Abstract
New trends in biomedical applications of the hybrid polymeric hydrogels, obtained by combining natural polymers with synthetic ones, have been reviewed. Homopolysaccharides, heteropolysaccharides, as well as polypeptides, proteins and nucleic acids, are presented from the point of view of their ability to form hydrogels with synthetic polymers, the preparation procedures for polymeric organic hybrid hydrogels, general physico-chemical properties and main biomedical applications (i.e., tissue engineering, wound dressing, drug delivery, etc.).
1. Introduction
Hydrogels can be classified by taking into consideration many factors, such as source; preparation methods; network structure (as permanent (chemically crosslinked or irreversible), and non-permanent (physically crosslinked or reversible, hydrogen-bonded hydrogels); dimensions (macrogels, microgels, nanogels); sensitivity to stimuli (such as physical, chemical, and biochemical stimuli); charge of polymer network (nonionic, ionic, zwitterion, and amphoteric); physical aspect (micro-/nanoparticle, film, matrix, gel, etc.); configuration (amorphous and semicrystalline); composition (homopolymeric, multipolymeric or heteropolymeric, copolymeric, and interpenetrating polymer networks, hybrids, composites); degradability (biodegradable, bioabsorbable, bioerodible, and degradable in a controlled manner) (Scheme 1) [1,2].
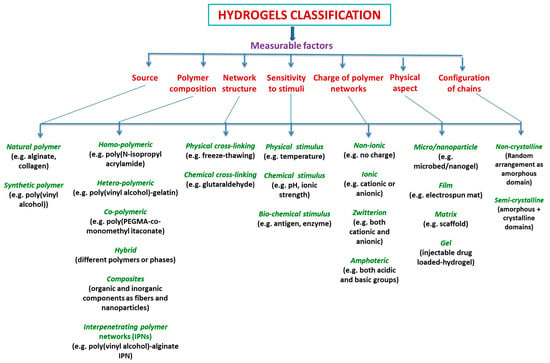
Scheme 1.
Classification of hydrogels [1,2].
Generally, hydrogels contain polar/charged functional groups which offer them hydrophilicity, water absorption capacity and, respectively, swelling in a certain medium, enhancement of their susceptibility to stimuli, etc. [3,4]. They can also differentiate in respect with their equilibrium swelling grade (SWD) as those low SWD hydrogels (20–50%), medium SWD hydrogels (50–90%), high SWD hydrogels (90–99.5%), and superabsorbent hydrogels (>99.5%) [5,6]. The hydrogels with high SWD show good permeability and biocompatibility [7] being preferred for use in the medical field.
Hybrid hydrogels definition is still debatable. They are defined either as a complex composed of hundreds of chemically or physically cross-linking nanogels [8], or it refers to systems combined with different polymers and/or with nanoparticles, such as plasmonic, magnetic, and carbonaceous nanoparticles, among others, or they are constituted by chemically, functionally, and morphologically distinct building blocks from at least two distinct classes of molecules, which can include biologically active polymers as polysaccharides and/or proteins, peptides, or nano/microstructures, interconnected via physical or chemical means [9]. Depending on the size and the nature of the building blocks, the hybridization can occur at molecular level or at microscopic scale [10,11].
For the purpose of this review, we refer only to the organic polymeric hybrid hydrogels containing natural polymers (Figure 1), defined according to the last definition and their medical applications (in medicine/nanomedicine).
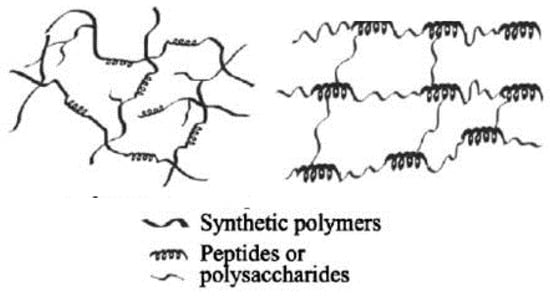
Figure 1.
Schematic representation of organic hybrid hydrogels systems (adapted from [11]).
Each medical application involves the unique choice of a combination of the component materials, with the goal to match both desired structural and functional properties which must effectively produce an advanced polymeric system, with a new profile [12]. One of the most relevant examples is the combination protein/other polymers. Such combinations can be resulted by polymerization or conjugation (click chemistry) with synthetic polymers resulting compatible hybrid hydrogels both in vitro and in vivo as it was demonstrated by cell differentiation, proliferation, migration studies and drug delivery, tissue engineering, wound healing applications [13,14], respectively or sequestration of growth factors from the surrounding medium [15]. Commonly, the hybrid hydrogels are heterogeneous and this property is important to assure cell adhesion, organization, and cell–cell interactions required for medical applications [16,17,18,19].
1.1. Polymers Used in Hybrid Hydrogels
There are four main types of natural biodegradable polymers used in hybrid hydrogels described in this review—Table 1, including [20]: (1) homopolysaccharides, as: cellulose and derivatives, pullulan, dextran, starch, etc.; (2) heteropolysaccharides from which can be mentioned: chitosan/chitin and their derivatives [21], dextran, agarose, alginic acid and alginates, hyaluronic acid (HA), chondroitin and derivative sulphates, heparin, pectin, etc. (3) polypeptides/proteins, such as gelatin, collagen, albumin, fibrin and fibrinogen, soy and whey proteins, silk, Matrigel™, etc., and genetically engineered proteins [22,23,24] (calmodulin (a calcium-binding protein), elastin-like polypeptides, leucine zipper) [25]; (4) deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) [26]. The protein/polysaccharide hybrid polymers like fibrin/cellulose, collagen/HA, gelatin/alginate and many others etc. were studied [27] and other many combination make now topics of undergoing researches. Lignin was also used [28,29]. Most of them are components of the extracellular matrix (ECM) in vivo. Their composition (bovine fibrinogen, rat tail collagen, etc.) may vary with source and processing method, being difficult to control their microstructures, properties and reproducibility between experiments.

Table 1.
Natural polymers used in organic hybrid hydrogels for medical applications.
Synthetic polymers commonly used in the hybrid hydrogels preparation can be classified into three main types: non-biodegradable [30,31], biodegradable [32], and bioactive polymers [33]. Most common synthetic polymers are: poly (lactic acid) (PLA), poly (ε-caprolactone) (PCL), poly(glycolic acid) (PGA) and copolymers [34], poly (ethylene glycol) (PEG) and poly(vinyl alcohol) (PVA) [35,36,37,38] to produce biodegradable hydrogels. Hydrogels may include vinyl monomers like 2-hydroxyethyl methacrylate (HEMA), N-isopropyl acrylamide (NIPAAm), 2-hydroxypropyl methacrylate (HPMA), acrylamide (AAm), acrylic acid (AAc) or macromers [37,38,39], methoxyl poly(ethylene glycol) (PEG), monoacrylates (mPEGMA or PEGMA), and diacrylates (PEGDA), ethylene glycol diacrylate (EGDA), Pluronic® polymers, etc. [39].
By combining the properties of synthetic and natural polymers to form hybrid hydrogels, a direct approach is created for bioactive hydrogel scaffolds for tissue engineering.
Comparatively with natural polymers, the synthetic polymers are easily synthesized even at large scale by polymerization, cross-linking, and functionalization (modification by block structures, by blending, copolymerization), their molecular structure, molecular weight, physical and chemical properties (mechanical strength, biodegradability [40,41]) are more reproducible, this aspect being critical for the medical applications mainly scaffolding. Unfortunately, applications of synthetic hydrogels as biomaterials are limited by their absence of bioactivity. The protein-polymer hybrid networks with complex abilities, including bioactivity, stimuli-responsiveness, catalytic activity, or ability to regulate cell behaviors have been/are created to overcome this limitation, maintaining good mechanical properties of materials [42,43,44,45,46].
1.1.1. Microgel
The term microgel describes a variety of particles that differ substantially in structure, physico-chemical properties, preparation and application and is interchangeably with terms such as nanogel, microsphere and macrogel depending on the numerous particle types falling within the broad sphere of nano-/microparticle shapes and sizes [47,48,49,50].
1.1.2. Hybrid Nanogels
Hybrid nanogels later developed are highly crosslinked nano-sized hydrogel systems [47,48] with diameter less than 100 nm [49,50] having a non-fluid colloidal/polymer network that combine the properties of both hydrogels and nanomaterials. The nanoscale provides a large surface area for bioconjugation, long time of circulation in blood, and the possibility of being actively or passively targeted to the desired site of action (e.g., tumor sites) [10]. Hybrid smart hydrogels/nanogels show the ability to respond to biomedically relevant changes like pH, temperature, ionic force/concentration, redox environment, light, glucose, magnetic field, electrical field, chemicals or specific biomarkers etc., by changing their volume, refractive index, and hydrophilicity/hydrophobicity etc. Micro- and nano-sized hydrogels are faster in responding to changes in their environment than their macroscopic or bulk counterparts and can be used more efficiently in medical and sensor applications [51].
1.1.3. Multifunctional Hybrid Nanogels
Multifunctional hybrid nanogels found applications in medical field/nanomedicine for continuous monitoring by optical sensing to mentioned stimuli in complex samples such as blood and bioreactor fluids as well as for intracellular imaging, contributing to the explanation of intricate biological processes, the development of novel diagnoses and therapy toward clinical applications. [52].
1.1.4. Hybrid Polymer Nanogel/Hydrogels
Hybrid polymer nanogel/hydrogels include interpenetrated networks (IPNs) and core-shell particles. The core-shell strategy is especially useful for targeting therapy, while the interpenetration allows the development of multiresponssive nanogels and the control of the drug release profile [53].
1.1.5. Physical Hydrogels
Physical hydrogels result by ionic and physical interactions, such as hydrogen bonds, coordination bonds, electrostatic and hydrophobic interactions in certain conditions and physico-chemical interactions (stereo-complexation, charge condensation, or supramolecular chemistry) [54]. By changing the temperature, pH, ionic strength or solvent composition, they form a homogeneous solution and re-gel when they return to their initial conditions, being reversible gels, generally unstable and mechanically weak [55]. The physical cross-links are also formed by crystallization, [56] between amphiphilic block and graft copolymers [57], and protein interactions [58]. Physically crosslinked hydrogels show stimuli-responsiveness and self-healing properties, but their mechanical strength is low and they often exhibit plastic flow [59].
1.1.6. Chemically or Covalently Crosslinked Hydrogels
Chemically or covalently crosslinked hydrogels with a permanently fixed shape at rest, exhibit a low fracture toughness and extensibility. Therefore, it is preferred to create both physically and covalently crosslinking hydrogels [60,61], resulting doubly-crosslinked hybrid gels that combine all mentioned properties [62]. Many double network (DN) hydrogels prepared by double chemically crosslinking or by hybrid physical/chemical crosslinking imply crosslinking agents, but they present toxicity which is an important disadvantage. Designing a new generation of DN gels comprising two non-covalent associated networks is a promising technique.
Kondo and coworkers [63] prepared a dually-crosslinked polymer gel with a very homogeneous network architecture, using a tetra-arm star-shaped poly(ethylene glycol) (PEG), PEG and poly(dimethylsiloxane) (PDMS) building blocks linked by orthogonal cross-coupling, The obtained network from hydrophilic and hydrophobic components regularly and uniformly distributed is non-covalent hydrophobic association whose strength is tuned by the molar ratio of the hydrophilic PEG and the hydrophobic PDMS segments [64].
1.1.7. Self-Assembling Hybrid Hydrogels
Self-assembling hybrid hydrogels containing peptides provide the desired biological functionality and biodegradability, are able to mimic biological structures and materials having direct biomedical applications, namely as carriers for drug and cell delivery (e.g., incorporation of bioactive sequences from natural proteins). To control mechanical, biocompatibility and degradation properties, the peptides are combined with polymeric networks [65,66] by chemical modification, covalently linking or non-covalent interactions between peptides and polymers [67].
Hybrid hydrogels self-assembled from graft copolymers via formation of coiled coil antiparallel heterodimers was also demonstrated [68], based on HPMA copolymers backbone and a pair of oppositely charged peptide grafts. The formation of these hybrid hydrogels was reversible [68]. A DNA/poly(lactic-co-glycolic acid) (PLGA) hybrid hydrogel (HDNA) was prepared for water-insoluble ophthalmic therapeutic delivery of dexamethasone and it may be applied in treatment of various eye diseases [69].
1.1.8. Interpenetrated and Semi-Interpenetrated Polymer Networks
To enhance the mechanical strength, the swelling/deswelling response, and to add new sensitivities to a nanogel, multicomponent networks as full IPNs and semi-IPNs (sIPNs) were prepared by simultaneous synthesis and sequential synthesis involving two or more polymers [70,71]. The reaction can take place in the presence of a crosslinking agent, in order to form a complete IPN or in the absence of the crosslinking initiator, to form a sIPN.
1.1.9. Core-Shell Polymer Networks
The most common techniques of synthesis of core-shell nanogels are the seed precipitation polymerization, crosslinking of amphiphilic micelles preformed by self-assembly or the reversible addition–fragmentation chain-transfer polymerization (RAFT) [72,73,74,75,76,77].
Several examples of hybrid polymeric hydrogel include:
- (1)
- PEG-modified natural polymers [11,78,79], like fibrinogen, heparin (Hep), dextran, HA, and albumin;
- (2)
- PNIPAAm-modified natural polymers, like collagen, chitosan, and alginate [80,81,82,83].
1.1.10. Supramolecular Hydrogel
Supramolecular hydrogel are builded by blocks of peptides and polymers by the coupling/conjugation of specific peptide sequences (cell adhesive and/or enzymatically cleavable) to polymer chains. In such a way is obtained controlled cell responses (adhesion, migration, differentiation) because the components can self-assembly into hybrid hydrogels either, as peptide-polymer conjugates or combining individual components. These will determine the properties of the hydrogels (as stiffness, mesh structure, responsiveness, and biocompatibility) [84], cooperative folding/unfolding transitions control over the structure formation at the nanometer level. The new produced materials may possess unprecedented levels of structural organization and novel properties [85]. By optimizing the amino acid sequence, responsive hybrid hydrogels tailor-made for a specific application may be designed. Hybrid peptide/polymer molecular hydrogel design and synthesis showed significant research progress to mimic the natural proteins molecular architectures, dynamic responsiveness, and cellular functions, combined with tunability and processability provided by the synthetic polymer constituents.
2. Preparation Procedures for Polymeric Hybrid Hydrogels
2.1. Routes to Obtain Hybrid Hydrogels
Crosslinking techniques can be: (i) physical crosslinking (achieved by using repeated freezing/thawing cycles and led to cryogels) by ionic interaction, complex coacervation or H-bonding; (ii) chemical crosslinking or grafting by polymerization, co-polymerization, chemical conversion (using crosslinking agents such as borates, glyoxal, glutaraldehyde, etc.), and (iii) irradiation crosslinking or grafting (electron beam or gamma radiation, depending on irradiation dose). The properties of hydrogels can be controlled by different parameters, such as structures, by cross-linking type, end density, and synthesis of polymers, while in the case of physical hydrogels, by environment conditions (as pH, temperature, ionic strength etc.).
Chemically cross-linked gels are obtained by radical polymerization/crosslinking, emulsion, reverse microemulsion, inverse miniemulsion, heating, irradiation (ultraviolet, high-energy radiation, especially gamma and electron beams), photolithographic chemical reactions via crosslinker as di-sulfide crosslinking, ionic, click chemistry (such as azide-alkyne cyclo-addition reactions, thiol-ene couplings, Diels-Alder reactions and tetrazine-norbornene chemistry), Schiff base crosslinking with a huge ensemble of reactions, such as Michael type reaction, Michaelis-Arbuzov reaction, and nucleophile addition [86], and enzymatic cross-linking [87]. Both chemical and physical cross-linking approaches are employed for hydrogels preparation [2].
A breakthrough toward the synthesis of complex structures with a high degree of functionality and compositional variety is the utilization as synthesis ways the controlled/living radical polymerization technique such as the catalytic atom (group) transfer radical polymerization (ATRP), degenerative chain transfer polymerization represented by iodine-mediated polymerization (RITP), and reversible addition-fragmentation chain transfer polymerization (RAFT) [88]. A new strategy of hybrid hydrogels synthesis entails the non-covalent attachment of genetically engineered coiled-coil protein motifs to hydrophilic synthetic HPMA copolymer backbone. The physical crosslinking was established by self-assembly of the coiled-coil domains [89].
2.1.1. Chemical Modifications
Chemical modifications involve a plenty of ligands which can be used for targeted drug delivery, stimulus responsive drug release or preparation of complex materials. The cross-linking of the hybrid network and conjugating proteins to the gel backbone as a platform for immobilizing functional proteins was reported by Lim et al. [90].
2.1.2. Functionalization
Hybrid hydrogels/nanogels can also be surface functionalized with specific ligands to achieve targeted therapy and reduce toxicity [91]. Functionalization is also important in order to create different types of macro/micro/nanogels morphologies, as hairy microgels, core-and-shell, hallow, multilayer microgels, [92] etc.
2.1.3. Stealth Functionalization
Hybrid nanosystems/nanogels for drug delivery and biomedical purposes need a non-secondary requirement, as their biocompatibility necessary both to reduce the inflammatory or the immune response of the organism, and to improve blood circulation lifetime, biodistribution, and bioavailability of the carried drugs and also to overcome the self-defense mechanisms present in the bloodstream of the host organism. To achieve this requirement the hybrid nanogels must be specifically designed. A very wide variety of architectures result by their decoration, modification, and functionalization, [93], or they can be modified by conjugation with both organic [94] and inorganic [95] types of nanoparticles and nanostructures. The morphologies of hybrid nanogels vary both with the particle type and the assembly technique, each component being either core or shell, of different size and architecture [96]. These variable morphologies may be obtained by chemical reactions or through physical crosslinking based on hydrogen bonds, ionic interactions, and other intermolecular bonds. Therefore, a proper surface decoration and its biocompatibility, is a parameter capable of strongly influencing the biodistribution together with the dimensions, the surface charge and the ligands interaction. Many stealth functionalizations exploit hydrophilic polymeric chains, as polyethylene glycols or chitosan.
2.1.4. PEGylation
PEGylation is a solution to increase the bioavailability of the decorated nanostructures and to extend the circulating lifetime [97]. After this modification a protein corona is formed around the antifouling PEG functionalization [98]. It will create a hindered zone around the nanoparticles and reduces the wrapping by plasma proteins and the subsequent uptake by macrophages PEGylation depends on many factors such as hydrophilicity of the PEG chains, molecular weight (MW) which vary from 2000 to 13,000 Da.
2.2. Processing Methods
Processing methods include [1]: solution casting/drying, theta gelation, freezing or freezing/pressurizing, freeze drying, emulsion freeze drying, inverse microemulsion polymerization technique, solution blowing, electrospinning, coagulation treatment, CO2-in-water emulsion, sol-gel method/thermal annealing, CO2 bubbles template freeze drying, high hydrostatic pressure [HHP] method, supercritical gel-drying. Other new synthesis methods include the implementation of click chemistry reactions [99], photo-patterning, and rapid prototyping, 3D printing for the facile production of hybrid hydrogels, self-assembly [100,101], the use of biological molecules and motifs to promote a desired cellular outcome, and the tailoring of kinetics and transport behavior to obtain desired biomedical outcomes [102]. 3D bioprinting of hydrogels is performed in accordance with the native tissue architecture therefore it is expected to result in a new generation of engineered tissues. Bakarich et al. [103] fabricated by a new 3D-printing approach an interesting material with good mechanical performance based on κ-carrageenan and poly(oxyalkylene amine) (Jeffamine) based ionic-covalent entanglement hydrogels. The carrageenan induced a fast gelation, a structural integrity to the hydrogel system and thermoresponsiveness, while the epoxy-amine reaction to form covalent bonding takes place an ambient temperature for covalent bond formation.
Hydrogels and their products can be obtained in a wide range of shapes as temporary or permanent shape, shape memory, smart shape memory, quadruple-shape, sponges, soft or rigid, stretchable, films, sheets, bilayer, micro/nanoparticles with defined shapes, ultrathin microcapsules, matrix, scaffolds, hollow cube, hemisphere, pyramid, cylindrical, twisted bundle, patches for wound dressing, artificial ear, nose, and many others.
3. Properties
The specific physico-chemical key properties of the hybrid hydrogels are: remarkable thermodynamic stability, elevated capacity of solubilization, mildness, density, swelling/deswelling, high-water content and permeability, low surface tension and relative low viscosity, stiffness, mesh structure and size, responsiveness, biocompatibility and biodegradability (so avoiding its accumulation in the organs), non-immunologic response and capability of undergoing vigorous sterilization techniques [48], as well as their tunable viscoelasticity and structural similarity to the ECM. Their properties can be fine-tuned through selection of the hydrogel components (chemical composition), hydrophobicity/hydrophilicity ratio, and cross-linking strategy, crosslinking density etc. Hydrogels are commonly considered as highly biocompatible, owing to the high-water content and also to the physico-chemical similarity with the native ECM. Chemically cross-linked synthetic polymeric hydrogels have higher mechanical properties compared to self-assembling (physically crosslinked) systems, thanks to the high molecular weight of polymer materials, but they lack biological functionality, while self-assembling hydrogels, formed through physical cross-links, allow minimally invasive implantation in the body.
3.1. Swelling
The swelling of hydrogels is a process occurring in three steps, namely: (a) diffusion of water molecules into hydrogel network, (b) hydration of polymeric chains and their relaxation and (c) expansion of crosslinked polymeric network. The primary and secondary bound water is uptaken by the network by its interaction with the polar and hydrophobic sites, respectively and then the network is imbibed with additional water which is named free water. Finally at an infinite dilution to a maximum, level equilibrium water content is reached. The determination of swelling behavior is the main assay to establish the hydrogel quality, as it is also a means to evaluate other properties as: crosslinking degree, mechanical properties, degradation rate, etc. Swelling properties of the stimuli responsive hydrogels are significantly changed by the modification in parameters of the surrounding environment (i.e., temperature, pressure, pH, solvent composition, ionic strength, electrical potential, etc.). The polymeric hybrid hydrogels exhibit biodegradability and biocompatibility, high permeability, to oxygen, nutrients, and to water-soluble metabolites, being promising carriers and for cells encapsulation. They resemble with natural soft tissues [41,104] being very useful in regenerative medicine, for tissue scaffold or therapeutic transfer systems, promoting cell attachment and proliferation [2].
3.2. Mechanical Properties
The mechanical properties can be varied and tuned by changing the crosslinking degree, or lowered by heating. To seed osteoblast cells, it is necessary a more stiff material than in the case of adipocyte culture, as for this is also requirement for the development of a heterogeneous prosthetic device, as substitute for the intervertebral disc. The elastic nature of hydrated gels has been found to minimize irritation to the surrounding tissues after implantation.
3.3. Responsiveness
Generally, hydrogels have weak mechanical properties and a slow or delayed response to external stimuli. Novel hydrogel designs substantially enhanced mechanical properties and by creating the superporous and comb-type grafted hydrogels fast responses to external stimuli were obtained as also was done by development of self-assembling hydrogels from hybrid graft copolymers with property-controlling protein domains, and genetically engineered triblock copolymers containing hydrogels.
The low interfacial tension between the gel surface and body fluid minimizes protein adsorption and cell adhesion, reducing the chances of negative immune reactions [105].
3.4. Porosity and Permeation
The average pore size, the pore size distribution, and the pore interconnections included together in the parameter called « tortuosity » are important factors for a hydrogel matrix. They are influenced by the composition and the crosslink density of the hydrogel polymer network. Pores can show different morphologies: they can be closed, open as a blind end or interconnected, again divided in cavities and throats.
Net charge of the polyelectrolyte hydrogel is determined by the initial concentration of the cationic and/or anionic monomer.
Crosslinking influences all the other properties of the hydrogels. By controlling the crosslinking degree, the materials with tunable and optimized properties destined to different applications can be obtained [106].
The micro-/nanogels are valuable materials as drug-delivery carriers because they show high loading capacity, good stability, and reversible volume change in response to environmental stimuli (such as pH, temperature, and glucose level) [93].
4. Applications
Hydrogels remain the most appealing candidates for tissue engineering scaffolds. The development of hybrid hydrogels constituted from different polymers is based on numerous resources and they are applied for regenerative medicine, tissue engineering (including: bone regeneration [107,108,109,110], cartilage tissue, vascular tissue, cardiac tissue, cardiovascular tissue, meniscus tissue, human prostate tissue, skin tissue/wound, and other tissues), wound healing, artificial cornea, drug/gene delivery, cancer cells, nucleus pulposus bioelectronic interfaces due to their structural similarity to the natural ECM, inherent biocompatibility, tunable viscoelasticity, tunable physical and mechanical properties, and their ability to form scaffolds for different tissues, high-water content and high permeability for oxygen and essential nutrients [11]. Biomedical applications of hydrogels as the first materials developed for uses inside the patient started from the decade of 70 s [111].
It is considered that the development of the hydrogels for medical applications known three steps [100,112]. The first generation of hydrogels is characterized by various crosslinking procedures involving the chemical modifications of a monomer or polymer with an initiator to develop materials with high swelling and good mechanical properties. The second generation of materials is that capable to respond to specific stimuli (temperature, pH, ionic strength, different external fields or concentration of specific bioactive molecules etc.), known as smart hydrogels. Finally, the research for the third generation of hydrogels was focused on the investigation and development of hybrid, stereo complexed materials (e.g., PEG-PLA interaction) with a wide spectrum of tunable properties and trigger stimuli [113,114]. This last stage aimed to develop the so called “smart hydrogels” with a variety of possible applications. Hybrid hydrogels based on both natural and synthetic polymers offer infinite possibility to cells encapsulation, as matrices for repairing and regenerating a wide variety of tissues and organs [115], are capable of responding to biological signals in vivo or remote triggers and other many possible applications in biomaterials, biomedicine and nanomedicine [116].
Other important applications are [102] (Scheme 2): wound dressing/healing, treatment of severe burns, drug delivery/controlled release, injectable hydrogels, vaccines, cancer treatment, autoimmune disease, neurodegenerative disease, anti-inflammatory, ophthalmology, etc.
Scheme 2.
Biomedical applications of hydrid hydrogels based on natural and synthetic polymers.
Particularized examples of medical applications of hybrid hydrogels are described in the following sections.
5. Homopolysaccharides-Based Hybrid Hydrogels
5.1. Ability of Homopolysaccharides to Form Hybrid Hydrogels
Homopolysaccharides (HP) are subdivided into straight chain and branched chain ones, into plant polysaccharides, animal polysaccharides, microbial/bacterial polysaccharides, and seaweed polysaccharides.
Most homopolysaccharides can form hydrogels due to their intrinsic properties and the gel formation is generally driven by physical interactions. Amongst the plant-derived homopolysaccharides, cellulose and its derivatives possess plentiful hydrophilic functional groups (such as hydroxyl, carboxyl, and aldehyde groups) in the backbone that can be used to prepare hydrogels [117]. Starch is the most abundant storage polysaccharide in plants and includes two main structural components, namely amylose and amylopectin. The synthesis of starch hydrogels is determined by important features such as gelatinization and retrogradation, which are in turn affected by amylose and amylopectin ratio [118]. The hydrogels obtained from native starch, pure starch components and their derivatives are hydrophilic and of great significance in the biomedical domain because of their good swelling capacity in water, biocompatibility and biodegradability [119]. Carrageenan (CG) family of polysaccharides are soluble in hot water (>60 °C) and forms thermoreversible gels in a process that is dependent on temperature (when dropped down to 30 °C–40 °C gelation occurs) and the type of ions [120]. Due to the structural resemblance to glycosaminoglycans (GAGs) (that is a component of natural extracellular matrix—ECM) and its fine physical functional properties, CG is extensively used in biomedical applications. Formation of gellan gum (GG) (a linear anionic exopolysaccharide) –based hydrogels takes place in the presence of mono-, di- and trivalent cations and depends on the temperature [121].
5.2. Biomedical Applications of Homopolysaccharides-Based Hydrogels
Homopolysaccharides native or modified with the various conjugates have been extensively used to develop organic hybrid hydrogels, for combating last-ditch biomedical challenges. In Table 2 are listed the several examples of components in homopolyssaccharide-based organic hybrid hydrogels, their synthesis pathways and medical applications.

Table 2.
Examples of homopolysaccharide-based organic hybrid hydrogels, their obtaining methods and medical applications.
5.2.1. Tissue Engineering
Multicomponent hydrogels based on PHEMA matrix and BC nanofibers were successfully prepared by in situ UV radical polymerization of HEMA monomer impregnated into wet BC nanofibrous structure. Biocompatibility tests demonstrated that BC-PHEMA hydrogels are non-toxic providing a favorable environment for proliferation of marrow stem cells isolated from rabbits (rMSCs)—Figure 2. Therefore, the obtained hydrogels can be seen as promising for application in the tissue engineering area, particularly in tissue replacement and wound healing [131].
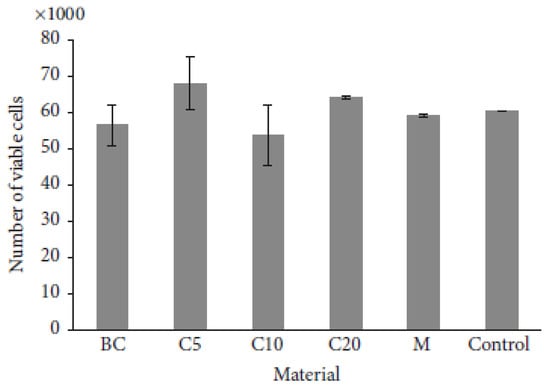
Figure 2.
Number of viable rMSCs afer 72 h of growth onto BC-PHEMA hydrogels determined by cell viability AlamarBlue Assay (Reprinted from [131], open access Hindawi).
PAAm/cellulose nanofibers (CNF) DN gels were synthesized by simply using an alkali treatment (15 wt % NaOH) at room temperature. Investigating the morphology of this DN gel it was noticed that the CNF network was embedded in the PAAm matrix, in this manner improving the strength of these hybrid gels. The obtained PAAm/CNF DN gels present notably improved mechanical properties that are proper for application as biomedical load-bearing gel materials [126]. Hydrogels based on PVA blended with cellulose (PVA-Cel) were obtained through FT cycles and were evaluated in terms of appropriateness as a part of a structure simulating the length scale dependence of human skin [123]. CMC-PEO hydrogels and porous gel films, with excellent biocompatibility, were prepared by mixing CMC-acrylate and PEO-hexa-thiols, as precursor solutions. The porous gel films were obtained by using ammonium bicarbonate particles as porogens, prior added in the precursor solutions. The obtained hydrogels and gel films show significant potential for tissue engineering applications [141]. Hashimoto et al. [159] has fabricated an amphiphilic crosslinked porous nanogel (NanoCliP), which self assembles, and presents the ability to embedded proteins, liposomes, and cells. This NanoCliP gel was synthetized using Michael reaction, by addition of a self-assembled nanogel of acryloyl group-modified cholesterol-bearing pullulan to pentaerythritol tetra (mercaptoethyl) polyoxyethylene, followed by freezing-induced phase separation. The in vivo tests show that the NanoCliP gel brings suitable features as a scaffold for tissue engineering, demonstrating improved cell infiltration, tissue ingrowth and neovascularization as observed from Figure 3.
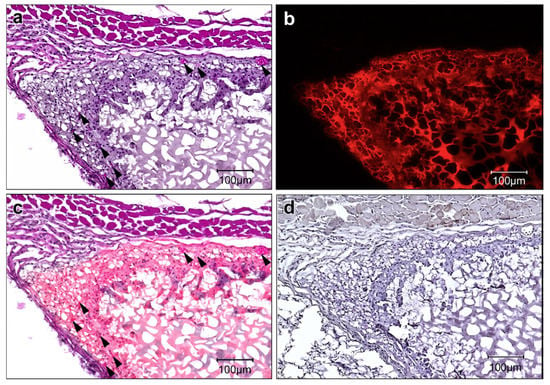
Figure 3.
Histology of subcutaneously implanted NanoCliP gel in mice. (a) H&E staining of the NanoCliP gel 4 weeks after transplantation. Arrows indicate the newly formed vessels in the NanoCliP gel. (b) The confocal LSM image of the rhodamine-labeled NanoCliP gel confirms that the NanoCliP gel has remained in situ. (c) Superimposed image of a and b shows the region containing the remaining NanoCliP gel. (d) F4/80 staining shows no monocytes and macrophages in or around the transplanted NanoCliP gel. Scale bar ¼ 100 mm: with permission from [159]. Copyright 2020 Elsevier.
Zhang et al. [161] fabricated PVA-i-CG based organic hybrid hydrogels, via a facile FT technique, as tissue engineering scaffolds. The hydrogels demonstrated increased pore structure stability, enhanced attachment and proliferation of ATDC5 cells, good hemocompatibility, and low adverse effects. Li et al. [172] has prepared a DN hydrogel GG/PEGDA by combining GG with PEGDA. The effects of viscoelasticity of GG/PEGDA DN hydrogel on the biological behavior of bone mesenchymal stem cells (BMSCs) were explored in vitro and in vivo. GG/PEGDA DN hydrogel shows excellent mechanical and relaxation properties which provide a favorable physical environment for cell proliferation and spreading, and induce chondrogenic differentiation. In another study was developed a DN hydrogel based on a GG gel and a poloxamer-Hep (PoH) network (PoH/GG DNH) to overcome the drawbacks of each gel network and to enhance the microenvironment for cell delivery. The DNH system was tested on bone marrow stem cells isolated from rabbits (rBMSCs) revealing that supported cell survival, maintained cell’s morphology and phenotype. The in vivo results have demonstrated that PoH/GG DNH endorse the cell distribution, adherence, and ECM production [173].
5.2.2. Wound Dressing
Gamma irradiated PVP/κ-CG based hydrogel obtained by gamma irradiation was intensively studied and applied as a biomaterial for wound dressing. This system presents several advantages such as a single step simultaneous sterilization and hydrogel formation, without the need of using initiator or crosslinker [169]. To enhance the poor mechanical strength of γ-irradiated PVA/PVP/κ-CG hydrogel, silk was added as a reinforcement agent [178]. PVP/κ-CG/PEG hydrogel dressing presents a long shelf life, have a high tensile strength, thus assuring an easy removal because it maintain its physical integrity. The advantages mentioned above make these systems to present increased patient compliance and are more effective than the commercially available ones [169]. PEG/GG hydrogel showed superior biocompatibility (N 90%), cell adhesion and improved cell growth compared to simple gellan gum hydrogel. In addition, reverse transcription polymerase chain reaction (RT-PCR) was used to confirm RPE-specific gene expression, and the result showed that it was positively influenced. As a result, it was observed that PEG/GG hydrogel promotes retinal regeneration compared to that of pure GG [171].
5.2.3. Drug Delivery
Superabsorbent polymer compositions (SAPCs) based on poly(acrylic acid-co-acrylamide-co-22-acrylamido-2-methyl-1-propanesulfonic acid)-grafted nanocellulose /poly(vinyl alcohol)-P(AA-co-AAm-co-AMPS)-g-NC/PVA, were obtained using graft copolymerization reaction, to create a system for amoxicillin drug delivery. The SAPCs drug delivery vehicle obtained was intended to apply for the treatment of peptic and duodenal ulcers induced by Helicobacter pylori [122]. Smart (thermo- and pH-responsive) microgel particles based on HPC-AAc and poly(l-glutamic acid-2-hydroxyethyl methacrylate) were synthetized by emulsion polymerization. The microgel was tested for controlled delivery of insulin, being noted that the system is resistant to gastric pH (1.2) and release insulin in a controlled manner at intestinal pH (6.8) [135]. By NIPAAm/CMC copolymerization were obtained copolymeric (CP) sIPN hydrogels, which were redox crosslinked using N,N′-methylenebisacrylamide (BIS) and N,N′-bis(acryloyl)cystamine (CBA). The hydrogels were tested for egg white protein lysozyme delivery at pH 1.2 while the system cross-linked with BIS showed higher swelling and maximum release [137]. A hydrogel system based on CMC and CMPVA grafted copolymer was developed by crosslinking with adipic dihydrazide. This copolymeric hybrid hydrogel was proposed as a carrier for drug delivery and as a scaffold for tissue engineering, based on its biocompatibility with the living cells and the fact that ensures outstanding survival rate at lower polymer concentration [138]. Hydrogels based on bacterial cellulose-g-poly(acrylic acid) that are stimuli-responsive were fabricated using electron beam irradiation and evaluated as oral delivery system for proteins (e.g., bovine serum albumin (BSA)). This method offers the advantage that no cross-linking agents are involved, thus overcoming the eventual toxic effects related to cross-linkers use [129]. Pandey et al. [128] using microwaves irradiation has developed hydrogels based on solubilized BC/AAm as a drug delivery system for theophylline. Different sets of BC-g-poly(acrylic acid-coacrylamide) hydrogels were obtained through microwave-assisted graft copolymerization using NaOH/urea as solvent system. These series of hydrogels have demonstrated a pH-sensitivity, which had influence on in vitro drug release profile, namely lower level of release in simulated gastric fluid (SGF) than in simulated intestinal fluid (SIF). This behavior indicates that the hydrogels may be efficient as a potential oral, controlled-release drug delivery system for the lower gastrointestinal (GI) tract [130]. Another hydrogel based on BC-g-PAA was prepared by electron beam irradiation technique. BSA was loaded into the BC-g-PAA hydrogel and showed low release in acidic SGF and higher penetration across the intestinal mucosa. The in vivo tests revealed that the hydrogel is biocompatible and non-toxic [129]. Ceresh et al. [146] obtained copolymeric hydrogels by graft-copolymerization of acrylic acid on three types of starch (potato, corn and rice starches) via 60Co-gamma irradiation. The starch-based hydrogels presented potential as prolonged drug (e.g., sodium salicylate and theophylline) delivery vehicles; in Figure 4 being illustrated the rate of release of theophylline from copolymeric hydrogels.
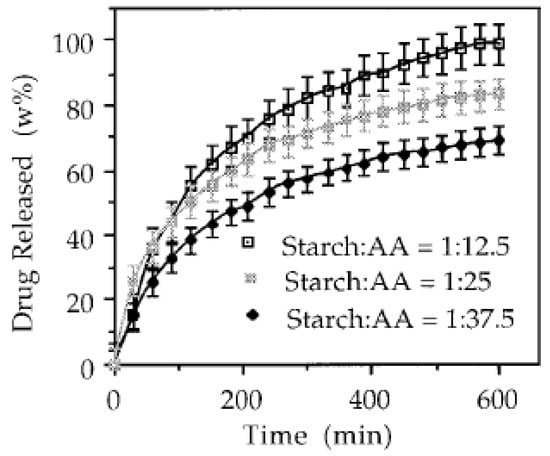
Figure 4.
Rate of release of theophylline from graft copolymers obtained from grafting starch with acrylic acid in weight ratios of 1:12.5; 1:25, and 1:37.5: used permission from [146]. Copyright 2020 John Wiley and Sons.
CMC functionalized with thiol groups (obtained by reaction with cysteamine in presence of 1-(3-dimethyl aminopropyl)-3-ethylcarbodiimide hydrochloride (EDC)) was cross-linked, using dithiothreitol, with norbornene immobilized tetra-arm PEG (PEG-Nor) forming CMC-PEG hydrogels. The presence of thiol-bearing CMC into hydrogel structure determined pH sensitivity of the gels, demonstrating improved swelling and faster release of loaded BSA protein at basic pH [140]. Moreover, thermo-responsive CMC-Nor hydrogels was developed by its crosslinking with a dithiol end functionalized PNIPAAm, determining temperature-induced shrinkage of the gel, at temperatures above the lower critical phase transition temperature (LCST) (around 32 °C) [179].
Bajpai and Saxena [142] performed potassium persulfate (KPS)-initiated graft copolymerization of AAc onto soluble starch in the presence of N,N′-methylene bisacrylamide (MBA) as the cross-linker. The hydrogels obtained were pH-sensitive and enzymatically degradable, exhibiting minimum swelling in an acidic pH and extensive swelling at pH 7.4 (i.e., simulating intestinal fluid). The behavior at acidic pH is determined by the formation of a complex hydrogen-bonded structure and at intestinal pH enzymatic degradation occurred along with the swelling controlled by chain-relaxation, being suitable for colon targeted drug delivery. Saboktakin et al. [149] have obtained pH-sensitive starch hydrogels by free radical graft copolymerization of PMAA onto CMS, using bisacrylamide as a crosslinking agent (CA) and persulfate as an initiator. The pH-responsive behavior of CMS-g-PMAA hydrogels is characterized by a transition between the swollen and the collapsed states that occurs at high and low pH. The CMS-based hydrogels were tested for drug delivery. Double hydrophilic thermo-responsive pullulan-g-PNIPAAm copolymers with two different molecular weights of thermosensitive grafts were synthesized and used for preparation of indomethacin-loaded nanoparticles by dialysis and nanoprecipitation method [180]. The sustained-release properties of poloxamer 407-based in situ gel were enhanced by the combination with CG, and present high potential to be used in vaginal in situ gel drug delivery systems with prolonged local residence and therefore for better clinical outcome [160]. Hamcerencu et al. [176] performed free radical grafting/polymerization of unsaturated esters (gellan maleate) with NIPAAm, using N,N′-methylenebisacrylamide as cross-linker, to design thermosensitive hydrogels. These hybrid hydrogels were tested for their swellability, in vitro loading and release of different drugs (e.g., adrenaline and chloramphenicol) and in vivo biocompatibility. By in vivo evaluation was not observed necrosis, calcification and acute inflammation, only the formation of a thin fibrous capsule around the implanted hydrogels, thus they being preliminary proposed for ophthalmic applications.
5.2.4. Other Biomedical Applications
The BC/PGA hydrogels were prepared by 60Co γ-irradiation crosslinking method. The BC nanofibers and PGA can form the multicomponent hydrogels with double crosslinking structure via γ-irradiation. The addition of BC increases compressive strength, storage modulus (G’) and the gel fraction but decreases the equilibrium swelling ratio of the BC/PGA composite hydrogels. The compressive strength and storage modulus of hydrogels increase 5 times and 10 times respectively at the irradiation dose of 50 kGy. Moreover, the BC/PGA hydrogels are non-toxic, indicating their safety for biomedical application [132]. By UV photo-crosslinking were obtained temperature sensitive hydrogels based on hemicellulose (Hce) obtained from acetic acid pulping of Eucalyptus and NIPAAm. The protocol involved two steps; firstly, a Hce derivative was synthetized by grafting MA to Hce that contains vinyl bonds within the side chains followed by UV photocrosslinking of Hce-MA with NIPAAm in LiCl/DMF solvent. The equilibrium swelling ratio and morphology of the hydrogels were dependent on environment temperature, implying their potential as smart materials for medical application [134]. All-trans retinoic acid aqueous gels composed of ι-CG and polyethylene oxide were proposed to be applied as a topical treatment of skin. In these gels, the PEO was selected for its high mucoadhesion property and spinnability, while ι-CG was chosed for its texture modification property and gelling feature. Combination of these components maximizes the optima properties of each entity by reducing the drawbacks of each individual polymer [162].
Deng et al. developed a novel κ-CG/PAAm (KC/PAAm) DN hydrogel through a dual physical-crosslinking strategy, with the ductile, hydrophobically associated PAAm being the first network, and the rigid potassium ion (K+) cross-linked KC being the second network. The DN (DPC-DN) hydrogels with optimized KC concentration exhibit excellent fracture tensile stress and toughness, comparable to those fully chemically linked DN hydrogels and physically-chemically cross-linked hybrid DN hydrogels. Additionally, DPC-DN demonstrated rapid self-recovery, remarkable notch-insensitivity, self-healing capability, as well as excellent cytocompatibility towards stem cells [167]. In a similar manner were obtained hybrid hydrogels based on Iota-Carrageenan and polyacrylamide to be used as matrix for silver nanoparticles designed for bacterial inactivation applications [181].
6. Heteropolysaccharides-Based Hybrid Hydrogels
6.1. Ability of Heteropolysaccharides to Form Hybrid Hydrogels
A biocompatible and biodegradable heteropolysaccharide that forms hydrogels by mixing with multivalent cations is the alginic acid [182,183]. Spherical core–shell gel-bead structures (or worms) were obtained by combining alginic acid with 1,3,2,4-di-(4-acylhydrazide)-benzylidenesorbitol (DBS-CONHNH2) [184]. The gels based on alginic acid proved to have important applications in domains like drug delivery and tissue engineering [185].
Microspheres (MS) of hybrid hydrogels that can adjust their mechanical properties and durability in function of the biological environment were obtained using SA with heterotelechelic PEG derivatives [186]. These hydrogels are appropriate for cell transplantation applications.
For enhancing the ability of liquid uptake and the mechanical properties of the hydrogels based on chitosan (CS), this natural polymer was associated with synthetic polymers or grafted with vinyl monomers, such as acrylic acid and acrylamide [187,188].
Chen et al. [189] prepared macroporous PVA/CS hydrogel sponges that showed higher antimicrobial and haemostatic activity than pure CS sponges.
Hyaluronic acid (HA) is abundant in connective, epithelial, and neural tissues [190]. HA macromolecules showed anti-inflammatory, immunosuppressive properties and block angiogenesis, while cleaved small fragments induce opposite behavior, enabling endothelial cells migration and angiogenesis [191]. Kim et al. [192] obtained PVA/HA hydrogel nanofibers by chemical crosslinking, using HCl and glutaraldehyde. They observed that the swelling ratio of these hydrogels is higher in respect with that corresponding to pure PVA hydrogel. A good biocompatibility of PVA/HA hydrogel nanofibers was evidenced by a higher cell adhesion at their surfaces, independent on the HA presence.
Heparin (Hep) has a high negative charge, the 3-D hydrogels based on it being used in tissue engineering, implantation, biosensor domain, drug delivery. Because Hep poses some safety problems (because it is often obtained from animal sources), analogous Hep-mimicking polymers and hydrogels obtained from synthetic sources were proposed.
The use of Hep in hydrogels by delivery growth-factors generates proliferation signals to cells because of its protein polysaccharide interactions closely mimicking the native structure and functioning of ECM [193].
Supramolecular hybrid hydrogels self-assembled were obtained from low-molecular-weight gelator (LMWG, which are small organic molecules which self-assemble in water or organic solvents, forming a 3D network that entraps the liquid phase resulting in gel formation) building blocks with the polymer gelator (PG) (e.g., calcium alginate) [184]. This type of hydrogel can be used in regenerative medicine [194], in controlled drug delivery [195], or in electronics devices as patterned conducting gels where they contact interface with living media. The components usually used for obtaining self-assembled multi-component hybrid hydrogels are: a pH activated LMWG, a temperature activated PG, an anionic biopolymer (such as Hep) and a cationic system capable of binding Hep.
6.2. Biomedical Applications of Heteropolysaccharides-Based Hybrid Hydrogels
Some examples of heteropolysaccharide-based hybrid hydrogels used in different biomedical domains are listed in the Table 3 where are also mentioned preparation methods and general properties.

Table 3.
Heteropolysaccharide-based hybrid hydrogel systems with biomedical applications.
6.2.1. Tissue Engineering
Generally, the scaffolds used in tissue engineering should have several properties, such as biocompatibility, cell proliferation, controlled swelling, ease of administration, antimicrobial, stability, porosity, adhesion, low immunogenicity, colonization of host cells without inducing any histological changes, integration with host tissues [249,250,251,252], biodegradability, bio mineralization, non-toxic degradation products, and also degradation of scaffolds should be inversely proportional to the rate of synthesis of the newly regenerated tissue [253].
Pok et al. [254] obtained 3D scaffolds of self-assembled PCL in a gelatin-CS hydrogel, for possible application in congenital heart defects. They observed similarities between the mechanical properties of the hydrogel with those of the native tissue, as well as migration of neonatal rat ventricular myocytes (NRVMs) [254]. Zhao et al. [228] synthesized hydrogel scaffolds by chemical crosslinking between quaternized CS and polyaniline, using oxidized dextran as cross-linker. The obtained hydrogels presented decreased cytotoxicity, higher antibacterial activity, and enhanced proliferation of C2C12 myoblast cells when compared with quaternized CS hydrogel. These hydrogels could be used for muscle, nerve, and cardiovascular repair [228]. PVA hydrogel was loaded on one side only with Hep for possible application in vascular tissue engineering [255], because release of Hep from PVA/Hep hydrogel can prevent clot formation.
HA-based hydrogels are often used in cartilage tissue engineering, because HA has an inhibitory effect on fibronectin fragment-mediated chondrocytic chondrolysis [256], inhibitory effects on prostaglandin synthesis, proteoglycan release [257], and degradation by enzymes and free radicals [258]. One of the main disadvantages of using HA in cartilage tissue engineering is its poor mechanical properties. That is why, this natural polymer has to be used together with various synthetic polymers, such as PNIPAAm and PEG [259].
Fan et al. [260] evaluated the potential of hybrid poly(lactic-co-glycolic acid)-gelatin/chondroitin/hyaluronate (PLGA-GCH) scaffolds in cartilage repair. It was observed that differentiated mesenchymal stem cells (MSCs) seeded on PLGA-GCH significantly increased the proliferation of MSCs and GAG synthesis compared with PLGA scaffolds.
Bichara et al. [261] developed a flexible PVA/SA hydrogel, using human nasal septum chondrocyte cells; the systems have been implanted into the subcutaneous environment of nude mice. In vivo tests showed deposition of collagen type II in the hydrogels, behavior that recommend this hydrogel type for reconstruction of craniofacial cartilage.
Kunisch et al. [262] prepared star PEG/Hep hydrogels trying to prevent mineralization of the upper cartilage zone, for inhibiting long-term progression of calcified cartilage into bone.
A thermo-sensitive copolymer hydrogel was obtained by grafting PNIPAAm onto HA. This system passed from a liquid-like behavior to an elastic gel-similar one, at 30 °C, this fact being useful for cell encapsulation in the hydrogel [263]. Another thermo-sensitive hydrogel was prepared using Pluronic and HA, this one being a potential candidate for applications as artificial vitreous substitute [264].
Self-healing hydrogels can be successfully used in drug/cell delivery or in 3D printing [265]. Self-healing hydrogels based on glycol chitosan and difunctionalized PEG (GC-DP) were also used for tissue repairs, in central nervous system [266], or for inducing blood capillary formation. In order to achieve this second purpose, a multicomponent hybrid hydrogel was obtained using an IPN of GC-DP and fibrin [267]. The hydrogel induced vascular endothelial cells to form capillary-like structures; injection of this hydrogel promoted angiogenesis in zebrafish and rescued the blood circulation in ischemic hindlimbs of mice.
Fares et al. prepared IPN and sIPN hydrogels, using a pectin grafted polycaprolactone (pectin-g-PCL) and a gelatin methacryloyl (GelMA) component [268]. The IPN hydrogels were characterized by cytocompatibility and, in the meantime, induced the growth of MC3T3-E1 preosteoblasts in vitro, proving that they are appropriate for different applications in tissue engineering.
A pectin-Fe3+/polyacrylamide hybrid DN hydrogel was developed by Niu et al. [269]. These hybrid DN hydrogels were characterized by very good mechanical properties (such as stiffness, fatigue resistance, notch-insensitivity), as well as a high-water absorption ability (85%). All these characteristics recommend this hydrogel type to be used in the load-bearing tissue repair field.
Injectable scaffolds are superior to preformed scaffolds in terms of improved patient’s compliance, ease of clinical implementation for the treatment of geometrically complex, and large lesions via minimally invasive techniques, such as arthroscopy [270]. This type of scaffolds can be used in minimally invasive surgical procedures; they completely fill the defect area and have good permeability, being hence promising biomaterials [271,272]. The technique can be effectively applied to deliver a wide range of bioactive agents, such as drugs, proteins, growth factors, and even living cells. For the development of such type of scaffolds, natural polymers were used (i.e., collagen, chitosan, gelatin, alginate, hyaluronan, chondroitin sulfate, pectin) [273]. In order to obtain in situ gelling systems, different techniques can be applied, such as photo-crosslinking, chemical crosslinking, enzymatic crosslinking, pH-induced gelation, temperature-induced gelation, ionic and hydrophobic interactions [259].
6.2.2. Wound Dressing
Due to its properties, CS proved to be an attractive candidate for treating wounds, even major burns [274,275].
PVA/HA membrane hydrogels were tested for wound dressing application, from the point of view of their biological properties and biocompatibility. Increasing the HA content in the hybrid hydrogels, a decreased migration and cell viability were observed, due to an increase in the viscosity of the PVA/HA system. In the absence of ampicillin, the obtained membrane hydrogels were active against Candida albicans, while when ampicillin was added, they proved to be effective also against Staphylococcus aureus, but not against Escherichia coli [276]. HA/PVPA/CS hydrogel designed for being used for skin wound healing showed antimicrobial activity against E. coli [277].
6.2.3. Drug Delivery
Hydrogels represent a drug delivery system class that has excelled as smart drug delivery [105,278]. Biocompatible, biodegradable hydrogels have been designed using natural polymers that are susceptible to enzymatic degradation, or using synthetic polymers that possess hydrolysable moieties. CS positive features e.g., hydrophilicity, functional amino groups, and a net cationic charge recommend its hydrogels for the intelligent drug delivery and of macromolecular compounds, such as peptides, proteins, antigens, oligonucleotides, and genes [220,279].
Wu et al. [280] developed hydrogel-based N-[(2-hydroxy-3-trimethylammonium) propyl] chitosan chloride (HTCC) and PEG for insulin release. Hydrogen bonds among amino groups present in insulin and hydroxyl groups present in PEG or HTCC allowed prolonged drug release. After spraying of formulation into nasal cavity, the solution formed gel at body temperature. This hydrogel system presented lower mucosal clearance and sustained in site targeted drug release. The results showed that the hydrogel can be used as nasal delivery carrier for protein or peptide drugs [280].
When oral drug delivery is not practicable, nasal administration of the CS hydrogels can be used for delivery of peptides and vaccines [281].
CS/PEG hydrogels were tested for drug delivery at the level of the gastrointestinal tract [282]. The release rate of the drug from the PE/CS systems was delayed when compared with those only with CS [283], maybe because PEG can improve the CS solubility [284], thus increasing its transfection efficiency when used as a gene carrier [285].
IPNs obtained from CS and PEO proved to be appropriate carriers for drug delivery systems specific for the stomach, being effective for Helicobacter pylori treatment [286].
For colon drug delivery, CS-polyacrylic acid (PAA) hybrid hydrogels were tested, these ones being biodegraded by colonic normal flora [287].
CS/PVA hybrid hydrogels were studied as potential systems for drug delivery, using as drug model: PTX, insulin, BSA; the properties of these hydrogels (such as antitumoral activity, period of drug release) were superior to those of other delivery systems [209,212].
Zhou et al. [288] developed a CS/poly(oligo ethylene glycol) system, used as a controlled drug release in the chemo-cryo cancer therapy.
Åhlén, Tummala, and Mihranyan [289] reported that contact lenses based on CS-PAA nanoparticles and PVA hybrid hydrogels had greater potential for extended release during 28 h.
Due to the highly negative effects of the usual chemotherapy treatments, polysaccharide hydrogels were tested as drug carriers, in order to obtain a controlled/localized drug release. As examples are the 3D CS/PVA hybrid hydrogels developed by Jamal et al., which described the great potential in inhibiting angiogenesis of these hybrid systems [290].
Islam and Yasin [291] developed CS/PVA porous hybrid hydrogels crosslinked with tetraethoxysilane as a drug delivery system for dexamethasone. By increasing the PVA concentration it was obtained a decrease of the swelling degree of the hydrogels. The pH media also affected the swelling degree, the minimum swelling being observed in acidic and basic media, and the maximum around a neutral pH. Hydrogels released around 9.4% dexamethasone during the first two hours, the released amount increasing up to six hours (Figure 5).
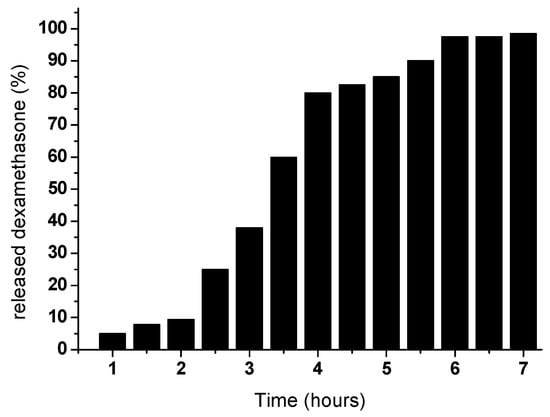
Figure 5.
Release behavior of dexamethasone from CS/PVA hydrogel (adapted from [291]).
Yang et al. [292] developed GC and DP for intra-tumoral therapy in vivo. GC-DP hydrogel containing antitumoral drug was injected into the disease site, for being released in situ. Moreover, the ionic GC-DP hydrogel exhibited microwave susceptibility to produce high-temperature hyperthermia for tumor ablation [293].
A thermoresponsive nano-sized chitosan-grafted PNIPAAm (CS-g-pN) hybrid hydrogels curcumin-loaded was developed as an advanced material that can be functionalized and optimized for targeted therapy and controlled delivery of small molecules and/or biomolecules [294].
For treating oral mucosa ulcer, one can use antibiotics, analgesics, adrenocortical hormones, and glucocorticoids, drugs that may induce undesirable side effects. That is why, Luo et al. prepared four different injectable CS based thermogels, using PNIPAAm and PAAm, synthesized by an in situ free radical polymerization procedure. Hybrid hydrogelswere tested from the point of view of antibacterial activity against Gram-negative (Escherichia coli) and Gram-positive (Staphylococcus aureus) bacteria, human gingival fibroblasts viability and growth, therapeutic effect, hemostatic activity [295].
All the four CS-based hydrogels proved antibacterial activity, the inhibition rate of the studied bacteria significantly increasing with increasing hydrogel concentration up to 5 wt%. The antibacterial activity was higher for the samples with a higher CS content (Figure 6a,b). In the meantime, all the four CS-based hybrid hydrogels induced no important toxicity towards human gingival fibroblasts and were characterized by good hemostatic properties. By comparison, the samples with the highest CS and PNIPAAm content (samples 1 and 2) were more effective in treating oral mucosa ulcer. Another important feature of some of them (i.e., 1, 2, and 4, see Figure 6) can reversibly form semi-solid gels at physiological temperature, being easily applied to oral cavity by injection.
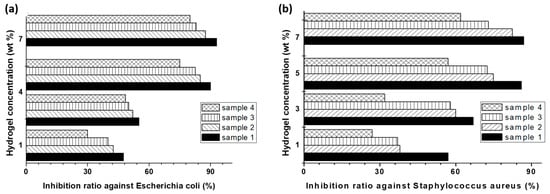
Figure 6.
Inhibition ratio against Escherichia coli (a) and Staphylococcus aureus (b) induced by different concentrations of CS-based hydrogels, after 16 h. Samples: 1 (CS-g-PNIPAAm with 30.6% CS and 69.4% PNIPAAm), 2 (CS-g-PNIPAAm with 25.3% CS and 74.7% PNIPAAm), 3 (CS-g-PAAm, with 22.7% CS), and 4 (CS-g-PNIPAAm-g-PAAm with 12.9% CS) (adapted from [295]).
The in vitro release of the heat shock protein 27 (HSP27) (protein that protects heart muscle for ischemic injuria) was released over a period of 14 days from a hybrid hydrogel PLGA/Alg containing also TAT peptide [296]. After injection of this system in a myocardial infarction model, some parameters describing the heart state were significantly improved.
SA/NIPAAm hydrogels, chemically crosslinked with N,N’-methylene bis-(acrylamide), that respond at the fluctuations in temperature and pH proved to be suitable for sustained drug release of paracetamol and theophylline [297], a better drug entrapment and a slower drug release being obtained.
Thermo-responsive hydrogels for injectable drug administration in chemotherapeutic treatment were reported by Chen et al., who prepared hexamethylene diisocyanate (HDI)-pluronic F 127 copolymer /HA systems [298]. Injectable hydrogels were also prepared by conjugating HA with two types of complementary single-stranded DNA (HA-DNAs) [299].
Both bFGF and vascular endothelial growth factor (VEGF) were entrapped in PVA/Hep hydrogels [300]. When compared with PVA gels, the hybrid PVA-Hep gels induced a significantly lower fraction of initial release of bFGF in the first 12 h, as well as a significantly decreased quantity of released bFGF, behavior observed for the entire followed period of time (Figure 7a). The same behavior was also evidenced when VEGF was encapsulated in PVA-Hep gels (Figure 7b). This fact suggests that PVA-Hep gels are appropriate for being used in controlled-drug release applications. In what is concerning the dual release of bFGF and VEGF, a synergistic effect was observed (Figure 7c).
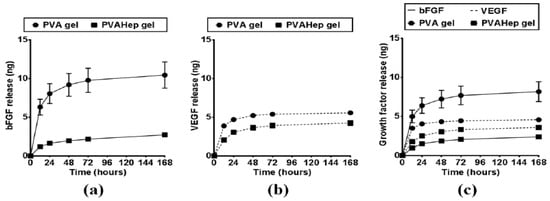
Figure 7.
Growth factor release profiles from PVA and PVA-Hep gels for a period of 7 days: (a) bFGF release, (b) VEGF release, and (c) dual release of bFGF (solid lines) and VEGF (dashed lines) (reprinted from [300], open access).
Hep-based injectable hydrogels can be used to mimic the extracellular matrix as promising drug delivery systems for postoperative chemotherapy, cell delivery carrier and the regeneration of damaged liver or other tissues. Hep-based nano-hydrogels are commonly applied for cancer cell-targeted delivery, as carriers for anti-fibrotic and anti-cancer agents and gene delivery [301]. Since Hep has high cost, dramatic loss in bioactivity and degradation when using covalent or non-covalent strategies for obtaining the hydrogels, interference with blood components), analogous Hep-mimicking (also called Hep-inspired) polymers are one of the current research hotspots to substitute the usage of Hep in the fabrication of hydrogels. Sulfonated polymers and sulfated glycosaminoglycan have been widely recognized as Hep-inspired components since they show similar bioactivity properties as Hep, such as anticlotting and antithrombotic activities, stabilization of growth factors, and promotion of angiogenesis. Between the main applications of the Hep-inspired hydrogels, one can mention: cell culture, loading of drugs/molecules, blood contacting applications.
7. Hybrid Proteins Based Hydrogels for Biomedical Applications
7.1. Ability of Proteins/Peptides to Form Hybrid Hydrogels
Protein-based materials are popular as engineering bioactive scaffolds because of their advantages in mimicking the extracellular environment [302]. Hydrogels-based on proteins are applied in biomedicine field as tissue engineering materials, drug delivery, etc., because they are easily degraded by the body and display a high biocompatibility.
Proteins can be employed as building blocks, systems embedded with particles, etc. In the design of most hybrid hydrogels composed with synthetic polymeric, peptides act as structural elements. They are generally used in hybrid systems for the improvement of mechanical properties of other polymers in the sense of malleability, and also to induce biodegradability and biocompatibility. Variety of functional groups belonging to proteins/peptides allows the physical cross-linking through hydrogen bonds, electric interactions, and/or π-π stacking.
Additionally, chemical crosslinked hybrid hydrogels can be formulated using protein—synthetic polymer couple. Common cross-linkers such as glutaraldehyde, formaldehyde and carbodiimide, have been widely used in the fabrication of protein-based hybrid hydrogels in the past decades [303]. Tetrakis (hydroxymethyl) phosphonium chloride (THPC), possessing four hydroxymethyl arms, is an effective, mild and low-cost cross-linker to make protein hydrogels. Various natural proteins, including BSA, gelatin, silk fibroin, milk protein, soy isolate protein, ovalbumin and lysozyme, could be cross-linked by THPC to form high strength hybrid hydrogels with rapid self-recovery properties [304]. Biocompatible protein crosslinkers of proanthocyanidin, a kind of naturally occurring polyphenol extracted mainly from plants [305] and genipin [306] as natural crosslinkers that can be used to obtain biocompatible hybrid hydrogels based on proteins. Multi-functional natural proteins macromonomers can be also used alone as macro-crosslinkers, eliminating the need for a conventional crosslinkers.
7.2. Properties of Proteins to Form Hybrid Hydrogels for Biomedical Applications
7.2.1. Collagen
Collagen an important insoluble protein of the human and of animal body is met in skin, connective tissue, cartilage and bones. Of all twenty-nine types of collagen that have been discovered, type I is frequently used as a component in the development of biomaterials. Collagen had gained a considerable reputation in the biomedical field due to its unique properties such as low immunogenicity, low toxicity, excellent biocompatibility, good safety, biodegradability, weak antigenicity and ability of skin, bone, or other tissue regeneration [307]. From the structural point of view, collagen based hybrid hydrogels can mimic the physiological ECM, stimulating cell migration, proliferation and adhesion, and providing bio-safe profiles and no chronic inflammatory response. Collagen hybrid hydrogels have been applied for the partial or the whole reconstruction and healing of different parts of human body including skin tissue [308], bone, cartilage [302], blood vessels, cornea [309], and brain parts [310].
7.2.2. Gelatin
Gelatin is ussualy extracted from animal (bovine, porcine) and fish (jelly fish, sea urchin) sources by acid (type A gelatin) or basic (type B gelatin) hydrolysis of collagen [311]. The physically gelatin gels are thermo-reversible, thus during cooling, the random coil structure is in part rebuilded to a triple helical structure [312]. The isoelectric point of type A gelatin varies between pH 7 and 9, while in case of type B gelatin, the isoelectric point is placed from pH 4.7 to 5.4. Cationic or anionic charged gelatin is usefful for loading therapeutic principles (molecules, drugs) through electrostatic interactions. In contrast with collagen, gelatin manifests good stability at high temperature in a broad interval of pH [313]. Synthetic polymers such as PEG and PVA are some of the most used for the obtaining of hybrid gelatin based hydrogels with application in almost all the medical domains.
7.2.3. Keratin
Keratin is a group of fibrous proteins that forms the bulk of cytoplasmic epithelia and epidermal structures and is abundant in hair, nails, wool, horns and feathers. Studies on keratin-based biomaterials are mainly focused on keratin extracted from wool and poultry feathers, but more especially from human hairs because being human-derived, the risk of immune response is reduced [314]. Two conformations can be found in keratin, α-helix and β-sheet. Like other naturally derived protein biomaterials, keratin possesses 14 types of amino acids, where cysteine plays an important role in the formation of disulfide bonds that influence the high mechanical strength of keratin.
When extracted reductively [315], the resulting material is known as kerateine (KTN) with thiol groups able of forming disulfide bonds. When extracted by oxidative means [316], is referred to as keratose (KOS). The thiol groups of the cysteine residues in KOS are “capped” as sulfonic acid residues and are unable to form disulfide bonds. These chemical differences in the proteins are known to affect the physical properties of biomaterials, particularly hydrogels, derived from keratins. KOS will form hydrogels through physical entanglements, but degrades rapidly due to the lack of covalent disulfide crosslinks. KTN persists much longer due to the presence of both physical entanglements and covalent disulfide crosslinks [317]. Unlike collagen, keratin-based hydrogels can stay stable and resistant to biodegradation in vivo for a longer period without being degraded by enzymes because there is no keratinase or other keratin-degrading enzymes in humans and animals bodies.
In the form of hydrogels, keratin scaffolds have porous gel walls and large voids which are suitable for the cells proliferation. When keratin is combined with synthetic polymers, generally keratin-based hybrid hydrogels are obtained with potentially applications in drug delivery systems or wound healing [318].
7.2.4. Bovine Serum Albumin
Bovine serum albumin (BSA) is frequently used as an active principle, but in some cases as a component in a hydrogel system. because its low cost, stability, specific ligand-binding properties and increased solubility [319].
7.2.5. Silk
Silk, a fiber protein produced by the silkworms, is composed of two main proteins called silk sericin (SS) (25% of the total weight of raw silk) and silk fibroin (SF) (75%) [320]. Sericins, amorphous and hydrophilic proteins, act as a gummy/adhesive substance that joins the fibroin filaments. This glue-like sericin protein gets wrap around the SF, what is hydrophobic, highly crystalline with an oriented structure [321]. Silk based biomaterials has various advantageous features, including excellent biocompatibility, controllable biodegradation, and desirable mechanical properties, are nontoxic, nonimmunogenic, and have been approved by the United States Food and Drug Administration (US FDA) for use in the human body for sustained-release drug delivery systems, bone and skin tissue regeneration and repair, biosensor, and 3D bioprinting [322]. It was demonstrated that SS plays a crucial role in the antibacterial process of wound treatment. The sol–gel transition of aqueous solution of SF is a natural process which without an exterior stimulus is quite long, usually a week to a month, which may limit its practical use [323]. Therefore, there are some factors that can stimulate and enhance the gelation kinetics of SF. For example, reducing the electrostatic repulsion by lowering the pH (<5), increasing the gelation temperature (>60 °C), increasing in protein sol-gel critical concentration (> 5–10% (w/v)), blending with polyhydric alcohol agents, or adding divalent ions (Ca2+) can decrease the gelation time by attenuating the hydrophobic interactions amongst protein chains [324]. Physical methods such as ultrasonication, or chemical crosslinking or a polyreaction of additive micromolecular agents, accelerate the gelation of fibroin. Nevertheless, these physically or chemically stimulated sol–gel transitions are almost irreversible. During the gelation process fibroin molecules rearrange from a random coil conformation in the sol state to an antiparallel β sheet conformation in the gelled state [325]. The presence of a large amount of Gly-Ala repeats units in fibroin favors the formation of β sheets which accelerate its gelation. Silk polymers can be engineered to produce thermo-sensitive hydrogels. At room temperature, the thermo-sensitive hydrogel may remain liquid but at body temperature exists as a hydrogel.
7.2.6. Resilin
Resilin is a pliable and extendable structural protein found in insects. It is named for its resilience to repeated rounds of stretching and relaxation [326]. Resilin has ability to store mechanical energy [327].
7.2.7. Whey Proteins
Whey proteins (WP) are globular milk-derived proteins and contain as major protein fractions β-lactoglobulin, α-lactalbumin and BSA. They are extremely inexpensive and abundantly available in various forms (concentrates—WPC, hydrolysates—WPH, and isolates—WPI). Heat treatment of an aqueous solution of whey protein isolate (WPI) above 60 °C results in its unfolding followed by the formation of new inter-and intra-protein bonds that create a three-dimensional gel network [328]. Biodegradability and ability of WPI to form a hydrogel without the use of chemical cross-linking agents makes it attractive for use in biomedical applications.
7.2.8. Soy Protein Isolate
Soy protein isolate (SPI), contains two major components: glycinin (52%) and conglycinin (35%), with hydrophobic components in the molecular structure [329]. Soy protein has advantages over the various types of natural proteins employed for biomedical applications due to its low price, nonanimal origin, and relatively long storage time and stability. Because of its globular structure, soy protein is more resistant to hydrolysis compared with coiled or helical structures. SPI is an electroactive protein with an increased content of polar amino acid moeties that produces charges in various conditions of pHs especially in strong acidic or basic pH [330], which recommend its to be used as a natural protein-based electroactive hydrogel for microsensor and actuator, particularly in the biomedical area. Due to their flexibility, SP materials could also be successfully processed into scaffolds by a 3D printing [331]. Residues resulted after the degradation process of SP based hydrogels were demonstrated to be non-toxic and also are capable to promote collagen deposition in cultures of fibroblast cells and to determine the mineralization in the presence of osteoblasts, hence sustaining the ideea that SP manifest intrinsic bioactivity [332].
Peptides derived from native soy protein may be employed as excellent building blocks to fabricate hybrid hydrogels [333]. The procedure includes the dissolving of native SPI in alkaline solution (such us urea) where it is denatured and unfolded to peptide chains. Thus, the unfolded peptide chains will induce an increase of protein solubility in water. Moreover, the exposed sulfhydryl and hydrophobic groups on the peptide chains will be employed for further chemical reactions. The use of SP chains as structural components into hydrogels have some advantages such as cell and growth factor or surface binding and electroactive characteristics [13].
Due to their various potential biomedical applications, hydrogels based on engineered proteins have attracted considerable interest [334]. Calmodulin (CaM) is a calcium-binding protein with an important role in the biological recognition being used as an element in stimuli-sensitive hydrogels. It has the ability to manifests a large conformational change on binding calcium, certain peptides, and the phenothiazine group of drugs (anti-psychotics). CaM undergoes two different types of conformational changes, an apo-state in the absence of a ligand, and a holo-state when it is bounded to a ligand [335]. Thus, in the presence of Ca2+, CaM undergoes a rapid transition from an extended dumbbell conformation to a collapsed (more constrictive) conformation in response to binding of ligands (small molecule drugs, peptides, a variety of proteins). When Ca2+ is removed from CaM, the protein changes from its bound conformation to native conformation. New classes of CaM based hydrogels should lead to a new breed of intelligent biomaterials that could find many applications in the field of responsive drug delivery systems, as well as in a variety of microfluidics systems and BioMEMS devices [22].
7.2.9. Elastin
Elastin represents a structural protein of the ECM providing tensile strength and elasticity. Natural elastin hardly has been used as a hydrogel in biomaterial field. An important requirement when using proteins as biomaterial is the purity. During the synthesis, elastin can be contaminated and may induce immunological responses. Additionally, elastin is insoluble and has a strong tendency to calcify, making purification even more difficult [336]. Consequently, soluble forms of elastin including tropoelastin [337], α-elastin [338], and elastin-like polypeptides (ELPs) [339] are frequently used to form crosslinked hydrogels.
The gelation process of ELPs is influenced by the temperature. When the temperature is reduced under a certain value, the hydrophobic groups are fenced by ordered water molecules from hydrophobic hydration, and ELPs became soluble. Above this transition temperature, the H2O molecules surrounding the ELPs become bulky being less-ordered, and thus the protein is collapsed. This leads to the folding and self-assembly of ELP, and consequently the gelation occurs [13,340]. No hydrogels based on elastin and synthetic polymers have been reported in literature.
Self-assembled Leucine zipper (LZ) was first studied by Petka et al. started with 1998 [341]. From that moment, LZ building blocks were involved in the devlopment of self-assembled protein based hydrogels, initiating remarkable possibility for using genetic engineering proteins to adjust the physical and functional properties of hybrid hydrogel materials [342]. The high flexibility of the random-coil like polypeptides incline to form intramolecular loops, conducting to an unwanted and accelerated erosion rate of the hydrogel matrix [343]. Peptides with different biological functions can be incorporated in the LZ protein backbone to create a functional chimeric protein or fusion proteins with self-assembling properties [344] for the use in tissue engineering applications. Shu et al. designed polypeptide-polymer conjugates using PEG chains covalently bounded to the exterior side chain of peptides forming tertiary structures, that could be leucine zipper [345]. This knowledge is usefull for the development of new self-assembled and responsive protein based hybrid hydrogels.
7.3. Biomedical Applications of Protein Based Hybrid Hydrogels
The main biomedical applications of the protein based hybrid hydrogels are summarized in Table 4 Some of the most recent (from the last 6 years) application in the biomedical field of protein based hydrogels in combination with a synthetic polymer are given in Table 5. As can be observed, most of the researchers are trying to find the most biocompatible way to develop a new biomaterial without the use of chemical cross-linkers (which are very toxics) even if the gelation process is formed by chemical or physical interactions.

Table 4.
Biomedical applications of protein based hybrid hydrogels in biomedical fields, preparation procedures, some properties and their type.

Table 5.
Recent developments in biomedical applications of protein based hybrid hydrogels.
7.3.1. Tissue Engineering
In situ forming hydrogel systems have attracted considerable interest as injectable scaffolds for tissue engineering and drug delivery due to their easy applications and minimally invasive injection procedure. By modification of gelatin with hydroxyphenyl propionic acid (HPA) and conjugation of 4-arm-polypropylene oxide−polyethylene oxide (4-Arm-PPO-PEO = Tetronic) with tyramines, Park et al. succeeded to prepare an injectable hydrogel by mixing the solutions of the two components in the presence of horseradish peroxidase (HRP) and hydrogen peroxide (H2O2) [379].
During the enzymatic coupling reaction under physiological conditions, HRP facilitated to the phenol molecules from both in modified gelatin and conjugate, to participate to the coupling reaction by interaction via C-C bond in ortho position or with C-O bonds at phenoxy oxygen. After the subcutaneous injection on mice, gelatin based hybrid hydrogel was quickly created allowing the natural tissues growth into the hydrogel network.
SF has also been considered as a candidate for 3D bioprinting, as the protein polymer chains can be physically crosslinked through intermolecular and intramolecular β-sheet structure formation via hydrophobic interactions to stabilize the materials without the need for chemical reactions or additives. Thus, SF/PEG hydrogels were studied as self-standing bioinks (biological ink) for 3D printing use in tissue engineering [380]. Mixing PEG with silk induces physical crosslinking and thus rapid gelation of silk and water insolubility. After subcutaneously implantation in mice, the bioink gel with and without fibroblast maintained shape and structure after 6 weeks, and a significant amount of cells remained alive in the gel matrix.
One single example of hybrid resilin based hydrogel have been reported in literature by McGann et al. that was composed of a resilin-like polypeptides (RLPs) and a multi-arm PEG macromer [381]. This hybrid hydrogel can be rapidly cross-linked through a Michael-type addition reaction between the thiols of cysteine residues on the RLP and vinyl sulfone groups on the multi-arm PEG. The obtained elastic and resilient hydrogels are capable to encapsulate human mesenchymal stem cells (hMSCs), are biodegradable and possess rubber-like properties that would be useful for mechanically-demanding tissue engineering applications, especially those aiming to remedy cardiovascular pathologies.
Liu et al. obtained photo-crosslinkable physical hydrogels with practicability in artificial ECM. Before the hydrogel synthesis step, this research group created macromers comprising a hydrophilic chain with a terminal self-assembling leucine zipper domain A and a terminal photoreactive acrylate group (PEGDA) [382]. LZ domain A was chosen due to its ability to undergoes tetrameric physical association that will allow the hydrophilic polymers to self-assemble into four-arm macromers. Then, the four-arm macromers were photo-crosslinked into hydrogels, where their biodegradability and ability to mimic nonproteolytically mediated cell migration and outgrowth by reversible opening and closing the 3D cell migration paths in biological systems, was demonstrated.
7.3.2. Bone Tissue Engineering
Sometimes, the injectable property of traditional protein based hydrogel is unsatisfactory, which cannot provide adaptable filling of lesion defects with irregular shapes. Thus, the adding to it of a synthetic polymer with propper properties can be the solution for these problems.
There are hydrogels that are not suitable for applications in musculoskeletal systems, because most of them often exhibit too weak mechanical properties owing to a decreased elastic modulus ranging from kPa to MPa, in comparison with the native bone tissue that exert a modulus between 1 and 20 GPa. Because human cells respond differently to various mechanical stresses, such as compression, tension and shear [383], it is essential for a hydrogel scaffold to support loads and movements when is used an implant. Fortunately, by growing the number density of crosslinks and the polymer concentration inside the network gel or by combining two or more IPNs, this way has allowed scientists to obtain hydrogels with increased stiffness. Microfabrication techniques such as electrospinning and 3D printing have also proven to be successfully used in the designing of hydrogels with strong and complex structures for bone engineering applications [384].
Liu et al. [385] obtained a biomimetic bone substitute comprising a type I collagen matrix gel in which poly(l-lactide-co-caprolactone) nanoyarns were incorporated before gelation using a water vortex as collector, instead of traditional rotating drums or dual metal collection rings, to produce aligned nanoyarns in order to increase the mechanical strength of the resulted hydrogels allowing in the same time the cell proliferative ability of collagen. The nanoyarns were short enough to eliminate the entanglements formation when they were suspended in the protein solution.
Gan et al. [386] studied the interpenetration between a primary network made of dextran and gelatin and a secondary network composed of PEG. The resulted hybrid hydrogels exhibited improved toughness and good proliferation, clustering and adhesion of the incorporated nucleus pulposus cells, when the proportion of the natural network was 4-fold greater than the synthetic one.
7.3.3. Cartilage Tissue Engineering
Several hybrid hydrogels have been developed as injectable scaffolds to mimic the ECM of cartilage. Wang et al. [387] obtained in situ injectable silk solutions of mixed silk and low-molecular-weight PEG formed hydrogels in less than 30 min when the concentration of PEG in the gel was 40–45%. A scaffold/nano- or micro-particles system is introduced for accelerated healing by sustained release of drugs/biomolecules or/and by rapid cell proliferation. Thus, gelatin can be part of the scaffold or of the particles. For example, Xu et al. reported the fabrication of the alginate-gelatin hydrogel microspheres using an electrospray technique, that were embedded with human bone marrow stromal cell (hBMSCs), and then seeded and assembled in 3D-printed PCL scaffolds for the fabrication of a mechanically stable and biologically supportive tissue engineering cartilage construct [388]. In Asadi et al. study [389], gelatin was used as a component of hydrogel scaffold. In this case, PCL−PEG−PCL nanoparticles loaded with transforming growth factor β1 (TGFβ1) were embedded in the gelatin hydrogel scaffolds and investigated as system for cartilage tissue engineering.
Lee et al. developed a strategy to engineer an auricular cartilage using SF and PVA hydrogel [390]. They demonstrated that an intact 3D ear-shaped auricular cartilage formed six weeks after the subcutaneous implantation of a chondrocyte-seeded 3D ear-shaped P50/S50 hydrogel in rats.
7.3.4. Wound Healing
An ideal scaffold for skin tissue regeneration is expected to be biocompatible, biodegradable where the degradation rate should be correlated with the regeneration time of the new tissue, and should have interconnected pores of appropriate size allowing cell attachment, migration, proliferation and vascularization to supply the necessary nutrients to the newly formed tissue [391]. Most of the hydrogels used for wound dressing are usually loaded with drug to increase the rate of healing of the skin. Shamloo et al. [214] prepared a PVA/chitosan/gelatin hydrogel embedded with PCL microspheres by a double-emulsion-solvent-evaporation method destined for accelerated wound healing by sustained release of bFGF. Electron beam irradiation (EBI) was used to prepare hybrid keratin based hydrogels in the presence of two synthetic polymers, namely PVA and polyethylenimine (PEI) that influence the gelation rate of keratin [392]. In this work, keratin was extracted from two natural sources, human hair and wool, using sulfitolysis reaction with sodium disulfite in order to cleave the cystine disulfide bonds and to form cysteine thiol. Because during EBI, the aqueous solution of obtained reduced keratin was not converted to gel, PVA was added to the solution inducing gel formation at an EBI dose of approximately 90 kGy. Besides, by the addition of a secondary synthetic polymer, namelly PEI, in the aqueous blend containing S-sulfo keratin and PVA, the gelation occurs at a much lower irradiation dose, up to 10 kGy. The gel forming may be assigned not only to the physical interaction between S-sulfo keratin chains, oxygen groups in PVA aqueous solution and the amine groups of PEI, but also to the keratin covalent crosslinking by EBI. Later, these hydrogels have been tested for wound healing [393] and was suggested that the treatment with these keratin-based hydrogels enhanced the production of new collagen and fibroblast proliferation during granulation tissue formation and the remodeling phase of wound healing. Synthetic polymers as PEGDA [394], PVA [395], poly(N-hydroxyethyl acrylamide) (PHEA) [396], PAAm [397] etc., were used in combination with silk sericin to obtain hybrid hydrogels that could be used especially for wound dressings and dermal reconstructions.
7.3.5. Drug and Molecule Delivery
Due to its biocompatibility and well-established safety profiles, hydrogels fabricated using collagen have been employed as delivery vehicles for therapeutic genes, which can direct and/or enhance the function of the transplanted or endogenous cells [398].
Krebs et al. [399] showed the collagen chains ability to locally load siRNA (small interfering RNA) and then release it in a controlled manner in order to extend the effect directly at the specific target. Peng et al. [400] tested collagen based hybrid hydrogels for the Id1-siRNA targeting and its delivery and sustained release for the treatment of gastric cancer. To improve the siRNA delivery by stimulating the target of Id1-siRNA into the defect cells and prolonging the silencing effect, cationic PEI was further engaged for scaffold modification.
Gelatin has been used for nanogels preparation for different biomedical applications [401]. In this respect, activated PEG methyl ether was grafted onto gelatin backbone to obtain the gelatin-PEG copolymer and then self-assembled to form nanogels (Figure 8) that were encapsulated with poor water soluble curcumin (CUR) for cancer treatment [402]. Nanogels improved the solubility of CUR in the aqueous environment, protected it from the hydrolytic degradation and also increased the amount of delivered CUR in a control release manner, thus enhancing the therapeutic efficacy of CUR.
Figure 8.
Representation for the preparation of curcumin-loaded gelatin—poly(ethylene glycol) (PEG) nanogels (reprinted from [402], open access).
Gelatins have been used in designing of different formulations suitable as vectors for gene delivery [403,404]. Rumschöttel et al. [405] were incorporated core-shell like spherical DNA/PEI polyplexes in gelatin hydrogels. After a μ-DSC study, it has concluded that by adding a third component, e.g., gelatin, the complex stability between the polycation PEI and the negatively charged DNA is influenced by its interaction with the polycation shell. Thus, the effect of the gelatin can be explained by the weakening of the electrostatic interactions in DNA/PEI polyplexes, due to the gelatin attachment to the PEI shell or partially takes off the PEI from the shell. Conversely, in the DNA/maltose-modified PEI polyplexes, a stronger interaction via H-bonding between maltose units and gelatin was confirmed.
A pH sensitive and partly-biodegradable sIPN hydrogel based on methacrylic acid, BSA, and PEG, with potential application as drug delivery systems, was prepared by UV initiated free radical polymerization [406]. Additionally, BSA was copolymerized with PEG to obtain high-water content (>96%) hydrogels [407] that be useful for the preparation of controlled release devices in the field of wound dressing.
In the field of controlled and sustained drug delivery systems, SF based hydrogels were tested using as model drugs curcumin [408] and hydrophobic/hydrophilic drugs, namely aspirin and indomethacin [409], where the synthetic part was represented by PVA and PLA-PEG-PLA copolymer, respectively.
WPI based hydrogels can be used as bioresponsive carriers for controlled release of biomolecules and drugs [410], as they exhibit good pH-sensitivity and protects the entrapped drug from degradation by its grafting onto synthetic polymers resulting co-polymers. An example was reported by Aderibigbe and Ndwabu [411] who prepared by simultaneous redox cross-linked polymerization an WPI-g-carbopol polyacrylamide based hydrogel loaded with pamidronate, (nitrogen containing bisphosphonate), to treat the skeletal disorders in children, osteoporosis and bone cancer. The hybrid hydrogels showed a pH dependent swelling behavior, being more swollen at high pH values.
8. Nucleic Acids Based Hybrid Hydrogels for Biomedical Applications
Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) are polynucleotides with distinct biological function. The specific bonding of DNA base pairs (for example guanine–cytosine, adenine–thymine for DNA and adenine–uracil in the case of RNA) provide the chemical foundation for genetics, being a powerful molecular recognition system [412,413]. The level of versatility and structural programmability of nucleic acids ranks them on a higher level in the field of biomedical applications compared to other natural or synthetic polymers. Since 2001 (according to SCOPUS database) there has been an intensification of research in the field of nucleic acids-based hydrogel development, especially due to the great progress in DNA synthesis and consequently the accessibility of larger amounts of DNA. Due to the outstanding properties of nucleic acids as hydrophilicity, biocompatibility, stimuli responsiveness, versatility and structural programmability, controlled biodegradability, which can be exploited in the development of new materials for numerous biomedical applications, this may be the subject of a separate review. In the recent published scientific literature there are already comprehensive reviews covering all aspects of obtaining and using hydrogels based on or containing nucleic acids in biomedical applications [414,415,416]. Hence, in the current review is presented just a short summary regarding the development of nucleic acids-containing hydrogels.
8.1. Nucleic Acids Ability to Form Hydrogels
DNA is an outstanding component for the obtaining of supramolecular hydrogels, especially because it is an amphiphilic bio-polylectrolyte capable to absorb large quantities of water and may induce in the incorporating material controllable properties, programmability and biocompatibility [415]. At this moment there are three approaches for using nucleic acids in the construction of hydrogels, namely: (1) as a building unit for hydrogel-based materials—representing the so called “all-DNA” hydrogels [417,418,419]; (2) as biocompatible cross-linkers combining nucleic acids with synthetic polymers, resulting hybrid hydrogels [420]; and (3) short DNA sequences (e.g., aptamers) that act as functional grafts to obtain tailorable hydrogels with unique bio-specificity [414,421,422].
Even though DNA-based hydrogels have numerous distinctive properties, their use in the biomedical field beyond conceptual demonstration is limited by a number of shortcomings, namely: small number of functional groups available (needed in further chemical modifications), highly negative net charge, and high costs of synthesis [414]. To overcome these drawbacks, the combination of nucleic acids with hydrophilic polymer scaffolds represents a promising approach. Polyamines show the ability to bind DNA by promoting its condensation, a process of paramount importance in medical applications, mainly in immunotherapy. Among the synthetic polyamines, poly(ethylene imine) (PEI) is one of the most prominent examples of cationic polymers capable to induce condensation of DNA [423]. PEI has a high charge density and by electrostatic interactions it can strongly bind to DNA leading to its compaction [415,424].
8.2. Biomedical Applications of Nucleic Acids-Containing Hybrid Hydrogels
8.2.1. Drug Delivery
Spherical DNA-based nanogels were synthesized by Costa et al. [425] using PEI as pDNA condensation agent. These nanogels showed very low size distribution, high loading capacity and interesting release kinetics of simultaneous delivery of pDNA and drugs. In a recent study, DNA was grafted with PCL to obtain hybrid nanogels that were then loaded with Cas9/single guide RNA complex. The resulted DNA based hybrid hydrogel can be used as carrier for gene editing tool delivery that provides excellent physiological stability against nuclease digestion [426].
8.2.2. Immunotherapy
Mimi et al. [427] have fabricated a gelatin PEI core shell nanogel via a two-stage synthesis. The resultant nanogels are highly uniform spherical particles and have a well-defined core shell nanostructure with a biodegradable gelatin core and a hairy and extended PEI shell. The resultant nanogels were able to completely condense siRNA, forming stable complexes that were capable of protecting the siRNA from enzymatic degradation. The gelatin PEI nanogels were four times less toxic than the native PEI, and were able to effectively deliver the siRNA into HeLa cells. Increasing the N/P ratio significantly improved the intracellular uptake efficiency of the siRNA. Li et al. [428] has proposed a flexible strategy to design well-defined reducible cationic nanogels (PGED-NGs) based on ethylenediamine (ED)-functionalized low-molecular-weight poly(glycidyl methacrylate) (PGMA) with friendly crosslinking reagents (α-lipoic acid). PGED-NGs could effectively complex pDNA and siRNA. Compared with pristine PGED, PGED-NGs exhibited much better performance of pDNA transfection. PGED-NGs also could efficiently transport metastasis-associated lung adenocarcinoma transcript 1 siRNA into hepatoma cells and significantly suppressed cancer cell proliferation and migration. After the photoisomerization of the Azo moiety on the DNA cross-linker, Kang et al. [429] succeded to develop photoresponsive hydrogels based on comb-shaped DNA-polyacrylamide conjugates that can be used for the DOX delivery in cancer therapy. More precisely, these hybrid hydrogels were capable to carry and then to release a large amount of active drug molecules (DOX) in a controllable manner due to their ability to transform in sol upon UV light irradiation, and subsequent inducing a very high rate of cancer cell death. siRNA based nanoparticle complexes have been embedded in PEG-PLA-dimethacrylate hydrogels in order to obtain biodegradable systems that exhibits high controlled tissue-specific localization and sustained gene delivery due to the hydrolytic degradation of ester bonds within the PLA crosslinks [430]. Negatively-charged siRNA was complexed to nanoparticles by its self-assembly with a poly(dimethylaminoethyl methacrylate)-b-poly-(dimethylaminoethyl methacrylate-co-propylacrylic acid-co-butyl methacrylate) diblock polymer.
8.2.3. Biosensing Applications
The analyte-triggered opening of the DNA-crosslinked structures and consequent dissociation of the gel networks can initiate the release of encapsulated signal substances to generate an output signal. Using different responsive DNA hydrogels in which enzymes, DNAzymes, or catalytic nanomaterials are entrapped, researchers have developed various colorimetric visual sensors and readout devices based on the sol-gel transition strategy for different kinds of biotargets, such as metal ions and glucose. Liu and co-workers [431] demonstrated that covalently crosslinked polyacrylamide hydrogels can be used as a platform to attach Hg2+-responsive DNA structures for the ultrasensitive detection and removal of Hg2+ [432]. Shape-memory DNA hydrogels that can reversibly respond to external stimuli have also been developed. Hu et al. designed bilayer hybrid hydrogels whose stiffness can be controlled by different triggers such us thermal and pH (i-motif) stimuli [433]. One of the two layers was consisted of a non-responsive hydrogel based on acrydite-DNA crosslinked polyacrylamide, and the second layer included a thermosensitive acrydite-DNA crosslinked PNIPAM based hydrogel. The two-layer hybrid hydrogels that exhibits controlled stimuli-induced shape transitions are promising for the use in the biomedical field as intelligent actuators. Similar studies, of shape memory hydrogels that include DNA and polyacrylamide were found in the scientific literature [434,435]. In Figure 9 is illustrated a photoresponsive hybrid hydrogel that reveals light-induced switchable stiffness functions. The hybrid hydrogel is based on pDNA crosslinked with polyacrylamide and stabilized by trans-azobenzene intercalator units.
Figure 9.
Schematic light-induced shape-memory transitions between the triangle-shaped, high-stiffness polyacrylamide-based hydrogel crosslinked by glucosamine–boronate ester bridges and trans-azobenzene stabilized duplex crosslinkers and the low-stiffness hydrogel crosslinked by the glucosamine–boronate esters only. Adapted from [435] Open Access, Copyright 2020, Royal Society of Chemistry.
9. Hybrid Hydrogels Containing Lignin for Biomedical Applications
Lignin hydrogels are considered to have a great potential for valorization of this abundant polyaromatic bio-polymer abundant in plants and which results as by-product in pulp and paper industry. The three phenolic sub-structures of the lignin structure, namely syringyl (S), guaiacyl (G), and p-hydroxyphenyl (H) units and also lignin derivatives (resulted by epoxidation, amination, hydroxyalkylation, nitration, halogenation, sulfomethylation, etc.) contain many different functional groups (hydroxyls, carboxyls, carbonyls and methoxyls) as active sites, in preparation of functional hydrogels both by physical crosslinking with hydrophilic polymers by H-bonding and also by, polymerization, copolymerization, ATRP and reversible addition-fragmentation transfer (RAFT) polymerization, chemical crosslinking, crosslinking grafted lignin and monomers etc. [436,437,438].
Lignin-based hydrogels had a rougher surface morphology and a porous structure related to an increasing lignin concentration. The water uptake and the water retention of lignin-based hydrogels depend on structure of the hydrogels, including the pores sizes and the surface morphology. Physical hybrid hydrogels showed self-healing capability due to the dynamic hydrogen bonding between lignin and hydrophilic polymers.
Increasing the content of lignin in hydrogels significantly improved the mechanical properties of the hydrogels [439] and they show excellent strength properties. The biodegradation of the lignin-based hydrogel depends on the crosslink density and the phenolics content in the hydrogel. The former is correlated with small pores, stronger cross-linked gels which had less accessibility for lignolytic fungi and actinomycetes whch induces high resistance against microbial attack than slightly cross-linked hydrogels. The phenolic substructures in hydrogels directly attacked the expressed enzyme systems of lignolytic fungi [440] so they protect plant from fungi invasion. Hydrogels containing lignin and its derivatives with acrylic acid, methacrylic and NIPAAm monomers exhibit external stimuli response as pH response, thermoresponsiveness and mechanical response. The self-healing property of lignin-based supramolecular lignin-based hydrogel with α-cyclodextrin and a hyper branch architecture was obtained that possessed a mechanical response feature. When the oscillation stain increased, the solid-like hydrogel translated into liquid-like, and the liquid-like hydrogel recovered to be solid-like via self-assembly when the oscillate stain decreased [441].
The biocompatibility, biodegradability, low toxicity and eco-friendliness of lignin-based hydrogels are main features which have determined their wide application as biomaterials in many fields as for controlled release of the functional materials including enzyme immobilization and drug delivery.
9.1. Bioactive Compounds Delivery
Lignin-based hydrogels could be used as biological carriers for human hepatocytes culture, because a large number of hepatocytes adhered to the pores of the lignin-based hydrogel, and a higher cell proliferation rate and metabolic activity was reported [442]. From lignin grafted polymers and hydrophilic polyurethane/acrylic monomer, a double-network hydrogel with tough and pH sensitive properties [443] was prepared and can be processed by fiber spinning, casting and 3D printing, this one being biocompatible with primary human dermal fibroblasts [444]. Lipase immobilized on cellulose/lignin hydrogel beads exhibited higher stability and activity [445].
A hydrophilic supramolecular complex of hemicellulose/oak lignin hydrogel with pectin embedded in the 3D structure was prepared and tested to deliver β-glucuronidase and estrogens [446].
A smart hydrogel of carboxylated lignin nanoparticles/PEG-poly(histidine) block copolymer/a cell-penetrating peptide loaded with a poorly water-soluble cytotoxic agent was evaluated for chemotherapeutic potential [447]. The reactivity of lignin with epichlorohydrine as curing agent was exploited to obtain superabsorbent hydrogels with cellulose and its derivatives, PVA, xanthan, which controlled release active substances as phenols and vanillin, active aroma ingredient, [28,448,449].
Acid activated lignosulphonate/poly(vinyl pyrrolidone) hydrogel is a good carrier for controlled release of amoxicillin [450].
9.2. Applications as Antimicrobial, Antioxidant, Antifungal materials
Lignin itself, as a complex natural polymer with phenolic groups, possess non-toxicity, antimicrobial, antioxidant and antifungal properties, which may enhance the potential application in food science and health care of its hydrogels [451,452]. Antibacterial and antioxidant agents like CS and Alg had been added into lignin-based hydrogels, which had great potential to be applied in biosensors and tissue engineering [220].
Modification of the lignin with triazole moiety enhances the antibiofilm and antimicrobial activities. A new hydrogel was prepared as an ointment for anti-infection, which had abilities to prevent infection of burn wound and could be used as an anti-inflammatory dressing and aid-healing material [453]. Lignin/CS hydrogels show a good potential applicability in wound healing, since these hydrogels present a good cell attachment and proliferation of NIH 3T3 mouse fibroblast [454]. Highly antibacterial lignin-based hydrogels for drug delivery have been recently reported against Staphylococcus aureus and Proteus mirabilis, behaviour demonstrated for hydrogels prepared with lignin, cellulose, hyaluronan, Gantrez S-97 (poly(methyl vinyl ether-co-maleic acid), poly(ethylene glycol) or glycerol and others [455,456,457].
10. Conclusions and Future Trends
Because of the large availability of materials used in organic hybrid polymeric hydrogels (both synthetic and natural polymers), the number of possible combinations is enormous, which explained the large number of publications only in the last decades (more than 2000 annually). The biomedical applications of the hydrogels started back multiple decades and their study is continuously growing.
In this review, some selective research studies have been summarized especially in the last two decades, for the preparation of natural polymers-containing hybrid hydrogels and their potential application in a wide range of medical applications. It was described both advantages and disadvantages of each hydrogel applied in different medical application. Desired hybrid hydrogels may be developed for targeted applications by making changes in composition, use of specific biomolecules, antimicrobial agents, use of suitable cells, and selecting suitable synthesis routes and processing techniques. The successful use of a polymeric hybrid hydrogel consists in creating a three-dimensional micro-/nano environment that represents a synthetic ECM for the cells, which should provide biodegradability, biocompatibility, pore interconnectivity to assure the penetration and absorption of nutrient, modulation of proliferation for successful reconstruction of organs, cell-adhesion and regeneration certain tissue. In the most recent researches, injectable hydrogels and 3D-bioprinted hybrid hydrogels allow successful their interaction with the cells of damaged tissues. The hybrid nano hydrogel materials are able to convert external stimuli signals to heat, highly oxidative species etc., which are helpful for combinatorial therapies and theranostics. By a simple hybridization of the components of the hybrid hydrogels smart multiresponsive materials can be obtained by synergistic combination of the best properties of both components, useful toward applications in nanomedicine which exhibit an excellent targetability, minimal side effects in treatments and diagnostic. The industrial application of the new hybrid hydro/nanogels materials is in its first steps and it need more relevant clinical data concerning their safety and efficacy in vivo. In Table 6 are listed few examples of hybrid nanogels/hydrogels evaluated in preclinical and clinical studies.

Table 6.
Hybrid nanogels/hydrogels in preclinical and clinical phase.
CHP nanogels have been used as an intranasal vaccine-delivery system [459,462,464] their clinical trials showed promising results for therapy-refractory esophageal cancer patients and HER2 expressing cancer patients. The CHPHER2 complex vaccine was safe, well tolerated and showed specific antibody responses (Phase I and II of trial). From the products already on the market that use a combination, one can mention the HYAFFTM esterified hyaluronic acid [465], produced by FIDIA Ltd.: LaserskinAutograft® (made of a HA-membrane with keratinocytes), and Hyalo-graft 3D® (made with HA, but with fibroblasts added). More preclinical studies are needed to provide convincing resources for supporting advanced clinical applications.
According to the Food and Agriculture Organization of the United Nations and World Health Organization, probiotics are “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host”. For probiotic delivery systems, biomaterials such as proteins (gelatin, casein or whey proteins), polysaccharides, as well as synthetic polymers, such as poly(d,l-lactic-co-glycolic acid), polyvinyl alcohol or Eudragit (poly(methacrylic acid-co-ethyl acrylate) 1:1) could be used [466].
The complex (synthetic and natural) polymeric hybrid hydrogels with functional domains or nano/microstructures that provide both improved mechanical and physical properties, tunable release kinetics for targeted drug therapy, mediated cell response, stimuli-responsive material behavior are under continuously development. Their use in clinical and research applications in biomedical practice, as drug discovery, drug/gene delivery, regenerative medicine, etc., is very promising. New and/or improve existing multi length-scale methodologies for predicting the properties of the materials based on those of the individual components, as a guide for experimental development is necessary. A better understanding of the interactions between components of the hybrid hydrogels will lead to a more efficient design and control of their mechanical performance, long-term stability, the hydrophilic/hydrophobic nature of the material for improved drug loading capacity and controlled release, or the precise control of shape variations induced by external stimuli, to name a few. They are used to impart to homopolysaccharides-based hydrogels stimuli responsiveness (e.g., pH, temperature), to improve their mechanical properties, etc. Is noticed a tendency of the research towards the variation of the methods of modifying the polysaccharides properties (by obtaining new copolymers, functionalization through various chemical reactions, using innovative techniques such as 3D printing or electrospinning) to meet the specific requirements of biomedical applications. Using composite hydrogels containing various inorganic nanoparticle is another direction with multiple possibilities to obtain materials with tunable properties and targeting applications directed to personalized medicine. This are/will be subject to other many reviews.
Author Contributions
Conceptualization, C.V., D.P., E.S., and M.B.; software, C.V., D.P., E.S., and M.B.; validation, C.V., D.P., E.S., and M.B.; writing—review and editing, C.V., D.P., E.S., and M.B.; supervision, C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We acknowledged support of MDPI offering us free APC for this review.
Conflicts of Interest
The authors declare no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Abbreviations
| AAc—acrylic acid | LCST—lower critical phase transition temperature |
| AAm—acrylamide | LMWG—low-molecular-weight gelator |
| Alg—alginate | MA—maleic anhydride |
| APS—ammonium persulfate | MAA—methacrylic acid |
| ATRP—atom transfer radical polymerization | MACMC—methacrylate carboxymethyl cellulose |
| BC—bacterial cellulose | MBA—N,N′-methylene bisacrylamide |
| bFGF—basic fibroblast growth factor | MS—microspheres |
| BIS—N,N-Methylene bisacrylamide | MW—molecular weight |
| JuanuaryBMSCs—bone mesenchymal stem cells | MGG—methacrylated gellan gum |
| BSA—bovine serum albumin | MA—maleic anhydride |
| CA—crosslinking agent | NanoCliP—nanogel-crosslinked porous |
| CaM—Calmodulin | NIPAAm—N-isopropylacrylamide |
| CBA—N,N′-bis(acryloyl)cystamine | PAA—polyacrylic acid |
| CBMA—carboxybetaine methacrylate | PAAm—polyacrylamide |
| CE-chitosan—carboxyethyl chitosan | PAN—polyacrylonitrile |
| CG—Carrageenan | PCL—poly(ε-caprolactone) |
| Cel—cellulose | PDEA—poly(diethylacrylamide) |
| CHP—cholesterol-bearing pullulan | PDMAEMA—poly 2-(dimethylamino) ethyl methacrylate |
| CHP—cholesteryl-modified pullulan | PDMS—poly(dimethylsiloxane) |
| CHPANG—acrylate group-modified cholesterol-bearing pullulan | PEG—poly (ethylene glycol) |
| CHPOA—acryloyl group modified-cholesterol-bearing pullulan | PEGDA—polyethylene glycol diacrylate |
| CMC—carboxymethyl cellulose | PEG-Nor—norbornene immobilized tetra-arm PEG |
| CM-chitosan—N-O-carboxymethyl chitosan | PEI—polyethylenimine |
| CMP—carboxymethyl pullulan | PEO—poly(ethylene oxide) |
| CMPVA—carboxymethyl polyvinyl alcohol | PET—poly (ethylene terephthalate) |
| CMS—carboxymethyl starch | PG—polymer gelator |
| CNCs—cellulose nanocrystals | PGA—poly(glycolic acid) |
| CNF—cellulose nanofiber | PHB—poly(3-hydroxybutyrate) |
| CPUNs—cationic polyurethane nanoparticles | PHEA—poly(N-hydroxyethyl acrylamide) |
| CS—chitosan | PHEMA—poly(2-hydroxyethyl methacrylate) |
| CTS—chondroitin sulfate | PLA—poly (lactic acid) |
| CUR—curcumin | PLGA—poly(lactic-co-glycolic acid) |
| DDS—drug delivery system | PLLA—poly(l-lactide) |
| DN—double network | PMAA—poly(methacrylic acid) |
| DNA—deoxyribonucleic acid | PMMA—poly(methyl methacrylate) |
| DP—difunctionalized PEG | PNIPAAm—poly(N-isopropylacrylamide) |
| DPC-DN—dual physically cross-linked double network | PoH—poloxamer-heparin |
| DTT—dithiothreitol | PPO—poly(propylene oxide) |
| EBI—electron beam irradiation | PPy—polypyrrole |
| ECM—extracellular matrix | PTX—paclitaxel |
| EDAC—1-ethyl-3-(3- dimethylaminopropyl) carbodiimide | PU—polyurethane |
| EDC—1-(3-Dimethyl aminopropyl)-3-ethylcarbodiimide Hydrochloride | PULMA—methacrylated pullulan |
| EDTA—ethylene diamine tetraacetic acid | PUU—polyurethane-urea |
| EGDA—ethylene glycol diacrylate | PVP—polyvinylpyrrolidone |
| EGDMA—ethylene glycol dimethacrylate | Q-chitosan—quaternary chitosan |
| ELPs—elastin-like polypeptides | rBMSCs—bone marrow stem cells isolated from rabbits |
| FK—feather keratin | rMSCs—marrow stem cells isolated from rabbits |
| FT—freeze–thawing | RAFT—reversible addition–fragmentation chain-transfer polymerization |
| GA—glutaraldehyde | RNA—ribonucleic acid |
| GAG—glycosaminoglycan | RITP—iodine-mediated polymerization |
| GC—glycol chitosan | RLPs—resilin-like polypeptides |
| GC-DP—hydrogels based on glycol chitosan and difunctionalized PEG | RT-PCR—reverse transcription polymerase chain reaction |
| GG—gellan gum | SA—sodium alginate |
| GI tract—gastrointestinal tract | SAPCs—superabsorbent polymer composites |
| HA—hyaluronic acid | SF—silk fibroin |
| Hce—hemicellulose | SGF—simulated gastric fluid |
| Hep—Heparin | SIF—simulated intestinal fluid |
| hBMSCs—human bone marrow stromal cell | sIPNs—semi-IPNs |
| HDI—hexamethylene diisocyanate | siRNA—small interfering RNA |
| HEC—hydroxyethyl cellulose | SP—soy protein |
| HEMA—2-hydroxyethyl methacrylate | SPI—soy protein isolate |
| HHP—high hydrostatic pressure | SPION—super paramagnetic iron oxide nanoparticles |
| HLC—human like collagen | SS—silk sericin |
| hMSCs—human mesenchymal stem cells | TEMED—N,N,N′,N′-tetramethylethylenediamine |
| hBMSCs—human bone marrow stromal cell | TG—transglutaminase |
| HP—homopolysaccharides | TGFβ1—transforming growth factor β1 |
| HPA—hydroxyphenyl propionic acid | |
| HPC—hydroxypropyl cellulose | THPC—tetrakis(hydroxymethyl)phosphonium chloride |
| HPMA—2-hydroxypropyl methacrylate | TIPS—thermally induced phase separation |
| HSP27—heat shock protein 27 | VEGF—vascular endothelial growth factor |
| HRP—horseradish peroxidase | VP—vinyl pyrrolidone |
| ICH—intracerebral hemorrhage | WP—whey protein |
| IPN—interpenetrated network | WPC—whey protein concentrates |
| KOS—keratose | WPH—whey protein hydrolysates |
| KTN—kerateine | WPI—whey protein isolates |
| KPS—potassium persulfate | XG—xanthan gum |
| LZ—leucine zipper | |
References
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Budama-Kilinc, Y.; Cakir-Koc, R.; Aslan, B.; Özkan, B.; Mutlu, H.; Üstün, E. Chapter 12: Hydrogels in Regenerative Medicine. In Biomaterials in Regenerative Medicine; Dobrzański, L.A., Ed.; IntechOpen: London, UK, 2018; pp. 277–301. [Google Scholar]
- Durmaz, S.; Okay, O. Acrylamide/2-acrylamido-2-methylpropane sulfonic acid sodium salt-based hydrogels: Synthesis and characterization. Polymer 2000, 41, 3693–3704. [Google Scholar] [CrossRef]
- Ekici, S.; Saraydin, D. Synthesis, characterization and evaluation of IPN hydrogels for antibiotic release. Drug Deliv. 2004, 11, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Swami, S.N. Radiation synthesis of polymeric hydrogels for swelling-controlled drug release studies. Ph.D. Thesis, Western Sydney University, Sydney, Australia, 2004. Available online: http://handle.uws.edu.au:8081/1959.7/698 (accessed on 7 January 2020).
- Katime, I.; Novoa, R.; Zuluaga, F. Swelling kinetics and release studies of theophylline and aminophylline from acrylic acid/n-alkyl methacrylate hydrogels. Eur. Polym. J. 2001, 37, 1465–1471. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, R.; Yang, S.; Guan, J.; Zhang, D.; Ma, Y.; Liu, H. Research progress of self-assembled nanogel and hybrid hydrogel systems based on pullulan derivatives. Drug Deliv. 2018, 25, 278–292. [Google Scholar] [CrossRef]
- Kopeček, J.; Tang, A.; Wang, C.; Stewart, R.J. De novo design of biomedical polymers. Hybrids from synthetic macromolecules and genetically engineered protein domains. Macomol. Symp. 2001, 174, 31–42. [Google Scholar] [CrossRef]
- Molina, M.; Asadian-Birjand, M.; Balach, J.; Bergueiro, J.; Miceliac, E.; Calderon, M. Stimuli-responsive nanogel composites and their application in nanomedicine. Chem. Soc. Rev. 2015, 44, 6161–6186. [Google Scholar] [CrossRef]
- Jia, X.; Kiick, K.L. Hybrid multicomponent hydrogels for tissue engineering. Macromol. Biosci. 2009, 9, 140–156. [Google Scholar] [CrossRef]
- Myung, D.; Waters, D.; Wiseman, M.; Duhamel, P.E.; Noolandi, J.; Ta, C.N.; Frank, C.W. Progress in the development of interpenetrating polymer network hydrogels. Polym. Adv. Technol. 2008, 19, 647–657. [Google Scholar] [CrossRef]
- Jonker, A.M.; Löwik, D.W.P.M.; van Hest, J.C.M. Peptide- and protein-based hydrogels. Chem. Mater. 2012, 24, 759–773. [Google Scholar] [CrossRef]
- Lau, H.K.; Kiick, K.L. Opportunities for multicomponent hybrid hydrogels in biomedical applications. Biomacromolecules 2015, 16, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Maisani, M.; Pezzoli, D.; Chassande, O.; Mantovani, D. Cellularizing hydrogel-based scaffolds to repair bone tissue: How to create a physiologically relevant micro-environment? J. Tissue Eng. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.A.; McCarthy, C.W.; Tyo, A.; Hubbard, K.; Fisher, H.; Altscheffel, J.; He, W.; Pinnaratip, R.; Lui, Y.; Lee, B.P.; et al. Development of an injectable nitric oxide releasing poly(ethylene) glycol-Fibrin adhesive hydrogel. ACS Biomater. Sci. Eng. 2019, 5, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Ann. N. Y. Acad. Sci. 2001, 944, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lin, H.; Rothrauff, B.B.; Yu, S.; Tuan, R.S. Multilayered polycaprolactone/gelatin fiber-hydrogel composite for tendon tissue engineering. Acta Biomater. 2016, 35, 68–76. [Google Scholar] [CrossRef]
- Patel, D.; Sharma, S.; Screen, H.R.C.; Bryant, S.J. Effects of cell adhesion motif, fiber stiffness, and cyclic strainon tenocyte gene expression in a tendon mimetic fiber composite hydrogel. Biochem. Biophys. Res. Commun. 2018, 499, 642–647. [Google Scholar] [CrossRef]
- Kopeček, J. Hydrogel Biomaterials: A Smart Future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, W.; Han, B.; Yang, C.; Ma, Q.; Song, F.; Bi, Q. An in situ formed biodegradable hydrogel for reconstruction of the corneal endothelium. Colloids Surf. B: Biointerfaces 2011, 82, 1–7. [Google Scholar] [CrossRef]
- Ehrick, J.D.; Deo, S.K.; Browning, T.W.; Bachas, L.G.; Madou, M.J.; Daunert, S. Genetically engineered protein in hydrogels tailors stimuli-responsive characteristics. Nat. Mater. 2005, 4, 298–302. [Google Scholar] [CrossRef]
- Sengupta, D.; Heilshorn, S.C. Protein-engineered biomaterials: Highly tunable tissue engineering scaffolds. Tissue Eng. Part B: Rev. 2010, 16, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Foo, W.P.C.T.; Lee, J.S.; Mulyasasmita, W.; Parisi-Amon, A.; Heilshorn, S.C. Two-component protein-engineered physical hydrogels for cell encapsulation. Proc. Natl. Acad. Sci. USA 2009, 106, 22067–22072. [Google Scholar]
- Sakai, S.; Hirose, K.; Taguchi, K.; Ogushi, Y.; Kawakami, K. An injectable, in situ enzymatically gellable, gelatin derivative for drug delivery and tissue engineering. Biomaterials 2009, 30, 3371–3377. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Cheng, E.; Yang, Y.; Chen, P.; Zhang, T.; Sun, Y.; Yang, Z.; Liu, D. Self-assembled DNA hydrogels with designable thermal and enzymatic responsiveness. Adv. Mater. 2011, 23, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Campbell, J.J.; Thian, E.S.; Watson, C.J.; Cameron, R.E. Collagen–hyaluronic acid scaffolds for adipose tissue engineering. Acta Biomater. 2010, 6, 3957–3968. [Google Scholar] [CrossRef]
- Raschip, I.E.; Hitruc, G.E.; Vasile, C.; Popescu, M.C. Effect of the lignin type on the morphology and thermal properties of xanthan/lignin hydrogels. Int. J. Biol. Macromol. 2013, 54, 230–237. [Google Scholar] [CrossRef]
- Raschip, I.E.; Hitruc, E.G.; Vasile, C. Semi-interpenetrating polymer networks containing polysaccharides. II. Xanthan/lignin network: A spectral and thermal characterization. High Perform. Polym. 2011, 23, 219–229. [Google Scholar] [CrossRef]
- Hejčl, A.; Sedý, J.; Kapcalová, M.; Toro, D.A.; Amemori, T.; Lesný, P.; Likavcanová-Mašínová, K.; Krumbholcová, E.; Prádný, M.; Michálek, J.; et al. HPMA-RGD hydrogels seeded with mesenchymal stem cells improve functional outcome in chronic spinal cord injury. Stem Cells Dev. 2010, 19, 1535–1546. [Google Scholar] [CrossRef]
- Beamish, J.A.; Zhu, J.; Kottke-Marchant, K.; Marchant, R.E. The effects of monoacrylated poly(ethylene glycol) on the properties of poly (ethylene glycol) diacrylate hydrogels used for tissue engineering. J. Biomed. Mater. Res. Part A 2010, 92, 441–450. [Google Scholar] [CrossRef]
- Zustiak, S.P.; Leach, J.B. Hydrolytically degradable poly (ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromolecules 2010, 11, 1348–1357. [Google Scholar] [CrossRef]
- Silva, A.K.A.; Richard, C.; Bessodes, M.; Scherman, D.; Merten, O.-W. Growth factor delivery approaches in hydrogels. Biomacromolecules 2008, 10, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.; Elisseeff, J.H. Hydrogels for musculoskeletal tissue engineering. Adv. Polym. Sci. 2006, 203, 95–144. [Google Scholar]
- Jiang, Z.; Hao, J.; You, Y.; Liu, Y.; Wang, Z.; Deng, X. Biodegradable and thermoreversible hydrogels of poly (ethylene glycol)-poly (ε-caprolactone-co-glycolide)-poly (ethylene glycol) aqueous solutions. J. Biomed. Mater. Res. Part A 2008, 87, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, M.; Singh, Y.; Gunaseelan, S.; Gao, D.; Stein, S.; Sinko, P.J. Biodegradable poly (ethylene glycol) hydrogels based on a self-elimination degradation mechanism. Biomaterials 2010, 31, 6675–6684. [Google Scholar] [CrossRef]
- Schmedlen, R.H.; Masters, K.S.; West, J.L. Photocrosslinkable polyvinyl alcohol hydrogels that can be modified with cell adhesion peptides for use in tissue engineering. Biomaterials 2002, 23, 4325–4332. [Google Scholar] [CrossRef]
- Ossipov, D.A.; Brännvall, K.; Forsberg-Nilsson, K.; Hilborn, J. Formation of the first injectable poly (vinyl alcohol) hydrogel by mixing of functional PVA precursors. J. Appl. Polym. Sci. 2007, 106, 60–70. [Google Scholar] [CrossRef]
- Higuchi, A.; Aoki, N.; Yamamoto, T.; Miyazaki, T.; Fukushima, H.; Tak, T.M.; Jyujyoji, S.; Egashira, S.; Matsuoka, Y.; Natori, S.H. Temperature-induced cell detachment on immobilized pluronic surface. J. Biomed. Mater. Res. Part A 2006, 79, 380–392. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef]
- Matsumoto, T.; Isogawa, Y.; Tanaka, T.; Kondo, A. Streptavidin-hydrogel prepared by sortase A-assisted click chemistry for enzyme immobilization on an electrode. Biosens. Bioelectron. 2018, 99, 56. [Google Scholar] [CrossRef]
- Ito, F.; Usui, K.; Kawahara, D.; Suenaga, A.; Maki, T.; Kidoaki, S.; Suzuki, H.; Taiji, M.; Itoh, M.; Hayashizaki, Y.; et al. Reversible hydrogel formation driven by protein-peptide-specific interaction and chondrocyte entrapment. Biomaterials 2009, 31, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.; Guan, D.; Ugaz, V.; Chen, Z. Intein-triggered artificial protein hydrogels that support the immobilization of bioactive proteins. J. Am. Chem. Soc. 2013, 135, 5290. [Google Scholar] [CrossRef] [PubMed]
- Krishna, O.D.; Kiick, K.L. Protein- and peptide-modified synthetic polymeric biomaterials. Biopolymers 2010, 94, 32–48. [Google Scholar] [CrossRef]
- Pamfil, D.; Vasile, C.; Tarțău, L.; Vereștiuc, L.; Poieată, A. pH-Responsive 2-hydroxyethyl methacrylate/citraconic anhydride-modified collagen hydrogels as ciprofloxacin carriers for wound dressings. J. Bioact. Compat. Polym. 2017, 32, 355–381. [Google Scholar] [CrossRef]
- Dorwal, D. Nanogels as Novel and versatile pharmaceutics. J. Pharm. Pharm. Sci. 2012, 4, 67–74. [Google Scholar]
- Yadav, H.K.S.; Al Halabi, N.A.; Alsalloum, G.A. Nanogels as Novel Drug Delivery Systems-A Review. J. Pharm. Pharm. Res. 2017, 1, 1–8. [Google Scholar]
- Tahara, Y.; Akiyoshi, K. Current advances in self-assembled nanogel delivery systems for immunotherapy. Adv. Drug Deliv. Rev. 2015, 95, 65–76. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Siegwart, D.J.; Srinivasan, A.; Horkay, F.; Hollinger, J.O.; Washburn, N.R.; Matyjaszewski, K. Nanostructured hybrid hydrogels prepared by a combination of atom transfer radical polymerization and free radical polymerization. Biomaterials 2009, 30, 5270–5278. [Google Scholar] [CrossRef]
- Sahiner, N.; Godbey, W.T.; McPherson, G.L.; John, V.T. Microgel, nanogel and hydrogel–hydrogel semi-IPN composites for biomedical applications: Synthesis and characterization. Colloid Polym. Sci. 2006, 284, 1121–1129. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, S. Hybrid micro-/nanogels for optical sensing and intracellular imaging. Nano Rev. 2010, 1, 5730. [Google Scholar] [CrossRef]
- Lohani, A.; Singh, G.; Sankar Bhattacharya, S.; Verma, A. Interpenetrating Polymer Networks as Innovative Drug Delivery Systems. J. Drug Deliv. 2014, 2014, 583612. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Ebara, M.; Kotsuchibashi, Y.; Narain, R.; Idota, N.; Kim, Y.J.; Hoffman, J.M.; Uto, K.; Aoyagit, T. Smart Biomaterials; Springer: Tokyo, Japan, 2014; ISBN 978-4-431-54400-5. [Google Scholar]
- Amini, A.A.; Nair, L.S. Injectable hydrogels for bone and cartilage repair. Biomed. Mater. 2012, 7, 024105. [Google Scholar] [CrossRef] [PubMed]
- Jin, R. Chapter 2: In-Situ Forming Biomimetic Hydrogels for Tissue Regeneration. In Biomedicine; Lin, C., Ed.; INTECH Open Access Publisher: London, UK, 2012; pp. 35–58. [Google Scholar]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Czarnecki, S.; Rossow, T.; Seiffert, S. Hybrid Polymer-Network Hydrogels with Tunable Mechanical Response. Polymers 2016, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Lin, P.; Ma, S.; Wang, X.; Zhou, F. Molecularly engineered dual-crosslinked hydrogel with ultrahigh mechanical strength, toughness, and good self-recovery. Adv. Mater. 2015, 27, 2054–2059. [Google Scholar] [CrossRef]
- Narita, T.; Mayumi, K.; Ducouret, G.; Hébraud, P. Viscoelastic properties of poly(vinyl alcohol) hydrogels having permanent and transient cross-links studied by microrheology, classical rheometry, and dynamic light scattering. Macromolecules 2013, 46, 4174–4183. [Google Scholar] [CrossRef]
- Kondo, S.; Hiroi, T.; Han, Y.S.; Kim, T.H.; Shibayama, M.; Chung, U.I.; Sakai, T. Reliable hydrogel with mechanical “fuse link” in an aqueous environment. Adv. Mater. 2015, 27, 7407–7411. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal chemistry: Fishing for selectivity in a sea of functionality. Angew. Chem. Int. Ed. 2009, 48, 6974–6998. [Google Scholar] [CrossRef]
- Kopeček, J.; Yang, J. Smart Self-Assembled Hybrid Hydrogel Biomaterials. Angew. Chem. Int. Ed. 2012, 51, 7396–7417. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.M.D.L.; Hemar, Y.; Cornish, J.; Brimble, M.A. Structure–mechanical property correlations of hydrogel forming β-sheet peptides. Chem. Soc. Rev. 2016, 45, 4797. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension–how 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, C.; Wang, C.; Kopeček, J. Refolding hydrogels self-assembled from N-(2-hydroxypropyl)methacrylamide graft copolymers by antiparallel coiled-coil formation. Biomacromolecules 2006, 7, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.; Sun, R.; Xia, K.; Zhang, Q.; Li, W.; Wang, F.; Zhang, X.; Ge, Z.; Wang, L.; Fan, C.; et al. DNA-Based Hybrid Hydrogels Sustain Water-Insoluble Ophthalmic Therapeutic Delivery against Allergic Conjunctivitis. ACS Appl. Mater. Interfaces 2019, 11, 26704–26710. [Google Scholar] [CrossRef] [PubMed]
- Mundargi, R.C.; Patil, S.A.; Kulkarni, P.V.; Mallikarjuna, N.N.; Aminabhavi, T.M. Sequential interpenetrating polymer network hydrogel microspheres of poly(methacrylic acid) and poly(vinyl alcohol) for oral controlled drug delivery to intestine. J. Microencapsul. 2008, 25, 228–240. [Google Scholar] [CrossRef]
- Liu, X.; Guo, H.; Zha, L. Study of pH/temperature dual stimuli-responsive nanogels with interpenetrating polymer network structure. Polym. Int. 2012, 61, 1144–1150. [Google Scholar] [CrossRef]
- Blackburn, W.H.; Dickerson, E.B.; Smith, M.H.; McDonald, J.F.; Lyon, L.A. Peptide-Functionalized Nanogels for Targeted siRNA Delivery. Bioconjug. Chem. 2009, 20, 960–968. [Google Scholar] [CrossRef]
- Schachschal, S.; Balaceanu, A.; Melian, C.; Demco, D.E.; Eckert, T.; Richtering, W.; Pich, A. Polyampholyte Microgels with Anionic Core and Cationic Shell. Macromolecules 2010, 43, 4331–4339. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, R.; Tao, W.; He, H.; Shui, S. Preparation of monodisperse HPMC/PAA hybrid nanogels via surfactant-free seed polymerization. Colloid Polym. Sci. 2013, 292, 317–324. [Google Scholar] [CrossRef]
- Nayak, S.; Lee, H.; Chmielewski, J.; Lyon, L.A. Folate-Mediated Cell Targeting and Cytotoxicity Using Thermoresponsive Microgels. J. Am. Chem. Soc. 2004, 126, 10258–10259. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, E.; Blackburn, W.; Smith, M.; Kapa, L.; Lyon, L.A.; McDonald, J. Chemosensitization of cancer cells by siRNA using targeted nanogel delivery. BMC Cancer 2010, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Lapeyre, V.; Ancla, C.; Catargi, B.; Ravaine, V. Glucose-responsive microgels with a core-shell structure. J. Colloid Interface Sci. 2008, 327, 316–323. [Google Scholar] [CrossRef]
- Zieris, A.; Prokoph, S.; Levental, K.R.; Welzel, P.B.; Grimmer, M.; Freudenberg, Y.; Werner, C. FGF-2 and VEGF functionalization of starPEG–heparin hydrogels to modulate biomolecular and physical cues of angiogenesis. Biomaterials 2010, 31, 7985–7994. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Moreira Teixeira, L.S.; Krouwels, A.; Dijkstra, P.J.; van Blitterswijk, C.A.; Karperien, M.; Feijen, J. Synthesis and characterization of hyaluronic acid–poly (ethylene glycol) hydrogels via Michael addition: An injectable biomaterial for cartilage repair. Acta Biomater. 2010, 6, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Griffith, M.; Li, Z.; Tanodekaew, S.; Sheardown, H.; Hakim, M.; Carlsson, D.J. Recruitment of multiple cell lines by collagen-synthetic copolymer matrices in corneal regeneration. Biomaterials 2005, 26, 3093–3104. [Google Scholar] [CrossRef] [PubMed]
- Nistor, M.T.; Vasile, C.; Tatia, R.; Chiriac, A. Hybrid collagen/pNIPAAM hydrogel nanocomposites for tissue engineering application. Colloid Polym. Sci. 2018, 296, 1555–1571. [Google Scholar]
- Nistor, M.T.; Chiriac, A.P.; Nita, L.E.; Vasile, C. Characterization of the semi-interpenetrated network based on collagen and poly(N-isopropyl acrylamide-co-diethylene glycol diacrylate). Int. J. Pharm. 2013, 452, 92–101. [Google Scholar] [CrossRef]
- Cheaburu, C.N.; Vasile, C. Responsive freeze-drying interpolymeric associations of alginic acid and PNIPAM. II. Transition and temperature dependence on pH and composition. Cell. Chem. Technol. 2008, 42, 207–212. [Google Scholar]
- Radvar, E.; Azevedo, H.S. Supramolecular Peptide/Polymer Hybrid Hydrogels for Biomedical Applications. Macromol. Biosci. 2019, 19, 1800221. [Google Scholar] [CrossRef]
- Vandermeulen, G.W.M.; Klok, H.A. Peptide/protein hybrid materials: Enhanced control of structure and impropved performance through conjugation of biological and synthetic polymers. Macromol. Biosci. 2004, 4, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Gulrez, S.K.H.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of Preparation, Characterisation and Applications. In Progress in Molecular and Environmental Bioengineering. Analysis and Modeling to Technology Applications; Carpi, A., Ed.; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 2007, 28, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Barner-Kowollik, C. Handbook of RAFT Polymerization; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2008; ISBN 978-3-527-31924-4. [Google Scholar]
- Wang, C.; Kopeček, J.; Stewart, R.J. Hybrid hydrogels crosslinked by genetically engineered coiled-coil block proteins. Biomacromolecules 2001, 2, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Jung, G.A.; Muckom, R.J.; Glover, D.J.; Clark, D.S. Engineering bioorthogonal protein–polymer hybrid hydrogel as a functional protein immobilization platform. Chem. Commun. 2019, 55, 806–809. [Google Scholar] [CrossRef]
- Sierra-Martin, B.; Fernandez-Barbero, A. Multifunctional hybrid nanogels for theranostic applications. Soft Matter 2015, 14, 8205–8216. [Google Scholar] [CrossRef] [PubMed]
- Sanson, N.; Rieger, J. Synthesis of nanogels/microgels by conventional and controlled radical crosslinking copolymerization. Polym. Chem. 2010, 1, 96577. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef]
- Wu, H.Q.; Wang, C.C. Biodegradable smart nanogels: A new platform for targeting drug delivery and biomedical diagnostics. Langmuir 2016, 32, 6211–6225. [Google Scholar] [CrossRef]
- Karg, M. Functional Materials Design through Hydrogel Encapsulation of Inorganic Nanoparticles: Recent Developments and Challenges. Macromol. Chem. Phys. 2016, 217, 242–255. [Google Scholar] [CrossRef]
- Eslami, P.; Rossi, F.; Fedeli, S. Review Hybrid Nanogels: Stealth and Biocompatible Structures for Drug Delivery Applications. Pharmaceutics 2019, 11, 71. [Google Scholar] [CrossRef]
- Grover, G.N.; Rao, N.; Christman, K.L. Myocardial Matrix-Polyethylene Glycol Hybrid Hydrogels for Tissue Engineering. Nanotechnology 2014, 25, 014011. [Google Scholar] [CrossRef] [PubMed]
- Foster, J. Chapter 12: PEGylation and BioPEGylation of Polyhydroxyalkanoates: Synthesis, Characterisation and Applications. In Biopolymers; Elnashar, M., Ed.; IntevhOpen: Rijeka, Croatia, 2010; pp. 243–256. [Google Scholar]
- Jiang, Y.; Chen, J.; Deng, C.; Suuronen, E.J.; Zhong, Z. Click hydrogels, microgels and nanogels: Emerging platforms for drug delivery and tissue engineering. Biomaterials 2014, 35, 4969–4985. [Google Scholar] [CrossRef] [PubMed]
- Chirani, N.; Yahia, L.H.; Gritsch, L.; Motta, F.L.; Chirani, S.; Faré, S. History and Applications of Hydrogels. J. Biomed. Sci. 2015, 4, 32. [Google Scholar] [CrossRef]
- Zhou, M.; Smith, A.M.; Das, A.K.; Hodson, N.W.; Collins, R.F.; Ulijn, R.V.; Gough, J.E. Self-assembled peptide-based hydrogels as scaffolds for anchorage-dependent cells. Biomaterials 2009, 30, 2523–2530. [Google Scholar] [CrossRef]
- Palmese, L.L.; Thapa, R.K.; Sullivan, M.O.; Kiick, K.L. Hybrid hydrogels for biomedical applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157. [Google Scholar] [CrossRef]
- Bakarich, S.E.; Balding, P.; Gorkin, R.; Spinks, G.M. Printed ioniccovalent entanglement hydrogels from carrageenan and an epoxy amine. RSC Adv. 2014, 4, 38088–38092. [Google Scholar] [CrossRef]
- Tan, H.; Marra, K.G. Injectable, Biodegradable Hydrogels for Tissue Engineering Applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Weber, L.M.; Lopez, C.G.; Anseth, K.S. Effects of PEG hydrogel crosslinking density on protein diffusion and encapsulated islet survival and function. J. Biomed. Mater. Res. A 2009, 90, 720–729. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Ota, M.S.; Shimoda, A.; Nakahama, K.; Akiyoshi, K.; Miyamoto, Y.; Iseki, S. Cholesteryl group- and acryloyl group-bearing pullulan nanogel to deliver BMP2 and FGF18 for bone tissue engineering. Biomaterials 2012, 33, 7613–7620. [Google Scholar] [CrossRef]
- Shimoda, A.; Chen, Y.; Akiyoshi, K. Nanogel containing electrospun nanofibers as a platform for stable loading of proteins. RSC Adv. 2016, 6, 40811–40817. [Google Scholar] [CrossRef]
- Shimoda, A.; Sawada, S.-i.; Akiyoshi, K. Intracellular protein delivery using self-assembled amphiphilic polysaccharide nanogels. In Intracellular Delivery II: Fundamentals and Applications; Prokop, A., Iwasaki, Y., Harada, A., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 265–274. [Google Scholar]
- Molinos, M.; Carvalho, V.; Silva, D.M.; Gama, F.M. Development of a Hybrid Dextrin Hydrogel Encapsulating Dextrin Nanogel As Protein Delivery Systems. Biomacromolecules 2012, 13, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Kwon, I.K.; Park, K. Hydrogels for delivery of bioactive agents: A historical perspective. Adv. Drug Deliv. Rev. 2013, 65, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J.; Boere, K.W.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Yom-Tov, O.; Neufeld, L.; Seliktar, D.; Bianco-Peled, H. A novel design of injectable porous hydrogels with in situ pore formation. Acta Biomater. 2014, 10, 4236–4246. [Google Scholar] [CrossRef] [PubMed]
- Abebe, D.G.; Fujiwara, T. Controlled thermoresponsive hydrogels by stereocomplexed PLA-PEG-PLAprepared via hybrid micelles of premixed copolymers with different PEG lengths. Biomacromolecules 2012, 13, 1828–1836. [Google Scholar] [CrossRef]
- Sefton, M.V.; May, M.H.; Lahooti, S.; Babensee, J.E. Making microencapsulation work: Conformal coating, immobilization gels and in vivo performance. J. Control. Release 2000, 65, 173–186. [Google Scholar] [CrossRef]
- Wang, L.; Guo, S.; Dong, J.; Cui, J.; Hao, J. Microgels in biomaterials and nanomedicines. Adv. Colloid Interface Sci. 2019, 266, 1–20. [Google Scholar] [CrossRef]
- Fu, L.-H.; Qi, C.; Ma, M.-G.; Wan, P. Multifunctional cellulose-based hydrogels for biomedical applications. J. Mater. Chem. B 2019, 7, 1541–1562. [Google Scholar] [CrossRef]
- Ismail, H.; Irani, M.; Ahmad, Z. Starch-based bydrogels: Present status and applications. Int. J. Polym. Mater. Polym. Biomat. 2013, 62, 411–420. [Google Scholar] [CrossRef]
- Sıvoli, L.; Perez, E.; Caraballo, D.; Rodrıguez, J.P.; Rodrıguez, D.; Moret, J.; Sojo, F.; Arvelo, F.; Tapia, M.; Colina, M.; et al. Cytocompatibility of a matrix of methylated cassava starch and chitosan. J. Cell Plast. 2013, 49, 507–520. [Google Scholar] [CrossRef]
- Kara, S.; Tamerler, C.; Bermek, H.; Pekcan, O. Cation effects on sol-gel and gel-sol phase transitions of κ-carrageenan-water system. Int. J. Biol. Macromol. 2003, 31, 177–185. [Google Scholar] [CrossRef]
- Osmałek, T.; Froelich, A.; Tasarek, S. Application of gellan gum in pharmacy and medicine. Int. J. Pharm. 2014, 466, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Rejeena, S.R. Poly(acrylic acid-co-acrylamide-co-2-acrylamido-2-methyl-1-propanesulfonic acid)-grafted nanocellulose/poly(vinyl alcohol) composite for the in vitro gastrointestinal release of amoxicillin. J. Appl. Polym. Sci. 2014, 131, 40699. [Google Scholar] [CrossRef]
- Morales Hurtado, M.; de Vriesa, E.G.; Zeng, X.; van der Heide, E. A tribo-mechanical analysis of PVA-based building-blocks for implementation in a 2D-layered skin model. J. Mech. Behav. Biomed. Mater. 2016, 62, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Li, G.; Lv, L.; Ji, H.; Li, Z.; Chen, S.; Chu, S. Effect of DMAEMA content and polymerization mode on morphologies and properties of pH and temperature double-sensitive cellulose-based hydrogels. J. Macromol. Sci. A 2020, 57, 207–216. [Google Scholar] [CrossRef]
- Yang, J.; Han, C.R. Mechanically viscoelastic properties of cellulose nanocrystals skeleton reinforced hierarchical composite hydrogels. ACS Appl. Mater. Interfaces 2016, 8, 25621–25630. [Google Scholar] [CrossRef]
- Chen, C.; Li, D.; Abe, K.; Yano, H. Formation of high strength double-network gels from cellulose nanofiber/polyacrylamide via NaOH gelation treatment. Cellulose 2018, 25, 5089–5097. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; McMullen, L.M.; McDermott, M.T.; Deng, H.; Du, Y.; Chen, L.; Zhang, L. Stretchable, tough, self-recoverable, and cytocompatible chitosan/cellulose nanocrystals/polyacrylamide hybrid hydrogels. Carbohyd. Polym. 2019, 222, 114977. [Google Scholar] [CrossRef]
- Pandey, M.; Mohamad, N.; Amin, M.C.I.M. Bacterial cellulose/acrylamide pH-sensitive smart hydrogel: Development, characterization, and toxicity studies in ICR mice model. Mol. Pharm. 2014, 11, 3596–3608. [Google Scholar] [CrossRef]
- Ahmad, N.; Amin, M.C.I.M.; Mahali, S.M.; Ismail, I.; Chuang, V.T.G. Biocompatible and mucoadhesive bacterial cellulose-g-poly(acrylic acid) for oral protein delivery. Mol. Pharm. 2014, 11, 4130–4142. [Google Scholar] [CrossRef] [PubMed]
- Mohd Amin, M.C.I.; Ahmad, N.; Pandey, M.; Jue Xin, C. Stimuli-responsive bacterial cellulose-g-poly(acrylic acid-co-acrylamide) hydrogels for oral controlled release drug delivery. Drug Dev. Ind. Pharm. 2014, 40, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Hobzova, R.; Hrib, J.; Sirc, J.; Karpushkin, E.; Michalek, J.; Janouskova, O.; Gatenholm, P. Embedding of bacterial cellulose nanofibers within PHEMA hydrogel matrices: Tunable stiffness composites with potential for biomedical applications. J. Nanomater. 2018, 2018, 5217095. [Google Scholar] [CrossRef]
- Dai, L.; Fang, B.; Liang, C.; Zeng, R.; Tu, M.; Zhao, J. Preparation of bacterial cellulose/poly γ-glutamic acid composite hydrogels via 60Co γ-irradiation crosslinking and their properties. Polym. Mater. Sci. Eng. 2017, 33, 167–171. [Google Scholar] [CrossRef]
- Alosmanov, R.; Wolski, K.; Zapotoczny, S. Grafting of thermosensitive poly(N-isopropylacrylamide) from wet bacterial cellulose sheets to improve its swelling drying ability. Cellulose 2017, 24, 285–293. [Google Scholar] [CrossRef]
- Yang, J.Y.; Zhou, X.S.; Fang, J. Synthesis and characterization of temperature sensitive hemicellulose-based hydrogels. Carbohyd. Polym. 2011, 86, 1113–1117. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, Z.; Zhang, A.; Chen, L.; He, C.; Zhuang, X.; Chen, X. Novel thermo- and pH-responsive hydroxypropyl cellulose- and poly (l-glutamic acid)-based microgels for oral insulin controlled release. Carbohyd. Polym. 2012, 89, 1207–1214. [Google Scholar] [CrossRef]
- Li, Q.; Gong, J.; Zhang, J. Rheological properties and microstructures of hydroxyethyl cellulose/poly(acrylic acid) blend hydrogels. J. Macromol. Sci. B 2015, 54, 1132–1143. [Google Scholar] [CrossRef]
- Dutta, S.; Samanta, P.; Dhara, D. Temperature, pH and redox responsive cellulose based hydrogels for protein delivery. Int. J. Biol. Macromol. 2016, 87, 92–100. [Google Scholar] [CrossRef]
- Dahlan, N.A.; Pushpamalar, J.; Veeramachineni, A.K.; Muniyandy, S. Smart hydrogel of carboxymethyl cellulose grafted carboxymethyl polyvinyl alcohol and properties studied for future material applications. J. Polym. Environ. 2018, 26, 2061–2071. [Google Scholar] [CrossRef]
- Salama, A.; El-Sakhawy, M.; Kamel, S. Carboxymethyl cellulose based hybrid material for sustained release of protein drugs. Int. J. Biol. Macromol. 2016, 93, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, Y.H.; Ki, C.S. Fabrication of PEG-carboxymethyl cellulose hydrogel by thiol-norbornene photo-click Chemistry. Int. J. Biol. Macromol. 2016, 83, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Bang, S.; Kim, S.; Jo, S.Y.; Kim, B.-C.; Hwang, Y.; Noh, I. Synthesis and in vitro characterizations of porous carboxymethyl cellulose-poly(ethylene oxide) hydrogel film. Biomater. Res. 2015, 19, 12. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Saxena, S. Enzymatically degradable and pH-sensitive hydrogels for colon-targeted oral drug delivery. I. Synthesis and characterization. J. Appl. Polym. Sci. 2004, 92, 3630–3643. [Google Scholar] [CrossRef]
- Zhang, X.; Zhuo, R. Synthesis of temperature-sensitive poly(N- isopropylacrylamide) hydrogel with improved surface property. J. Colloid Interface Sci. 2000, 223, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.M.; Wang, G.H.; Lu, H.W. A new class of starch based hydrogels incorporating acrylamide and vinyl pyrrolidone: Effects of reaction variables on water sorption behavior. J. Bioact. Compat. Polym. 2005, 20, 2005. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Tan, Y.; Xu, K.; Lu, C.; Wang, P. In situ hydrogel constructed by starch-based nanoparticles via a Schiff base reaction. Carbohydr. Polym. 2014, 110, 87–94. [Google Scholar] [CrossRef]
- Geresh, S.; Gilboa, Y.; Peisahov-Korol, J.; Gdalevsky, G.; Voorspoels, J.; Remon, J.P.; Kost, J. Preparation and characterization of bioadhesive grafted starch copolymers as platforms for controlled drug delivery. J. Appl. Polym. Sci. 2002, 86, 1157–1162. [Google Scholar] [CrossRef]
- Zhai, M.; Yoshii, F.; Kume, T.; Hashim, K. Syntheses of PVA/Starch grafted hydrogels by irradiation. Carbohydr. Polym. 2002, 50, 295–303. [Google Scholar] [CrossRef]
- Bhuniya, S.P.; Rahman, S.; Satyanand, A.J.; Gharia, M.M.; Dave, A.M. Novel route to synthesis of allyl starch and biodegradable hydrogel by copolymerizing allyl-modified starch with methacrylic acid and acrylamide. J. Polym. Sci. A: Polym. Chem. 2003, 41, 1650–1658. [Google Scholar] [CrossRef]
- Saboktakin, M.R.; Maharramov, A.; Ramazanov, M.A. pH-sensitive starch hydrogels via free radical graft copolymerization, synthesis and properties. Carbohydr. Polym. 2009, 77, 634–638. [Google Scholar] [CrossRef]
- Seo, S.; Lee, C.S.; Jung, Y.S.; Na, K. Thermo-sensitivity and triggered drug release of polysaccharide nanogels derived from pullulan-g-poly(L-lactide) copolymers. Carbohydr. Polym. 2012, 87, 1105–1111. [Google Scholar] [CrossRef]
- Shitole, A.A.; Raut, P.W.; Khandwekar, A.; Sharma, N.; Baruah, M. Design and engineering of polyvinyl alcohol based biomimetic hydrogels for wound healing and repair. J. Polym. Res. 2019, 26, 201. [Google Scholar] [CrossRef]
- Baron, R.I.; Culica, M.E.; Biliuta, G.; Bercea, M.; Gherman, S.; Zavastin, D.; Ochiuz, L.; Avadanei, M.; Coseri, S. Physical hydrogels of oxidized polysaccharides and poly(vinyl alcohol) for wound dressing applications. Materials 2019, 12, 1569. [Google Scholar] [CrossRef]
- Masci, G.; Bontempo, D.; Crescenzi, V. Synthesis and characterization of thermoresponsive N-isopropylacrylamide/methacrylated pullulan hydrogels. Polymer 2002, 43, 5587–5593. [Google Scholar] [CrossRef]
- Asmarandei, I.; Fundueanu, G.; Cristea, M.; Harabagiu, V.; Constantin, M. Thermo- and pH sensitive interpenetrating poly(N-isopropylacrylamide)/carboxymethyl pullulan network for drug delivery. J. Polym. Res. 2013, 20, 293. [Google Scholar] [CrossRef]
- Morimoto, N.; Winnik, F.M.; Akiyoshi, K. Botryoidal assembly of cholesteryl−pullulan/poly(N-isopropylacrylamide) nanogels. Langmuir 2007, 23, 217–223. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Kang, E.-C.; Kurumada, S.; Sunamoto, J.; Principi, T.; Winnik, F.M. Controlled association of amphiphilic polymers in water: Thermosensitive nanoparticles formed by self-assembly of hydrophobically modified pullulans and poly(Nisopropylacrylamides). Macromolecules 2000, 33, 3244–3249. [Google Scholar] [CrossRef]
- Hasegawa, U.; Sawada, S.; Shimizu, T.; Kishida, T.; Otsuji, E.; Mazda, O.; Akiyoshi, K. Raspberry-like assembly of cross-linked nanogels for protein delivery. J. Control. Release 2009, 140, 312–317. [Google Scholar] [CrossRef]
- Cinay, G.E.; Erkoc, P.; Alipour, M.; Hashimoto, Y.; Sasaki, Y.; Akiyoshi, K.; Kizilel, S. Nanogel-integrated pH responsive composite hydrogels for controlled drug delivery. ACS Biomater. Sci. Eng. 2017, 3, 370–380. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Mukai, S.; Sawada, S.; Sasaki, Y.; Akiyoshi, K. Nanogel tectonic porous gel loading biologics, nanocarriers, and cells for advanced scaffold. Biomaterials 2015, 37, 107–115. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.-Y.; Wei, G.; Lu, W.-Y. Effect of carrageenan on poloxamer-based in situ gel for vaginal use: Improved in vitro and in vivo sustained-release properties. Eur. J. Pharm. Sci. 2009, 37, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ye, L.; Cui, M.; Yang, B.; Li, J.; Sun, H.; Yao, F. Physically crosslinked poly (vinyl alcohol)-carrageenan composite hydrogels: Pore structure stability and cell adhesive ability. RSC Adv. 2015, 5, 78180–78191. [Google Scholar] [CrossRef]
- Kawata, K.; Hanawa, T.; Endo, N.; Suzuki, M.; Oguchi, T. Formulation study on retinoic acid gel composed of iota-carrageenan, polyethylene oxide and Emulgen®408. Chem. Pharm. Bull. 2012, 60, 825–830. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kulkarni, R.V.; Boppana, R.; Krishna Mohan, G.; Mutalik, S.; Kalyane, N.V. pH-responsive interpenetrating network hydrogel beads of poly(acrylamide)-g-carrageenan and sodium alginate for intestinal targeted drug delivery: Synthesis, in vitro and in vivo evaluation. J. Colloid Interface Sci. 2012, 367, 509–517. [Google Scholar] [CrossRef]
- Chan, A.H.; Boughton, P.C.; Ruys, A.J.; Oyen, M.L. An interpenetrating network composite for a regenerative spinal disc application. J. Mech. Behav. Biomed. Mater. 2017, 65, 842–848. [Google Scholar] [CrossRef]
- Chen, J.; Liu, M.; Jin, S.; Liu, H. Synthesis and characterization of κ- carrageenan/poly(N,N-diethylacrylamide) semi-interpenetrating polymer network hydrogels with rapid response to temperature. Polym. Adv. Technol. 2008, 17, 395–418. [Google Scholar] [CrossRef]
- Mohanan, A.; Vishalakshi, B.; Ganesh, S. Swelling and diffusion characteristics of stimuli-responsive N-isopropylacrylamide and κ-carrageenan semi-IPN hydrogels. Int. J. Polym. Mater. Polym. Biomat. 2011, 60, 787–798. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, M.; Sun, D.; Hou, Y.; Li, Y.; Dong, T.; Wang, X.; Zhang, L.; Yang, W. Dual physically cross-linked κ-carrageenan-based double network hydrogels with superior self-healing performance for biomedical application. ACS Appl. Mater. Interfaces 2018, 10, 37544–37554. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Hooshyar, Z.; Rastgo, F. Kappa carrageenan-g-poly (acrylic acid)/SPION nanocomposite as a novel stimuli-sensitive drug delivery system. Colloid Polym. Sci. 2013, 291, 2791–2803. [Google Scholar] [CrossRef]
- De Silva, D.A.; Hettiarachchi, B.U.; Nayanajith, L.D.C.; Milani, M.D.Y.; Motha, J.T.S. Development of a PVP / kappa-carrageenan / PEG hydrogel dressing for wound healing applications in Sri Lanka. J. Natl. Sci. Found. Sri Lanka 2011, 39, 25–33. [Google Scholar] [CrossRef]
- Bakarich, S.E.; Pidcock, G.C.; Balding, P.; Stevens, L.; Calvert, P.; Panhuis, M. Recovery from applied strain in interpenetrating polymer network hydrogels with ionic and covalent cross-links. Soft Matter 2012, 8, 9985–9988. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, D.; Jeong, Y.W.; Choi, M.J.; Lee, G.W.; Thangavelu, M.; Song, J.E.; Khang, G. Engineering retinal pigment epithelial cells regeneration for transplantation in regenerative medicine using PEG/Gellan gum hydrogels. Int. J. Biol. Macromol. 2019, 130, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, D.; Hu, D.; Zhu, S.; Pan, C.; Jiao, Y.; Li, L.; Luo, B.; Zhou, C.; Lu, L. Stress-relaxing double-network hydrogel for chondrogenic differentiation of stem cells. Mater. Sci. Eng.: C 2020, 107, 110333. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Choi, O.K.; Lee, J.; Noh, J.; Lee, S.; Park, A.; Rim, M.A.; Reis, R.L.; Khang, G. Evaluation of double network hydrogel of poloxamer-heparin/gellan gum for bone marrow stem cells delivery carrier. Colloid Surf. B 2019, 181, 879–889. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Aminabhavi, T.M. Development of novel interpenetrating network gellan gum-poly(vinyl alcohol) hydrogel microspheres for the controlled release of carvedilol. Drug Dev. Ind. Pharm. 2005, 31, 491–503. [Google Scholar] [CrossRef]
- Hamcerencu, M.; Desbrieres, J.; Khoukh, A.; Popa, M.; Riess, G. Synthesis and characterization of new unsaturated esters of gellan gum. Carbohydr. Polym. 2008, 71, 92–100. [Google Scholar] [CrossRef]
- Hamcerencu, M.; Desbrieres, J.; Popa, M.; Riess, G. Thermo-sensitive gellan maleate/N-isopropylacrylamide hydrogels: Initial “in vitro” and “in vivo” evaluation as ocular inserts. Polym. Bull. 2020, 77, 741–755. [Google Scholar] [CrossRef]
- Sahraro, M.; Barikani, M.; Daemi, H. Mechanical reinforcement of Gellan gum polyelectrolyte hydrogels by cationic polyurethane soft nanoparticles. Carbohydr. Polym. 2018, 187, 102–109. [Google Scholar] [CrossRef]
- Wu, M.; Bao, B.; Yoshii, F.; Makuuchi, K. Irradiation of crosslinked, poly(vinyl alcohol) blended hydrogel for wound dressing. J. Radioanal. Nucl. Chem. 2001, 250, 391–395. [Google Scholar] [CrossRef]
- Dadoo, N.; Landry, S.B.; Bomar, J.D.; Gramlich, W.M. Synthesis and spatiotemporal modification of biocompatible and stimuli-responsive carboxymethyl cellulose hydrogels using thiol-norbornene chemistry. Macromol. Biosci. 2017, 17, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.; Bucatariu, S.; Stoica, I.; Fundueanu, G. Smart nanoparticles based on pullulan-g-poly(N-isopropylacrylamide) for controlled delivery of indomethacin. Int. J. Biol. Macromol. 2017, 94, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Jayaramudu, T.; Raghavendra, G.M.; Varaprasad, K.; Sadiku, R.; Ramam, K.; Raju, K.M. Iota-Carrageenan-based biodegradable Ag0 nanocomposite hydrogels for the inactivation of bacteria. Carbohydr. Polym. 2013, 95, 188–194. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Newsom, J.P.; Payne, K.A.; Krebs, M.D. Microgels: Modular, tunable constructs for tissue regeneration. Acta Biomater. 2019, 88, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Piras, C.C.; Slavik, P.; Smith, D.K. Self-Assembling Supramolecular Hybrid Hydrogel Beads, Supramolecular Chemistry. Angew. Chem. Int. Ed. 2020, 59, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.M.P.; Hay, L.L.; Smith, D.K. Multi-component hybrid hydrogels – understanding the extent of orthogonal assembly and its impact on controlled release. Chem. Sci. 2017, 8, 6981–6990. [Google Scholar] [CrossRef]
- Passemard, S.; Szabó, L.; Noverraz, F.; Montanari, E.; Gonelle-Gispert, C.; Bühler, L.H.; Wandrey, C.; Gerber-Lemaire, S. Synthesis Strategies to Extend the Variety of Alginate-Based Hybrid Hydrogels for Cell Microencapsulation. Biomacromolecules 2017, 18, 2747–2755. [Google Scholar] [CrossRef]
- Chaterji, S.; Kwon, I.K.; Park, K. Smart polymeric gels: Redefining the limits of biomedical devices. Prog. Polym. Sci. 2007, 32, 1083–1122. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar]
- Chen, C.; Liu, L.; Huang, T.; Wang, Q.; Fang, Y.E. Bubble template fabrication of chitosan/poly(vinyl alcohol) sponges for wound dressing applications. Int. J. Biol. Macromol. 2013, 62, 188–193. [Google Scholar] [CrossRef]
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U.B.G. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Truong, N.F.; Segura, T. Design of cell-matrix interactions in hyaluronic acid hydrogel scaffolds. Acta Biomater. 2014, 10, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.O.; Akada, Y.; Kai, W.; Kim, B.S.; Kim, I.S. Cells Attachment Property of PVA Hydrogel Nanofibers Incorporating Hyaluronic Acid for Tissue Engineering. J. Biomater. Nanobiotechnol. 2011, 2, 353–360. [Google Scholar] [CrossRef]
- Benoit, D.S.; Durney, A.R.; Anseth, K.S. The effect of heparin-functionalized PEG hydrogels on three-dimensional human mesenchymal stem cell osteogenic differentiation. Biomaterials 2007, 28, 66–77. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Skilling, K.J.; Citossi, F.; Bradshaw, T.D.; Ashford, M.; Kellam, B.; Marlow, M. Insights into low molecular mass organic gelators: A focus on drug delivery and tissue engineering applications. Soft Matter 2014, 10, 237–256. [Google Scholar] [CrossRef]
- Cho, S.H.; Oh, S.H.; Lee, J.H. Fabrication and characterization of porous alginate/polyvinyl alcohol hybrid scaffolds for 3D cell culture. J. Biomater. Sci. Polym. Ed. 2005, 16, 933–947. [Google Scholar] [CrossRef]
- Kim, J.O.; Park, J.K.; Kim, J.H.; Jin, S.G.; Yonga, C.S.; Li, D.X.; Choi, J.Y.; Woo, J.S.; Yoo, B.K.; Lyoo, W.S.; et al. Development of polyvinyl alcohol–sodium alginate gel matrix-based wound dressing system containing nitrofurazone. Int. J. Pharm. 2008, 359, 79–86. [Google Scholar] [CrossRef]
- Kim, J.O.; Choi, J.Y.; Park, J.K.; Kim, J.H.; Jin, S.G.; Chang, S.W.; Li, D.X.; Hwang, M.-R.; Woo, J.S.; Kim, J.-A.; et al. Development of Clindamycin-Loaded Wound Dressing with Polyvinyl Alcohol and Sodium Alginate. Biol. Pharm. Bull. 2008, 31, 2277–2282. [Google Scholar] [CrossRef]
- Stone, S.A.; Gosavi, P.; Athauda, T.J.; Ozer, R.R. In situ citric acid crosslinking of alginate/polyvinyl alcohol electrospun nanofibers. Mater. Lett. 2013, 112, 32–35. [Google Scholar] [CrossRef]
- Tarun, K.; Gobi, N. Calcium alginate/PVA blended nano fiber matrix for wound dressing. Indian J. Fibre Text. Res. 2012, 37, 127–132. [Google Scholar]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar]
- Kumar, A.; Lee, Y.; Kim, D.; Rao, K.M.; Kim, J.; Park, S.; Haider, A.; Lee, D.H.; Han, S.S. Effect of crosslinking functionality on microstructure, mechanical properties, and in vitro cytocompatibility of cellulose nanocrystals reinforced poly (vinyl alcohol)/sodium alginate hybrid scaffolds. Int. J. Biol. Macromol. 2017, 95, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Anjum, F.; Lienemann, P.S.; Metzger, S.; Biernaskie, J.; Kallos, M.S.; Ehrbar, M. Enzyme responsive GAG-based natural-synthetic hybrid hydrogel for tunable growth factor delivery and stem cell differentiation. Biomaterials 2016, 87, 104–117. [Google Scholar] [CrossRef]
- Zhao, L.; Gwon, H.-J.; Lim, Y.-M.; Nho, Y.-C.; Kim, S.Y. Hyaluronic acid/chondroitin sulfate-based hydrogel prepared by gamma irradiation technique. Carbohydr. Polym. 2014, 102, 598–605. [Google Scholar] [CrossRef]
- Huang, X.; Brazel, C.S. Review On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- El-Salmawi, K.M. Gamma radiation-induced crosslinked PVA/chitosan blends for wound dressing. J. Macromol. Sci. Part A: Pure Appl. Chem. 2007, 44, 541–545. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Q.; Chen, X.; Yu, F.; Zhu, Z. Investigation of PVA/ws-chitosan hydrogels prepared by combined gama-irradiation and freeze–thawing. Carbohydr. Polym. 2008, 73, 401–408. [Google Scholar] [CrossRef]
- Yang, X.; Yang, K.; Wu, S.; Chen, X.; Yu, F.; Li, J.; Ma, M.; Zhu, Z. Cytotoxicity and wound healing properties of PVA/ws Chitosan/glycerol hydrogels made by irradiation followed by freeze–thawing. Radiat. Phys. Chem. 2010, 79, 606–611. [Google Scholar] [CrossRef]
- Jiang, Y.; Meng, X.; Wu, Z.; Qi, X. Modified chitosan thermosensitive hydrogel enables sustained and efficient anti-tumor therapy via intratumoral injection. Carbohydr. Polym. 2016, 144, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Su, W.Y.; Leu, T.L.; Yang, M.C. Evaluation of chitosan/PVA blended hydrogel membranes. J. Membr. Sci. 2004, 236, 39–51. [Google Scholar] [CrossRef]
- Sung, J.H.; Hwang, M.R.; Kim, J.O.; Lee, J.H.; Kim, Y.I.; Kim, J.H.; Change, S.W.; Jin, S.G.; Kim, J.A.; Lyoo, W.S.; et al. Gel characterization and in vivo evaluation of minocycline-loaded wound dressing with enhanced wound healing using polyvinyl alcohol and chitosan. Int. J. Pharm. 2010, 392, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Zhang, Y.; Zhao, X.; Shan, C.; Zu, S.; Wang, K.; Li, Y.; Ge, Y. Preparation and Characterization of Chitosan–polyvinyl Alcohol Blend Hydrogels for the Controlled Release of Nano-Insulin. Int. J. Biol. Macromol. 2012, 50, 82–87. [Google Scholar] [CrossRef]
- Charernsriwilaiwat, N.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Electrospun Chitosan/polyvinyl Alcohol Nanofibre Mats for Wound Healing. Int. Wound J. 2014, 11, 215–222. [Google Scholar] [CrossRef]
- Shamloo, A.; Sarmadi, M.; Aghababaie, Z.; Vossoughi, M. Accelerated full-thickness wound healing via sustained bFGF delivery based on a PVA/chitosan/gelatin hydrogel incorporating PCL microspheres. Int. J. Pharm. 2018, 537, 278–289. [Google Scholar] [CrossRef]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and Characterization of Chitosan/gelatin/PVA Hydrogel for Wound Dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef]
- Tsai, R.-Y.; Kuo, T.-Y.; Hung, S.-C.; Lin, C.-M.; Hsien, T.-Y.; Wang, D.-M.; Hsieh, H.-J. Use of Gum Arabic to Improve the Fabrication of Chitosan–gelatin-based Nanofibers for Tissue Engineering. Carbohydr. Polym. 2015, 115, 525–532. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Garcia-Carvajal, Z.; Jimenez-Palomar, I.; Jimenez-Avalos, J.; Espinosa-Andrews, H. Development of Gelatin/chitosan/PVA Hydrogels: Thermal stability, water state, viscoelasticity, and cytotoxicity assays. J. Appl. Polym. Sci. 2018, 136, 47149. [Google Scholar] [CrossRef]
- Khan, F.; Tare, R.S.; Oreffo, R.O.C.; Bradley, M. Versatile biocompatible polymer hydrogels: Scaffolds for cell growth. Angew. Chem. Int. Ed. 2009, 48, 978–982. [Google Scholar] [CrossRef]
- Bhattarai, N.; Ramay, H.R.; Gunn, J.; Matsen, F.A.; Zhang, M.Q. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J. Control. Release 2005, 103, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Hong, Y.; Gong, Y.; Gao, C.; Shen, J. Collagen-coated polylactide microcarriers/chitosan hydrogel composite: Injectable scaffold for cartilage regeneration. J. Biomed. Mater. Res. Part A 2008, 85, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Moztarzadeh, F.; Mohandesi, J.A. Investigating the effect of chitosan on hydrophilicity and bioactivity of conductive electrospun composite scaffold for neural tissue engineering. Int. J. Biol. Macromol. 2018, 121, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Elcin, Y.M.; Dixit, V.; Gitnick, G. Hepatocyte attachment on biodegradable modified chitosan membranes: In vitro evaluation for the development of liver organoids. Artif. Organs 1998, 22, 837–846. [Google Scholar] [CrossRef]
- Zhao, L.; Mitomo, H.; Zhai, M.; Yoshii, F.; Nagasawa, N.; Kume, T. Synthesis of Antibacterial PVA/CM-chitosan Blend Hydrogels with Electron Beam Irradiation. Carbohydr. Polym. 2003, 53, 439–446. [Google Scholar] [CrossRef]
- Afshari, M.J.; Sheikh, N.; Afarideh, H. PVA/CM-chitosan/honey hydrogels prepared by using the combined technique of irradiation followed by freeze-thawing. Radiat. Phys. Chem. 2015, 113, 28–35. [Google Scholar] [CrossRef]
- Xiao, C.; Zhou, G. Synthesis and properties of degradable poly(vinyl alcohol) hydrogel. Polym. Degrad. Stab. 2003, 81, 297–301. [Google Scholar] [CrossRef]
- Ignatova, M.; Starbova, K.; Manolova, N.; Rashkov, I. Electrospun nano-fibre mats with antibacterial properties from quaternized chitosan and poly(vinyl alcohol). Carbohydr. Res. 2006, 341, 2098–2107. [Google Scholar] [CrossRef]
- Zhao, X.; Li, P.; Guo, B.; Ma, P.X. Antibacterial and conductive injectable hydrogels based on quaternized chitosan-graft-polyaniline/oxidized dextran for tissue engineering. Acta Biomater. 2015, 26, 236–248. [Google Scholar] [CrossRef]
- Fan, L.; Yang, J.; Wu, H.; Hu, Z.; Yi, J.; Tong, J.; Zhu, X. Preparation and Characterization of Quaternary Ammonium Chitosan Hydrogel with Significant Antibacterial Activity. Int. J. Biol. Macromol. 2015, 79, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Cui, Z.K.; Kim, P.J.; Jung, L.Y.; Lee, M. Design of hydrogels to stabilize and enhance bone morphogenetic protein activity by heparin mimetics. Acta Biomater. 2018, 72, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.X.; Liu, F.Y.; Cheng, J.; Gu, J.X.; Dan, S.; Liu, C.Y.; Qu, X.Z.; Yang, Z.Z. Dually responsive injectable hydrogel prepared by in situ cross-linking of glycol chitosan and benzaldehyde-capped PEO-PPO-PEO. Biomacromolecules 2010, 11, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Lee, S.Y.; Joung, Y.K.; Na, J.S.; Lee, M.C.; Park, K.D. Thermosensitive chitosan–Pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomater. 2009, 5, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Choi, W.I.; Kim, Y.H.; Tae, G. Brain-targeted delivery of protein using chitosan- and RVG peptide-conjugated, pluronic-based nano-carrier. Biomaterials 2013, 34, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Yoo, H.S. Pluronic/chitosan hydrogels containing epidermal growth factor with wound-adhesive and photo-crosslinkable properties. J. Biomed. Mater. Res. Part A 2010, 95, 564–573. [Google Scholar] [CrossRef]
- Farahani, B.V.; Ghasemzaheh, H.; Afraz, S. Intelligent semi-IPN chitosan-PEG-PAAm hydrogel for closed-loop insulin delivery and kinetic modeling. RSC Adv. 2016, 6, 26590–26598. [Google Scholar] [CrossRef]
- Li, Q.; Williams, C.G.; Sun, D.D.N.; Wang, J.; Leong, K.; Elisseeff, J.H.J. Photocrosslinkable polysaccharides based on chondroitin sulfate. Biomed. Mater. Res. 2004, 68, 28–33. [Google Scholar] [CrossRef]
- Zhang, C.; Dong, Q.; Liang, K.; Zhou, D.; Yang, H.; Liu, X.; Xu, W.; Zhou, Y.; Xiao, P. Photopolymerizable thiol-acrylate maleiated hyaluronic acid/thiol-terminated poly(ethylene glycol) hydrogels as potential in-situ formable scaffolds. Int. J. Biol. Macromol. 2018, 119, 270–277. [Google Scholar] [CrossRef]
- Sharma, B.; Williams, C.G.; Khan, M.; Manson, P.; Elisseeff, J.H. In vivo Chondrogenesis of Mesenchymal Stem Cells in a Photopolymerized Hydrogel. Plast. Reconstr. Surg. 2007, 119, 112–120. [Google Scholar] [CrossRef]
- Bae, K.H.; Yoon, J.J.; Park, T.G. Fabrication of Hyaluronic Acid Hydrogel Beads for Cell Encapsulation. Biotechnol. Prog. 2006, 22, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, S.; Sivashanmugam, A.; Mohandas, A.; Janarthanan, R.; Iyer, S.; Nair, S.V.; Jayakumar, R. Injectable deferoxamine nanoparticles loaded chitosan–hyaluronic acid coacervate hydrogel for therapeutic angiogenesis. Colloids Surf. B Biointerfaces 2018, 161, 129–138. [Google Scholar]
- Na, K.; Kim, S.; Woo, D.G.; Sun, B.K.; Yang, H.N.; Chung, H.M.; Park, K.H. Combination material delivery of dexamethasone and growth factor in hydrogel blended with hyaluronic acid constructs for neocartilage formation. J. Biomed. Mater. Res. Part A 2007, 83, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Leone, G.; Consumi, M.; Lamponi, S.; Bonechi, C.; Tamassi, G.; Donati, A.; Rossi, C.; Magnani, A. Hybrid PVA-xanthan gum hydrogels as nucleus pulposus substitutes. Int. J. Polym. Materi. Polym. Biomater. 2018, 68, 681–690. [Google Scholar] [CrossRef]
- Elizalde-Peña, E.A.; Zarate-Triviño, D.G.; Nuño-Donlucas, S.M.; Medina-Torres, L.; Gough, J.E.; Sanchez, I.C.; Villaseñor, F.; Luna-Barcenas, G. Synthesis and characterization of a hybrid (chitosan-g-glycidyl methacrylate)–xanthan hydrogel. J. Biomater. Sci. Polym. Ed. 2013, 24, 1426–1442. [Google Scholar] [CrossRef]
- Shah, S.S.; Kim, M.; Cahill-Thompson, K.; Tae, G.; Revzin, A. Micropatterning of bioactive heparin-based hydrogels. Soft Matter 2011, 7, 3133–3140. [Google Scholar] [CrossRef]
- Kim, M.; Shin, Y.; Hong, B.-H.; Kim, Y.-J.; Chun, J.-S.; Tae, G.; Kim, Y.H. In vitro Chondrocyte Culture in a Heparin-Based Hydrogel for Cartilage Regeneration. Tissue Eng. Part C: Meth. 2009, 16, 1–10. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.Y.; Jones, C.N.; Revzin, A.; Tae, G. Heparin-based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes. Biomaterials 2010, 31, 3596–3603. [Google Scholar] [CrossRef]
- Goh, M.C.; Hwang, Y.; Tae, G. Epidermal growth factor loaded heparin-based hydrogel sheet for skin wound healing. Carbohydr. Polym. 2016, 147, 251–260. [Google Scholar] [CrossRef]
- Xu, H.-L.; Tian, F.-R.; Xiao, J.; Chen, P.-P.; Xu, J.; Fan, Z.-L.; Yang, J.-J.; Lu, C.-T.; Zhao, Y.-Z. Sustained-release of FGF-2 from a hybrid hydrogel of heparin-poloxamer and decellular matrix promotes the neuroprotective effects of proteins after spinal injury. Int. J. Nanomed. 2018, 13, 681–694. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu, A.A.; Sheikh, J. A review on chitosan centred scaffolds and their applications in tissue engineering. Int. J. Biol. Macromol. 2018, 116, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.G.; Camargo, S.E.A.; Bishop, T.T.; Popat, K.C.; Kipper, M.J.; Martins, A.F. Pectin-chitosan membrane scaffold imparts controlled stem cell adhesion and proliferation. Carbohydr. Polym. 2018, 197, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Patil, R.; Bahadur, P. Polysaccharide based scaffolds for soft tissue engineering applications. Polymers 2019, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional Hydrogels With Tunable Structures and Properties for Tissue Engineering Applications. Front. Chem. 2018, 6, 499. [Google Scholar] [CrossRef]
- Qasim, S.B.; Husain, S.; Huang, Y.; Pogorielov, M.; Deineka, V.; Lyndin, M.; Rawlinson, A.; Rehman, I.U. In vitro and in vivo Degradation Studies of Freeze Gelated Porous Chitosan Composite Scaffolds for Tissue Engineering Applications. Polym. Degrad. Stab. 2017, 136, 31–38. [Google Scholar] [CrossRef]
- Pok, S.; Myers, J.D.; Madihally, S.V.; Jacot, J.G. A multilayered scaffold of a chitosan and gelatin hydrogel supported by a PCL core for cardiac tissue engineering. Acta Biomater. 2013, 9, 5630–5642. [Google Scholar] [CrossRef]
- Negishi, J.; Nam, K.; Kimura, T.; Hashimoto, Y.; Funamoto, S.; Higami, T.; Fujisato, S.; Kishida, A. Fabrication of a heparin–PVA complex hydrogel for application as a vascular access. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1426–1433. [Google Scholar] [CrossRef]
- Homandberg, G.A.; Hui, F.; Wen, C.; Kuettner, K.E.; Williams, J.M. Hyaluronic acid suppresses fibronectin fragment mediated cartilage chondrolysis: I. In vitro. Osteoarthr. Cartil. 1997, 5, 309–319. [Google Scholar] [CrossRef]
- Shimazu, A.; Jikko, A.; Iwamoto, M.; Koike, T.; Yan, W.; Okada, Y.; Shinmei, M.; Nakamura, S.; Kato, Y. Effects of hyaluronic acid on the release of proteoglycan from the cell matrix in rabbit chondrocyte cultures in the presence and absence of cytokines. Arthritis Rheum. 1993, 36, 247–253. [Google Scholar] [CrossRef]
- Morris, E.A.; Wilcon, S.; Treadwell, B.V. Inhibition of interleukin 1-mediated proteoglycan degradation in bovine articular cartilage explants by addition of sodium hyaluronate. Am. J. Vet. Res. 1992, 53, 1977–1982. [Google Scholar]
- Balakrishnan, B.; Banerjee, R. Biopolymer-Based Hydrogels for Cartilage Tissue Engineering. Chem. Rev. 2011, 111, 4453–4474. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Hu, Y.; Zhang, C.; Li, X.; Lv, R.; Qin, L.; Zhu, R. Cartilage regeneration using mesenchymal stem cells and a PLGA-gelatin/chondroitin/hyaluronate hybrid scaffold. Biomaterials 2006, 27, 4573–4580. [Google Scholar] [CrossRef] [PubMed]
- Bichara, D.A.; Zhao, X.; Hwang, N.S.; Bodugoz-Senturk, H.; Yaremchuk, M.J.; Randolph, M.A.; Muratoglu, O.K. Porous Poly(vinyl alcohol)-Alginate Gel Hybrid Construct for Neocartilage Formation Using Human Nasoseptal Cells. J. Surg. Res. 2010, 163, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Kunisch, E.; Knauf, A.-K.; Hesse, E.; Freudenberg, U.; Werner, C.; Bothe, F. StarPEG/heparin-hydrogel based in vivo engineering of stable bizonal cartilage with a calcified bottom layer. Biofabrication 2018, 11, 015001. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ramirez, C.M.; Miljkovic, N.; Li, H.; Rubin, J.P.; Marra, K.G. Thermosensitive injectable hyaluronic acid hydrogel for adipose tissue engineering. Biomaterials 2009, 30, 6844–6853. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-K.; Chen, K.-H.; Kuan, C.-Y. The synthesis and characterization of a thermally responsive hyaluronic acid/Pluronic copolymer and an evaluation of its potential as an artificial vitreous substitute. J. Bioact. Compat. Pol. 2013, 28, 355–367. [Google Scholar] [CrossRef]
- Liu, Y.; Hsu, S. Synthesis and Biomedical Applications of Self-healing Hydrogels. Front. Chem. 2018, 6, 449. [Google Scholar] [CrossRef]
- Tseng, T.C.; Tao, L.; Hsieh, F.Y.; Wei, Y.; Chiu, I.M.; Hsu, S.H. An injectable, self-healing hydrogel to repair the central nervous system. Adv. Mater. 2015, 27, 3518–3524. [Google Scholar] [CrossRef]
- Hsieh, F.-Y.; Tao, L.; Wei, Y.; Hsu, S.H. A novel biodegradable self healing hydrogel to induce blood capillary formation. Npg Asia Mater. 2017, 9, e363. [Google Scholar] [CrossRef]
- Fares, M.M.; Sani, E.S.; Lara, R.P.; Oliveira, R.B.; Khademhosseini, A.; Annabi, N. Interpenetrating network gelatin methacryloyl (GelMA) and pectin-g-PCL hydrogels with tunable properties for tissue engineering. Biomater. Sci. 2018, 6, 2938–2950. [Google Scholar] [CrossRef]
- Niu, R.; Qin, Z.; Ji, F.; Xu, M.; Tian, X.; Li, J.; Yao, F. Hybrid pectin-Fe3+/polyacrylamide double network hydrogels with excellent strength, high stiffness, superior toughness and notch-insensitivity. Soft Matter 2017, 13, 9237–9245. [Google Scholar] [CrossRef] [PubMed]
- Temenoff, J.S.; Mikos, A.G. Injectable biodegradable materials for orthopedic tissue engineering. Biomaterials 2000, 21, 2405–2412. [Google Scholar] [CrossRef]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8, 1–16. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef] [PubMed]
- Chircov, C.; Grumezescu, A.M.; Bejenaru, L.E. Hyaluronic acid-based scaffolds for tissue engineering. Rom. J. Morphol. Embryol. 2018, 59, 71–76. [Google Scholar] [PubMed]
- Baican, M.; Vasile, C. Chitosan containing biomaterials for tissue engineering applications. In Advances in Polymers for Biomedical Applications; Deepak, P., Bhuvanesh, G., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2018; pp. 221–279. [Google Scholar]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, A.; Kamoun, E.A.; El-Eisawy, R.; El-Fakharany, E.M.; Taha, T.H.; El-Damhougy, B.K.; Abdelhai, F. Poly(vinyl alcohol)-hyaluronic acid membranes for wound dressing applications: Synthesis and in vitro bio-evaluations. J. Braz. Chem. Soc. 2015, 26, 1466–1474. [Google Scholar] [CrossRef]
- Phuc, D.H.; Hiep, N.T.; Phuc Chau, N.D.; Hoai, N.T.T.; Khon, H.C.; Toi, V.V.; Hai, N.D.; Bao, B.C. Fabrication of hyaluronan-poly(vinylphosphonic acid)-chitosan hydrogel for wound healing application. Int. J. Polym. Sci. 2016, 2016, 6723716. [Google Scholar]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef]
- Wu, J.; Wei, W.; Wang, L.Y.; Su, Z.G.; Ma, G.H. A thermosensitive hydrogel based on quaternized chitosan and poly(ethylene glycol) for nasal drug delivery system. Biomaterials 2007, 28, 2220–2232. [Google Scholar] [CrossRef] [PubMed]
- Dyer, A.M.; Hinchcliffe, M.; Watts, P.; Castile, J.; Jabbal-Gill, I.; Nankervis, R.; Smith, A.; Illum, L. Nasal delivery of insulin using novel chitosan based formulations: A comparative study in two animal models between simple chitosan formulations and chitosan nanoparticles. Pharm. Res. 2002, 19, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Buranachai, T.; Praphairaksit, N.; Muangesin, N. Chitosan/polyethylene glycol beads crosslinked with tripolyphosphate and glutaraldehyde for gasterointestinal drug delivery. AAPS Pharm. Sci. Tech. 2010, 11, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Hassani, N.A.; Abdouss, M.; Faghihi, S. Synthesis and evaluation of PEG-O-chitosan nanoparticles for delivery of poor water soluble drugs: Lbuprofen. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 41, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Yoshida, C.; Murakami, Y. Design of novel sheet-shaped chitosan hydrogel for wound healing: A hybrid biomaterial consisting of both PEG-grafted chitosan and crosslinkable polymeric micelles acting as drug containers. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3697–3703. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.; Kurisawa, M.; Chung, J.E.; Yang, Y.Y. Synthesis and characterization of chitosan-g-poly (ethylene glycol)-folate as a non-viral carrier for tumor-targeted gene delivery. Biomaterials 2007, 28, 540–549. [Google Scholar] [CrossRef]
- Patel, V.; Amiji, M. Preparation and characterization of freeze-dried chitosan-poly (ethylene oxide) hydrogels for site-specific antibiotic delivery in the stomach. Pharm. Res. 1996, 13, 588–593. [Google Scholar] [CrossRef]
- Gong, S.; Tu, H.; Zheng, H.; Xu, H.; Yin, Y. Chitosan-g-PAA hydrogels for colon-specific drug delivery: Preparation, swelling behavior and in vitro degradability. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2010, 25, 248–251. [Google Scholar] [CrossRef]
- Zhou, T.; Xiao, C.; Fan, J.; Chen, S.; Shen, J.; Wu, W.; Zhou, S. A nanogel of on-site tunable pH-response for efficient anticancer drug delivery. Acta Biomater. 2013, 9, 4546–4557. [Google Scholar] [CrossRef]
- Åhlén, M.; Tummala, G.K.; Mihranyan, A. Nanoparticle-loaded hydrogels as a pathway for enzymetriggered drug release in ophthalmic applications. Int. J. Pharm. 2018, 536, 73–81. [Google Scholar]
- Jamal, A.; Shahzadi, L.; Ahtzaz, S.; Zahid, S.; Chaudhry, A.A.; Rehman, I.U.; Yar, M. Identification of anti-cancer potential of doxazocin: Loading into chitosan based biodegradable hydrogels for on-site delivery to treat cervical cancer. Mater. Sci. Eng. C. Mater. Biol. Appl. 2018, 82, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Yasin, T. Controlled Delivery of Drug from pH Sensitive Chitosan/poly (vinyl Alcohol) Blend. Int. J. Biol. Macromol. 2012, 88, 1055–1060. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.S.; Gou, Y.Z.; Wang, X.; Zhao, X.M.; Tao, L. Improving tumor chemotherapy effect using an injectable self-healing hydrogel as drug carrier. Polym. Chem. 2017, 8, 3071–3076. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, D.; Yan, H.; Tao, L.; Wei, Y.; Li, Y.; Wang, X.; Zhao, W.; Zhang, Y.; Zhao, L.; et al. An injectable ionic hydrogel inducing high temperature hyperthermia for microwave tumor ablation. J. Mater. Chem. B 2017, 5, 4110–4120. [Google Scholar] [CrossRef]
- Luckanagul, J.A.; Pitakchatwong, C.; Bhuket, P.R.N.; Muangnoi, C.; Rojsitthisak, P.; Chirachanchai, S.; Wang, Q.; Rojsitthisak, P. Chitosan-based polymer hybrids for thermo-responsive nanogel delivery of curcumin. Carbohydr. Polym. 2018, 181, 1119–1127. [Google Scholar] [CrossRef]
- Luo, Z.; Xue, K.; Zhang, X.; Lim, J.Y.C.; Lai, X.; Young, D.J.; Zhang, Z.-X.; Wu, Y.-L.; Loh, X.J. Thermogelling chitosan-based polymers for the treatment of oral mucosa ulcers. Biomater. Sci. 2020, 8, 1364–1379. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cha, M.-J.; Lim, K.S.; Kim, J.-K.; Lee, S.-K.; Kim, Y.-H.; Hwang, K.-C.; Lee, K.Y. Injectable microsphere/hydrogel hybrid system containing heat shock protein as therapy in a murine myocardial infarction model. J. Drug Target. 2013, 21, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, R.P.; Oprea, A.M.; Vasile, C. A drug delivery system based on stimuli-responsive alginate/N-isopropylacryl amide hydrogel. Cell. Chem. Technol. 2009, 43, 251–262. [Google Scholar]
- Chen, Y.-Y.; Wu, H.-C.; Sun, J.-S.; Dong, G.-C.; Wang, T.-W. Injectable and thermoresponsive self-assembled nanocomposite hydrogel for long-term anticancer drug delivery. Langmuir 2013, 29, 3721–3729. [Google Scholar] [CrossRef]
- Fujita, S.; Hara, S.; Hosono, A.; Sugihara, S.; Uematsu, H.; Suye, S. Hyaluronic Acid Hydrogel Crosslinked with Complementary DNAs. Hindawi Adv. Polym. Technol. 2020, 2020, 1470819. [Google Scholar] [CrossRef]
- Roberts, J.J.; Farrugia, B.L.; Green, R.A.; Kovacina, J.R.; Martens, P.J. In situ formation of poly(vinyl alcohol)–heparin hydrogels for mild encapsulation and prolonged release of basic fibroblast growth factor and vascular endothelial growth factor. J. Tissue Eng. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Ji, H.; Qian, Y.; Wang, Q.; Liu, X.; Zhao, W.; Zhao, C. Heparin-based and heparin-inspired hydrogels: Size-effect, gelation and biomedical applications. J. Mater. Chem. B 2019, 7, 1186–1208. [Google Scholar] [CrossRef]
- Li, J.; Chen, G.; Xu, X.; Abdou, P.; Jiang, Q.; Shi, D.; Gu, Z. Advances of injectable hydrogel-based scaffolds for cartilage regeneration. Regen. Biomater. 2019, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Sun, X.; Chen, J.; Lei, Y. Natural or Natural-Synthetic Hybrid Polymer-Based Fluorescent Polymeric Materials for Bio-imaging-Related Applications. Appl. Biochem. Biotechnol. 2017, 183, 461–487. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tang, Z.; Zhu, L.; Lu, S.; Chen, F.; Tang, C.; Sun, H.; Yang, J.; Qin, G.; Chen, Q. Natural protein-based hydrogels with high strength and rapid self-recovery. Int. J. Biol. Macromol. 2019, 141, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cai, Z.; Wei, Q.; Wang, D.; Wu, J.; Tan, Y.; Lu, J.; Ai, H. Proanthocyanidin-crosslinked collagen/konjac glucomannan hydrogel with improved mechanical properties and MRI trackable biodegradation for potential tissue engineering scaffolds. Mater. Chem. B 2020, 8, 316–331. [Google Scholar] [CrossRef]
- Eslahi, N.; Simchi, A.; Mehrjoo, M.; Shokrgozar, M.A.; Bonakdar, S. Hybrid cross-linked hydrogels based on fibrous protein/block copolymers and layered silicate nanoparticles: Tunable thermosensitivity, biodegradability and mechanical durability. RSC Adv. 2016, 6, 62944. [Google Scholar] [CrossRef]
- Pamfil, D.; Schick, C.; Vasile, C. New Hydrogels Based on Substituted Anhydride Modified Collagen and 2-Hydroxyethyl Methacrylate. Synthesis and Characterization. Ind. Eng. Chem. Res. 2014, 53, 11239–11248. [Google Scholar] [CrossRef]
- Lotz, C.; Schmid, F.F.; Oechsle, E.; Monaghan, M.; Walles, H.; Groeber-Becker, F.K. A Crosslinked Collagen Hydrogel Matrix Resisting Contraction to Facilitate Full-thickness Skin Equivalents. ACS Appl. Mater. Interfaces 2017, 9, 20417–20425. [Google Scholar] [CrossRef]
- Dinescu, S.; Albu Kaya, M.; Chitoiu, L.; Ignat, S.; Kaya, D.A.; Costache, M. Collagen-Based Hydrogels and Their Applications for Tissue Engineering and Regenerative Medicine. In Cellulose-Based Superabsorbent Hydrogels. Polymers and Polymeric Composites: A Reference Series; Mondal, M., Ed.; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Ucar, B.; Humpel, C. Collagen for brain repair: Therapeutic perspectives. Neural. Regen. Res. 2018, 13, 595–598. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Farris, S.; Song, J.; Huang, Q. Alternative Reaction Mechanism for the Cross-Linking of Gelatin with Glutaraldehyde. J. Agric. Food Chem. 2010, 58, 998–1003. [Google Scholar] [CrossRef]
- Nagarajan, S.; Radhakrishnan, S.; Kalkura, S.N.; Balme, S.; Miele, P.; Bechelany, M. Overview of Protein-Based Biopolymers for Biomedical Application. Macromol. Chem. Phys. 2019, 220, 1900126. [Google Scholar] [CrossRef]
- Ni, N.; Dumont, M.-J. Protein-Based Hydrogels Derived from Industrial Byproducts Containing Collagen, Keratin, Zein and Soy. Waste Biomass Valor. 2017, 8, 285–300. [Google Scholar] [CrossRef]
- Neves, N.M.; Reis, R.L. Biomaterials from Nature for Advanced Devices and Therapies; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- de Guzman, R.C.; Merrill, M.R.; Richter, J.R.; Hamzi, R.I.; Greengauz-Roberts, O.K.; Van Dyke, M.E. Mechanical and biological properties of keratose biomaterials. Biomaterials 2011, 32, 8205–8217. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.J.; Hyzy, S.L.; Haque, S.; Olson, L.C.; Boyan, B.D.; Saul, J.M.; Schwartz, Z. Effects of Tunable Keratin Hydrogel Erosion on rhBMP-2 Release, Bioactivity, and Bone Induction. Tissue Eng. Part A 2018, 24, 1616–1630. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, P.; Gupta, A.; Sharma, S. Synthesis of wound-healing keratin hydrogels using chicken feathers proteins and its properties. Int. J. Pharm. Sci. 2017, 9, 171–178. [Google Scholar] [CrossRef]
- Ma, X.; Sun, X.; Hargrove, D.; Chen, J.; Song, D.; Dong, Q.; Lu, X.; Fan, T.; Fu, Y.; Lei, Y. A Biocompatible and Biodegradable Protein Hydrogel with Green and Red Autofluorescence: Preparation, Characterization and In vivo Biodegradation Tracking and Modeling. Sci. Rep. 2016, 6, 19370. [Google Scholar] [CrossRef]
- Pandey, V.; Haider, T.; Jain, P.; Gupta, P.N.; Soni, V. Silk as a leading-edge biological macromolecule for improved drug delivery. J. Drug Deliv. Sci. Technol. 2020, 55, 101294. [Google Scholar] [CrossRef]
- Liu, B.; Song, Y.W.; Jin, L.; Wang, Z.J.; Pu, D.Y.; Lin, S.Q.; Zhou, C.; You, H.J.; Ma, Y.; Li, J.M.; et al. Silk structure and degradation. Colloids Surf. B Biointerfaces 2015, 131, 122–128. [Google Scholar] [CrossRef]
- Li, Z.; Song, J.; Zhang, J.; Hao, K.; Liu, L.; Wu, B.; Zheng, X.; Xiao, B.; Tong, X.; Dai, F. Topical application of silk fibroin-based hydrogel in preventing hypertrophic scars. Colloids Surf. B Biointerfaces 2020, 185, 110735. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Wu, F.; Xing, T.; Yadavalli, V.K.; Kunduc, S.C.; Lu, S. A silk fibroin hydrogel with reversible sol–gel transition. RSC Adv. 2017, 7, 24085. [Google Scholar] [CrossRef]
- Xu, M. Development of protein based hydrogels as encapsulation matrices for Lactobacillus casei ATCC 393. Master’s Thesis, McGill University, Macdonald Campus, Montreal, QC, Canada, 2015. Available online: https://escholarship.mcgill.ca/concern/theses/c247dw09t?locale=en (accessed on 24 February 2020).
- Nagarkar, S.; Nicolai, T.; Chassenieux, C.; Lele, A. Structure and gelation mechanism of silk hydrogels. Phys. Chem. Chem. Phys. 2010, 12, 3834–3844. [Google Scholar] [CrossRef] [PubMed]
- Abascal, N.C.; Regan, L. The past, present and future of protein based materials. Open Biol. 2018, 8, 180113. [Google Scholar] [CrossRef]
- Wong, D.C.; Pearson, R.D.; Elvin, C.M.; Merritt, D.J. Expression of the rubber-like protein, resilin, in developing and functional insect cuticle determined using a Drosophila anti-rec 1 resilin antibody. Dev. Dyn. 2012, 241, 333–339. [Google Scholar] [CrossRef]
- de Castro, R.J.S.; Domingues, M.A.F.; Ohara, A.; Okuro, P.K.; dos Santos, J.G.; Brexó, R.P.; Sato, H.H. Whey protein as a key component in food systems: Physicochemical properties, production technologies and applications. Food Struct. 2017, 14, 17–29. [Google Scholar] [CrossRef]
- Tansaz, S.; Boccaccini, A.R. Biomedical applications of soy protein: A brief overview. Mater. Res. Part A 2016, 104, 553–569. [Google Scholar] [CrossRef]
- Tian, K.; Shao, Z.; Chen, X. Natural electroactive hydrogel from soy protein isolation. Biomacromolecules 2010, 11, 3638–3643. [Google Scholar] [CrossRef]
- Lin, H.; Hsieh, F.; Tseng, C.; Hsu, S. Preparation and characterization of biodegradable polyurethane hydrogel and the hybrid gel with soy protein for 3D cell-laden bioprinting. J. Mater. Chem. B 2016, 4, 6694–6705. [Google Scholar] [CrossRef]
- Meikle, S.T.; Standen, G.; Salvage, J.; De Santis, R.; Nicolais, L.; Ambrosio, L.; Santin, M. Synthesis and Characterization of Soybean-Based Hydrogels with an Intrinsic Activity on Cell Differentiation. Tissue Eng. Part A 2012, 18, 1932–1939. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. Recent Advances in Edible Polymer Based Hydrogels as a Sustainable Alternative to Conventional Polymers. J. Agric. Food Chem. 2018, 66, 6940–6967. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kong, N.; Laver, B.; Liu, J. Hydrogels Constructed from Engineered Proteins. Small 2016, 12, 973–987. [Google Scholar] [CrossRef] [PubMed]
- Cook, W.J.; Walter, L.J.; Walter, M.R. Drug binding by calmodulin: Crystal structure of a calmodulin-trifluoperazine complex. Biochemistry 1994, 33, 15259–15265. [Google Scholar] [CrossRef] [PubMed]
- Daamen, W.F.; Hafmans, T.; Veerkamp, J.H.; van Kuppevelt, T.H. Comparison of five procedures for the purification of insoluble elastin. Biomaterials 2001, 22, 1997–2005. [Google Scholar] [CrossRef]
- Mithieux, S.M.; Rasko, J.E.J.; Weiss, A.S. Synthetic elastin hydrogels derived from massive elastic assemblies of self-organized human protein monomers. Biomaterials 2004, 25, 4921–4927. [Google Scholar] [CrossRef]
- Leach, J.B.; Wolinsky, J.B.; Stone, P.J.; Wong, J.Y. Crosslinked alpha-elastin biomaterials: Towards a processable elastin mimetic scaffold. Acta Biomater. 2005, 1, 155–164. [Google Scholar] [CrossRef]
- Lim, D.W.; Nettles, D.L.; Setton, L.A.; Chilkoti, A. In situ cross-linking of elastin-like polypeptide block copolymers for tissue repair. Biomacromolecules 2008, 9, 222–230. [Google Scholar] [CrossRef][Green Version]
- Haider, M.; Megeed, Z.; Ghandehari, H. Genetically engineered polymers: Status and prospects for controlled release. J. Control. Release 2004, 95, 1–26. [Google Scholar] [CrossRef]
- Petka, W.A.; Harden, J.L.; McGrath, K.P.; Wirtz, D.; Tirrell, D.A. Reversible hydrogels from self-assembling artificial proteins. Science 1998, 281, 389–392. [Google Scholar] [CrossRef]
- Banta, S.; Wheeldon, I.R.; Blenner, M. Protein Engineering in the Development of Functional Hydrogels. Annu. Rev. Biomed. Eng. 2010, 12, 167–186. [Google Scholar] [CrossRef]
- Cao, Y.; Li, H. Engineering tandem modular protein based reversible hydrogels. Chem. Commun. 2008, 4144–4146. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ravindran, S.; Yin, Z.; George, A. 3-D self-assembling leucine zipper hydrogel with tunable properties for tissue engineering. Biomaterials 2014, 35, 5316–5326. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.Y.; Tan, C.; DeGrado, W.F.; Xu, T. New design of helix bundle peptide-polymer conjugates. Biomacromolecules 2008, 9, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Posritong, S.; Flores Chavez, R.; Chu, T.G.; Bruzzaniti, A. A Pyk2 inhibitor incorporated into a PEGDA-gelatin hydrogel promotes osteoblast activity and mineral deposition. Biomed. Mater. 2019, 14, 025015. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, M.; Wang, J.; Zhang, W.; Lu, W.; Gao, Y.; Zhang, B.; Guo, Y. Development of a Photo-Crosslinking, Biodegradable GelMA/PEGDA Hydrogel for Guided Bone Regeneration Materials. Materials 2018, 11, 1345. [Google Scholar] [CrossRef]
- Singh, D.; Zo, S.M.; Singh, D.; Han, S.S. Interpenetrating Alginate on Gelatin- Poly (2-hydroxyethyl Methacrylate) as a Functional Polymeric Matrix for Cartilage Tissue Engineering. Int. J. Polym. Mater. Po. 2019, 68, 551–563. [Google Scholar] [CrossRef]
- Kim, D.H.; Heo, S.-J.; Shin, J.-W.; Mun, C.W. Preparation of Thermosensitive Gelatin-Pluronic Copolymer for Cartilage Tissue Engineering. Macromol. Res. 2010, 18, 387–391. [Google Scholar] [CrossRef]
- Han, L.; XU, J.; LU, X.; Gan, D.; Wang, Z.; WANG, K.F.; Zhang, H.; Yuan, H.; Weng, J. Biohybrid methacrylated gelatin/ polyacrylamide hydrogels for cartilage repair. J. Mater. Chem. B 2017, 5, 731–741. [Google Scholar] [CrossRef]
- Saghebasl, S.; Davaran, S.; Rahbarghazi, R.; Montaseri, A.; Salehi, R.; Ramazani, A. Synthesis and in vitro evaluation of thermosensitive hydrogel scaffolds based on (PNIPAAm-PCL-PEG-PCL-PNIPAAm)/Gelatin and (PCL-PEGPCL)/ Gelatin for use in cartilage tissue engineering. J. Biomater. Sci. Polym. Ed. 2018, 29, 1185–1206. [Google Scholar] [CrossRef]
- Temofeew, N.A.; Hixon, K.R.; McBride-Gagyi, S.H.; Sell, S.A. The fabrication of cryogel scaffolds incorporated with poloxamer 407 for potential use in the regeneration of the nucleus pulposus. J. Mater. Sci. Mater. Med. 2017, 28, 36. [Google Scholar] [CrossRef]
- Marin, S.; Albu Kaya, M.G.; Ghica, M.V.; Dinu-Pîrvu, C.; Popa, L.; Udeanu, D.I.; Mihai, G.; Enachescu, M. Collagen-Polyvinyl Alcohol-Indomethacin Biohybrid Matrices as Wound Dressings. Pharmaceutics 2018, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Fan, D.; Duan, Z.; Zhu, C.; Fu, R.; Li, X. Non-stick hemostasis hydrogels as dressings with bacterial barrier activity for cutaneous wound healing. Mater. Sci. Eng. C 2019, 105, 110118. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.R.; Ghasemi-Mobarakeh, L.; Gharibi, H.; Meamar, R.; Ajalloueian, F.; Chronakis, I.S. Surface modification of poly (ethylene terephthalate) fabric by soy protein isolate hydrogel for wound dressing application. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 1–9. [Google Scholar] [CrossRef]
- Snyders, R.; Shingel, K.I.; Zabeida, O.; Roberge, C.; Faure, M.-P.; Martinu, L.; Klemberg-Sapieha, J.E. Mechanical and microstructural properties of hybrid poly(ethylene glycol)–soy protein hydrogels for wound dressing applications. J. Biomed. Mater. Res. Part A 2007, 83, 88–97. [Google Scholar] [CrossRef]
- Demeter, M.; Virgolici, M.; Vancea, C.; Scarisoreanu, A.; Kaya, M.G.A.; Meltzer, V. Network structure studies on γ–irradiated Collagen–PVP superabsorbent hydrogels. Radiat. Phys. Chem. 2017, 131, 51–59. [Google Scholar] [CrossRef]
- Noppakundilograt, S.; Choopromkaw, S.; Kiatkamjornwong, S. Hydrolyzed collagen-grafted-poly[(acrylic acid)-co-(methacrylic acid)] hydrogel for drug delivery. J. Appl. Polym. Sci. 2018, 135, 45654. [Google Scholar] [CrossRef]
- Pupkaite, J.; Rosenquist, J.; Hilborn, J.; Samanta, A. Injectable Shape-Holding Collagen Hydrogel for Cell Encapsulation and Delivery Cross-linked Using Thiol-Michael Addition Click Reaction. Biomacromolecules 2019, 20, 3475–3484. [Google Scholar] [CrossRef]
- Bini, R.A.; Silva, M.F.; Varanda, L.C.; da Silva, M.; Dreiss, C.A. Soft nanocomposites of gelatin and poly(3-hydroxybutyrate) nanoparticles for dual drug release. Colloids Surf. B Biointerfaces 2017, 157, 191–198. [Google Scholar] [CrossRef]
- Lai, T.; Yu, J.; Tsai, W. Gelatin Methacrylate/Carboxybetaine Methacrylate Hydrogels with Tunable Crosslinking for Controlled Drug Release. J. Mater. Chem. B 2016, 4, 2304–2313. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Mohan, A.M. Novel pH sensitive dual drug loaded-gelatin methacrylate/methacrylic acid hydrogel for the controlled release of antibiotics. Int. J. Biol Macromol. 2018, 110, 167–178. [Google Scholar] [CrossRef]
- Yu, M.; Yao, Q.; Zhang, Y.; Chen, H.; He, H.; Zhang, Y.; Yin, T.; Tang, X.; Xu, H. Core/shell PLGA microspheres with controllable in vivo release profile via rational core phase design. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Pan, S.; Yin, X.; He, Y.-F.; Li, T.; Wang, R.-M. pH-Sensitive keratin-based polymer hydrogel and its controllable drug-release behavior. J. Appl. Polym. Sci. 2015, 132, 41572. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, Y.; Liao, M. pH and temperature-responsive IPN hydrogels based on soy protein and poly(N-isopropylacrylamide-co-sodium acrylate). J. Appl. Polym. Sci. 2015, 131, 39781. [Google Scholar] [CrossRef]
- He, N.; Chen, X.; Wang, L.; Wen, J.; Li, Y.; Cao, Q.; Liu, Z.; Li, B. Fabrication of composite hydrogels based on soy protein isolate and their controlled globular protein delivery. Glob. Chall. 2019, 3, 1900030. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Mhlwatika, Z. Dual release kinetics of antimalarials from soy protein isolate-carbopol-polyacrylamide based hydrogels. J. Appl. Polym. Sci. 2016, 133, 43918. [Google Scholar] [CrossRef]
- Zhu, Q.; Gong, Y.; Guo, T.; Deng, J.; Ji, J.; Wang, B.; Hao, S. Thermo-sensitive keratin hydrogel against iron-induced brain injury after experimental intracerebral hemorrhage. Int. J. Pharm. 2019, 566, 342–351. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Vialli, N.; Fuh, J.Y.H.; Lu, W.F. Conductive collagen/PPy-b-PCL hydrogel for bioprinting of neural tissue constructs. Int. J. Bioprint. 2019, 5, 229. [Google Scholar] [CrossRef]
- Chen, K.; Fan, X.; Tang, K.; Wan, G.; He, X.; Li, X.; Chen, Q.; Shen, M.; Lv, Y.; Wang, F. A morphology-controllable collagen/poly(2-hydroxyethyl methacrylate) porous hydrogel with paraffin microsphere as template. ACS Appl. Bio. Mater. 2018, 1, 1311–1318. [Google Scholar] [CrossRef]
- Wang, C.; Chen, C.; Guo, M.; Li, B.; Han, F.; Chen, W. Stretchable collagen-coated polyurethane-urea hydrogel seeded with bladder smooth muscle cells for urethral defect repair in a rabbit model. J. Mater. Sci.: Mater. Med. 2019, 30, 135. [Google Scholar] [CrossRef]
- Wakuda, Y.; Nishimoto, S.; Suye, S.; Fujita, S. Native collagen hydrogel nanofibres with anisotropic structure using core-shell electrospinning. Sci. Rep. 2018, 8, 6248. [Google Scholar] [CrossRef]
- Sarkar, J.; Kamble, S.C.; Patil, R.; Kumar, A.; Gosavi, S.W. Gelatin interpenetration in poly N-isopropylacrylamide network reduces the compressive modulus of the scaffold: A property employed to mimic hepatic matrix stiffness. Biotechnol. Bioeng. 2020, 117, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Gu, L. Hydrolyzed polyacrylonitrile-blend-soy protein hydrogel fibers: A study of structure and dynamic pH response. Polym. Int. 2009, 58, 66–73. [Google Scholar] [CrossRef]
- Vishnoi, T.; Singh, A.; Teotia, A.K.; Kumar, A. Chitosan-Gelatin-Polypyrrole Cryogel Matrix for Stem Cell Differentiation into Neural Lineage and Sciatic Nerve Regeneration in Peripheral Nerve Injury Model. ACS Biomater. Sci. Eng. 2019, 5, 3007–3021. [Google Scholar] [CrossRef]
- Li, G.; Kong, Y.; Zhao, Y.; Zhao, Y.; Zhang, L.; Yang, Y. Fabrication and characterization of polyacrylamide/silk fibroin hydrogels for peripheral nerve regeneration. J. Biomater. Sci. Polym. Ed. 2015, 26, 899–916. [Google Scholar] [CrossRef]
- Sui, Z.; King, W.J.; Murphy, W.L. Dynamic Materials Based on a Protein Conformational Change. Adv. Mater. 2007, 19, 3377–3380. [Google Scholar] [CrossRef]
- Tan, H.; Jin, D.; Qu, X.; Liu, H.; Yin, M.; Liu, C. A PEG-Lysozyme hydrogel harvests multiple functions as a fit-to-shape tissue sealant for internal-use of body. Biomaterials 2019, 192, 392–404. [Google Scholar] [CrossRef]
- Park, K.M.; Lee, Y.; Son, J.Y.; Oh, D.H.; Lee, J.S.; Park, K.D. Synthesis and characterizations of in situ cross-linkable gelatin and 4-arm-PPO-PEO hybrid hydrogels via enzymatic reaction for tissue regenerative medicine. Biomacromolecules 2012, 13, 604–611. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, J.; Liu, M.; Wang, H.; Li, C.; Rodriguez, M.J.; Li, G.; Wang, X.; Kaplan, D.L. 3D Bioprinting of Self-Standing Silk-Based Bioink. Adv. Healthcare Mater. 2018, 7, 1701026. [Google Scholar] [CrossRef]
- McGann, C.L.; Akins, R.E.; Kiick, K.L. Resilin-PEG hybrid hydrogels yield degradable elastomeric scaffolds with heterogeneous microstructure. Biomacromolecules 2016, 17, 128–140. [Google Scholar] [CrossRef]
- Liu, B.; Lewis, A.K.; Shen, W. Physical Hydrogels Photo-Cross-Linked from Self-Assembled Macromers for Potential Use in Tissue Engineering. Biomacromolecules 2009, 10, 3182–3187. [Google Scholar] [CrossRef]
- Visser, J.; Melchels, F.P.W.; Jeon, J.E.; van Bussel, E.M.; Kimpton, L.S.; Byrne, H.M.; Dhert, W.J.A.; Dalton, P.D.; Hutmacher, D.W.; Malda, J. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 2015, 6, 6933. [Google Scholar] [CrossRef] [PubMed]
- De Mori, A.; Fernández, M.P.; Blunn, G.; Tozzi, G.; Roldo, M. 3D Printing and Electrospinning of Composite Hydrogels for Cartilage and Bone Tissue Engineering. Polymers 2018, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhan, J.; Su, Y.; Wu, T.; Ramakrishna, S.; Liao, S.; Mo, X. Injectable hydrogel incorporating with nanoyarn for bone regeneration. J. Biomater. Sci. Polym. Ed. 2014, 25, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Li, P.; Wang, L.; Mo, X.; Song, L.; Xu, Y.; Zhao, C.; Ouyang, B.; Tu, B.; Luo, L.; et al. An interpenetrating network-strengthened and toughened hydrogel that supports cell-based nucleus pulposus regeneration. Biomaterials 2017, 136, 12–28. [Google Scholar] [CrossRef]
- Wang, X.; Partlow, B.; Liu, J.; Zheng, Z.; Su, B.; Wang, Y.; Kaplan, D.L. Injectable silk-polyethylene glycol hydrogels. Acta Biomater. 2015, 12, 51–61. [Google Scholar] [CrossRef]
- Xu, Y.; Peng, J.; Richards, G.; Lu, S.; Eglin, D. Optimization of electrospray fabrication of stem cell-embedded alginate-gelatin microspheres and their assembly in 3D- printed poly(ε-caprolactone) scaffold for cartilage tissue engineering. J. Orthop. Transl. 2019, 18, 128–141. [Google Scholar] [CrossRef]
- Asadi, N.; Alizadeh, E.; Bakhshayesh, A.R.D.; Mostafavi, E.; Akbarzadeh, A.; Davaran, S. Fabrication and in vitro Evaluation of Nanocomposite Hydrogel Scaffolds Based on Gelatin/PCL−PEG−PCL for Cartilage Tissue Engineering. ACS Omega. 2019, 4, 449–457. [Google Scholar] [CrossRef]
- Lee, J.M.; Sultan, M.T.; Kim, S.H.; Kumar, V.; Yeon, Y.K.; Lee, O.J.; Park, C.H. Artificial Auricular Cartilage Using Silk Fibroin and Polyvinyl Alcohol Hydrogel. Int. J. Mol. Sci. 2017, 18, 1707. [Google Scholar] [CrossRef]
- Bektas, C.K.; Kimiz, I.; Urkmez, A.S.; Hasirci, V.; Hasirci, N. A Bilayer Scaffold Prepared from Collagen and Carboxymethyl Cellulose for Skin Tissue Engineering Applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 1764–1784. [Google Scholar] [CrossRef]
- Park, M.; Kim, B.-S.; Shin, H.K.; Park, S.-J.; Kim, H.-Y. Preparation and characterization of keratin-based biocomposite hydrogels prepared by electron beam irradiation. Mater. Sci. Eng. C 2013, 33, 5051–5057. [Google Scholar] [CrossRef]
- Park, M.; Shin, H.K.; Kim, B.-S.; Kim, M.J.; Kim, I.-S.; Park, B.-Y.; Kim, H.-Y. Effect of discarded keratin-based biocomposite hydrogels on the wound healing process in vivo. Mater. Sci. Eng. C 2015, 55, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Punyamoonwongsa, P.; Klayya, S.; Sajomsang, W.; Kunyanee, C.; Aueviriyavit, S. Silk sericin semi-interpenetrating network hydrogels based on PEG-Diacrylate for wound healing treatment. Int. J. Polym. Sci. 2019, 2019, 4740765. [Google Scholar] [CrossRef]
- Aramwit, P.; Sereemaspun, A.; Yamdech, R. Sericin Ameliorates the Properties of Poly(VinylAlcohol) Hydrogel Prepared by Simple Repeated Freeze-Thaw Process without the Use of Chemical Crosslinking. IJRS 2018, 4, 6–11. [Google Scholar] [CrossRef]
- Ross, S.; Yooyod, M.; Limpeanchob, N.; Mahasaranon, S.; Suphrom, N.; Ross, G.M. Novel 3D porous semi-IPN hydrogel scaffolds of silk sericin and poly(N-hydroxyethyl acrylamide) for dermal reconstruction. Express Polym. Lett. 2017, 11, 719–730. [Google Scholar] [CrossRef]
- Kundu, B.; Kundu, S.C. Silk sericin/polyacrylamide in situ forming hydrogels for dermal reconstruction. Biomaterials 2012, 33, 7456–7467. [Google Scholar] [CrossRef] [PubMed]
- Youngblood, R.L.; Truong, N.F.; Segura, T.; Shea, L.D. It’s All in the Delivery: Designing Hydrogels for Cell and Non-viral Gene Therapies. Mol. Ther. 2018, 26, 2087–2106. [Google Scholar] [CrossRef]
- Krebs, M.D.; Jeon, O.; Alsberg, E. Localized and sustained delivery of silencing RNA from macroscopic biopolymer hydrogels. J. Am. Chem. Soc. 2009, 131, 9204–9206. [Google Scholar] [CrossRef]
- Peng, H.; Yang, H.; Song, L.; Zhou, Z.; Sun, J.; Du, Y.; Lu, K.; Li, T.; Yin, A.; Xu, J.; et al. Sustained delivery of siRNA/PEI complex from in situ forming hydrogels potently inhibits the proliferation of gastric cancer. J. Exp. Clin. Cancer Res. 2016, 35, 57. [Google Scholar] [CrossRef]
- Pamfil, D.; Vasile, C. Chapter 4: Nanogels of natural polymers. In Polymer Gels: Science and Fundamentals. Gels Horizons: From Science to Smart Materials; Thakur, V., Thakur, M.K., Voicu, S., Eds.; Springer Nature: Singapore, 2018; pp. 71–110. [Google Scholar] [CrossRef]
- Tran, D.-H.N.; Nguyen, T.H.; Vo, T.N.N.; Pham, L.P.T.; Vo, D.M.H.; Nguyen, C.K.; Bach, L.G.; Nguyen, D.H. Self-assembled poly(ethylene glycol) methyl ether-grafted gelatin nanogels for efficient delivery of curcumin in cancer treatment. J. Appl. Polym. Sci. 2019, 136, 47544. [Google Scholar] [CrossRef]
- Ge, J.; Min, S.-H.; Kim, D.M.; Lee, D.C.; Park, K.C.; Yeom, Y.I. Selective gene delivery to cancer cells secreting matrix metalloproteinases using a gelatin/polyethylenimine/DNA complex. Biotechnol. Bioprocess. Eng. 2012, 17, 160–167. [Google Scholar] [CrossRef]
- Truong-Le, V.L.; Walsh, S.M.; Schweibert, E.; Mao, Q.H.; Guggino, W.B.; August, J.T.; Leong, K.W. Gene transfer by DNA-gelatin nanospheres. Arch. Biochem. Biophys. 1999, 361, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Rumschöttel, J.; Baus, S.; Kosmella, S.; Appelhans, D.; Koetz, J. Incorporation of DNA/PEI polyplexes into gelatin/chitosan hydrogel scaffolds: A μ-DSC study. Compos. Interfaces 2018, 25, 1–11. [Google Scholar] [CrossRef]
- Chao, G.; Deng, H.; Huang, Q.; Jia, W.; Huang, W.; Gu, Y.; Tan, H.; Fan, L.; Liu, C.; Huang, A.; et al. Preparation and Characterization of pH Sensitive Semi-interpenetrating Network Hydrogel Based on Methacrylic Acid, Bovine Serum Albumin (BSA), and PEG. J. Polym. Res. 2006, 13, 349–355. [Google Scholar] [CrossRef]
- Gayet, J.-C.; Fortier, G. High water content BSA-PEG hydrogel for controlled release device: Evaluation of the drug release properties. J. Control. Release 1996, 38, 177–184. [Google Scholar] [CrossRef]
- Li, X.; Qin, J.; Ma, J. Silk fibroin/poly (vinyl alcohol) blend scaffolds for controlled delivery of curcumin. Regen. Biomater. 2015, 2, 97–105. [Google Scholar] [CrossRef]
- Zhong, T.; Jiang, Z.; Wang, P.; Bie, S.; Zhang, F.; Zuo, B. Silk fibroin/copolymer composite hydrogels for the controlled and sustained release of hydrophobic/hydrophilic drugs. Int. J. Pharm. 2015, 494, 264–270. [Google Scholar] [CrossRef]
- Farooq, M.A.; Aquib, M.; Ghayas, S.; Bushra, R.; Khan, D.H.; Parveen, A.; Wang, B. Whey protein: A functional and promising material for drug delivery systems recent developments and future prospects. Polym. Adv. Technol. 2019, 30, 2183–2191. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Ndwabu, S. Evaluation of whey protein isolate-graft-carbopolpolyacrylamide pH-sensitive composites for controlled release of pamidronate. Polym. Bull. 2017, 74, 5129–5144. [Google Scholar] [CrossRef]
- Seeman, N. DNA in a material world. Nature 2003, 421, 427–431. [Google Scholar] [CrossRef]
- Stoleru, E.; Vasile, C. Chapter 6: Nucleic acids-based bionanomaterials for drug and gene therapy. In Micro and Nano Technologies; Vasile, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 235–259. [Google Scholar]
- Gačanin, J.; Synatschke, C.V.; Weil, T. Biomedical applications of DNA-based hydrogels. Adv. Funct. Mater. 2020, 30, 1906253. [Google Scholar] [CrossRef]
- Costa, D.; Valente, A.J.M.; Queiroz, J. Chapter 13: DNA-Based Hydrogels: An Approach for Multifunctional Bioapplications. In Hydrogels, Gels Horizons: From Science to Smart Materials; Thakur, V.K., Thakur, M.K., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2018. [Google Scholar]
- Lia, J.; Mo, L.; Lu, C.-H.; Fu, T.; Yang, H.-H.; Tan, W. Functional nucleic acid-based hydrogels for bioanalytical and biomedical applications. Chem. Soc. Rev. 2016, 45, 1410–1431. [Google Scholar] [CrossRef] [PubMed]
- Um, S.H.; Lee, J.B.; Park, N.; Kwon, S.Y.; Umbach, C.C.; Luo, D. Enzyme-catalysed assembly of DNA hydrogel. Nat. Mater. 2006, 5, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.; Xing, Y.; Chen, P.; Yang, Y.; Sun, Y.; Zhou, D.; Xu, L.; Fan, Q.; Liu, D. A pH-Triggered, fast-responding DNA hydrogel. Angew. Chem. Int. Ed. 2009, 48, 7660–7663. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Li, J.; Wu, C.; Yan, F.; Li, X.; Zhang, S. A self-assembled RNA-triple helix hydrogel drug delivery system targeting triple-negative breast cancer. J. Mater. Chem. B 2020. [Google Scholar] [CrossRef]
- Lin, D.C.; Yurke, B.; Langrana, N.A. Mechanical properties of a reversible, DNA-crosslinked polyacrylamide hydrogel. J. Biomech. Eng. 2004, 126, 104–110. [Google Scholar] [CrossRef]
- Soontornworajit, B.; Zhou, J.; Zhang, Z.; Wang, Y. Aptamer-functionalized in situ injectable hydrogel for controlled protein release. Biomacromolecules 2010, 11, 2724. [Google Scholar] [CrossRef]
- Li, S.; Chen, N.; Gaddes, E.R.; Zhang, X.; Dong, C.; Wang, Y. A Drosera-bioinspired hydrogel for catching and killing cancer cells. Sci. Rep. 2015, 5, 14297. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Zeng, X.; Sun, Y.-X.; Zhuo, R.-X. Bioactive materials in gene therapy. In Bioactive Materials in Medicine; Zhao, X., Courtney, J.M., Qian, H., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2011. [Google Scholar]
- Korolev, N.; Lyubartsev, A.; Nordenskiold, L. Cation-induced polyelectrolyte-polyelectrolyte attraction in solutions of DNA and nucleosome core particles. Adv. Colloid Interface Sci. 2010, 158, 32–47. [Google Scholar] [CrossRef]
- Costa, D.; Valente, A.J.M.; Queiroz, J. Plasmid DNA nanogels as photoresponsive materials for multifunctional bio-applications. J. Biotechnol. 2015, 20, 98–104. [Google Scholar] [CrossRef][Green Version]
- Ding, F.; Huang, X.; Gao, X.; Xie, M.; Pan, G.; Li, Q.; Song, J.; Zhu, X.; Zhang, C. A Non-cationic Nucleic Acid Nanogel for the Delivery of CRISPR/Cas9 Gene Editing Tool. Nanoscale 2019, 11, 17211–17215. [Google Scholar] [CrossRef]
- Mimi, H.; Ho, K.; Siu, Y.; Wu, A.; Li, P. Polyethyleneimine-based core-shell nanogels: A promising siRNA carrier for argininosuccinate synthetase mRNA knockdown in HeLa cells. J. Control. Release 2012, 158, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Li, R.Q.; Wu, W.; Song, H.Q.; Ren, Y.; Yang, M.; Li, J.; Xu, F.J. Well-defined reducible cationic nanogels based on functionalized low-molecular-weight PGMA for effective pDNA and siRNA delivery. Acta Biomater. 2016, 41, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Liu, H.; Zhang, X.; Yan, J.; Zhu, Z.; Peng, L.; Yang, H.; Kim, Y.; Tan, W. Photoresponsive DNA-Cross-Linked Hydrogels for Controllable Release and Cancer Therapy. Langmuir 2011, 27, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Benoit, D.S.W. Degradable poly(ethylene glycol) (PEG)-based hydrogels for spatiotemporal control of siRNA/nanoparticle delivery. J. Control. Release 2018, 287, 58–66. [Google Scholar] [CrossRef]
- Li, F.; Lyu, D.; Liu, S.; Guo, W. DNA Hydrogels and Microgels for Biosensing and Biomedical Applications. Adv. Mater. 2019, 32, 1–9. [Google Scholar] [CrossRef]
- Dave, N.; Huang, P.-J.J.; Chan, M.Y.; Smith, B.D.; Liu, J. Regenerable DNA-functionalized hydrogels for ultrasensitive, instrument-free mercury(II) detection and removal in water. J. Am. Chem. Soc. 2010, 132, 12668. [Google Scholar] [CrossRef]
- Hu, Y.; Kahn, J.S.; Guo, W.; Huang, F.; Fadeev, M.; Harries, D.; Willner, I. Reversible Modulation of DNA-Based Hydrogel Shapes by Internal Stress Interactions. J. Am. Chem. Soc. 2016, 138, 16112–16119. [Google Scholar] [CrossRef]
- Guo, W.; Lu, C.-H.; Orbach, R.; Wang, F.; Qi, X.-J.; Cecconello, A.; Seliktar, D.; Willner, I. pH-Stimulated DNA Hydrogels Exhibiting Shape-Memory Properties. Adv. Mater. 2015, 27, 73–78. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Fadeev, M.; Li, Z.; Wulf, V.; Tian, H.; Willner, I. Chemical and photochemical DNA “gears” reversibly control stiffness, shape-memory, self-healing and controlled release properties of polyacrylamide hydrogels. Chem. Sci. 2019, 10, 1008–1016. [Google Scholar] [CrossRef]
- Rico-García, D.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Hernández-Olmos, S.L.; Guerrero-Ramírez, G.L.; Vilas-Vilela, J.L. Lignin-Based Hydrogels: Synthesis and Applications. Polymers 2020, 12, 81. [Google Scholar] [CrossRef]
- Meng, Y.; Lu, J.; Cheng, Y.; Li, Q.; Wang, H. Lignin-based hydrogels: A review of preparation, properties, and application. Int. J. Biol. Macromol. 2019, 135, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Penaranda, J.E.; Sabino, M.A. Effect of the presence of lignin or peat in IPN hydrogels on the sorption of heavy metals. Polym. Bull. 2010, 65, 495–508. [Google Scholar] [CrossRef]
- Jin, C.; Song, W.; Liu, T.; Xin, J.; Hiscox, W.C.; Zhang, J.; Liu, G.; Kong, Z. Temperature and pH responsive hydrogels using methacrylated lignosulfonate cross-linker: Synthesis, characterization, and properties. ACS Sustain. Chem. Eng. 2018, 6, 1763–1771. [Google Scholar] [CrossRef]
- Passauer, L.; Halles, T.; Baucker, E.; Ciesielski, G.; Lebioda, S.; Hamer, U. Biodegradation of hydrogels from oxyethylated lignins in model soils. ACS Sustain. Chem. Eng. 2015, 3, 1955–1964. [Google Scholar] [CrossRef]
- Kai, D.; Low, Z.W.; Liow, S.S.; Karim, A.A.; Ye, H.; Jin, G.; Li, K.; Loh, X.J. Development of lignin supramolecular hydrogels with mechanically responsive and self-healing properties. ACS Sustain. Chem. Eng. 2015, 3, 2160–2169. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, Q.; Xie, Y.; Li, J.; Chen, X. Preparation of biocompatible hydrogel from lignin-carbohydrate complex (LCC) as cell carriers. Bioresources 2017, 12, 8490–8504. [Google Scholar]
- Naficy, S.; Spinks, G.M.; Wallace, G.G. Thin, tough, pH-sensitive hydrogel films with rapid load recovery. Acs Appl. Mater. Interfaces 2014, 6, 4109–4114. [Google Scholar] [CrossRef]
- Oveissi, F.; Naficy, S.; Le, T.Y.L.; Fletcher, D.F.; Dehghani, F. Tough and processable hydrogels based on lignin and hydrophilic polyurethane. ACS Appl. Bio. Mater. 2018, 1, 2073–2081. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.H.; Won, K.; Choi, J.W.; Kim, Y.H.; Kim, H.J.; Yang, Y.-H.; Lee, S.H. Wood mimetic hydrogel beads for enzyme immobilization. Carbohydr. Polym. 2015, 115, 223–229. [Google Scholar] [CrossRef]
- Borisenkov, M.F.; Karmanov, A.P.; Kocheva, L.S.; Markov, P.A.; Istomina, E.I.; Bakutova, L.A.; Litvinets, S.G.; Martinson, E.A.; Durnev, E.A.; Vityazev, F.V.; et al. Adsorption of beta-glucuronidase and estrogens on pectin/lignin hydrogel particles. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 433–441. [Google Scholar] [CrossRef]
- Figueiredo, P.; Ferro, C.; Kemell, M.; Liu, Z.; Kiriazis, A.; Lintinen, K.; Florindo, H.F.; Yli-Kauhaluoma, J.; Hirvonen, J.; Kostiainen, M.A.; et al. Functionalization of carboxylated lignin nanoparticles for targeted and pH-responsive delivery of anticancer drugs. Nanomedicine 2017, 12, 2581–2596. [Google Scholar] [CrossRef] [PubMed]
- Ciolacu, D.; Oprea, A.M.; Anghel, N.; Cazacu, G.; Cazacu, M. New cellulose-lignin hydrogels and their application in controlled release of polyphenols. Mater. Sci. Eng. C 2012, 32, 452–463. [Google Scholar] [CrossRef]
- Wu, L.; Huang, S.; Zheng, J.; Qiu, Z.; Lin, X.; Qin, Y. Synthesis and characterization of biomass lignin-based PVA super-absorbent hydrogel. Int. J. Biol. Macromol. 2019, 140, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Z.; Guo, X.; He, Q. Ultrasonic-assisted synthesis of sodium lignosulfonate-grafted poly(acrylic acid-co-poly (vinyl pyrrolidone)) hydrogel for drug delivery. RSC Adv. 2016, 6, 35550–35558. [Google Scholar] [CrossRef]
- Spasojevic, D.; Zmejkoski, D.; Glamoclija, J.; Nikolic, M.; Sokovic, M.; Milosevic, V.; Jaric, I.; Stojanovic, M.; Marinkovic, E.; Barisani-Asenbauer, T.; et al. Lignin model compound in alginate hydrogel: A strong antimicrobial agent with high potential in wound treatment. Int. J. Antimicrob. Agents 2016, 48, 732–735. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Effect of cellulose and lignin on disintegration, antimicrobial and antioxidant properties of PLA active films. Int. J. Biol. Macromol. 2016, 89, 360–368. [Google Scholar] [CrossRef]
- Mahata, D.; Jana, M.; Jana, A.; Mukherjee, A.; Mondal, N.; Saha, T.; Sen, S.; Nando, G.B.; Mukhopadhyay, C.K.; Chakraborty, R.; et al. Lignin-graft-polyoxazoline conjugated triazole a novel anti-infective ointment to control persistent inflammation. Sci. Rep. 2017, 7, 46412–46417. [Google Scholar] [CrossRef]
- Ravishankar, K.; Venkatesan, M.; Preeth, R.; Mahalingam, A. Biocompatible hydrogels of chitosan-alkali lignin for potential wound healing applications. Mater. Sci. Eng. C 2019, 102, 447–457. [Google Scholar] [CrossRef]
- Zmejkoski, D.; Spasojevic, D.; Orlovska, I.; Kozyrovska, N.; Sokovic, M.; Glamoclija, J.; Dmitrovic, S.; Matovic, B.; Tasic, N.; Maksimovic, V.; et al. Bacterial cellulose-lignin composite hydrogel as a promising agent in chronic wound healing. Int. J. Biol. Macromol. 2018, 118, 494–503. [Google Scholar] [CrossRef]
- Musilová, L.; Mrácek, A.; Kovalcik, A.; Smolka, P.; Minarík, A.; Humpolícek, P.; Vícha, R.; Ponížil, P. Hyaluronan hydrogels modified by glycinated Kraft lignin: Morphology, swelling, viscoelastic properties and biocompatibility. Carbohydr. Polym. 2018, 181, 394–403. [Google Scholar] [CrossRef]
- Larrañeta, E.; Imízcoz, M.; Toh, J.X.; Irwin, N.J.; Ripolin, A.; Perminova, A.; Dom, J.; Rodr, A.; Donnelly, R.F. Synthesis and Characterization of Lignin Hydrogels for Potential Applications as Drug Eluting Antimicrobial Coatings for Medical Materials. Sustain. Chem. Eng. 2018, 6, 9037–9046. [Google Scholar] [CrossRef]
- Alles, N.; Soysa, N.S.; Hussain, M.D.A.; Tomomatsu, N.; Saito, H.; Baron, R.; Morimoto, N.; Aoki, K.; Akiyoshi, K.; Ohya, K. Polysaccharide nanogel delivery of a TNF-α aand RANKL antagonist peptide allows systemic prevention of bone loss. Eur. J. Pharm. Sci. 2009, 37, 83–88. [Google Scholar] [CrossRef]
- Uenaka, A.; Wada, H.; Isobe, M.; Saika, T.; Tsuji, K.; Sato, E.; Sato, S.; Noguchi, Y.; Kawabata, R.; Yasuda, T.; et al. T cell immunomonitoring and tumor responses in patients immunized with a complex of cholesterol-bearing hydrophobized pullulan (CHP) and NY-ESO-1 protein. Cancer Immunol. 2007, 7, 9–20. [Google Scholar]
- Kageyama, S.; Kitano, S.; Hirayama, M.; Nagata, Y.; Imai, H.; Shiraishi, T.; Akiyoshi, K.; Scott, A.M.; Murphy, R.; Hoffman, E.W.; et al. Humoral immune responses in patients vaccinated with 1-146 HER2 protein complexed with cholesteryl pullulan nanogel. Cancer Sci. 2008, 99, 601–607. [Google Scholar] [CrossRef]
- Kitano, S.; Kageyama, S.; Nagata, Y.; Miyahara, Y.; Hiasa, A.; Naota, H.; Okumura, S.; Imai, H.; Shiraishi, T.; Masuya, M.; et al. HER2-specific T-cell immune responses in patients vaccinated with truncated HER2 protein complexed with nanogels of cholesteryl pullulan. Clin. Cancer Res. 2006, 12, 7397–7405. [Google Scholar] [CrossRef]
- Kawabata, R.; Wada, H.; Isobe, M.; Saika, T.; Sato, S.; Uenaka, A.; Miyata, H.; Yasuda, T.; Doki, Y.; Noguchi, Y.; et al. Antibody response against NY-ESO-1 in CHP-NY-ESO-1 vaccinated patients. Int. J. Cancer 2007, 120, 2178–2184. [Google Scholar] [CrossRef]
- Shingel, K.I.; Di Stabille, L.; Marty, J.P.; Faure, M.P. Inflammatory inert poly(ethylene glycol)–protein wound dressing improves healing responses in partial- and full-thickness wounds. Int. Wound J. 2006, 3, 332–342. [Google Scholar] [CrossRef]
- Nochi, T.; Yuki, Y.; Takahashi, H.; Sawada, S.-I.; Mejima, M.; Kohda, T.; Harada, N.; Kong, I.G.; Sato, A.; Kataoka, N.; et al. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat. Mater. 2010, 9, 572–578. [Google Scholar] [CrossRef]
- Kanokpanont, S.; Damrongsakkul, S.; Ratanavaraporn, J.; Aramwit, P. An innovative bi-layered wound dressing made of silk and gelatin for accelerated wound healing. Int. J. Pharm. 2012, 436, 141–153. [Google Scholar] [CrossRef]
- Kwiecień, I.; and Kwiecień, M. Application of Polysaccharide-Based Hydrogels as Probiotic Delivery Systems. Gels 2018, 4, 47. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).