Formulation and Optimization of Nanoemulsions Using the Natural Surfactant Saponin from Quillaja Bark
Abstract
1. Introduction
2. Results and Discussion
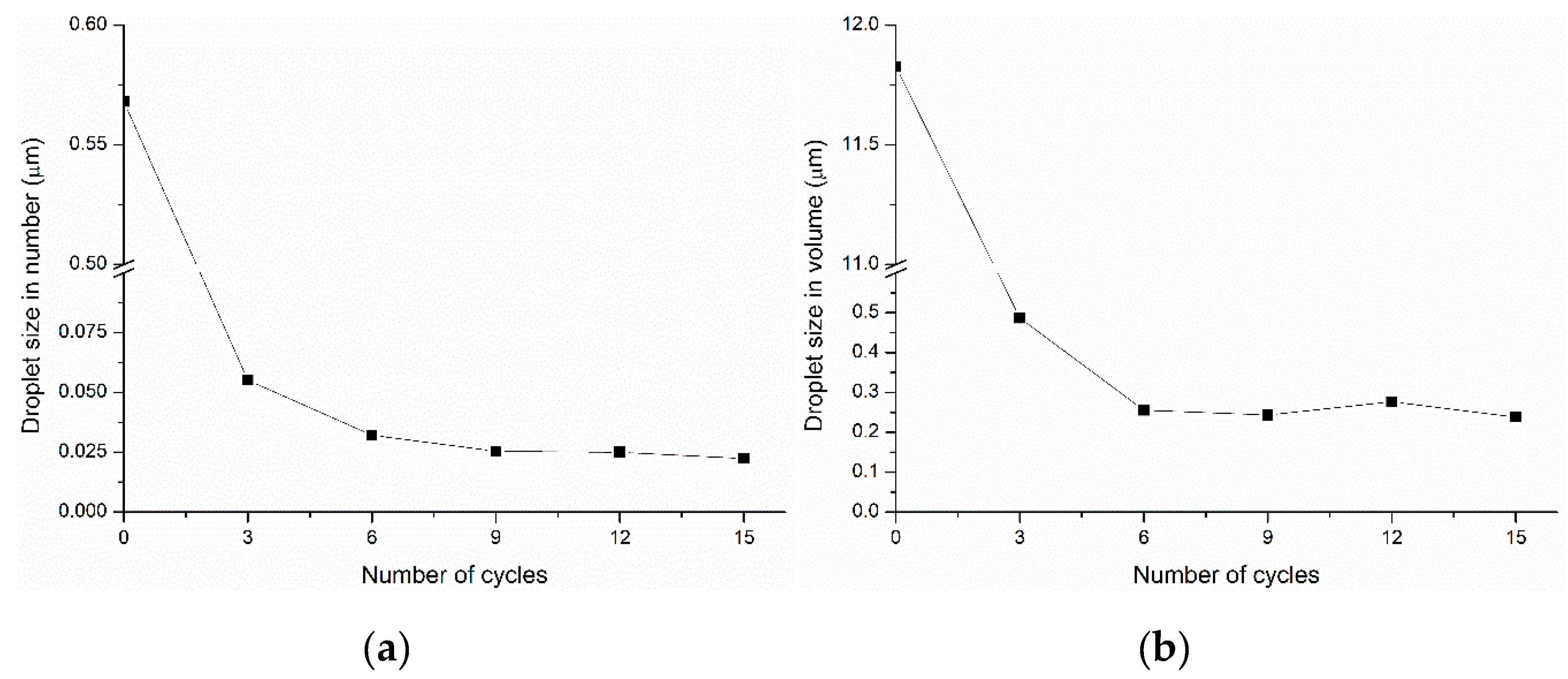
2.1. Optimization of Emulsion Preparation Approach
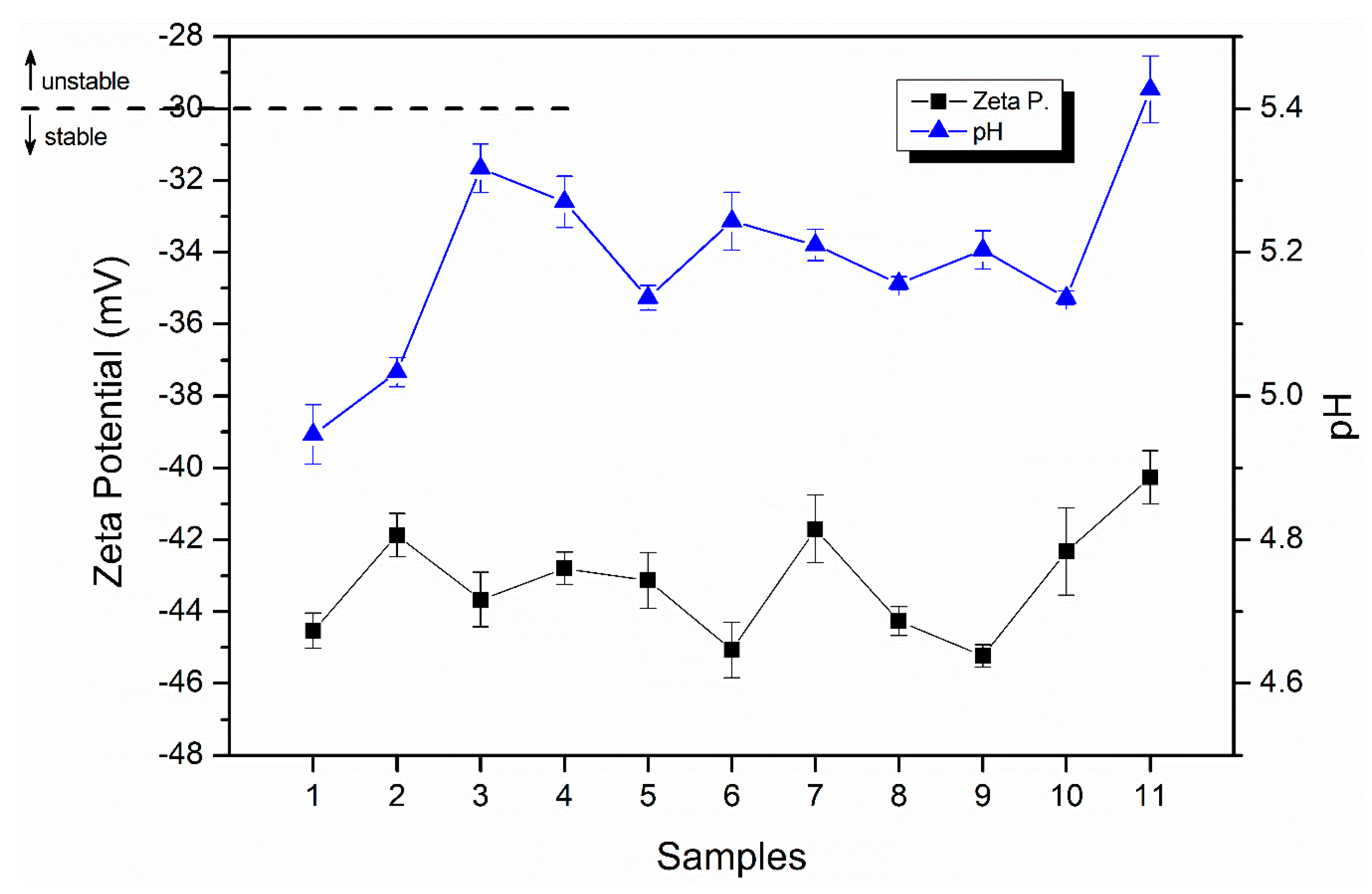
2.2. Characterization of Emulsions
2.3. Statistical Analysis
3. Materials and Methods
3.1. Materials
3.2. Emulsion Preparation and Stability
3.3. pH Measurements
3.4. Zeta Potential
3.5. Particle Size Determination
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Reichert, C.L.; Salminen, H.; Badolato Bönisch, G.; Schäfer, C.; Weiss, J. Concentration effect of Quillaja saponin—Co-surfactant mixtures on emulsifying properties. J. Colloid Interface Sci. 2018, 519, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Kregiel, D.; Berlowska, J.; Witonska, I.; Antolak, H.; Proestos, C.; Babic, M.; Babic, L.; Zhang, B. Saponin-Based, Biological-Active Surfactants from Plants. In Application and Characterization of Surfactants; InTechOpen: Rijeka, Croatia, 2017; pp. 183–205. [Google Scholar]
- Shen, P.; Zhang, R.; Julian, D.; Park, Y. Nanoemulsion-based delivery systems for testing nutraceutical efficacy using Caenorhabditis elegans: Demonstration of curcumin bioaccumulation and body-fat reduction. Food Res. Int. 2019, 120, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Schoener, A.L.; Zhang, R.; Lv, S.; Julian, D. Fabrication of plant-based vitamin D3-fortified nanoemulsions: Influence of carrier oil type on vitamin bioaccessibility. Food Funct. 2019, 10, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Food Emulsions Principles, Practices, and Techniques, 3rd ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Froelich, A.; Osmałek, T.; Snela, A.; Kunstman, P.; Jadach, B.; Olejniczak, M.; Roszak, G.; Białas, W. Novel microemulsion-based gels for topical delivery of indomethacin: Formulation, physicochemical properties and in vitro drug release studies. J. Colloid Interface Sci. 2017, 507, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Dasgupta, S.; Patel, D.K.; Ramani, Y.R.; Ghosh, S.K.; Mazumder, B. Nanoemulsion as a Carrier for Topical Delivery of Aceclofenac. Adv. Nanomater. Nanotechnol. 2013, 143, 1–19. [Google Scholar]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef]
- Yukuyama, M.N.; Ghisleni, D.D.M.; Pinto, T.J.A.; Bou-Chacra, N.A. Nanoemulsion: Process selection and application in cosmetics—A review. Int. J. Cosmet. Sci. 2016, 38, 13–24. [Google Scholar] [CrossRef]
- Farahin, A.W.; Yusoff, F.M.; Basri, M.; Nagao, N.; Shariff, M. Use of microalgae: Tetraselmis tetrathele extract in formulation of nanoemulsions for cosmeceutical application. J. Appl. Phycol. 2019, 31, 1743–1752. [Google Scholar] [CrossRef]
- Gesztesi, J.-L.; Santos, L.M.; de Hennies, P.T.; Macian, K.A. Oil-in-Water Nanoemulsion, a Cosmetic Composition and a Cosmetic Product Comprising it, a Process for Preparing Said Nanoemulsion. CA2585259C, 6 August 2013. [Google Scholar]
- Shaker, D.S.; Ishak, R.A.H.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A Review on Mechanisms for the Transdermal Delivery of Hydrophobic and Hydrophilic Drugs. Sci. Pharm. 2019, 87, 17. [Google Scholar] [CrossRef]
- Yang, Y.; Leser, M.E.; Sher, A.A.; McClements, D.J. Formation and stability of emulsions using a natural small molecule surfactant: Quillaja saponin (Q-Naturale®). Food Hydrocoll. 2013, 30, 589–596. [Google Scholar] [CrossRef]
- Ahmad, J.; Gautam, A.; Komath, S.; Bano, M.; Garg, A.; Jain, K. Topical Nanoemulgel for Skin Disorders: Formulation Approach and Characterization. Recent Pat. Antiinfect. Drug Discov. 2019, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Leite, C.; Coelho, J.; Muehlmann, L.A.; Azevedo, R.; Sousa, M. Microemulsions as Platforms for Transdermal Delivery of Hydrophilic Drugs—A Review. Curr. Nanosci. 2018, 14, 170–178. [Google Scholar] [CrossRef]
- McClements, D.J.; Bai, L.; Chung, C. Recent Advances in the Utilization of Natural Emulsifiers to Form and Stabilize Emulsions. Annu. Rev. Food Sci. Technol. 2017, 8, 205–236. [Google Scholar] [CrossRef] [PubMed]
- Taarji, N.; Rabelo da Silva, C.A.; Khalid, N.; Gadhi, C.; Hafidi, A.; Kobayashi, I.; Neves, M.A.; Isoda, H.; Nakajima, M. Formulation and stabilization of oil-in-water nanoemulsions using a saponins-rich extract from argan oil press-cake. Food Chem. 2018, 246, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Mcclements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Ralla, T.; Salminen, H.; Edelmann, M.; Dawid, C.; Hofmann, T.; Weiss, J. Sugar Beet Extract (Beta vulgaris L.) as a New Natural Emulsifier: Emulsion Formation. J. Agric. Food Chem. 2017, 65, 4153–4160. [Google Scholar] [CrossRef]
- Mitra, S.; Dungan, S.R. Micellar Properties of Quillaja Saponin. 1. Effects of Temperature, Salt, and pH on Solution Properties. J. Agric. Food Chem. 1997, 45, 1587–1595. [Google Scholar] [CrossRef]
- Ralla, T.; Herz, E.; Salminen, H.; Edelmann, M.; Dawid, C.; Hofmann, T.; Weiss, J. Emulsifying Properties of Natural Extracts from Panax ginseng L. Food Biophys. 2017, 12, 479–490. [Google Scholar] [CrossRef]
- Ralla, T.; Salminen, H.; Tuosto, J.; Weiss, J. Original article Formation and stability of emulsions stabilised by Yucca saponin extract. Int. J. Food Sci. Technol. 2017, 53, 1381–1388. [Google Scholar] [CrossRef]
- Reichert, C.L.; Salminen, H.; Weiss, J. Quillaja Saponin Characteristics and Functional Properties. Annu. Rev. Food Sci. Technol. 2019, 10, 43–73. [Google Scholar] [CrossRef]
- Stanimirova, R.; Marinova, K.; Tcholakova, S.; Denkov, N.D.; Stoyanov, S.; Pelan, E. Surface Rheology of Saponin Adsorption Layers. Langmuir 2011, 27, 12486–12498. [Google Scholar] [CrossRef] [PubMed]
- De Faria, J.T.; de Oliveira, E.B.; Minim, V.P.R.; Minim, L.A. Performance of Quillaja bark saponin and b -lactoglobulin mixtures on emulsion formation and stability. Food Hydrocoll. 2017, 67, 178–188. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations) & WHO (World Health Organization). Codex Alimentarius—International food Standards: List of Codex Specifications for Food Additives. 2016. Available online: http://www.fao.org/fileadmin/user_upload/jecfa_additives/docs/monograph3/additive-368.pdf (accessed on 15 February 2020).
- Golemanov, K.; Tcholakova, S.; Denkov, N.; Pelan, E.; Stoyanov, S.D. Surface shear rheology of saponin adsorption layers. Langmuir 2012, 28, 12071–12084. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, K.; Kezwon, A.; Lewandowska, J.; Marcinkowski, K. Effect of β-casein on surface activity of Quillaja bark saponin at fluid/fluid interfaces. Food Hydrocoll. 2014, 34, 208–216. [Google Scholar] [CrossRef]
- Kezwon, A.; Wojciechowski, K. Interaction of Quillaja bark saponins with food-relevant proteins. Adv. Colloid Interface Sci. 2014, 209, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Argin, S.; Ozilgen, M.; McClements, D.J. Formation and stabilization of nanoemulsion-based vitamin E delivery systems using natural surfactants: Quillaja saponin and lecithin. J. Food Eng. 2014, 142, 57–63. [Google Scholar] [CrossRef]
- Zhu, Z.; Wen, Y.; Yi, J.; Cao, Y.; Liu, F.; McClements, D.J. Comparison of natural and synthetic surfactants at forming and stabilizing nanoemulsions: Tea saponin, Quillaja saponin, and Tween 80. J. Colloid Interface Sci. 2019, 536, 80–87. [Google Scholar] [CrossRef]
- Maali, A.; Mosavian, M.T.H. Preparation and Application of Nanoemulsions in the Last Decade (2000–2010). J. Dispers. Sci. Technol. 2013, 34, 92–105. [Google Scholar] [CrossRef]
- Ali, A.; Mekhloufi, G.; Huang, N.; Agnely, F. β-lactoglobulin stabilized nanemulsions—Formulation and process factors affecting droplet size and nanoemulsion stability. Int. J. Pharm. 2016, 500, 291–304. [Google Scholar] [CrossRef]
- Barradas, T.N.; de Campos, V.E.B.; Senna, J.P.; dos Santos Cerqueira Coutinho, C.; Tebaldi, B.S.; Silva, K.G.d.H.e; Mansur, C.R.E. Development and characterization of promising o/w nanoemulsions containing sweet fennel essential oil and non-ionic sufactants. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 214–221. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed]
- Gurpreet, K.; Singh, S.K. Review of Nanoemulsion Formulation and Characterization Techniques. Indian J. Pharm. Sci. 2018, 80, 781–789. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar]
- Rebolleda, S.; Sanz, M.T.; Benito, J.M.; Beltrán, S.; Escudero, I.; San-josé, M.L.G. Formulation and characterization of wheat bran oil-in-water nanoemulsions. Food Chem. 2015, 167, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Abe, M. Measurement Techniques and Practices of Colloid and Interface, 1st ed.; Springer: Singapore, 2019. [Google Scholar]
- Mandenius, C.-F.; Brundin, A. Review: Biocatalysts and Bioreactor Design. Biotechnol. Prog. 2008, 24, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, D.C. Response Surface Methods and Other Approaches to Process Optimization. In Design and Analysis of Experiments; John Wiley & Sons: New York, NY, USA, 1997. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |



| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| O/W (w/w) | 20/80 | 30/70 | 30/70 | 10/90 | 10/90 | 20/80 | 10/90 | 10/90 | 20/80 | 30/70 | 30/70 |
| Surfactant (wt%) | 1.0 | 0.5 | 1.5 | 0.5 | 1.5 | 1.0 | 0.5 | 1.5 | 1.0 | 1.5 | 0.5 |
| Saponin/Glycerol (w/w) | 75/25 | 100 | 50/50 | 50/50 | 50/50 | 75/25 | 100 | 100 | 75/25 | 100 | 50/50 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Size number (nm) | 20.67 | 19.03 | 17.97 | 18.06 | 18.29 | 19.17 | 17.98 | 18.36 | 18.31 | 18.50 | 17.74 |
| Size volume (µm) | 2.09 | 3.05 | 2.15 | 4.31 | 1.79 | 1.47 | 1.18 | 1.12 | 1.25 | 1.25 | 2.97 |
| Source | Particle Size in Volume (µm) | Zeta Potential (mV) | ||
|---|---|---|---|---|
| β Coefficient (Coded Factors) | p-Value | β Coefficient (Coded Factors) | p-Value | |
| Model | ---- | 0.0006 | ---- | 0.0161 |
| Intercept | +222.90 | ---- | −42.50 | ---- |
| X1—oil (%) | +12.71 | 0.0280 | +0.47 | 0.0217 |
| X2—surfactant (%) | −65.09 | 0.0003 | −0.85 | 0.0042 |
| X3—saponin/glycerol (%) | −57.72 | 0.0004 | −0.04 | 0.74891 |
| X1X3 | +37.42 | 0.0013 | −0.12 | 0.34051 |
| X2X3 | +18.44 | 0.0102 | +0.09 | 0.47301 |
| X1X2X3 | −43.01 | 0.0009 | +0.65 | 0.0091 |
| Curvature | ----- | 0.0007 | ----- | 0.0013 |
| Lack of fit | ---- | 0.9293 | ---- | 0.8425 |
| Quality parameter | Value | Value | ||
| Adequate Precision | 41.833 | 18.030 | ||
| R2 | 0.9973 | 0.9757 | ||
| R2adj | 0.9920 | 0.9272 | ||
| R2pred | 0.9932 | 0.9082 | ||
| Variable | Symbol | Coded (Xi) Variable Level | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Oil (%) | x1 | 10 | 20 | 30 |
| Surfactant (%) | x2 | 0.5 | 1.0 | 1.5 |
| Saponin/glycerol (%) | x3 | 50 | 75 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreiner, T.B.; Santamaria-Echart, A.; Ribeiro, A.; Peres, A.M.; Dias, M.M.; Pinho, S.P.; Barreiro, M.F. Formulation and Optimization of Nanoemulsions Using the Natural Surfactant Saponin from Quillaja Bark. Molecules 2020, 25, 1538. https://doi.org/10.3390/molecules25071538
Schreiner TB, Santamaria-Echart A, Ribeiro A, Peres AM, Dias MM, Pinho SP, Barreiro MF. Formulation and Optimization of Nanoemulsions Using the Natural Surfactant Saponin from Quillaja Bark. Molecules. 2020; 25(7):1538. https://doi.org/10.3390/molecules25071538
Chicago/Turabian StyleSchreiner, Tatiana B., Arantzazu Santamaria-Echart, Andreia Ribeiro, António M. Peres, Madalena M. Dias, Simão P. Pinho, and Maria Filomena Barreiro. 2020. "Formulation and Optimization of Nanoemulsions Using the Natural Surfactant Saponin from Quillaja Bark" Molecules 25, no. 7: 1538. https://doi.org/10.3390/molecules25071538
APA StyleSchreiner, T. B., Santamaria-Echart, A., Ribeiro, A., Peres, A. M., Dias, M. M., Pinho, S. P., & Barreiro, M. F. (2020). Formulation and Optimization of Nanoemulsions Using the Natural Surfactant Saponin from Quillaja Bark. Molecules, 25(7), 1538. https://doi.org/10.3390/molecules25071538












