Catalytic Effect of Hydrogen Bond on Oxhydryl Dehydrogenation in Methanol Steam Reforming on Ni(111)
Abstract
1. Introduction
2. Computational Methods
3. Results and Discussion
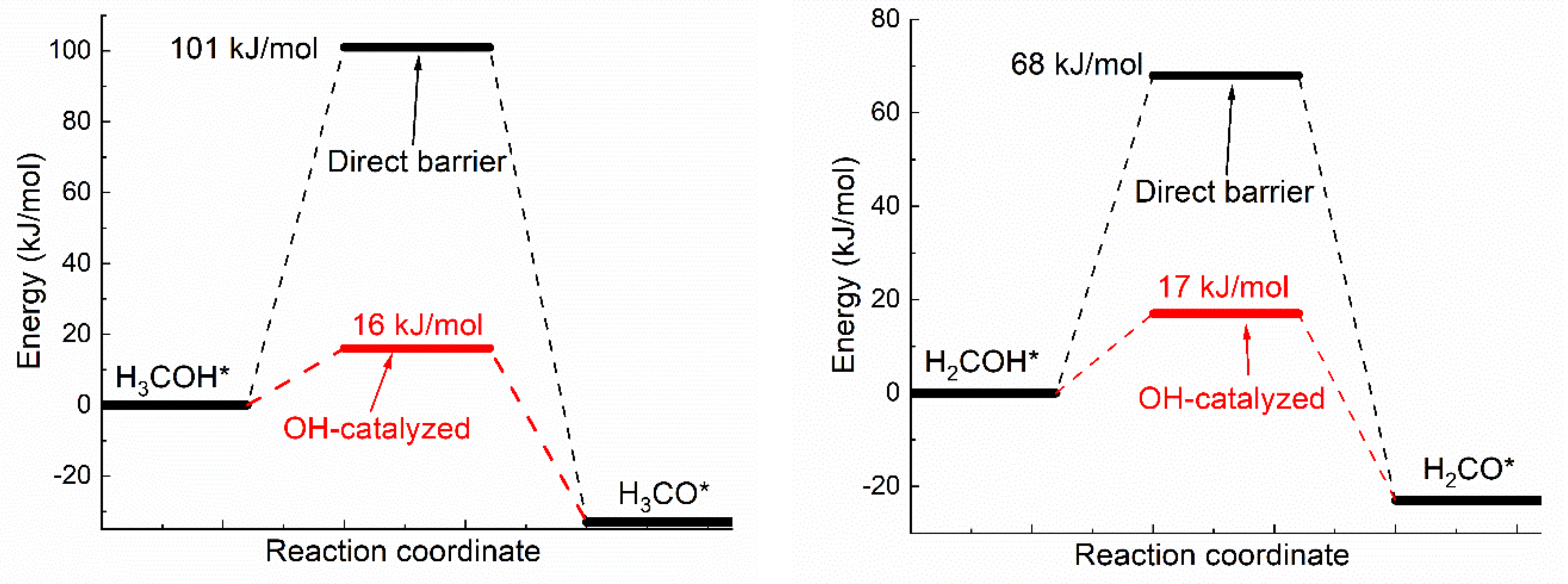
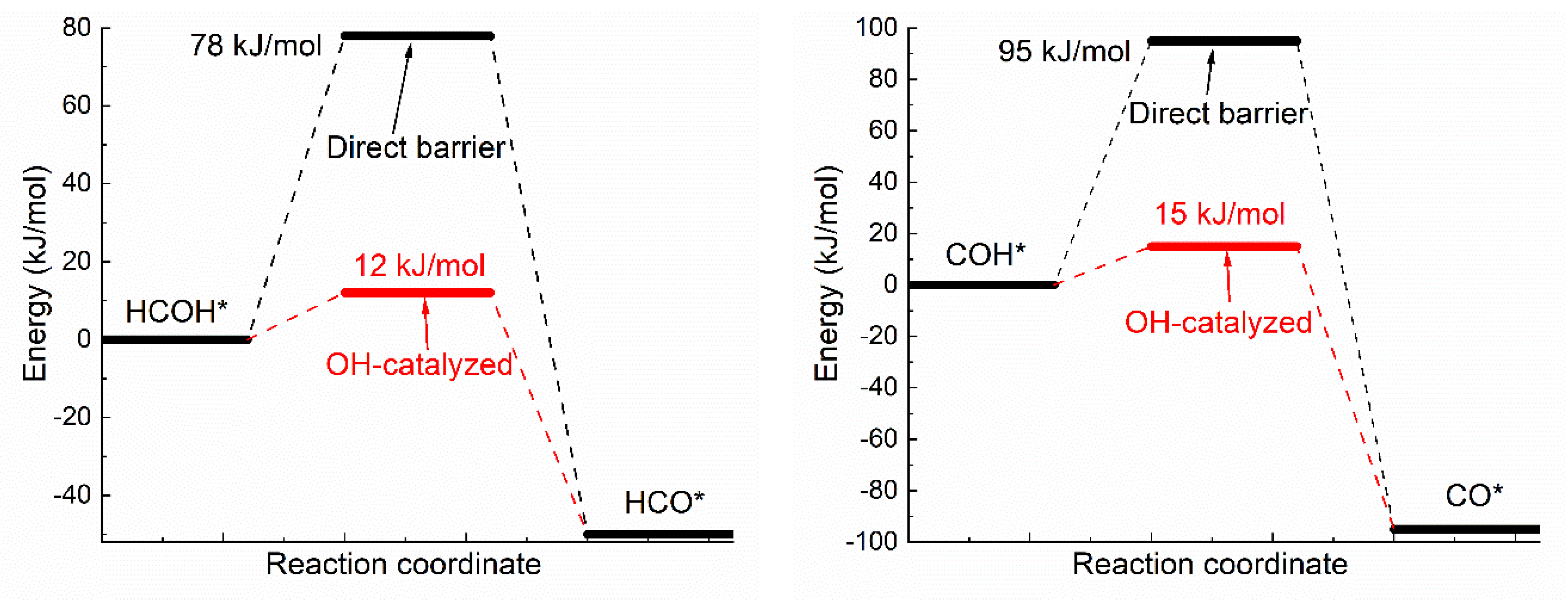
3.1. Oxhydryl Dehydrogenation of HxCOH*
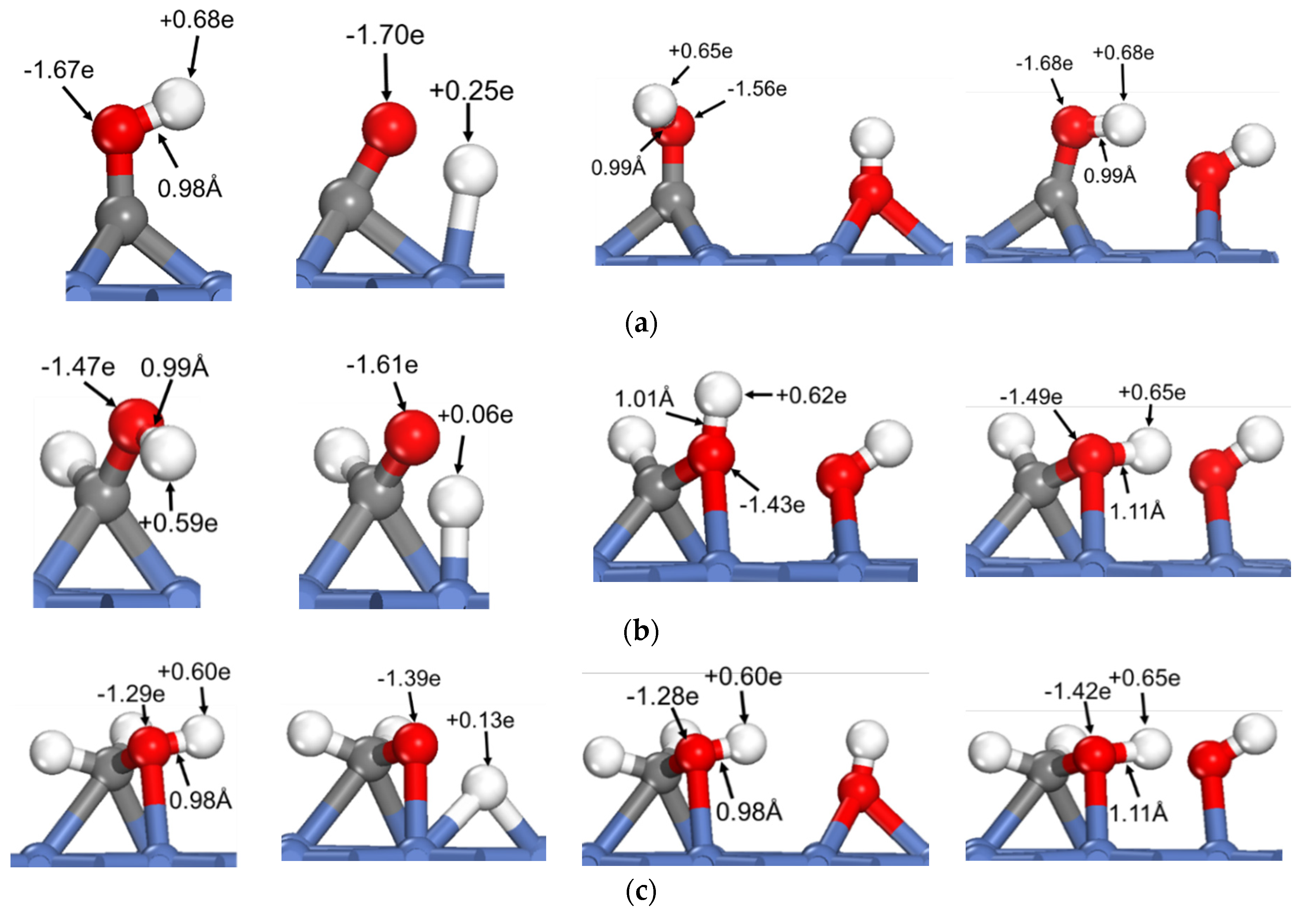
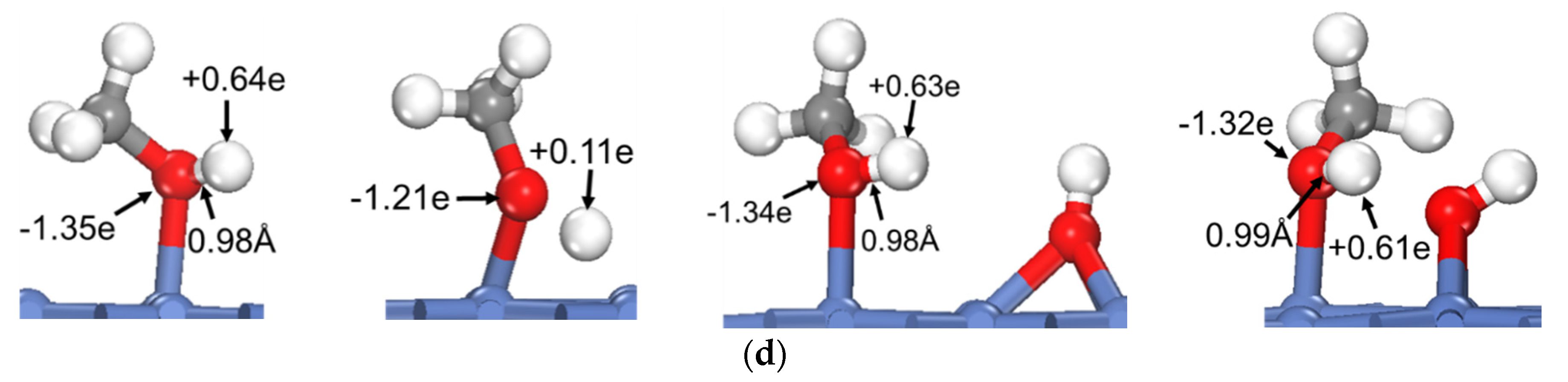
3.2. Dehydrogenation of OH* Assisted by H-Bond of O–H⋯OH and O–H⋯N
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Herdem, M.S.; Sinaki, M.Y.; Farhad, S.; Hamdullahpur, F. An overview of the methanol reforming process: Comparison of fuels, catalysts, reformers, and systems. Int. J. Energy Res. 2019, 43, 5076–5105. [Google Scholar] [CrossRef]
- Khzouz, M.; Gkanas, E.I.; Du, S.; Wood, J. Catalytic performance of Ni-Cu/Al2O3 for effective syngas production by methanol steam reforming. Fuel 2018, 232, 672–683. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Paxinou, A.; Słowik, G.; Neophytides, S.; Avgouropoulos, G. Steam reforming of methanol over nanostructured Pt/TiO2 and Pt/CeO2 catalysts for fuel cell applications. Catalysts 2018, 8, 544. [Google Scholar] [CrossRef]
- Trimm, D.L.; Önsan, Z.I. Onboard fuel conversion for hydrogen-fuel-cell-driven vehicles. Catal. Rev. 2001, 43, 31–84. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, S.; Johnston-Peck, A.; Xu, W.; Rodriguez, J.A.; Senanayake, S.D. Methanol steam reforming over Ni-CeO2 model and powder catalysts: Pathways to high stability and selectivity for H2/CO2 production. Catal. Today 2018, 311, 74–80. [Google Scholar] [CrossRef]
- Lu, J.; Li, X.; He, S.; Han, C.; Wan, G.; Lei, Y.; Chen, R.; Liu, P.; Chen, K.; Zhang, L.; et al. Hydrogen production via methanol steam reforming over Ni-based catalysts: Influences of Lanthanum (La) addition and supports. Int. J. Hydrogen Energy 2017, 42, 3647–3657. [Google Scholar] [CrossRef]
- Kaftan, A.; Kusche, M.; Laurin, M.; Wasserscheid, P.; Libuda, J. KOH-promoted Pt/Al2O3 catalysts for water gas shift and methanol steam reforming: An operando DRIFTS-MS study. Appl. Catal. B 2017, 201, 169–181. [Google Scholar] [CrossRef]
- Lin, S.; Xie, D.; Guo, H. Methyl formate pathway in methanol steam reforming on copper: Density functional calculations. ACS Catalysis 2011, 1, 1263–1271. [Google Scholar] [CrossRef]
- Zuo, Z.-J.; Wang, L.; Han, P.-D.; Huang, W. Insights into the reaction mechanisms of methanol decomposition, methanol oxidation and steam reforming of methanol on Cu(111): A density functional theory study. Int. J. Hydrogen Energy 2014, 39, 1664–1679. [Google Scholar] [CrossRef]
- Wang, S.-S.; Su, H.-Y.; Gu, X.-K.; Li, W.-X. Differentiating intrinsic reactivity of Copper, Copper–Zinc Alloy, and Copper/Zinc Oxide interface for methanol steam reforming by first-principles theory. J. Phys. Chem. C 2017, 121, 21553–21559. [Google Scholar] [CrossRef]
- Wang, S.-S.; Gu, X.-K.; Su, H.-Y.; Li, W.-X. First-principles and microkinetic simulation studies of the structure sensitivity of Cu catalyst for methanol steam reforming. J. Phys. Chem. C 2018, 122, 10811–10819. [Google Scholar] [CrossRef]
- Smith, G.K.; Lin, S.; Lai, W.; Datye, A.; Xie, D.; Guo, H. Initial steps in methanol steam reforming on PdZn and ZnO surfaces: Density functional theory studies. Surf. Sci. 2011, 605, 750–759. [Google Scholar] [CrossRef]
- Krajčí, M.; Tsai, A.P.; Hafner, J. Understanding the selectivity of methanol steam reforming on the (1 1 1) surfaces of NiZn, PdZn and PtZn: Insights from DFT. J. Catal. 2015, 330, 6–18. [Google Scholar] [CrossRef]
- Chen, W.; Cubuk, E.D.; Montemore, M.M.; Reece, C.; Madix, R.J.; Friend, C.M.; Kaxiras, E. A comparative ab initio study of anhydrous dehydrogenation of linear-chain alcohols on Cu(110). J. Phys. Chem. C 2018, 122, 7806–7815. [Google Scholar] [CrossRef]
- Lausche, A.C.; Abild-Pedersen, F.; Madix, R.J.; Nørskov, J.K.; Studt, F. Analysis of sulfur-induced selectivity changes for anhydrous methanol dehydrogenation on Ni(100) surfaces. Surf. Sci. 2013, 613, 58–62. [Google Scholar] [CrossRef]
- Kramer, Z.C.; Gu, X.-K.; Zhou, D.D.Y.; Li, W.-X.; Skodje, R.T. Following molecules through reactive networks: Surface catalyzed decomposition of methanol on Pd(111), Pt(111), and Ni(111). J. Phys. Chem. C 2014, 118, 12364–12383. [Google Scholar] [CrossRef]
- Du, P.; Wu, P.; Cai, C. Mechanism of methanol decomposition on the Pt3Ni(111) surface: DFT study. J. Phys. Chem. C 2017, 121, 9348–9360. [Google Scholar] [CrossRef]
- Liao, T.-W.; Yadav, A.; Ferrari, P.; Niu, Y.; Wei, X.-K.; Vernieres, J.; Hu, K.-J.; Heggen, M.; Dunin-Borkowski, R.E.; Palmer, R.E.; et al. Composition-tuned Pt-skinned PtNi bimetallic clusters as highly efficient methanol dehydrogenation catalysts. Chem. Mater. 2019, 31, 10040–10048. [Google Scholar] [CrossRef]
- Luo, W.; Asthagiri, A. Density functional theory study of methanol steam reforming on Co(0001) and Co(111) surfaces. J. Phys. Chem. C 2014, 118, 15274–15285. [Google Scholar] [CrossRef]
- Kim, D.K.; Iglesia, E. Isotopic and kinetic assessment of the mechanism of CH3OH-H2O catalysis on supported Copper clusters. J. Phys. Chem. C 2008, 112, 17235–17243. [Google Scholar] [CrossRef]
- Lee, J.K.; Ko, J.B.; Kim, D.H. Methanol steam reforming over Cu/ZnO/Al2O3 catalyst: Kinetics and effectiveness factor. Appl. Catal., A 2004, 278, 25–35. [Google Scholar] [CrossRef]
- Blaylock, D.W.; Zhu, Y.-A.; Green, W.H. Computational investigation of the thermochemistry and kinetics of steam methane reforming over a multi-faceted nickel catalyst. Top. Catal. 2011, 828–844. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Klimes, J.; Bowler, D.R.; Michaelides, A. Chemical accuracy for the van der Waals density functional. J. Phys. Condens. Matter 2010, 22, 022201. [Google Scholar] [CrossRef]
- Klimes, J.; Bowler, D.R.; Michaelides, A. Van der Waals density functionals applied to solids. Phys. Rev. B 2011, 83. [Google Scholar] [CrossRef]
- Carrasco, J.; Klimes, J.; Michaelides, A. The role of van der Waals forces in water adsorption on metals. J. Chem. Phys. 2013, 138, 024708. [Google Scholar] [CrossRef] [PubMed]
- Press, W.H.; Flannery, B.P.; Teukolsky, S.A.; Vetterling, W.T. Numerical Recipes: The Art of Scientific Computing; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar]
- Henkelman, G.; Jonsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef]
- Henkelman, G.; Jonsson, H. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J. Chem. Phys. 1999, 111, 7010–7022. [Google Scholar] [CrossRef]
- Sanville, E.; Kenny, S.D.; Smith, R.; Henkelman, G. Improved grid-based algorithm for Bader charge allocation. J. Comput. Chem. 2007, 28, 899–908. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef]
- Wang, G.C.; Zhou, Y.H.; Morikawa, Y.; Nakamura, J.; Cai, Z.S.; Zhao, X.Z. Kinetic mechanism of methanol decomposition on Ni(111) surface: A theoretical study. J. Phys. Chem. B 2005, 109, 12431–12442. [Google Scholar] [CrossRef]
- Zhou, Y.-H.; Lv, P.-H.; Wang, G.-C. DFT studies of methanol decomposition on Ni(100) surface: Compared with Ni(111) surface. J. Mol. Catal. A Chem. 2006, 258, 203–215. [Google Scholar] [CrossRef]
- Greeley, J.; Mavrikakis, M. Methanol decomposition on Cu(111): A DFT study. J. Catal. 2002, 208, 291–300. [Google Scholar] [CrossRef]
- Huang, Y.; He, X.; Chen, Z.X. Density functional study of methanol decomposition on clean and O or OH adsorbed PdZn(111). J. Chem. Phys. 2013, 138, 184701. [Google Scholar] [CrossRef]
- Ke, C.; Lin, Z. Density functional theory based micro- and macro-kinetic studies of Ni-catalyzed methanol steam reforming. Catalysts 2020, 10, 349. [Google Scholar] [CrossRef]
- Ke, C.; Lin, Z. Elementary reaction pathway study and a deduced macrokinetic model for the unified understanding of Ni-catalyzed steam methane reforming. React. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Che, F.L.; Gray, J.T.; Ha, S.; McEwen, J.S. Catalytic water dehydrogenation and formation on nickel: Dual path mechanism in high electric fields. J. Catal. 2015, 332, 187–200. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, C.; Lin, Z. Catalytic Effect of Hydrogen Bond on Oxhydryl Dehydrogenation in Methanol Steam Reforming on Ni(111). Molecules 2020, 25, 1531. https://doi.org/10.3390/molecules25071531
Ke C, Lin Z. Catalytic Effect of Hydrogen Bond on Oxhydryl Dehydrogenation in Methanol Steam Reforming on Ni(111). Molecules. 2020; 25(7):1531. https://doi.org/10.3390/molecules25071531
Chicago/Turabian StyleKe, Changming, and Zijing Lin. 2020. "Catalytic Effect of Hydrogen Bond on Oxhydryl Dehydrogenation in Methanol Steam Reforming on Ni(111)" Molecules 25, no. 7: 1531. https://doi.org/10.3390/molecules25071531
APA StyleKe, C., & Lin, Z. (2020). Catalytic Effect of Hydrogen Bond on Oxhydryl Dehydrogenation in Methanol Steam Reforming on Ni(111). Molecules, 25(7), 1531. https://doi.org/10.3390/molecules25071531





