion. Reaction of CuI with quinaldic acid and the crystal structure of tris-(2-carboxyquinoline)triiododicopper(I) monohydrate. Polyhedron 2001, 20, 2583–2587. [Google Scholar] [CrossRef]
- Kamau, P.; Jordan, R.B. Complex formation constants for the aqueous copper(I)−acetonitrile system by a simple general method. Inorg. Chem. 2001, 40, 3879–3883. [Google Scholar] [CrossRef] [PubMed]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Nimmermark, A.; Öhrström, L.; Reedijk, J. Metal-ligand bond lengths and strengths: Are they correlated? A detailed CSD analysis. Z. Kristallogr. Cryst. Mater. 2013, 228, 311–317. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 21, 3885–3896. [Google Scholar] [CrossRef]
- Stilinović, V.; Užarević, K.; Cvrtila, I.; Kaitner, B. Bis(morpholine) hydrogen bond pincer—A novel series of heteroleptic Cu(II) coordination compounds as receptors for electron rich guests. CrystEngComm 2012, 14, 7493–7501. [Google Scholar] [CrossRef]
- Martins, N.D.; Ramos Silva, M.; Silva, J.A.; Matos Beja, A.; Sobral, A.J.F.N. (Benzoato-κ2O,O′)(quinoline-2-carboxylato-κ2N,O)(quinoline-2-carboxylic acid-κ2N,O)copper(II). Acta Crystallogr. Sect. E 2008, 64, m829–m830. [Google Scholar] [CrossRef]
- Kumar, S.; Garg, S.; Sharma, R.P.; Venugopalan, P.; Tenti, L.; Ferretti, V.; Nivelle, L.; Tarpin, M.; Guillon, E. Four monomeric copper(II) complexes of non-steroidal anti-inflammatory drug Ibuprofen and N-donor ligands: Syntheses, characterization, crystal structures and cytotoxicity studies. New J. Chem. 2017, 41, 8253–8262. [Google Scholar] [CrossRef]
- Bowmaker, G.A.; Di Nicola, C.; Marchetti, F.; Pettinari, C.; Skelton, B.W.; Somers, N.; White, A.H. Synthesis, spectroscopic and structural characterization of some novel adducts of copper(II) salts with unidentate nitrogen bases. Inorg. Chim. Acta 2011, 375, 31–40. [Google Scholar] [CrossRef]
- Menabue, L.; Saladini, M.; Battaglia, L.P.; Bonamartini Corradi, A. The factors stabilizing square-planar geometries in σ-bonding amine adducts: Crystal and molecular structure of bis(N-tosyl-β-alaninato)bis(piperidine)copper(II). Inorg. Chim. Acta 1987, 138, 127–130. [Google Scholar] [CrossRef]
- Clegg, J.K.; Hayter, M.J.; Jolliffe, K.A.; Lindoy, L.F.; McMurtrie, J.C.; Meehan, G.V.; Neville, S.M.; Parsons, S.; Tasker, P.A.; Turner, P.; et al. New discrete and polymeric supramolecular architectures derived from dinuclear Co(II), Ni(II) and Cu(II) complexes of aryl-linked bis-β-diketonato ligands and nitrogen bases: Synthetic, structural and high pressure studies. Dalton Trans. 2010, 39, 2804–2815. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lian, Z.-X. catena-Poly[[[2-(2-chlorophenoxy)-N′-(2-oxidobenzylidene-κO)acetohydrazidato-κ2N′,O]copper(II)]-μ-morpholine-κ2N:O]. Acta Crystallogr. Sect. C 2013, 69, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-T.; Tang, H.-L.; Lu, S.-M.; Ye, Q.-Y.; Huang, X.-H.; Huang, C.-C.; Hu, X.-L.; Zheng, S.-T. Delicate modulated assembly of a new kind of trinuclear copper(II) motif governed by N-containing agents. CrystEngComm 2014, 16, 9792–9799. [Google Scholar] [CrossRef]
- Segl’a, P.; Palicová, M.; Koman, M.; Mikloš, D.; Melník, M. Synthesis, spectral properties and crystal structures of copper(II) isonicotinates, first example of uncoordinated isonicotinates. Inorg. Chem. Commun. 2000, 3, 120–125. [Google Scholar] [CrossRef]
- Sharma, R.P.; Singh, A.; Saini, A.; Venugopalan, P.; Molinari, A.; Ferretti, V. Controlling the ligating behaviour of biologically important p-hydroxybenzoate towards copper(II) by the use of nitrogen bases: Synthesis, characterization and single crystal X-ray structure determination of [trans-Cu(en)2(H2O)2](L1)2·2H2O and [cis-Cu(L1)2(L2)2] where en = ethylenediamine, L1 = p-hydroxybenzoate, L2 = 3-picoline. J. Mol. Struct. 2009, 923, 78–84. [Google Scholar] [CrossRef]
- Das, B.; Baruah, J.B. Cooperativity on selective products in one pot reactions of 2,6-pyridinedicarboxylic acid and ethylenediamine with metal ions. Inorg. Chem. Commun. 2010, 13, 350–352. [Google Scholar] [CrossRef]
- Keene, T.D.; Hursthouse, M.B.; Price, D.J. Recurrent H-bond graph motifs between metal tris-ethylenediamine cations and uncoordinated oxalate anions: Fitting a three pin plug into a two pin socket. CrystEngComm 2012, 14, 116–123. [Google Scholar] [CrossRef][Green Version]
- Das Neves Gomes, C.; Jacquet, O.; Villiers, C.; Thuéry, P.; Ephritikhine, M.; Cantat, T. A diagonal approach to chemical recycling of carbon dioxide: Organocatalytic transformation for the reductive functionalization of CO2. Angew. Chem., Int. Ed. 2012, 51, 187–190. [Google Scholar] [CrossRef]
- Zevaco, T.A.; Görls, H.; Dinjus, E. Synthesis, spectral and structural characterization of the zinc carboxylate [Zn(2-quinolinecarboxylato)2(1-methylimidazole)2]. Inorg. Chim. Acta 1998, 269, 283–286. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Colthup, N.B.; Daly, L.H.; Wiberley, S.E. Introduction to Infrared and Raman Spectroscopy, 3rd. ed.; Academic Press: San Diego, CA, USA, 1990; pp. 332–334, 339–340. [Google Scholar]
- Williams, D.B.G.; Lawton, M. Drying of organic solvents: Quantitative evaluation of the efficiency of several desiccants. J. Org. Chem. 2010, 75, 8351–8354. [Google Scholar] [CrossRef] [PubMed]
- Agilent. CrysAlis PRO; Agilent Technologies Ltd.: Yarnton, UK, 2014. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C 2015, 71, 9–18. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
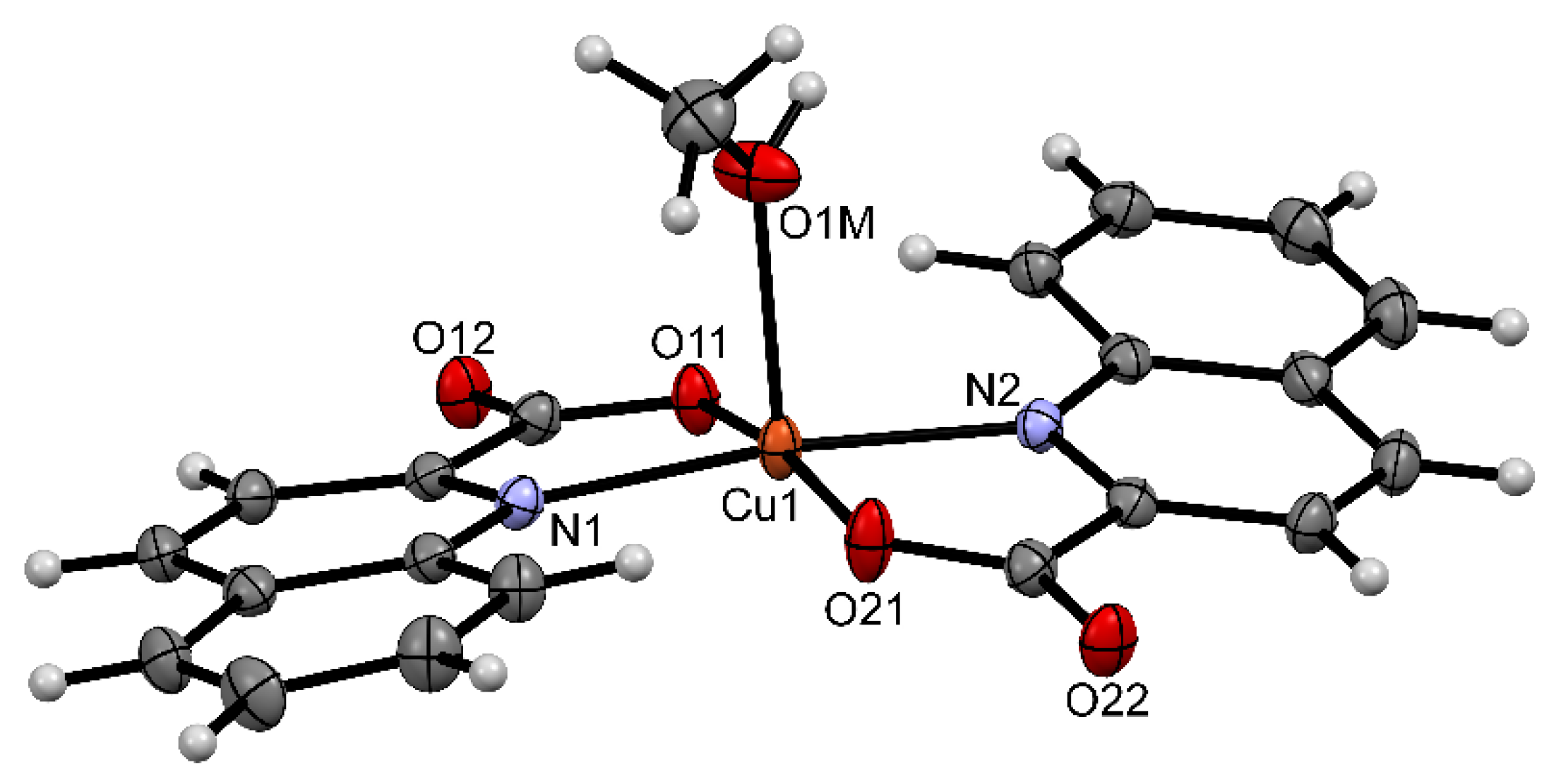
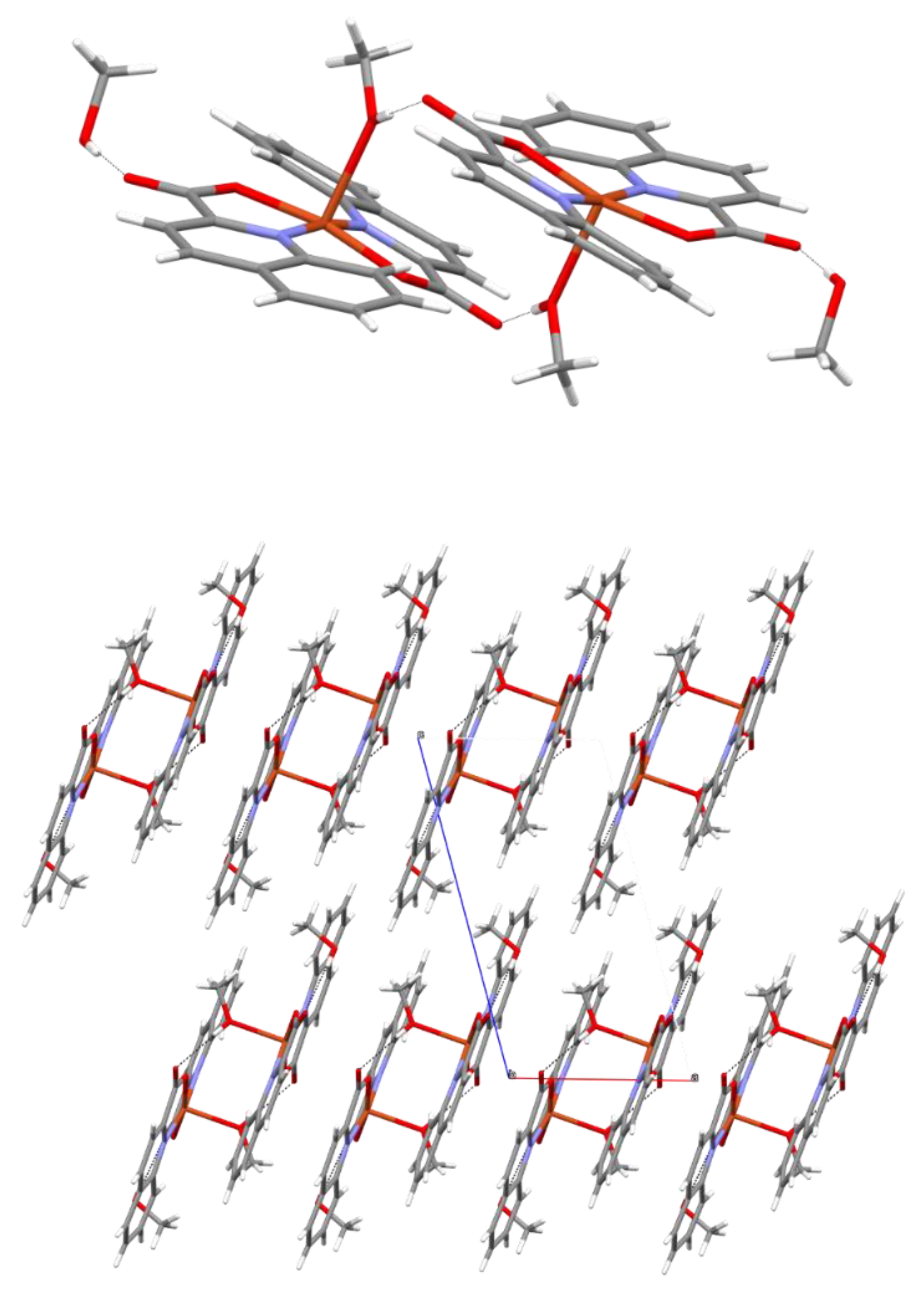
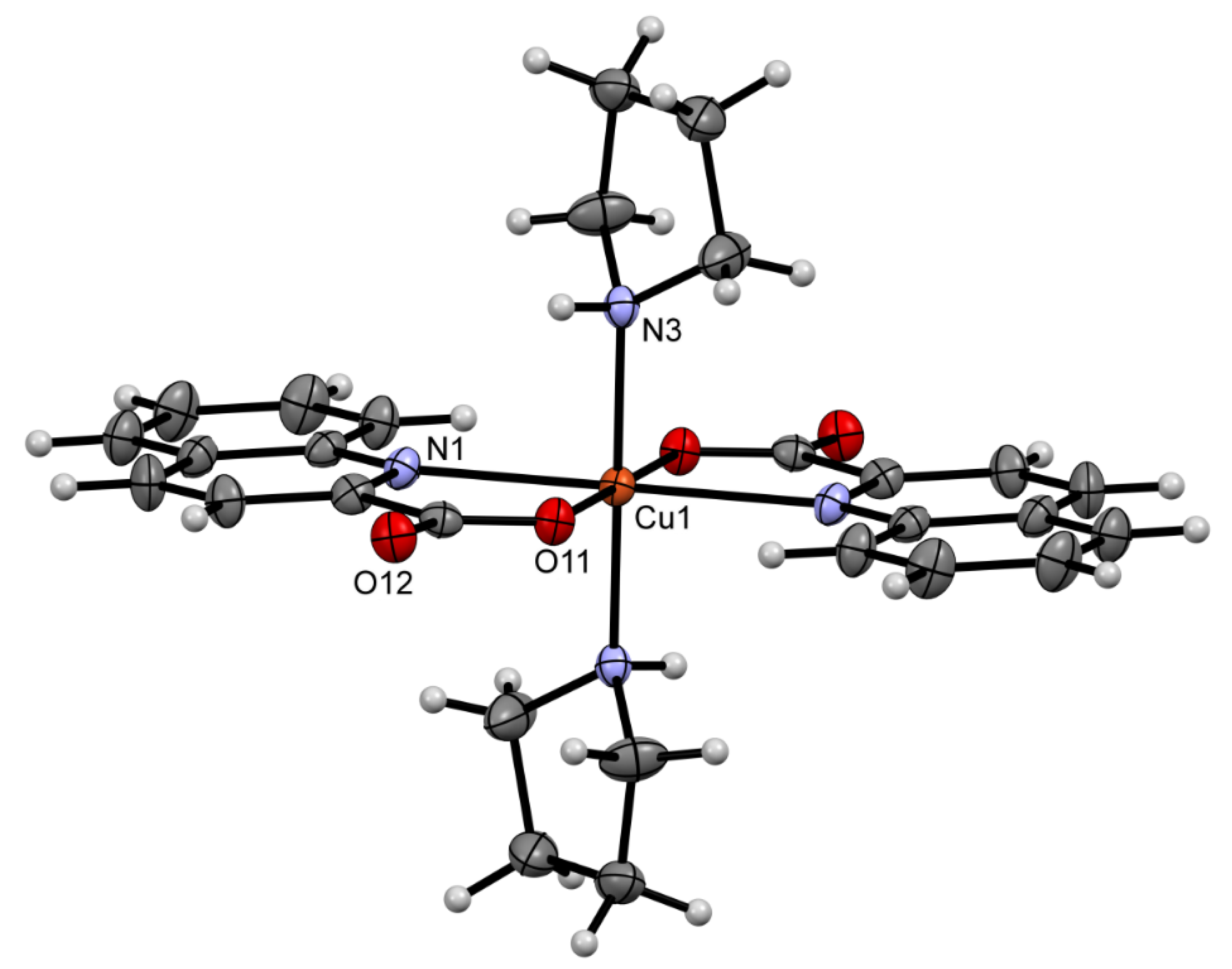
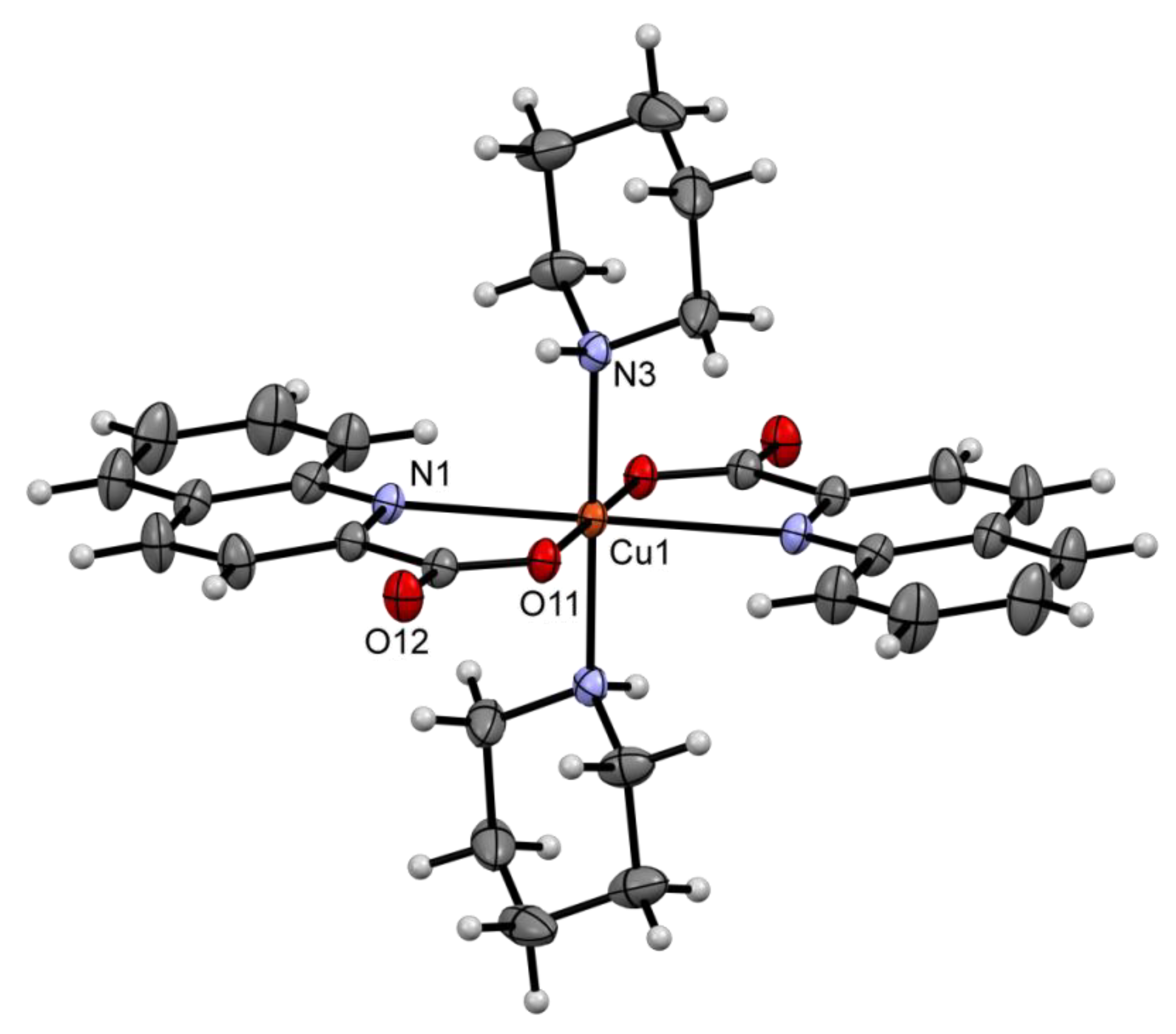
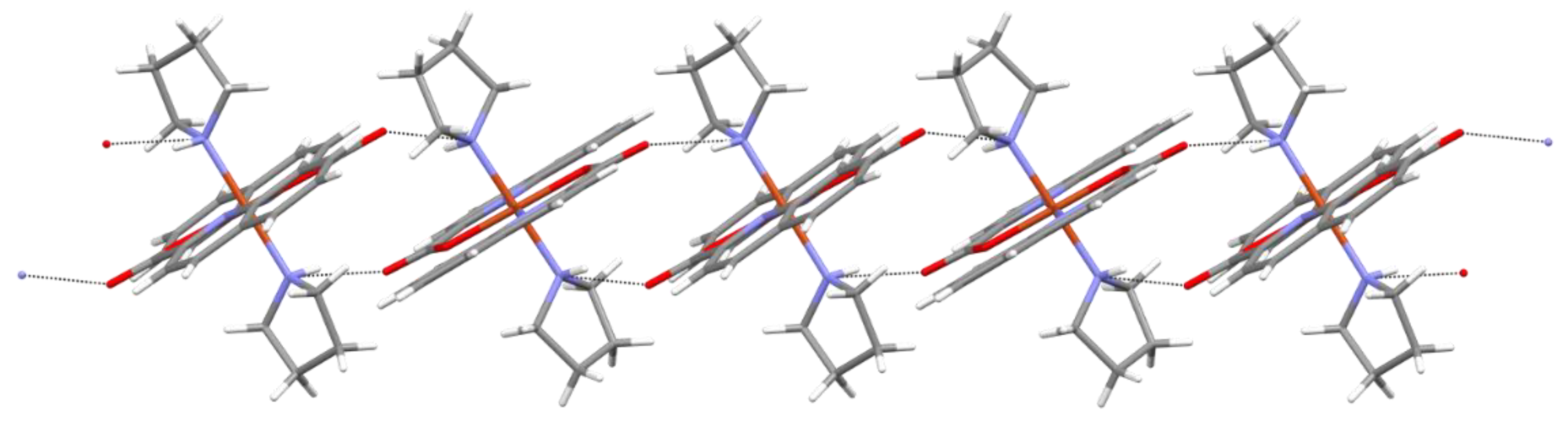
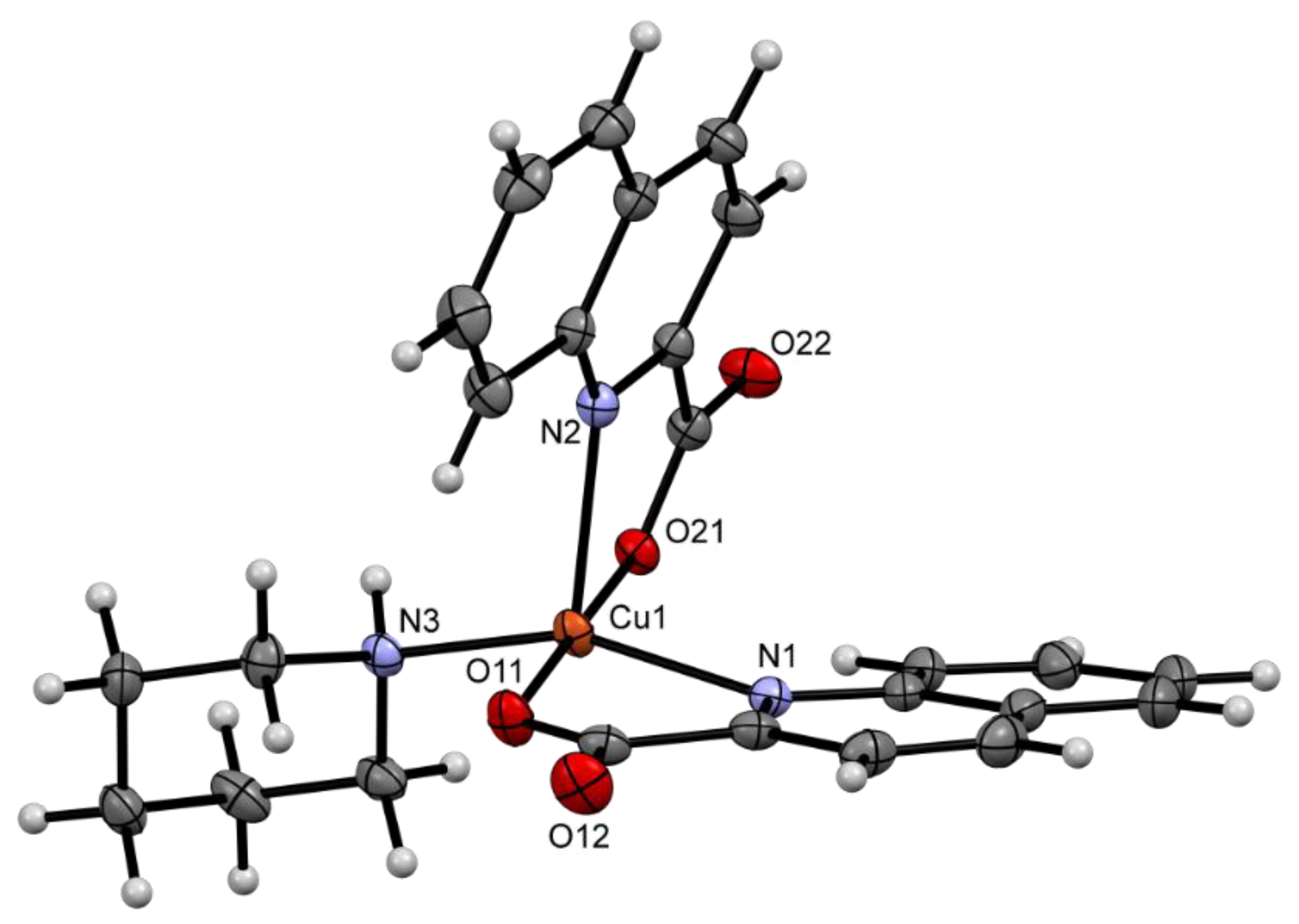
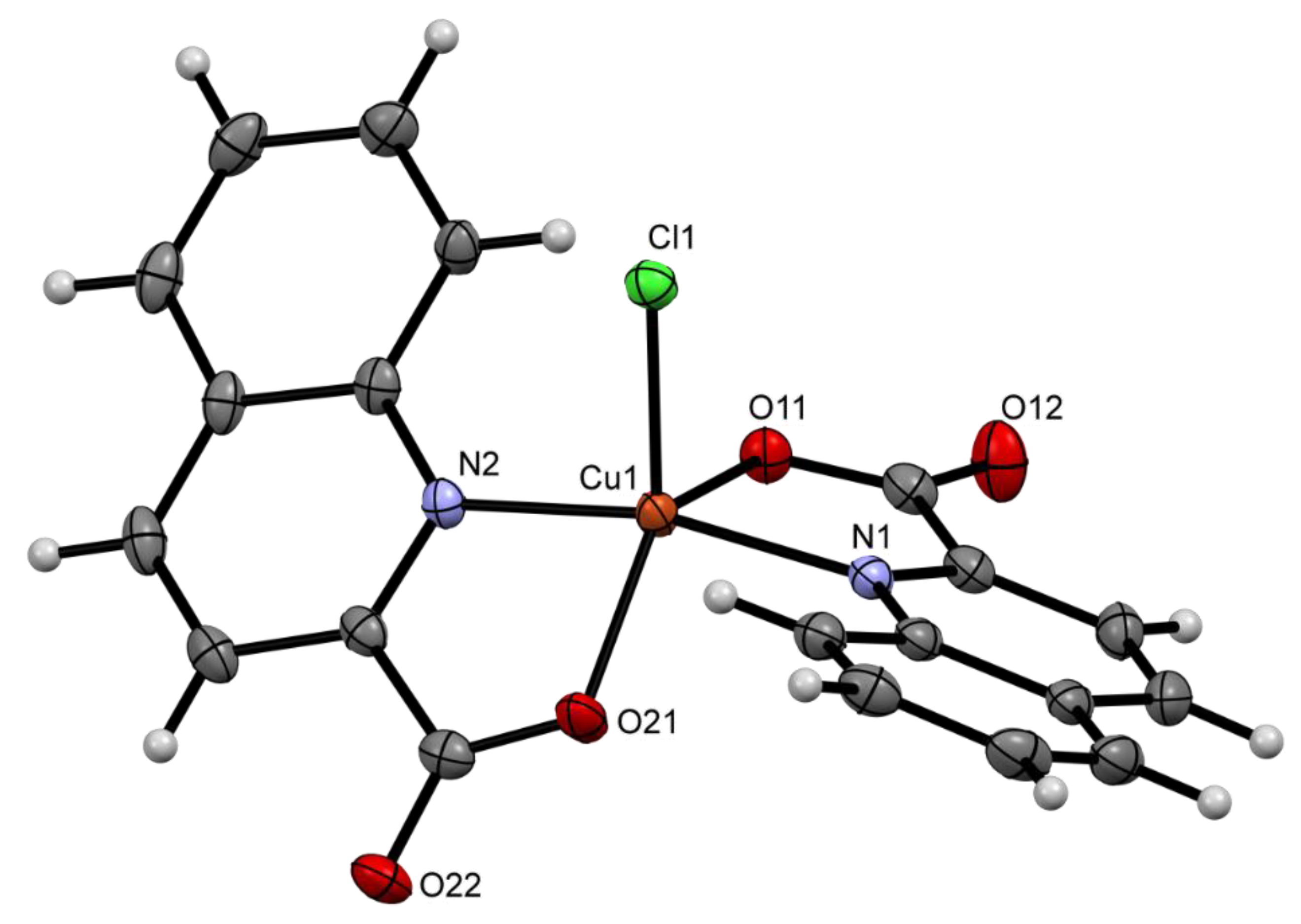
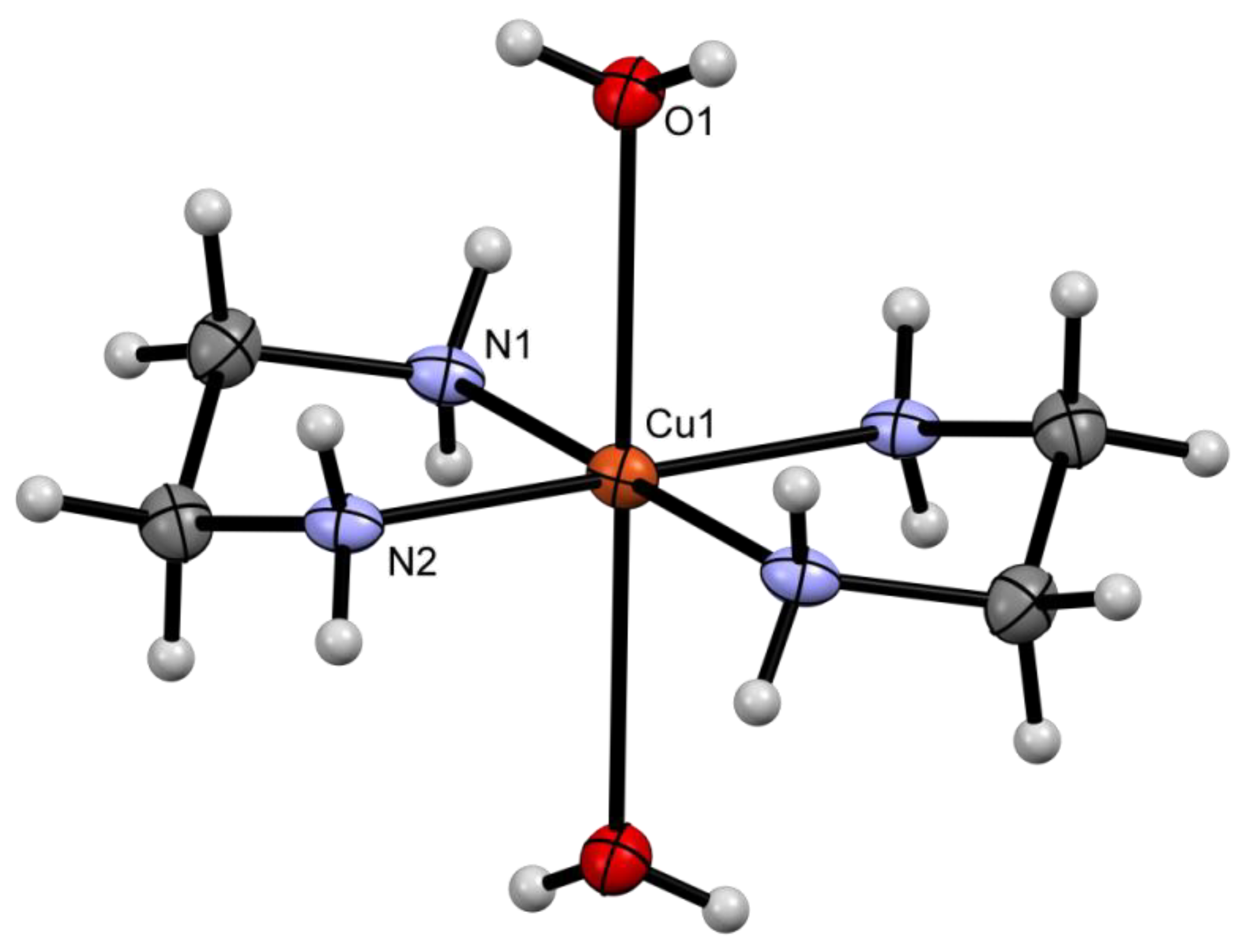
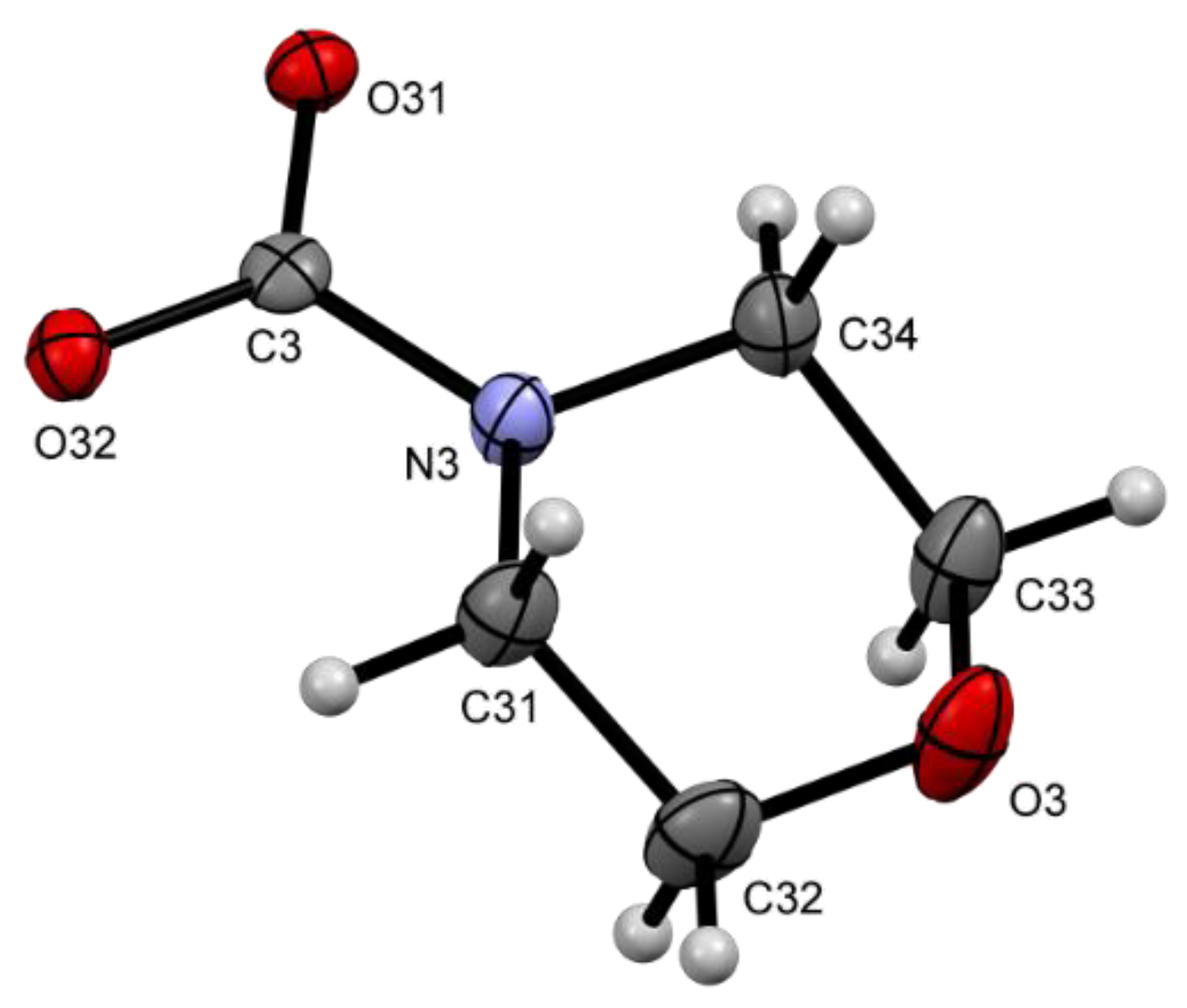
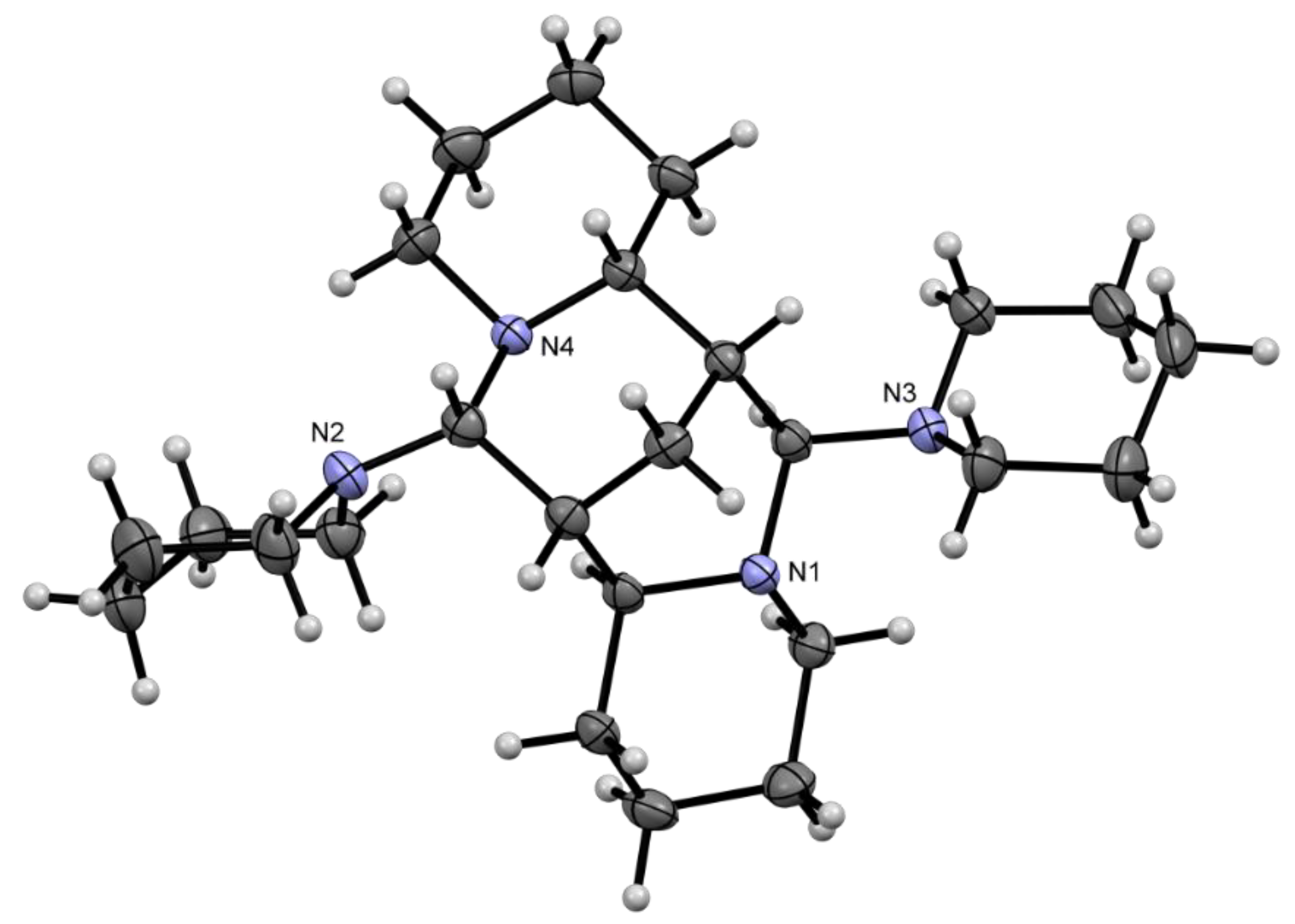
| Compound | Donor Set | τ a | Cu–N(quin−) | Cu–O(quin−) | L | Cu–L |
|---|---|---|---|---|---|---|
| 1 | N2O3 | 0.05 | 2.0994(15), 2.0836(15) | 1.9112(12), 1.9081(12) | CH3OH | 2.2806(14) |
| 2 | N4O2 | – | 2.3648(19), 2.3755(18) | 2.0440(15), 2.0279(14) | pyro | 2.0312(19), 2.0238(19) |
| 3 | N2O2Cl | 0.56, 0.71 | 1.9865(17), 1.9974(17), 1.9896(17), 2.0093(17) | 2.0774(15), 1.9947(15), 2.0365(14), 2.0162(14) | Cl− | 2.3811(6), 2.3483(6) |
| 4 | N4O2 | – | 2.387(3), 2.406(3) | 2.010(2), 1.987(2) | morph | 2.064(3), 2.074(3) |
| 6 | N3O2 | 0.36 | 2.1065(13), 2.2635(14) | 1.9246(12), 1.9373(11) | pipe | 2.0291(13) |
| 7 | N4O2 | – | 2.4161(14), 2.3659(14) | 1.9864(12), 2.0035(12) | pipe | 2.0727(15), 2.0833(15) |
| 8 | N4O2 | – | 2.3894(13) | 1.9839(10) | pipe | 2.0899(13) |
| 9 | N4O2 | – | 2.3805(12) | 1.9787(10) | pipe | 2.0921(12) |
| 11 | N2O2Cl | 0.51, 0.77 | 1.9960(12), 2.0262(12), 2.0022(12), 1.9898(13) | 2.0327(11), 2.0307(11), 2.0541(11), 2.0046(11) | Cl− | 2.3332(4), 2.3854(4) |
| Compound | νas(COO−) | νs(COO−) | ∆ a |
|---|---|---|---|
| [Cu(quin)2(H2O)] | 1631 | 1372, 1344 | 287 |
| [Cu(quin)2(CH3OH)]∙CH3OH (1) | 1645 | 1380, 1371, 1364, 1346 | 299 |
| [Cu(quin)2(pyro)2] (2) | 1620 | 1363, 1321 | 299 |
| (pyroH)[Cu(quin)2Cl] (3) | 1622, 1615 | 1374, 1365, 1357, 1344 | 278 |
| [Cu(quin)2(morph)2] (4) | 1618 | 1377, 1363 | 255 |
| [Cu(quin)2(pipe)] (6) | 1676, 1651 | 1355, 1341 | 335 |
| [Cu(quin)2(pipe)2] (7) | 1626 | 1364, 1358, 1346 | 280 |
| [Cu(quin)2(pipe)2]∙CH3CN (8) | 1626 | 1355, 1344 | 282 |
| [Cu(quin)2(pipe)2]∙CH3CH2CN (9) | 1624 | 1362, 1345 | 279 |
| (pipeH)[Cu(quin)2Cl] (11) | 1611 | 1367, 1355, 1345 | 266 |
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| Empirical formula | C22H20CuN2O6 | C28H30CuN4O4 | C24H22ClCuN3O4 | C28H30CuN4O6 | C14H36CuN6O8 | C25H23CuN3O4 |
| Formula weight | 471.94 | 550.10 | 515.43 | 582.10 | 480.03 | 493.00 |
| Crystal system | triclinic | monoclinic | triclinic | monoclinic | triclinic | monoclinic |
| Space group | P −1 | P 21/n | P −1 | P 21/n | P −1 | P 21/c |
| T [K] | 150.0 | 150.00(10) | 150.00(10) | 150.00(10) | 150.00(10) | 150.00(10) |
| λ [Å] | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| a [Å] | 7.0319(2) | 10.7782(5) | 9.2917(3) | 10.8070(6) | 6.1497(4) | 13.6876(7) |
| b [Å] | 10.7743(2) | 17.4803(8) | 14.8463(6) | 17.7249(13) | 6.9128(5) | 10.0952(4) |
| c [Å] | 14.0575(5) | 13.4856(6) | 16.5238(6) | 13.4503(7) | 14.5118(8) | 16.4802(9) |
| α [°] | 105.630(3) | 90 | 102.208(3) | 90 | 80.946(5) | 90 |
| β [°] | 104.177(3) | 91.801(4) | 99.808(3) | 91.991(5) | 89.732(5) | 106.995(5) |
| γ [°] | 93.338(3) | 90 | 92.838(3) | 90 | 65.307(7) | 90 |
| V [Å3] | 985.59(5) | 2539.5(2) | 2186.63(14) | 2574.9(3) | 552.24(7) | 2177.77(19) |
| Z | 2 | 4 | 4 | 4 | 1 | 4 |
| Dcalc [g/cm3] | 1.590 | 1.439 | 1.566 | 1.502 | 1.443 | 1.504 |
| μ [mm−1] | 1.153 | 0.902 | 1.159 | 0.900 | 1.040 | 1.042 |
| Collected reflections | 16,837 | 15,440 | 19,241 | 15,411 | 9645 | 20,410 |
| Unique reflections | 4520 | 6814 | 10,079 | 6603 | 2983 | 5898 |
| Observed reflections | 3968 | 4457 | 7902 | 3330 | 2614 | 5007 |
| Rint | 0.0329 | 0.0399 | 0.0293 | 0.0725 | 0.0539 | 0.0352 |
| R1 (I > 2σ(I)) | 0.0297 | 0.0420 | 0.0339 | 0.0563 | 0.0371 | 0.0332 |
| wR2 (all data) | 0.0733 | 0.1117 | 0.0793 | 0.1274 | 0.0858 | 0.0901 |
| 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|
| Empirical formula | C30H34CuN4O4 | C32H37CuN5O4 | C33H39CuN5O4 | C25H44N4 | C25H24ClCuN3O4 |
| Formula weight | 578.15 | 619.20 | 633.23 | 400.64 | 529.46 |
| Crystal system | monoclinic | monoclinic | monoclinic | triclinic | triclinic |
| Space group | P 21/c | P 21/n | P 21/n | P −1 | P −1 |
| T [K] | 150.00(10) | 150.00(10) | 150.00(10) | 150.00(10) | 150.00(10) |
| λ [Å] | 1.54184 | 1.54184 | 0.71073 | 0.71073 | 1.54184 |
| a [Å] | 10.9999(2) | 11.8229(2) | 14.0265(6) | 9.6952(5) | 9.2658(2) |
| b [Å] | 13.6491(2) | 7.60560(10) | 7.5246(4) | 11.4886(6) | 14.8807(3) |
| c [Å] | 19.0017(3) | 16.2266(3) | 14.4151(7) | 12.0529(8) | 17.1665(5) |
| α [°] | 90 | 90 | 90 | 64.013(6) | 101.324(2) |
| β [°] | 103.652(2) | 90.451(2) | 90.172(4) | 75.608(5) | 99.732(2) |
| γ [°] | 90 | 90 | 90 | 89.605(4) | 90.224(2) |
| V [Å3] | 2772.29(8) | 1459.05(4) | 1521.42(13) | 1160.85(13) | 2285.78(10) |
| Z | 4 | 2 | 2 | 2 | 4 |
| Dcalc [g/cm3] | 1.385 | 1.316 | 1.382 | 1.146 | 1.539 |
| μ [mm−1] | 1.457 | 1.384 | 0.764 | 0.068 | 2.749 |
| Collected reflections | 14,885 | 6018 | 7848 | 14,513 | 28,590 |
| Unique reflections | 5618 | 2937 | 3985 | 5341 | 9361 |
| Observed reflections | 4711 | 2749 | 3394 | 3465 | 8533 |
| Rint | 0.0376 | 0.0206 | 0.0202 | 0.0394 | 0.0267 |
| R1 (I > 2σ(I)) | 0.0419 | 0.0356 | 0.0315 | 0.0573 | 0.0297 |
| wR2 (all data) | 0.1237 | 0.0951 | 0.0854 | 0.1536 | 0.0799 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modec, B.; Podjed, N.; Lah, N. Beyond the Simple Copper(II) Coordination Chemistry with Quinaldinate and Secondary Amines. Molecules 2020, 25, 1573. https://doi.org/10.3390/molecules25071573
Modec B, Podjed N, Lah N. Beyond the Simple Copper(II) Coordination Chemistry with Quinaldinate and Secondary Amines. Molecules. 2020; 25(7):1573. https://doi.org/10.3390/molecules25071573
Chicago/Turabian StyleModec, Barbara, Nina Podjed, and Nina Lah. 2020. "Beyond the Simple Copper(II) Coordination Chemistry with Quinaldinate and Secondary Amines" Molecules 25, no. 7: 1573. https://doi.org/10.3390/molecules25071573
APA StyleModec, B., Podjed, N., & Lah, N. (2020). Beyond the Simple Copper(II) Coordination Chemistry with Quinaldinate and Secondary Amines. Molecules, 25(7), 1573. https://doi.org/10.3390/molecules25071573